Abstract
The modulation of the activity of DNA methyltransferases (DNMTs) represents a crucial epigenetic mechanism affecting gene expressions or DNA repair mechanisms in the cells. Aberrant modifications in the function of DNMTs are a fundamental event and part of the pathogenesis of human cancer. Phytochemicals, which are biosynthesized in plants in the form of secondary metabolites, represent an important source of biomolecules with pleiotropic effects and thus provide a wide range of possible clinical applications. It is well documented that phytochemicals demonstrate significant anticancer properties, and in this regard, rapid development within preclinical research is encouraging. Phytochemicals affect several epigenetic molecular mechanisms, including DNA methylation patterns such as the hypermethylation of tumor-suppressor genes and the global hypomethylation of oncogenes, that are specific cellular signs of cancer development and progression. This review will focus on the latest achievements in using plant-derived compounds and plant-based diets targeting epigenetic regulators and modulators of gene transcription in preclinical and clinical research in order to generate novel anticancer drugs as sensitizers for conventional therapy or compounds suitable for the chemoprevention clinical setting in at-risk individuals. In conclusion, indisputable anticancer activities of dietary phytochemicals linked with proper regulation of DNA methylation status have been described. However, precisely designed and well-controlled clinical studies are needed to confirm their beneficial epigenetic effects after long-term consumption in humans.
1. Introduction
DNA methylation is a common mechanism of gene silencing through transcriptional repression. Global DNA methylation is a characteristic event in the cell strongly linked with certain processes, such as X-chromosome inactivation, the repression of repeated elements, or genomic imprinting [1]. The initiation, promotion, and progression of carcinogenesis are regulated by both genetic and epigenetic events [2]. In the last decade, epigenetic modulations have been increasingly recognized as important targets of research in oncology. Epigenetic alterations, including DNA methylation status, chemical modifications of histones, or RNA mechanisms (e.g., microRNAs and long noncoding RNAs), are responsible for the control of gene expression by regulating gene transcription [3]. Most DNA methylation, catalyzed by DNA methyltransferases (DNMTs), represents essential physiological steps that ensure both cellular and tissue homeostasis, playing an irreplaceable role in numerous key functions of cells [4,5]. However, aberrant DNA methylation also contributes to pathologic processes in organisms, and DNA methylation is one of the frequent and key epigenetic mechanisms that regulates processes associated with neoplastic transformation in eukaryotic cells, i.e., changes in apoptosis, proliferation, cell cycles, differentiation, and invasiveness, so it is therefore important in the development of cancer [6]. Based on changes in these processes, cancer cells can gain substantial resistance to anticancer drugs or chemopreventive agents, and thus resistance to multiple drugs may develop in cancer patients, or it could initiate a neoplastic transformation in healthy individuals [7,8].
Importantly, the dysregulation of DNA methylation patterns is a frequently recognized cellular event during both the initiation and late stages of oncogenesis. To date, numerous research groups have established a crucial role of both hypermethylation of tumor suppressor genes and global hypomethylation of oncogenes in cancer development and progression. Both processes are clinically valid, diagnostic, and prognostic markers in cancer disease [9,10]. Furthermore, there is evidence suggesting that cancer-associated DNA hypomethylation could increase the instability of the genome via the activation of retrotransposons of otherwise silent genomic regions [11,12]. On the other hand, hypermethylation of the promoter regions of tumor-suppressor genes demonstrates its particular role in neoplastic transformation of the cell [13,14]. Hypermethylation of promoters may lead to the silencing of genes affecting important cellular signaling pathways. Some of them are considered to be hallmarks of cancer. These include aberrant cell cycle regulation, the induction of apoptosis, DNA repair machinery, or the control of other key cancer-related molecular networks. The overall impact of DNA methylation on the functioning of human organisms is multifactorial and is directed by certain endogenous and exogenous factors, such as age, gender, ethnic group, stage of cancer, environmental stress factors, lifestyle, drugs, and dietary factors. More and more research data have supported the hypothesis that specific environmental factors during critical developmental stages may influence the later risk of carcinogenesis in part via persistent reprogramming of DNA methylation patterns [15].
Bioactive phytochemicals, which are widely available and usually show minimal toxicity in an organism, have been intensively evaluated for their presumptive role as epigenetic modulators of the activity of genes and their consequent potential for use in cancer chemoprevention and/or therapy [3,16]. In this regard, “nutri-epigenetics” have gained considerable attention within oncology research, because in contrast to genetic changes, epigenetic alterations are reversible and thus potentially serve as an important tool to affect the initiation of carcinogenesis.
1.1. Source of Data
Research data published as English-language articles from the PubMed database were collected and analyzed. Relevant studies were retrieved through the use of “epigenetics or DNA methylation status or DNA hypomethylation or DNA hypermethylation or cancer or phytochemicals or plant natural substances or plant food or diet or cancer cells or animal model or clinical study” as either keywords or a medical subject heading (MeSH) term in searches of the PubMed bibliographic database. We focused primarily on recent scientific papers from the years 2014–2019.
1.2. Aim of the Study
We aimed to highlight the role of plant-derived compounds (phytochemicals) and plant-based diets in targeting changes in DNA methylation status associated with all stages of carcinogenesis, as well as its potential use in a chemoprevention clinical setting. The core of this review is the conclusion of data from preclinical and clinical research and an assessment of whether and how dietary phytochemicals modulate aberrant DNA methylation processes in cancer cells.
2. The Role of DNA Methylation Patterns as Potential Diagnostic/Prognostic Markers of Carcinogenesis
All processes of promoter hypermethylation/hypomethylation or global hypomethylation [17] interfere with so-called “methylation patterns”. These patterns, in contrast to normal tissue, change during tumorigenesis, making it a basis for whole-genome methylation studies. Using numerous molecular approaches, numerous methylation biomarkers have been discovered that have the potential to predict specific diagnoses, prognoses, therapeutic targets, as well as therapeutic responses [18].
The list of potential biomarkers, based on changes in DNA methylation, is long [19]. Several genes have been found aberrantly methylated in several types of tumors, and most of them could potentially serve as important prognostic or diagnostic biomarkers. However, besides for the MGMT gene, available studies are still experimental. Hypermethylation of one of the first epigenetic biomarkers that should be implemented in the clinic, the GSTP gene, may be used for the early diagnosis of prostate cancer. In combination with the detection of DNA methylation status in other tumor suppressor genes (TSGs) (i.e., AOX1, APC, EDNRB), the sensitivity of a prostate cancer diagnosis substantially increases [19,20]. Studies using DNA methylation as a prognostic biomarker have identified several aggressive tumors with an increased risk of rapid progression and/or relapse (RASSF1 in breast, prostate, squamous cervical cancer; CDH1 in squamous cervical cancer; APC in breast cancer; CDH1 in squamous cervical cancer; CDKN2A in colorectal cancer) [19,21,22]. DNA methylation in certain genes could also represent an excellent biomarker for predicting the response to a treatment. The hypermethylation of the MGMT repair gene in glioblastomas causes the gene to silence, which reduces its repair activity and the removal of alkyl groups, thus predicting a response to the chemotherapeutic agent [19,23]. However, the altered methylation profile of many TSG promoter regions appears to have multiple functions as methylation markers: For example, the hypermethylation of the BRCA1 repair gene should serve as (i) a diagnostic marker where a methylation signature is proposed to predict a sporadic risk of developing breast cancer in BRCA1 mutation carriers; (ii) a prognostic marker that stratifies and predicts death in advanced diagnosis; (iii) a therapeutic marker that is also suggested to determine the sensitivity of breast and ovarian cancer to a cross-linking agent [19,20]. LINE-1 is often considered a surrogate pattern for global DNA methylation, and its overexpression caused by promoter hypomethylation leads to a less favorable prognosis in non-smallcell lung carcinoma and gastrointestinal stromal tumors [22,24].
In terms of progression and metastatic spreading, an aberrant methylation plays a key role in predicting recurrence and potential therapeutic strategies. There has been evidence in experimental studies that hypermethylation of the Bin1 gene (also reduced expression identified in breast cancer tissues) is associated with poor prognosis in esophageal squamous cell carcinoma (ESCC) patients [25]. Reduced expression of the Bin1 gene is associated with metastasis (MTS) in lymph nodes, resistance to apoptosis, and an epithelial-to-mesenchymal transition (EMT) of ESCC cells in vitro and in vivo. Using demethylation agents, Bin1 gene expression was restored, thereby inhibiting EMT and MTS diffusion through the suppression of the PTEN/AKT signaling pathway, paving the way for use as a novel therapeutic tool [26]. Experimental analyses of breast cancer-specific biomarkers revealed the aberrant methylation status of seven genes (BRCA1, DAPK1, MSH2, CDKN2A, PGR, PRKCDBP, RANKL) that have an eminent effect on MTS spreading, making them perspective prognostic markers for breast cancer relapse risk [27].
The elimination of DNA repair genes by hypermethylation results in the accumulation of DNA damage and thus promotes cancer formation. The hypermethylation of DNA repair genes appears to be specific, compared to the aberrant methylation of other TSGs, in that it not only incites carcinogenesis, but also affects the susceptibility of tumors to chemotherapy based on DNA-damaging agents destroying tumor cells [28]. The epigenetic inactivation of DNA repair genes in tumors was demonstrated in several DNA repair pathways: nucleotide excision repair (NER), mismatch repair pathways (MMRs), and homologous recombination (HR) [29]. The methylation of DNA repair genes such as MGMT (mentioned above), BRCA1, and ERCC1 has raised the therapeutic responsiveness of various cancer types within experimental studies, giving potential therapeutic benefits to patients [30]. Moreover, the epigenetic shutdown of the MMR MLH1 gene function is associated with increased tumor resistance to chemotherapeutic agents [28,31]. These experimental data are promising for the development of individual cancer treatment strategies depending on the methylation status of a patient’s DNA repair genes.
3. Regulatory Mechanisms Controlling DNA Methyltransferase Activity
As mentioned previously, methylation is catalyzed by enzymes from the DNMT family, including DNMT1, DNMT3a, and DNMT3b. DNMT3a and DNMT3b mediate de novo methylation, which is essential for genome regulation and development [32]. DNMT1 represents a dominant enzyme with a role in the maintenance of methylation patterns in newly synthesized DNA strands during replication of the genetic material in the cell [33]. Alterations in the enzymatic activity of methyltransferases are responsible for the transcriptional silencing of multiple genes [34]. Based on the crucial role of DNMT1 in the maintenance of the methylation status of DNA and subsequent regulation of gene expression, there are various control mechanisms associated with regulation and stability of the enzyme, including intrinsic and extrinsic factors that directly or indirectly affect DNMT bioactivity. Intrinsic factors controlling the activity of DNMTs represent a cascade at transcriptional as well as at post-transcriptional regulation. Transcriptional regulation of DNMTs consists of signaling pathway cascades, including RAS-AP-1, PI3/PKB, pRb/E2F, or P53/SP1 [35,36,37]. Their abnormal induction leads to an increased level of DNMTs and is associated with cancer development. Other mechanisms contributing to methyltransferase activity and stability are related to post-transcriptional auto-inhibitory regulation via the Replication Focus Targeting Sequence (RFTS) domain, which is crucial for the targeting of DNMT1 into replication loci, and the CXXC domain, which is responsible for binding to an unmethylated CpG site. Both domains are localized to a regulatory N-terminal region, and their interactions with the catalytic C-terminal region are important in the modulation of DNMT activity. Additionally, the N-terminal domain has a vital function as a mediator of interactions with DNA, proteins, or substrates [38,39]. Extrinsic factors participate in the modulation of stability and activity of DNMTs through interactions with the outer environment, representing various regulatory molecules that affect the regulation of enzyme activity at a post-translation level [40]. For instance, the regulation of DNMTs at a post-translation level is available allosterically via an interaction with molecular partners, such as PCNA, UHRF1, or USP7 [41,42]. Moreover, extrinsic factors, including nutritional elements and other bioactive molecules, have to be considered in the modulation of DNMT activity globally or in specific CpG sites.
The Effects of Phytochemicals on DNA Methylation Patterns
Throughout history, plant-derived food was widely used in the prevention or treatment of various diseases [43,44,45]. The association between cancer and aberrant epigenetic modifications together with subsequent gene defects [46] began to be of interest in cancer research, especially at the end of the 20th century [46,47,48]. Considerable progress in the field of epigenetic has been connected to the development of various methods available to determine the methylation profiles of DNA [49]. Therefore, the study of epigenetic alterations, including the evaluation of the methylation status of genes, became one of the major areas of cancer research [50], with their potential reversibility making them promising targets for cancer preventive or therapeutic strategies [46]. Accordingly, evaluations of phytochemicals affecting cancer-related epigenetic modifications in the first years of the 21st century led to the demonstration of the protective efficacy of genistein [51], trans-resveratrol [52], or tea-derived phenolic epigallocatechin-3-gallate (EGCG) [53] against aberrations in DNA methylation in vitro or in vivo. However, the rapid progression concerning the beneficial effects of phytochemicals in epigenetic-associated changes implied in the carcinogenesis has gained increased interest in the last decade.
Recent evidence has suggested a linkage between diet and methyltransferase activity [39]. There are three dominant options associated with nutrients and the methylation of DNA, including provision substrates important for methylation, alterations in enzymatic activity contributing to a one-carbon cycle, and the provision of cofactors modulating DNMT activity [54]. The modulation of the activity of methyltransferases by dietary intervention can be mediated via regulation of S-adenosylmethionine (SAM) synthesis. SAM represents the key donor of a methyl group and is a crucial substrate in methylation. Importantly, SAM is synthesized in a methionine cycle, in which numerous enzymes and essential nutrients present in food, including folate, methionine, choline, betaine, and vitamins, participate. These nutrients act as precursors in the methionine cycle and directly contribute to the generation of SAM. Therefore, a deficiency of the above-mentioned nutrients is associated with a dysregulation of the SAM level, leading to a lack of substrates for DNMT activity, thus dramatically altering DNA methylation and resulting in global hypomethylation [55]. Our knowledge about the role of nutrients acting as methyl donors comes from animal studies, in which the intake of a low-methyl donor diet was significantly associated with global hypomethylation and vice versa [56,57]. Accordingly, methyl donors from the diet are associated with the alteration of methylation status, but there are many uncertainties about doses and the duration of dietary exposure resulting in epigenetic changes of DNA [58]. As described previously, DNMTs require SAM as a cofactor. However, the activity of SAM may be modulated by dietary intervention, leading to the modulation of its concentration in the cell. Moreover, the activity and stability of methyltransferases is inhibited directly and competitively through interaction with natural compounds, including EGCG, a phenolic from green tea, genistein from soybean, or myricetin as the representative of dietary flavonoids [59,60,61]. A current study indicated that DNMT action could be disrupted by EGCG in human colon cancer cells via its binding to the active pocket of the enzyme [60]. Moreover, dietary isoflavone genistein was proposed as an antagonist of DNMT1 in MCF7 and MDA-MB-231 breast cancer cell lines [62]. Furthermore, myricetin (with a pyrogallol moiety structurally similar to a gallic moiety of EGCG) significantly inhibited human DNMT1 and prokaryotic SssI DNMT1 [39]. Importantly, cross-talk between nutrients and methylation status may lead to modifications of one-carbon metabolism through the regulation of enzymes participating in a methionine cycle. One-carbon metabolism represents a complex of biochemical processes with unique enzymes and corresponding coenzymes leading to the formation or utilization of methyl groups. The importance of the cycle lays in its ability to produce SAM from methionine and ATP [63,64]. One-carbon metabolism comprises methionine and folate cycles, which are tightly interconnected. Vitamins, such as B2, B6, and B12, play significant role during the enzymatic cascade of the one-carbon metabolic pathways. Interestingly, these cofactors regulate enzymes from the folate cycle that are associated with SAM bioavailability. Therefore, the above-mentioned cofactors of the folate cycle act as catalyzers of enzymatic activity, leading to a conversion of homocysteine (Hcy) into methionine, which is further converted via methionine adenosyl-transferase (MAT) into SAM. Subsequently, SAM is converted via glycine N-methyltransferase (GNMT) into S-adenosyl-homocysteine (SAH) [55]. In summary, numerous studies have focused on the cross-connection between diet and patterns of DNA methylation via one-carbon metabolism, donors of methyl groups from the diet, or the enzymatic activity of DNMTs. Importantly, all previously described regulatory mechanisms can operate synergistically in time, indicating the complexity and robustness of nutritional epigenomics processes. Figure 1 summarizes the mechanisms and effects of phytochemicals on DNA methylation status in the cell.
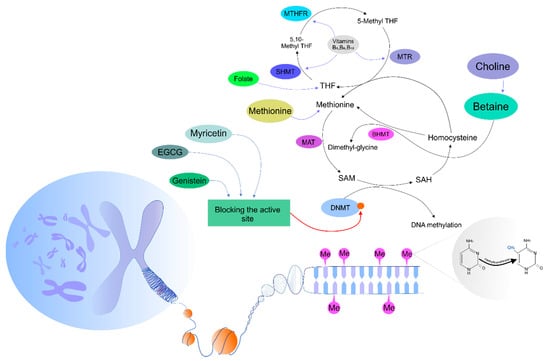
Figure 1.
Mechanisms of phytochemicals affecting DNA methylation processes in the cell. DNMTs, DNA methyltransferases; SAM, S-adenosylmethionine; SAH, S-adenosyl-L-homocysteine; MAT, methionine adenosyltransferase; BHMT, betaine-homocysteine methyltransferase; THF, tetrahydrofolate; MTR, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; SHMT, serine hydroxymethyltransferase.
4. Preclinical Cancer Research
Epigenetic therapy is a novel clinical approach focused on the modulation of aberrant epigenetic changes, presumably at cancer-related genes, by chemicals [65,66]. Regarding the clinical concept of continually developing and enhancing the effectiveness of anticancer therapy and prevention, the use of phytochemicals with significant epigenetic properties has gained considerable interest over the last decades. Current findings from preclinical oncology research using cell lines and/or animal models have contributed to new knowledge in the field of plant-derived compounds as potential epigenetic agents with substantial effects on DNA methylation status to protect various cell types against neoplastic transformation.
4.1. In Vitro Studies
Several recent preclinical studies using cancer cell lines have demonstrated the modulation of DNA methylation patterns after treatment with phytochemicals. DNA methyltransferase (DNMT) activity was greatly reduced in MDA-MB-231 and MCF-7 cells after treatment with proanthocyanidins from grape seeds in combination with trans-resveratrol [67]. In addition, this combination of phytochemicals synergistically decreased cell viability and post-treatment cell proliferation in both cell lines and induced apoptosis in MDA-MB-231 cells with a noticeable increase in the ratio of Bax/Bcl-2 expression. In another study using the HT29 cell line, indicaxanthin, a plant pigment present in beets, showed antiproliferative activity and induced demethylation in the promoters of certain methylation-silenced tumor-suppressor genes (i.e., p16INK4a, GATA4, and ESR1) that are involved in colorectal carcinogenesis [68]. Indicaxanthin also upregulated the expression of genes involved in DNA demethylation: Moreover, in silico molecular modeling revealed stable binding of this phyto-substance at the DNMT1 catalytic site. Jiang et al. [69] documented that glabridin, a phytochemical from the root of Glycyrrhiza glabra, enhanced the expression of miR-148a through DNA demethylation in MDA-MB-231 and Hs-578T breast cancer cell lines. Consequently, miR-148a blocked the expression/activation of SMAD2 (via targeting the SMAD2-3′-UTR) and thus restored epithelial characteristics, adhesive abilities, and cancer stem cell-like properties. Wong et al. [70] aimed to evaluate the genome-wide effects of sulforaphane (SFN) and 3,3′-diindolylmethane (DIM) on DNMT1, DNMT3A, and DNMT3B activity and promoter methylation status in normal prostate epithelial cells and LnCAP and PC3 prostate cancer cell lines. For all three prostate cell lines, the authors revealed widespread changes in promoter methylation patterns in response to SFN or DIM interventions. Importantly, SFN and DIM reversed many of the cancer-associated methylation alterations, including aberrantly methylated genes that are dysregulated or are highly involved in cancer progression, such as C–C chemokine receptor type 4 (CCR4), transforming growth factor-β1 receptor type I (TGFBR1), cysteine-rich angiogenic inducer 61 (CYR61), and C-X-C chemokine receptor type 4 (CXCR4). Suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor used in the treatment of cutaneous T-cell lymphoma, was administered in combination with EGCG, a DNA methyltransferase (DNMT) inhibitor, against MDA-MB-231 and MDA-MB-157 (triple-negative) breast cancer cell lines. The combined therapy decreased the activity of DNMTs. In this regard, the combination of compounds positively affected the expression of oncogenic miR-221/222, tumor suppressors p27 and PTEN, and ERα. An increased ratio in E-cadherin/N-cadherin gene expression indicated a more epithelial phenotype. These data pointed to the reduced migration of triple-negative breast cancer cells [71]. DNMT1, as the major enzyme responsible for the maintenance of the DNA methylation pattern, can potentially be used as an anticancer target. In this regard, solamargine, a poisonous chemical compound occurring in plants of the Solanaceae family, has been described as regulating EP4 downstream c-Jun via ERK1/2-mediated attenuation of DNMT1 activity in human nonsmall-cell lung cancer in vitro [72].
There are some reports that have demonstrated an increased sensitivity of cancer to conventional therapy after the administration of phytochemicals. Szarc Vel Szic et al. [73] showed that withaferin A (WA) downregulated HER2/PR/ESR-dependent gene expression interactions and thus repressed aggressive triple-negative MDA-MB-231 breast cancer cells with a specific DNA hypomethylation profile. In contrast to the DNA demethylating agent 5-aza-2′-deoxycytidine, WA treatment of MDA-MB-231 cells affected an epigenetic signaling network through gene-specific DNA hypermethylation of tumor oncogenes. This included a urokinase-type plasminogen activator, ADAM metallopeptidase domain 8, tumor necrosis factor (ligand) superfamily member 12, and genes related to mitochondrial metabolism (malic enzyme 3, ME3) and enzymes of cell detoxification, such as glutathione S-transferase mu 1. Based on this, WA may represent an attractive natural compound for the treatment of triple-negative breast cancer (TNBC), with the potential to improve therapeutic sensitivity to conventional drugs via epigenetic mechanisms. Another study revealed that physiologic concentrations of dietary phytochemicals, such as curcumin, DIM, EGCG, genistein, or indole-3-carbinol (I3C), via changes in DNA methylation, altered the gene expression of cadherin-11, p21Cip1, urokinase-type plasminogen activator, and interleukin-6 and consequently reduced the growth and apoptosis of MDA-MB-231 cancer cells [74]. The authors concluded that future research focused on the modulation of specific gene expression and cellular pathways by dietary agents is necessary and might offer a clinical tool to enhance the sensitivity of cancer cells to anticancer drugs. Li et al. [75] aimed to evaluate in vitro and in vivo epigenetic effects of genistein on ERα reactivation in MDA-MB-231 breast cancer cells. Moreover, the authors evaluated the expression of DNMT1 and HDAC1 as the most important epigenetic enzymes accompanied with the expression of changes of ERα. Changes in enzymatic activities of DNMTs in vitro and in vivo after genistein treatment pointed to the fact that DNA methylation may modify ERα expression via the regulation of DNMT-involved transcription. These data suggest that DNA methylation plays a role in genistein-induced ERα reactivation in ER-negative BC cells and thus induces the sensitivity of BC cells to endocrine therapy, e.g., with tamoxifen [75]. All above-mentioned results are summarized in Table 1.

Table 1.
DNA methylation patterns in cancer cell lines after treatment with plant natural compounds. SAHA: Suberoylanilide hydroxamic acid.
4.2. In Vivo Studies
The importance of naturally occurring plant-derived compounds in the modulation of methylation patterns has been supported by numerous studies using animal models. Dietary phenolics such as curcumin, which is a major compound of turmeric, are associated with beneficial impacts on health via antioxidant and anti-inflammatory features [76]. There have been several studies focusing on the role of curcumin in epigenetic regulations, including methylation. Du et al. [77] evaluated the effect of curcumin (peritoneal administration as solution in dose 100 mg/kg) on the reactivation of the tumor-suppressor gene RASSF1 in female athymic nu/nu mice in experimental mammary carcinogenesis using inoculated MCF-7 cells. Curcumin decreased tumor size, which correlated with specific CpG site hypomethylation of the RASSF1 promoter [77]. Similarly, curcumin significantly decreased the methylation status of the RARβ promoter region, leading to gene reactivation in female BALB/c nude mice in a lung cancer experimental model [78]. Moreover, in an experimental model of acute myeloid leukemia in female athymic nu/nu mice, a decreased expression of DNMT1 was found after treatment with curcumin [79]. Furthermore, an effect of curcumin associated with DNA methylation was detected in prostate tumorigenesis in TRAMP mice. Curcumin intervention decreased the methylation status of the Nrf2 promoter region [80]. Genistein possesses numerous biological activities, including antioxidant, anti-inflammatory, and anticipative features. Importantly, there has been evidence suggesting a role of genistein in the modulation of DNA methylation patterns [81]. Genistein was associated with the reactivation of estrogen receptor-α (ERα) as a consequence of lowered expression of DNMT1 in a breast cancer experimental model in virgin female immunodeficiency nu/nu mice [75]. Genistein as an epigenetic modifier also decreased tumor growth and methylation status in the CDH5 promoter region via the downregulation of DNMT3 in a neuroblastoma model, thus acting as an inhibitor of DNA methyltransferase activity in vivo [82]. Trans-resveratrol is a nutritional compound associated with numerous therapeutic effects, including the regulation of cancer initiation and progression [83]. This phytochemical demonstrated significant antineoplastic properties in experimental mammary carcinogenesis via the downregulation of DNMT3 expression in ACI rats [84]. Kaempferol is a flavonoid occurring in many vegetables and fruits that demonstrates health benefits for humans [85]. The administration of kaempferol led to the downregulation of DNMT3 and the demethylation of more than 100 DNA methylation positions associated with genes in an experimental model of bladder carcinogenesis in vivo [86]. The dietary chalcone isoliquiritigenin, which is found mainly in licorice root, significantly inhibited breast cancer progression via the demethylation of the WIF1 promoter region, which correlated with the inhibition of DNMT1 in a xenograft mouse model [87]. A lot of preclinical in vivo studies have focused on the mixture of phytochemicals in plants and their impact on the regulation of methylation patterns in various malignancies. Our group demonstrated that the mixture of phytochemicals present in Thymus vulgaris L. is significantly linked with tumor-preventive effects in an animal model of chemically induced breast carcinoma. Thyme haulm was administrated in a diet, and an evaluation of the DNA methylation status of five promoters of tumor-suppressor genes (ATM, PITX2, RASSF1, PTEN, and TIMP3) showed significant decreases in methylation patterns in vivo after intervention with thyme [88]. Furthermore, our previous experiment using the same animal model focused on the epigenetic modulation of clove buds and showed a demethylation effect on the promoter of the RASSF1 tumor suppressor in mammary tumors in vivo [89]. Black raspberries (BRBs) represent a mixture of vitamins, minerals, fiber, anthocyanin, and other compounds, showing antioxidant and anti-inflammatory properties in cancer [90]. Dietary administration of BRBs led to the demethylation of promoters of tumor-suppressor genes, including WIF1, SOX17, and GKI, in precancerous colon cancer in vivo [91]. Moreover, BRBs showed a demethylation effect via the reduction of the methylation status of the Sfr4 promoter region in a rat esophageal squamous cell papilloma model. This gene plays a role as an antagonist of WNT signaling pathways involved in the development of human squamous cell carcinoma [92]. An overview of phytochemicals targeting DNA methylation in cancer animal studies is shown in Table 2.

Table 2.
Phytochemicals targeting DNA methylation in cancer animal model studies.
5. Dietary Intervention and DNA Methylation Patterns in Cancer Clinical Research
As stated above, human malignancies are characterized by aberrant changes in DNA methylation, mainly hyper- or hypomethylation. The tumor cell genome may be globally hypomethylated, or the hypermethylation can occur in numerous CpG islands associated with gene promoters [17,93]. Several clinical trials have investigated the role of phytochemicals or other micronutrients as anticancer agents, with their efficacy mediated via the modulation of changes in DNA methylation. Due to the fact that DNA methylation is catalyzed via DNMTs [93], their inhibitors are important anticancer agents. Synthetic DNMT inhibitors, such as 5-aza-2′-deoxycytidine (5Aza-C), are narrow in their activity and may cause adverse toxic events. On the contrary, dietary phytochemicals show fewer side effects, are widely available, and act as direct or indirect DNMT inhibitors [94]. Majid et al. [95] evaluated the efficacy of genistein and the DNA methyltransferase inhibitor 5Aza-C on the expression of BTG3 through analysis of promoter methylation and chromatin modifications in prostate cancer cell lines and tissue samples obtained from patients of radical prostatectomy. Importantly, the BTG3 promoter region was hypermethylated in both cell lines and tumor samples when compared to the control. Nevertheless, the administration of genistein (50 µM) led to the demethylation of the hypermethylated BT3G promoter and thus reactivated expression of the gene, although to a lesser extent than of 5Aza-C [95]. Furthermore, premenopausal women with no history of atypia, in situ, or invasive breast cancer were included in the study, which dealt with the effects of soy isoflavone and circulating genistein on estrogenic markers and methylation levels of five cancer-related genes. Subsequently, the administration of isoflavones for one menstrual cycle in healthy premenopausal women led to significant alterations in the methylation levels of RARβ2 and CCND2 in the breast tissue. Importantly, these changes were dependent on the levels of circulating genistein due to the close link between the decrease in RARβ2 methylation and a post-treatment genistein level less than 600 ng/mL and the decrease in CCND2 methylation and post-treatment genistein less than 200 ng/mL. Paradoxically, post-treatment with a greater level of genistein led to an increase in the methylation of both genes. Despite statistically significant alterations in the methylation status, the biological importance of these changes is uncertain, but at least different mechanisms of action for high and low doses of isoflavones is suggested [96]. Moreover, a dose-dependent effect of trans-resveratrol on a hypermethylated promoter was demonstrated in a study conducted on subjects with an increased risk of breast cancer. An increase in the levels of serum trans-resveratrol was significantly associated with a decrease in methylation of RASSF-1α, while these changes in methylation were related to a decrease in systematic and breast-specific prostaglandin (PGE2) expression [97].
Folates are water-soluble vitamins that function as methyl donors in a one-carbon metabolism cycle [98] and thus modulate the stability of DNA. Consequently, the regulation of DNA biosynthesis, repair, and methylation is highly dependent upon the intake of folate in the diet [99,100], while aberrant changes in folate metabolism may contribute to the development of cancer [101]. Actually, RARB, BRCA1, and RASSF1A are genes frequently methylated in breast cancer. A study analyzing an association between folate and other one-carbon-related nutrients (including vitamins B2, B6, and B12) and methionine and promoter hypermethylation and the expression of these genes was conducted on Iranian women with primary breast cancer. The results revealed that the dietary intake of folate and cobalamin was inversely associated with the methylation status of RARB and BRCA1. Therefore, a low intake of folate and cobalamin correlated with the age-dependent tendency of promoter regions of these genes to be hypermethylated in tumors. However, a high dietary intake of riboflavin and pyridoxine was found to be a determinant of the RARB methylated promoter when compared to consumers of low amounts and thus contribute to the development of breast tumors. The results of the study thus suggest the possibility of age-dependent selectivity of dietary components on hypermethylation status [102]. Colacino et al. [103] evaluated the effects of the pretreatment dietary intake of micronutrients involved in one-carbon metabolism, antioxidants, and food groups abundant in these nutrients on DNA methylation in newly diagnosed head and neck patients. The analysis considered the effect of diet on the methylation of individual CpG regions of selected genes (RARB, C4B, GML, PTHR1, MLF1, THPO, CHGA, XIST, LCN2, SRC, SFN, PTHLH, LMO2, SIN3B, TNFSF8, HGF, HOXA11) and revealed that subjects reporting in the highest quartile of folate, vitamin B12, and vitamin A intake showed decrease in promoter methylation of tumor suppressors when compared to subjects in the lowest quartile, as was also demonstrated in subjects reporting a high intake of cruciferous vegetables, which is a rich source of folate [103]. Similarly, low dietary folate led to the methylation of p16INK4A, the methylation silencing being related to functional polymorphism in methylene tetrahydrofolate reductase, a folate metabolism enzyme. The results suggest that there may be a connection between the low intake of folate, a well-known risk factor for head and neck squamous cell carcinoma (HNSCC), and the inactivation of p16INK4A in HNSCC [104]. Interestingly, there have been other studies supporting the relation between folate intake and a decrease in the methylation of tumor-suppressive genes. It is supposed that DNA methylation in colorectal cancer is also associated with physiological and environmental factors, including the involvement of one-carbon metabolism. Therefore, the level of promoter methylation was evaluated in several genes in colorectal cancer samples and healthy adjacent tissues of subjects confirmed for colorectal cancer. The results showed a link between plasma folate levels below normal and a higher level of methylation of the hMLH1 promoter region [101]. On the contrary, the contribution of dietary factors involved in one-carbon metabolism with aberrant DNA methylation was evaluated in women diagnosed with primary incident breast cancer. However, no association between the dietary intake of folate, vitamins B2, B6, and B12, and methionine and promoter methylation of E-cadherin, p16, and RAR-β2 genes was found [105].
Apart from changes in DNA methylation in gene promoters, global DNA hypomethylation is also closely linked to carcinogenesis [93,106]. Folate and vitamin B12 deficiency and global DNA hypomethylation was observed in subjects with squamous cell lung cancer when comparing samples of tumor and adjacent tissue, which appeared to be normal from the same patients [107]. Due to the fact that low folate status increases the risk of colorectal cancer, the effects of folic acid supplementation on DNA biomarkers were evaluated in colonocytes from adenomatous polyps and tissue adjacent to the former polyp site. Overall, folic acid supplementation decreased global DNA hypomethylation, increased tissue folate, and decreased the uracil misincorporation ratio when compared to a placebo, thus improving the DNA biomarkers of cancer risk [108]. Additionally, a proper dietary pattern is surely included in the notion of a lifestyle and may also influence DNA methylation patterns, as was demonstrated by Delgado-Cruzata et al. [106], who analyzed the impact of lifestyle preferences on the epigenetic indicators of blood DNA methylation level, including global DNA methylation by the LUMA assay targeting CCGC sequences throughout the genome, DNA methylation of the repetitive element LINE-1, and tandem repeat Sat2. After all, it was observed that dietary modification and weight loss generally attributed to a healthier lifestyle affected changes in global DNA methylation in Hispanic, Afro-American, and Afro-Caribbean breast cancer survivors. Specifically, DNA methylation of LINE-1 was increased when compared to baseline after 6 and 12 months. Moreover, positive associations between alterations in the diet pattern, including changes in vegetable, protein, and total caloric intake, LUMA DNA methylation, and the intake of fruit, leading to positive changes in DNA methylation of LINE-1, were also observed [106]. As the LINE-1 was hypomethylated in tumor tissues of colon cancer, the epigenetic changes in the surrounding tissue should also be investigated. Therefore, an analysis of the impact of serum vitamin D and obesity on global hypomethylation was performed in the visceral adipose tissue from patients with and without colorectal cancer. Interestingly, the DNA methylation of LINE-1 was positively associated with the level of vitamin D and negatively with BMI, and the insulin resistance index with serum vitamin D was the main variable of LINE-1 variance [109].
The dietary intake of genistein, trans-resveratrol, folate, and various vitamins contributes to changes in DNA methylation. The results of clinical trials are summarized in Table 3. Significantly, several clinical studies have shown that proper dietary intake of these nutrients led to the remodeling of aberrant changes in DNA methylation in cancer patients, healthy subjects, and subjects with increased risk of developing cancer. However, results from the overall analysis of available clinical studies are not as clear, as there are unexpected but surely interesting findings suggesting the relation between micronutrient efficacy and the dose or age of study participants. Evidence from clinical trials has shown the positive effects of dietary intervention on DNA methylation patterns. Nonetheless, we highly support clinical research being further extended so that results that are more concrete can be obtained and progress in anticancer research can be made.

Table 3.
Clinical trials describing the effects of phytochemicals on DNA methylation patterns in cancer disease.
6. Conclusions and Perspectives
To date, it has been well described that epigenetic alterations dynamically contribute to cancer pathogenesis. The preclinical and clinical evidence has demonstrated that plant-based molecules may decrease the risk of the development of certain types of neoplasia. Importantly, clinical studies have clearly indicated that the regular dietary intake of phytochemicals can lead to the remodeling of aberrant changes in DNA methylation in cancer patients and high-risk or healthy subjects. Specifically, these natural molecules can potentially show benefits due to their reverting activities in the epigenetic modulations of oncogenes and tumor-suppressor genes, as well as in their ability to modify and reinstate the expression of cellular protein levels. In addition, it is proposed that phytochemicals enhance the anticancer activity of conventional drugs used in clinical practice. Demethylating agents (such as inhibitors of DNMTs) modify gene expression by reversing the aberrant epigenetic alterations that are characteristic features of the running carcinogenesis. Numerous laboratories have documented that natural compounds or whole-plant foods sensitize tumor cells through different epigenetic targets, including oncogenes and tumor suppressor genes, as well as DNMTs and other epigenetic mechanisms, such as chemical modifications of histone molecules and changes in the expression of miRNA or long noncoding RNA. In addition, the combination of isolated phytochemicals or plant foods with chemotherapeutic agents could provide an additive or synergistic anticancer effect, enhancing the final therapeutic outcome. An understanding of the complexity of epigenetic targets and epigenetic molecular signaling pathways to elicit the efficacy of plant natural compounds and their mechanisms that are involved in tumorigenesis, especially in cancers with a poor prognosis (i.e., highly aggressive and invasive lesions that demonstrate resistance to conventional therapies), should be beneficial for clinical oncologists. The role of the epigenetic diet and its impact on the cancer epigenome is highly clinically relevant. To this end, recently published clinical data provide an excellent example on the functional link between the metabolism of homocysteine as a well-known epigenetic modulator and the etiology of highly aggressive premenopausal triple-negative breast cancer (TNBC): As a matter of fact, specifically a mild increase in total homocysteine, usually resulting from dietary habits, but not hyperhomocysteinemia as a genetic deficit, has been attributed to the TNBC pathology [110]. Consequently, the mild epigenetic modulation of tumor suppressors resulting in cancer reprogramming and persistent promotion pressure toward metastatic disease have been hypothesized. Contextually, an identification of the transformation-specific multiomic signature is essential for predictive diagnostics and targeted treatments [110,111,112,113]. In consensus, an advanced approach through predictive, preventive, and personalized medicine is considered to be the medicine of the future in overall cancer management [114].
It is necessary to establish more comprehensive studies focused on the epigenetics diet that will be able to reveal important translational information for cancer prevention and combinational therapy. Follow-up studies are essential to evaluate the safety profile of doses used, the route of administration, tissue distribution, and the bioavailability of natural plant compounds administered alone or in combination with other anticancer drugs with the aim to attain the best clinical outcomes. In addition, future research should be directed toward different clinical settings in humans, using a personalized approach to establish the potential of phytochemicals in individuals. Finally, a deeper understanding of the global patterns of epigenetic changes caused by natural plant compounds in cancer cells will lead to designing improved clinical strategies to prevent and cure cancer disease.
Author Contributions
K.J., P.K., M.S., A.L., D.V., O.B, and B.B.K. conducted a literature search and wrote the manuscript; P.K., K.S., D.B., M.A., and T.K.K. provided skilled assistance and supervised the overall preparation of the manuscript; M.S. proposed and prepared the figure; T.B. contributed to the conception of the idea, the literature search, and manuscript editing; P.Z., J.D., and D.B. revised the manuscript with critical reviews and comments.
Acknowledgments
This research was funded by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic under contract nos. VEGA 1/0136/19, 1/0124/17, the Slovak Research and Development Agency under contract no. APVV-16-0021, and also CEPV II (Center of Excellence for Perinatology II), ITMS: 26220120036, 26220120036, 26220120036, 26220120036, 26220120036, 26220120036, 262201200.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; La Vega, H.A.-D.; De La Cruz, O.N.H.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Longo, F.; Giuliano, M.; Sabbatino, F.; Favia, G.; Ionna, F.; Addeo, R.; Scarpati, G.D.V.; Di Lorenzo, G.; Pisconti, S.; et al. Epigenetic control of gene expression: Potential implications for cancer treatment. Crit. Rev. Oncol. 2017, 111, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, T. DNA Methylation Reprogramming during Mammalian Development. Genes 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Gujar, H.; Weisenberger, D.J.; Liang, G. The Roles of Human DNA Methyltransferases and Their Isoforms in Shaping the Epigenome. Genes 2019, 10, 172. [Google Scholar] [CrossRef]
- Liu, P.; Shen, J.K.; Xu, J.; Trahan, C.A.; Hornicek, F.J.; Duan, Z. Aberrant DNA methylations in chondrosarcoma. Epigenomics 2016, 8, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Liu, Y.; Lv, J.; Ding, H.; Zhang, X.A.; Shao, L.; Yang, N.; Cheng, H.; Sun, L.; Zhu, D.; et al. ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells. Oncotarget 2016, 7, 20966–20980. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.-Y.; Wang, F. Association between DNA methylation and multidrug resistance in human glioma SHG-44 cells. Mol. Med. Rep. 2015, 11, 43–52. [Google Scholar] [CrossRef][Green Version]
- Ng, J.M.-K.; Yu, J. Promoter Hypermethylation of Tumour Suppressor Genes as Potential Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 2472–2496. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, H.; Li, H.; Li, X.; Yang, S. DNA methylation as an early diagnostic marker of cancer (Review). Biomed. Rep. 2014, 2, 326–330. [Google Scholar] [CrossRef]
- Van Tongelen, A.; Loriot, A.; De Smet, C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017, 396, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenetics 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xiong, X.; Zhang, L. Promoter hypermethylation of RARβ2, DAPK, hMLH1, p14, and p15 is associated with progression of breast cancer. Medicine 2018, 97, e13666. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Yamashita, K.; Watanabe, M. Analysis of DNA Methylation Status in Bodily Fluids for Early Detection of Cancer. Int. J. Mol. Sci. 2017, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.; Issa, J.-P.J. The promise of epigenetic therapy: Reprogramming the cancer epigenome. Curr. Opin. Genet. Dev. 2017, 42, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Defining Driver DNA Methylation Changes in Human Cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef]
- Hao, X.; Luo, H.; Krawczyk, M.; Wei, W.; Wang, W.; Wang, J.; Flagg, K.; Hou, J.; Zhang, H.; Yi, S.; et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 7414–7419. [Google Scholar] [CrossRef]
- Witte, T.; Plass, C.; Gerhäuser, C. Pan-cancer patterns of DNA methylation. Genome Med. 2014, 6, 2652. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Li, J.; Liu, H.; Wang, F.; Wei, Y.; Su, J.; Zhang, D.; Liu, T.; Zhang, Y. The Identification of Specific Methylation Patterns across Different Cancers. PLoS ONE 2015, 10, e0120361. [Google Scholar] [CrossRef]
- Holubeková, V.; Mendelová, A.; Grendár, M.; Meršaková, S.; Kapustová, I.; Jašek, K.; Vaňochová, A.; Danko, J.; Lasabová, Z. Methylation pattern of CDH1 promoter and its association with CDH1 gene expression in cytological cervical specimens. Oncol. Lett. 2016, 12, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Jašek, K.; Kasubova, I.; Holubekova, V.; Stanclova, A.; Plank, L.; Lasabová, Z. Epigenetics: An alternative pathway in GISTs tumorigenesis. Neoplasma 2018, 65, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, J.; Wang, W.; Wang, D. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol. Lett. 2017, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Vinciguerra, M.; Agodi, A. LINE-1 Hypomethylation in Blood and Tissue Samples as an Epigenetic Marker for Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e109478. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, H.; Wang, Y.; Wang, T.; Wang, M.; Ma, M.; Duan, Y.; Meng, X.; Liu, L. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int. J. Cancer 2015, 137, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Jia, Y.; Wang, Y.; Han, X.; Duan, Y.; Lv, W.; Ma, M.; Liu, L. Methylation decreases the Bin1 tumor suppressor in ESCC and restoration by decitabine inhibits the epithelial mesenchymal transition. Oncotarget 2017, 8, 19661–19673. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Melnikov, A.A.; Levenson, V.; Guerra, E.; Simeone, P.; Alberti, S.; Deng, Y. A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Julsing, J.R.; Peters, G.J. Methylation of DNA repair genes and the efficacy of DNA targeted anticancer treatment. Oncol. Discov. 2014, 2, 3. [Google Scholar] [CrossRef]
- Wojtczyk-Miaskowska, A.; Presler, M.; Michajlowski, J.; Matuszewski, M.; Schlichtholz, B. Gene Expression, DNA Methylation and Prognostic Significance of DNA Repair Genes in Human Bladder Cancer. Cell. Physiol. Biochem. 2017, 42, 2404–2417. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Herman, J.G.; Guo, M. The clinical value of aberrant epigenetic changes of DNA damage repair genes in human cancer. Oncotarget 2016, 7, 37331–37346. [Google Scholar] [CrossRef] [PubMed]
- Kašubová, I.; Kalman, M.; Jašek, K.; Burjanivová, T.; Malicherová, B.; Vaňochová, A.; Meršaková, S.; Lasabová, Z.; Plank, L. Stratification of patients with colorectal cancer without the recorded family history. Oncol. Lett. 2019, 17, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Daito, T.; Sasaki, Y.; Chung, Y.H.; Xing, X.; Pondugula, S.; Swamidass, S.J.; Wang, T.; Kim, A.H.; Yano, H. Inhibition of DNA Methyltransferases Blocks Mutant Huntingtin-Induced Neurotoxicity. Sci. Rep. 2016, 6, 31022. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.J.; Wee, C.P.P.; Gao, Z. DNA Methyltransferase Activity Assays: Advances and Challenges. Theranostics 2016, 6, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Heiland, D.H.; Ferrarese, R.; Claus, R.; Dai, F.; Masilamani, A.P.; Kling, E.; Weyerbrock, A.; Kling, T.; Nelander, S.; Carro, M.S. c-Jun-N-terminal phosphorylation regulates DNMT1 expression and genome wide methylation in gliomas. Oncotarget 2017, 8, 6940–6954. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-L.; Yin, Y.-R.; Xie, C.-R.; Zhang, S.; Zhao, W.-X.; Pan, C.; Wang, X.-M.; Yin, Z.-Y. Mechanistic and biological significance of DNA methyltransferase 1 upregulated by growth factors in human hepatocellular carcinoma. Int. J. Oncol. 2015, 46, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-A.; Tsai, Y.-T.; Lin, R.-K.; Hsu, H.-S.; Chen, C.-Y.; Wang, Y.-C. Deregulation of p53 and RB Transcriptional Control Leads to Overexpression of DNA Methyltransferases in Lung Cancer. J. Cancer Res. Pr. 2014, 1, 14–27. [Google Scholar] [CrossRef]
- Xie, S.; Qian, C. The Growing Complexity of UHRF1-Mediated Maintenance DNA Methylation. Genes 2018, 9, 600. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.-X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Wang, H. Long noncoding RNAs in DNA methylation: New players stepping into the old game. Cell Biosci. 2016, 6, 89. [Google Scholar] [CrossRef]
- Jeltsch, A.; Jurkowska, R.Z. Allosteric control of mammalian DNA methyltransferases—A new regulatory paradigm. Nucleic Acids Res. 2016, 44, 8556–8575. [Google Scholar] [CrossRef] [PubMed]
- Yarychkivska, O.; Tavana, O.; Gu, W.; Bestor, T.H. Independent functions of DNMT1 and USP7 at replication foci. Epigenetics Chromatin 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Bai, X.-Y.; Wang, C.-H. Traditional Chinese Medicine: A Treasured Natural Resource of Anticancer Drug Research and Development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, N.M.; Mortazavian, A.M.; Monfared, A.B.; Sohrabvandi, S. Phytochemicals in Cancer Prevention: A Review of the Evidence. Iran. J. Cancer Prev. 2017, 10, e7219. [Google Scholar]
- Shukla, S.; Meeran, S.M.; Katiyar, S.K. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014, 355, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Merlo, A.; Mao, L.; Lapidus, R.G.; Issa, J.P.; Davidson, N.E.; Sidransky, D.; Baylin, S.B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995, 55, 4525–4530. [Google Scholar] [PubMed]
- Jones, P.A. DNA methylation errors and cancer. Cancer Res. 1996, 56, 2463–2467. [Google Scholar]
- Laird, P.W.; Jaenisch, R. DNA methylation and cancer. Hum. Mol. Genet. 1994, 3, 1487–1495. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Bullock, M. DNA Methylation Analysis: Choosing the Right Method. Biology 2016, 5, 3. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef]
- Day, J.K.; Desbordes, C.; Zhuang, Y.; Newton, L.G.; Nehra, V.; Forsee, K.M.; Besch-Williford, C.; Huang, T.H.-M.; Lubahn, D.B.; Bauer, A.M.; et al. Genistein Alters Methylation Patterns in Mice. J. Nutr. 2002, 132, 2419S–2423S. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Sauter, E. Resveratrol induced DNA methylation in ER+ breast cancer. Cancer Res. 2005, 65, 647. [Google Scholar]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Llanos, A.A.; Dumitrescu, R.G.; Brasky, T.M.; Liu, Z.; Mason, J.B.; Marian, C.; Makambi, K.H.; Spear, S.L.; Kallakury, B.V.S.; Freudenheim, J.L.; et al. Relationships among folate, alcohol consumption, gene variants in one-carbon metabolism and p16INK4a methylation and expression in healthy breast tissues. Carcinogenesis 2015, 36, 60–67. [Google Scholar] [CrossRef]
- Farkas, S.A.; Befekadu, R.; Hahn-Strömberg, V.; Nilsson, T.K. DNA methylation and expression of the folate transporter genes in colorectal cancer. Tumor Boil. 2015, 36, 5581–5590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Naifeng, Z. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 2015, 7, 100. [Google Scholar] [CrossRef]
- Morris, J.; Moseley, V.R.; Cabang, A.B.; Coleman, K.; Wei, W.; Garrett-Mayer, E.; Wargovich, M.J. Reduction in promotor methylation utilizing EGCG (epigallocatechin-3-gallate) restores RXRα expression in human colon cancer cells. Oncotarget 2016, 7, 35313–35326. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F.; Roustazadeh, A.; Golestan, F. Effect of Genistein in Comparison with Trichostatin A on Reactivation of DNMTs Genes in Hepatocellular Carcinoma. J. Clin. Transl. Hepatol. 2018, 6, 141–146. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Li, J.; Hao, D.; Wang, L.; Wang, H.; Wang, Y.; Zhao, Z.; Li, P.; Deng, C.; Di, L.-J. Epigenetic targeting drugs potentiate chemotherapeutic effects in solid tumor therapy. Sci. Rep. 2017, 7, 4035. [Google Scholar] [CrossRef]
- Subramaniam, D.; Thombre, R.; Dhar, A.; Anant, S. DNA Methyltransferases: A Novel Target for Prevention and Therapy. Front. Oncol. 2014, 4, 80. [Google Scholar] [CrossRef]
- Gao, Y.; Tollefsbol, T.O. Combinational Proanthocyanidins and Resveratrol Synergistically Inhibit Human Breast Cancer Cells and Impact Epigenetic–Mediating Machinery. Int. J. Mol. Sci. 2018, 19, 2204. [Google Scholar] [CrossRef]
- Naselli, F.; Belshaw, N.J.; Gentile, C.; Livrea, M.A.; CaraDonna, F.; Tutone, M.; Tesoriere, L. Phytochemical Indicaxanthin Inhibits Colon Cancer Cell Growth and Affects the DNA Methylation Status by Influencing Epigenetically Modifying Enzyme Expression and Activity. J. Nutr. Nutr. 2015, 8, 114–127. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Mu, J.; Hu, C.; Zhou, M.; Wang, X.; Si, L.; Ning, S.; Li, Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol. Carcinog. 2016, 55, 929–940. [Google Scholar] [CrossRef]
- Wong, C.P.; Hsu, A.; Buchanan, A.; Palomera-Sanchez, Z.; Beaver, L.M.; Houseman, E.A.; Williams, D.E.; Dashwood, R.H.; Ho, E. Effects of Sulforaphane and 3,3′-Diindolylmethane on Genome-Wide Promoter Methylation in Normal Prostate Epithelial Cells and Prostate Cancer Cells. PLoS ONE 2014, 9, 86787. [Google Scholar] [CrossRef]
- Lewis, K.A.; Jordan, H.R.; Tollefsbol, T.O. Effects of SAHA and EGCG on Growth Potentiation of Triple-Negative Breast Cancer Cells. Cancers 2018, 11, 23. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Q.; Xiao, Q.; Yang, L.; Hann, S.S. Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine-inhibited growth of lung cancer cells. J. Cell. Mol. Med. 2017, 21, 222–233. [Google Scholar] [CrossRef]
- Szic, K.S.V.; Diddens, J.; Gerhäuser, C.; Declerck, K.; Crans, R.A.J.; Scherf, D.B.; Berghe, W.V. Epigenetic silencing of triple negative breast cancer hallmarks by Withaferin A. Oncotarget 2017, 8, 40434–40453. [Google Scholar]
- Moiseeva, E.P.; Almeida, G.M.; Jones, G.D.D.; Manson, M.M. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol. Cancer Ther. 2007, 6, 3071–3079. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Du, L.; Xie, Z.; Wu, L.-C.; Chiu, M.; Lin, J.; Chan, K.K.; Liu, S.; Liu, Z. Reactivation of RASSF1A in Breast Cancer Cells by Curcumin. Nutr. Cancer 2012, 64, 1228–1235. [Google Scholar] [CrossRef]
- Jiang, A.; Wang, X.; Shan, X.; Li, Y.; Wang, P.; Jiang, P.; Feng, Q. Curcumin Reactivates Silenced Tumor Suppressor Gene RARβ by Reducing DNA Methylation. Phytother. Res. 2015, 29, 1237–1245. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.-C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.; Chiu, M.; Wang, Q.-E.; et al. Curcumin Down-Regulates DNA Methyltransferase 1 and Plays an Anti-Leukemic Role in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e55934. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, Y.; Wu, T.-Y.; Shu, L.; Lee, J.H.; Kong, A.-N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef]
- Hu, P.; Ma, L.; Wang, Y.-G.; Ye, F.; Wang, C.; Zhou, W.-H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Huang, Y.; Huang, X.; Xu, L.; Lv, Z. Genistein demethylates the promoter of CHD5 and inhibits neuroblastoma growth in vivo. Int. J. Mol. Med. 2012, 30, 1081–1086. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA Effects of Resveratrol on Mammary Tumors vs. Normal Tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, A Flavonoid Compound from Gynura Medica Induced Apoptosis and Growth Inhibition in Mcf-7 Breast Cancer Cell. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Wang, Y.; Xie, X.; Shen, J.; Peng, C.; You, J.; Peng, F.; Tang, H.; Guan, X.; et al. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 2015, 6, 9854–9876. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Zubor, P. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Kruzliak, P.; Mojzis, J.; Vybohova, D.; Adamkov, M.; Jasek, K.; Lasabová, Z.; et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J. Cell. Mol. Med. 2017, 21, 2837–2851. [Google Scholar] [CrossRef]
- Kresty, L.A.; Mallery, S.R.; Stoner, G.D. Black raspberries in cancer clinical trials: Past, present and future. J. Berry Res. 2016, 6, 251–261. [Google Scholar] [CrossRef]
- Wang, L.-S.; Kuo, C.-T.; Huang, T.H.-M.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.-W. Black Raspberries Protectively Regulate Methylation of Wnt Pathway Genes in Precancerous Colon Tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Gu, F.; Dombkowski, A.; Wang, L.-S.; Stoner, G.D. Black raspberries demethylate Sfrp4, a WNT pathway antagonist, in rat esophageal squamous cell papilloma. Mol. Carcinog. 2016, 55, 1867–1875. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Bayat, S.; Shekari Khaniani, M.; Choupani, J.; Alivand, M.R.; Mansoori Derakhshan, S. HDACis (class I), cancer stem cell, and phytochemicals: Cancer therapy and prevention implications. Biomed Pharmacother. 2018, 97, 1445–1453. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer 2010, 116, 66–76. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Shi, H.; Hewett, J.E.; Ruhlen, R.L.; Macdonald, R.S.; Rottinghaus, G.E.; Chen, Y.-C.; Sauter, E.R. Soy Isoflavones Have an Antiestrogenic Effect and Alter Mammary Promoter Hypermethylation in Healthy Premenopausal Women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Liu, J.; Ward, R.L. Folate and One-Carbon Metabolism and Its Impact on Aberrant DNA Methylation in Cancer. Adv. Genet. 2010, 71, 79–121. [Google Scholar]
- Pentieva, K.; Lees-Murdock, D.J.; Walsh, C.P.; Irwin, R.E.; Cassidy, T.; McLaughlin, M.; Prasad, G.; McNulty, H. The interplay between DNA methylation, folate and neurocognitive development. Epigenomics 2016, 8, 863–879. [Google Scholar]
- Lendoiro, E.; Russell, W.; Bestwick, C.; Bermano, G.; Duthie, S. Folate and genomic stability: Differential effect of methylated and oxidised folate on DNA damage and ROS production in human colon fibroblasts. Proc. Nutr. Soc. 2018, 77, 77. [Google Scholar] [CrossRef]
- Coppedè, F.; Migheli, F.; Lopomo, A.; Failli, A.; Legitimo, A.; Consolini, R.; Fontanini, G.; Sensi, E.; Servadio, A.; Seccia, M.; et al. Gene promoter methylation in colorectal cancer and healthy adjacent mucosa specimens: Correlation with physiological and pathological characteristics, and with biomarkers of one-carbon metabolism. Epigenetics 2014, 9, 621–633. [Google Scholar] [CrossRef]
- Pirouzpanah, S.; Taleban, F.-A.; Mehdipour, P.; Atri, M. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J. Mol. Med. 2015, 93, 917–934. [Google Scholar] [CrossRef]
- Colacino, J.A.; Arthur, A.E.; Dolinoy, D.C.; Sartor, M.A.; Duffy, S.A.; Chepeha, D.B.; Bradford, C.R.; Walline, H.M.; McHugh, J.B.; D’Silva, N.J.; et al. Pretreatment dietary intake is associated with tumor suppressor DNA methylation in head and neck squamous cell carcinomas. Epigenetics 2012, 7, 883–891. [Google Scholar] [CrossRef]
- Kraunz, K.S.; Hsiung, D.; McClean, M.D.; Liu, M.; Osanyingbemi, J.; Nelson, H.H.; Kelsey, K.T. Dietary folate is associated with p16(INK4A) methylation in head and neck squamous cell carcinoma. Int. J. Cancer 2006, 119, 1553–1557. [Google Scholar] [CrossRef]
- Tao, M.-H.; Mason, J.B.; Marian, C.; McCann, S.E.; Platek, M.E.; Millen, A.; Ambrosone, C.; Edge, S.B.; Krishnan, S.S.; Trevisan, M.; et al. Promoter Methylation of E-Cadherin, p16, and RAR-β2Genes in Breast Tumors and Dietary Intake of Nutrients Important in One-Carbon Metabolism. Nutr. Cancer 2011, 63, 1143–1150. [Google Scholar] [CrossRef]
- Delgado-Cruzata, L.; Zhang, W.; McDonald, J.A.; Tsai, W.Y.; Valdovinos, C.; Falci, L.; Wang, Q.; Crew, K.D.; Santella, R.M.; Hershman, D.L.; et al. Dietary Modifications, Weight Loss, and Changes in Metabolic Markers Affect Global DNA Methylation in Hispanic, African American, and Afro-Caribbean Breast Cancer Survivors12. J. Nutr. 2015, 145, 783–790. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Johanning, G.L.; Macaluso, M.; Whiteside, M.; Oelschlager, D.K.; Heimburger, D.C.; Grizzle, W.E. Localized Folate and Vitamin B-12 Deficiency in Squamous Cell Lung Cancer Is Associated with Global DNA Hypomethylation. Nutr. Cancer 2000, 37, 99–107. [Google Scholar] [CrossRef]
- O’Reilly, S.L.; McGlynn, A.P.; McNulty, H.; Reynolds, J.; Wasson, G.R.; Molloy, A.M.; Strain, J.; Weir, D.G.; Ward, M.; McKerr, G.; et al. Folic Acid Supplementation in Postpolypectomy Patients in a Randomized Controlled Trial Increases Tissue Folate Concentrations and Reduces Aberrant DNA Biomarkers in Colonic Tissues Adjacent to the Former Polyp Site. J. Nutr. 2016, 146, 933–939. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Morcillo, S.; Crujeiras, A.B.; Sánchez-Alcoholado, L.; Clemente-Postigo, M.; Torres, E.; Tinahones, F.J.; Macias-Gonzalez, M. Association between serum 25-hydroxyvitamin D and global DNA methylation in visceral adipose tissue from colorectal cancer patients. BMC Cancer 2019, 19, 93. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Filep, N.; Yeghiazaryan, K.; Blom, H.J.; Hofmann-Apitius, M.; Kuhn, W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: Lessons for predictive, preventive and personalised medicine. Amino Acids 2018, 50, 383–395. [Google Scholar] [CrossRef]
- Fröhlich, H.; Patjoshi, S.; Yeghiazaryan, K.; Kehrer, C.; Kuhn, W.; Golubnitschaja, O. Premenopausal breast cancer: Potential clinical utility of a multi-omics based machine learning approach for patient stratification. EPMA J. 2018, 9, 175–186. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Cheng, T.; Zhan, X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017, 8, 51–60. [Google Scholar] [CrossRef]
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).