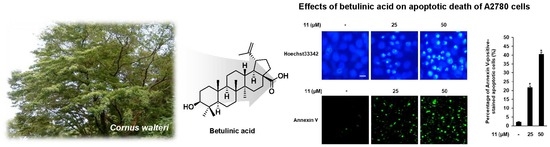
Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Cell Culture
2.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Viability Assay
2.6. Cell Staining with Hoechst 33342
2.7. Image-Based Cytometric Assay
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results and Discussions
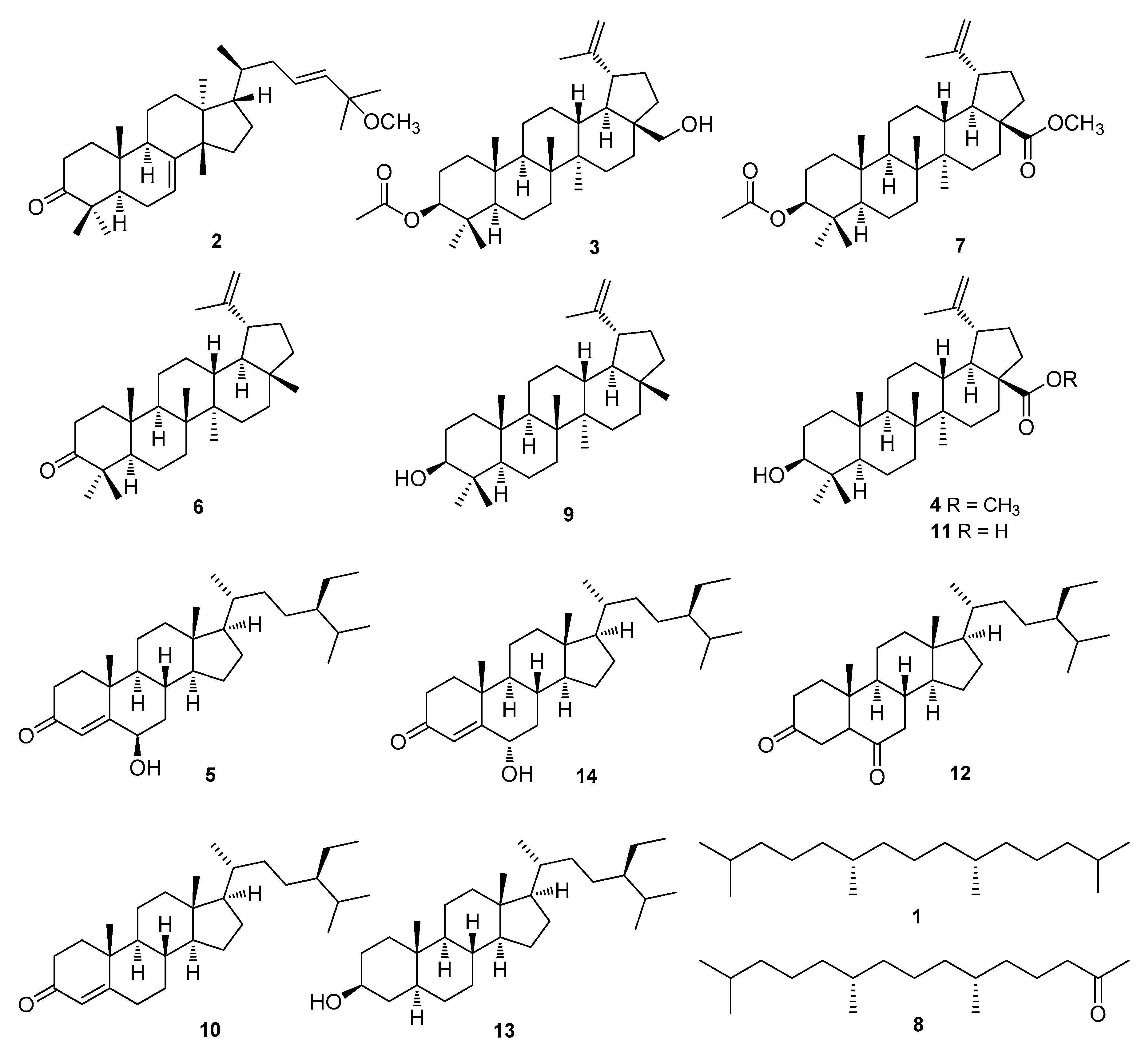
3.1. Isolation and Identification of Compounds 1–14
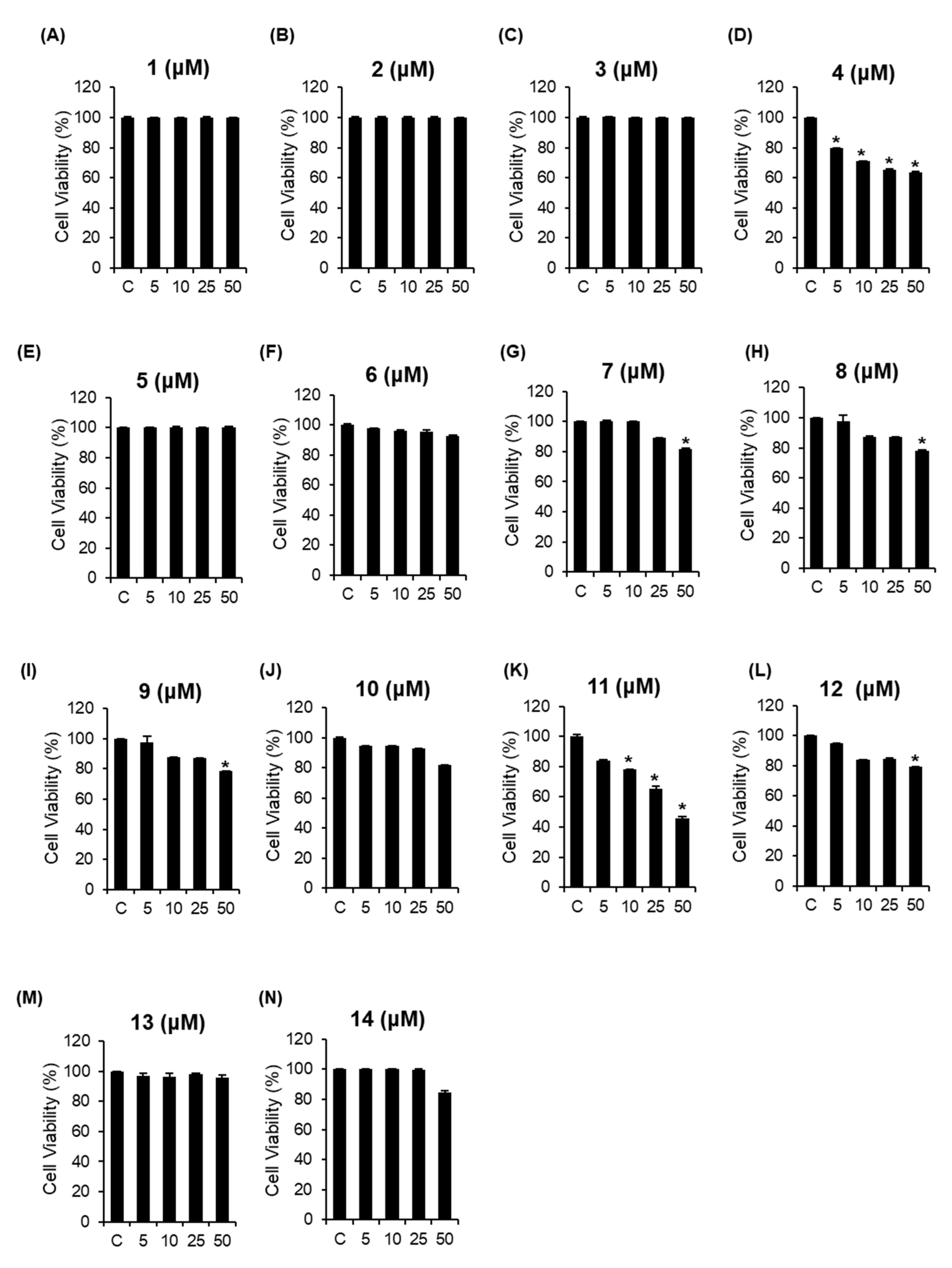
3.2. Cytotoxic Effects of the Isolated Compounds 1–14 on A2780 Human Ovarian Carcinoma Cells
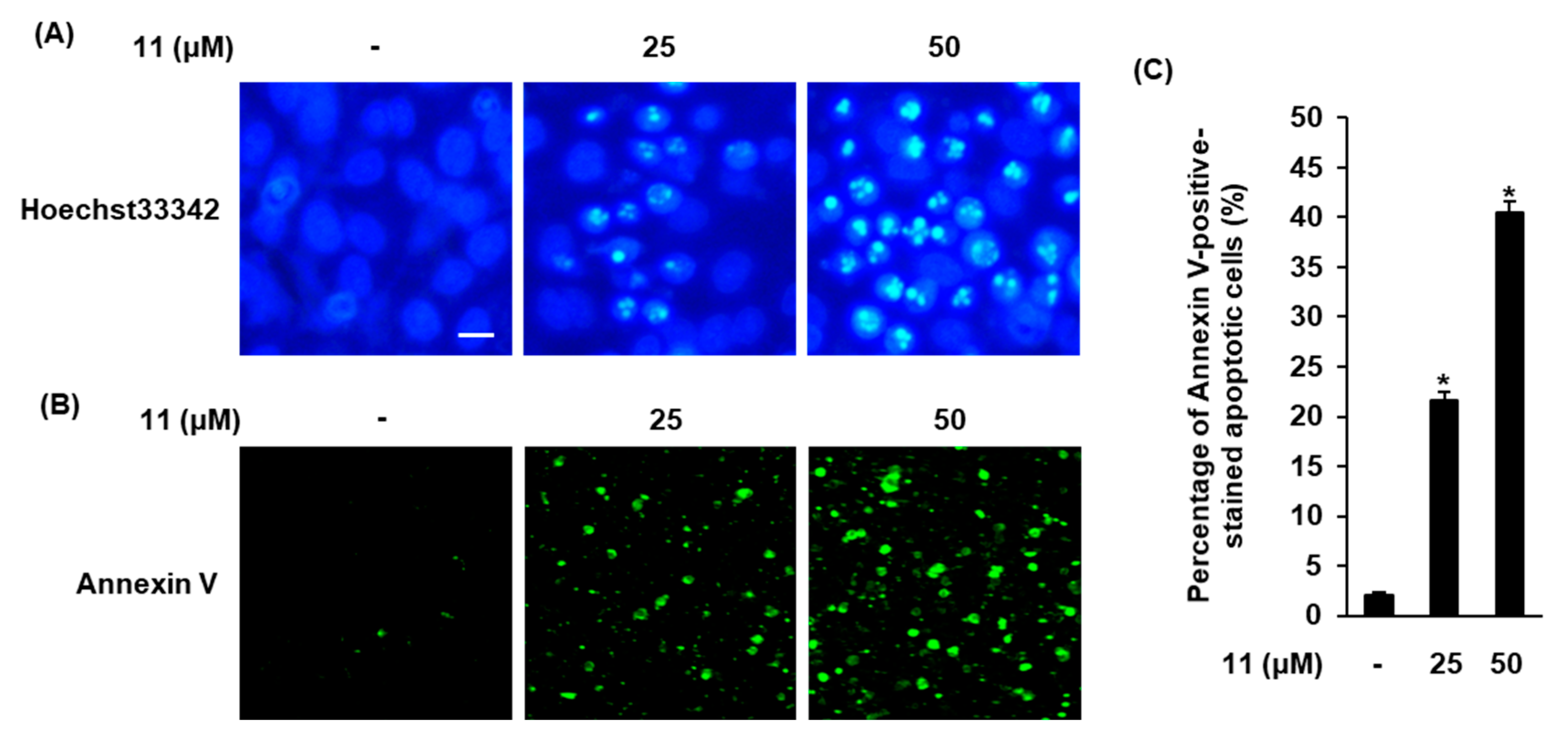
3.3. Effects of Compound 11 on Apoptotic Death of A2780 Cells
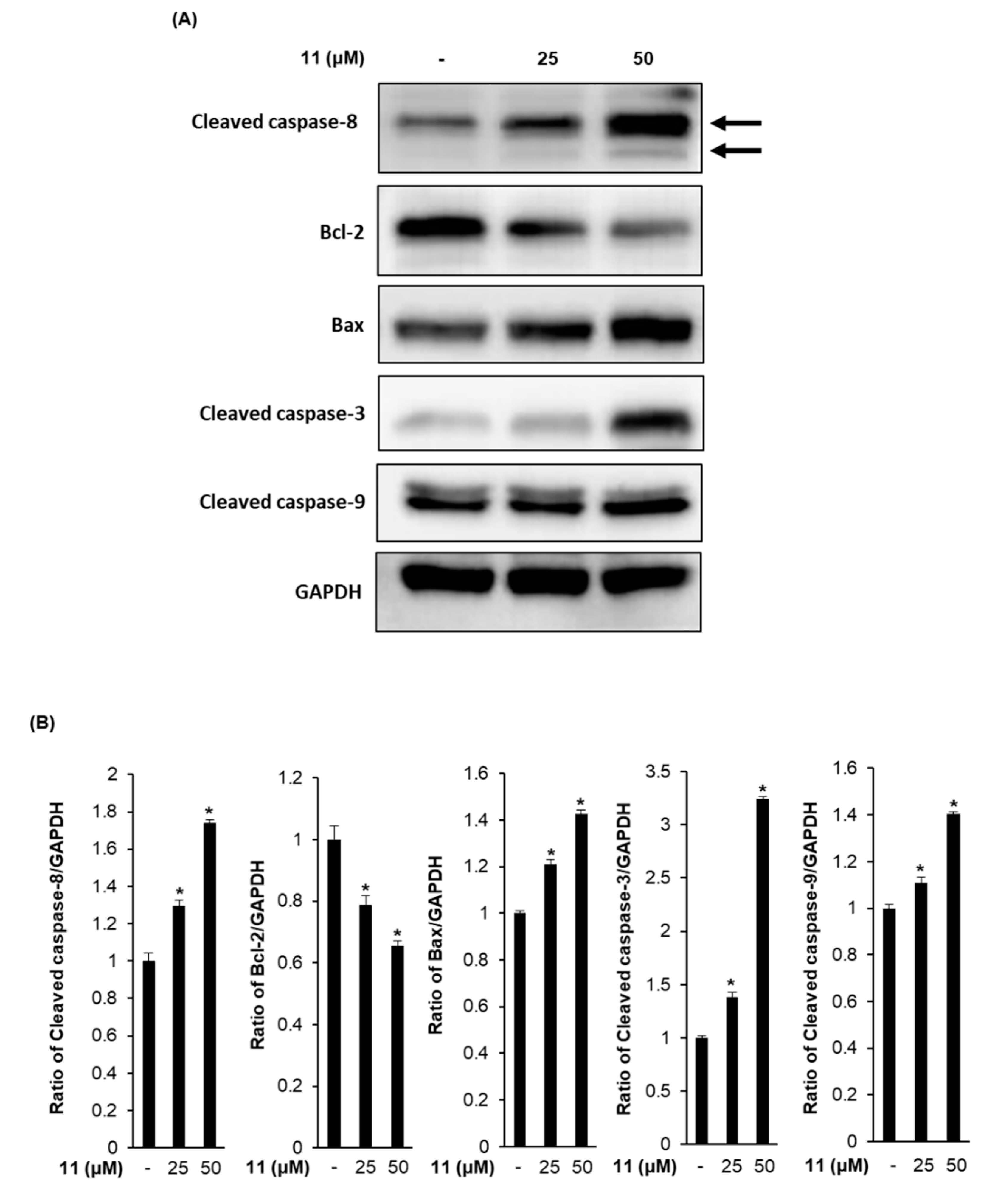
3.4. Effects of Compound 11 on the Expression of Apoptosis-Related Proteins in A2780 Human Ovarian Carcinoma Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Bansal, A.; Patel, F.D.; Sharma, S.C. Radiotherapy for ovarian cancers-redefining the role. Asian Pac. J. Cancer Prev. 2014, 15, 4759–4763. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, A.; Karagiannis, V. Epithelial ovarian cancer. risk factors, screening and the role of prophylactic oophorectomy. Hippokratia 2007, 11, 63–66. [Google Scholar] [PubMed]
- Mitra, S.; Dash, R. Natural products for the management and prevention of breast cancer. Evid. Based Complement. Alternat. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Jung, T.K.; Kim, M.J.; Yoon, K.S. Protective effect of Cornus walteri Wangerin leaf against UVB irradiation induced photoaging in human reconstituted skin. J. Ethnopharmacol. 2016, 193, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Park, W.Y.; Hwang, B.Y.; Oh, G.J.; Kang, S.J.; Lee, K.S.; Ro, J.S. Phenolic compounds from the stem bark of Cornus walteri Wanger. Korean J. Pharmacogn. 1998, 29, 217–224. [Google Scholar]
- Lee, S.H.; Yoon, K.R.; Lee, E.; Cha, Y.Y. Anti-inflammatory effect of Cornus walteri. J. Orient. Physiol. Pathol. 2011, 25, 982–988. [Google Scholar]
- Park, W.H.; Cha, Y.Y. Effect of stem bark extracts of Cornus walteri Wanger on the lipid lowering, anti-oxidative activity and concentration of proinflammatory cytokines in rat fed high fat diet. J. Orient. Rehabil. Med. 2009, 19, 59–78. [Google Scholar]
- Lee, S.R.; Park, Y.J.; Han, Y.B.; Lee, J.C.; Lee, S.; Park, H.; Lee, H.; Kim, K.H. Isoamericanoic Acid B from Acer tegmentosum as a Potential Phytoestrogen. Nutrients 2018, 10, 1915. [Google Scholar] [CrossRef]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Roh, H.; Song, S.; Ryoo, R.; Pang, C.; Baek, K.; Kim, K.H. Cytotoxic constituents from the sclerotia of Poria cocos against human lung adenocarcinoma cells by inducing mitochondrial apoptosis. Cells 2018, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Roh, H.S.; Baek, K.H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Kim, Y.C.; Lee, K.R. Tirucallane triterpenoids from Cornus walteri. J. Nat. Prod. 2011, 74, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Shin, Y.J.; Choi, S.U.; Lee, K.R. New cytotoxic δ-valerolactones from Cornus walteri. Bull. Korean Chem. 2011, 32, 2443–2445. [Google Scholar] [CrossRef]
- Dalling, D.K.; Pugmire, R.J.; Grant, D.M.; Hull, W.E. The use of high-field carbon-13 NMR spectroscopy to characterize chiral centers in isopranes. Magn. Reson. Chem. 1986, 24, 191–198. [Google Scholar] [CrossRef]
- Santos, R.C.; Salvador, J.A.R.; Marin, S.; Cascante, M. Novel semisynthetic derivatives of betulin and betulinic acid with cytotoxic activity. Bioorg. Med. Chem. 2009, 17, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef]
- Della Greca, M.; Monaco, P.; Previtera, L. Studies on aquatic plants. Part XVI. Stigmasterols from Typha latifolia. J. Nat. Prod. 1990, 53, 1430–1435. [Google Scholar] [CrossRef]
- Puapairoj, P.; Naengchomnong, W.; Kijjoa, A.; Pinto, M.M.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.S.; Herz, W. Cytotoxic activity of lupane-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Med. 2005, 71, 208–213. [Google Scholar] [CrossRef]
- Urban, M.; Sarek, J.; Klinot, J.; Korinkova, G.; Hajduch, M. Synthesis of A-seco derivatives of betulinic acid with cytotoxic activity. J. Nat. Prod. 2004, 67, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, C.; Mao, Z.; Gopinathan, M.B.; Rehder, K.; Brinton, R.D. Design, synthesis, and estrogenic activity of a novel estrogen receptor modulator-a hybrid structure of 17β-estradiol and vitamin E in hippocampal neurons. J. Med. Chem. 2007, 50, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Sholichin, M.; Yamasaki, K.; Kasai, R.; Tanaka, O. Carbon-13 nuclear magnetic resonance of lupane-type triterpenes, lupeol, betulin and betulinic acid. Chem. Pharm. Bull. 1980, 28, 1006–1008. [Google Scholar] [CrossRef]
- Zhang, D.D.; Yang, J.; Luo, J.F.; Li, X.N.; Long, C.L.; Wang, Y.H. New aporphine alkaloids from the aerial parts of Piper semiimmersum. J. Asian Nat. Prod. Res. 2018, 20, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Hattori, T.; Hamaguchi, N.; Masuda, K.; Takano, A.; Shiojima, K. Fern constituents: Dryocrassyl formate, sitostanyl formate and 12α-hydroxyfern-9(11)-ene from Cyathea podophylla. Chem. Pharm. Bull. 2003, 51, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Zheng, C.J.; Li, X.B.; Chen, G.Y.; Han, C.R.; Chen, W.H.; Song, X.P. Two new lanostane triterpenoids from the branches and leaves of Polyalthia obliqua. Molecules 2014, 19, 7621–7628. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.M.T.; Cao, T.Q.; Woo, M.H.; Min, B.S.; Weon, K.Y. Cytotoxic Triterpenoids from the Fruits of Ligustrum japonicum. Nat. Prod. Sci. 2018, 24, 93–98. [Google Scholar] [CrossRef]
- Wang, X.; Su, G.Y.; Zhao, C.; Qu, F.Z.; Wang, P.; Zhao, Y.Q. Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J. Ginseng Res. 2018, 42, 133–143. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Noriko, Y.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced nephrotoxicity in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; An, J.H.; Son, Y.K.; Yeo, J.H.; Song, K.S. The Cytotoxic Constituents of Betula platyphylla and their Effects on Human Lung A549 Cancer Cells. Nat. Prod. Sci. 2018, 24, 219–224. [Google Scholar] [CrossRef]
- Abbro, L.; Dini, L. Common morphological features of apoptotic cell blebs. Ital. J. Zool. 2003, 70, 297–299. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a008656. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Kominami, K.; Nakabayashi, J.; Nagai, T.; Tsujimura, Y.; Chiba, K.; Kimura, H.; Miyawaki, A.; Sawasaki, T.; Yokota, H.; Manabe, N.; et al. The molecular mechanism of apoptosis upon caspase-8 activation: Quantitative experimental validation of a mathematical model. BBA Mol. Cell Res. 2012, 1823, 1825–1840. [Google Scholar] [CrossRef] [Green Version]
- Belloc, F.; Belaud-Rotureau, M.A.; Lavignolle, V.; Bascans, E.; Braz-Pereira, E.; Durrieu, F.; Lacombe, F. Flow cytometry detection of caspase 3 activation in preapoptotic leukemic cells. Cytometry 2000, 40, 151–160. [Google Scholar] [CrossRef]
- Bagci, E.Z.; Vodovotz, Y.; Billiar, T.R.; Ermentrout, G.B.; Bahar, I. Bistability in apoptosis: Roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys. J. 2006, 90, 1546–1559. [Google Scholar] [CrossRef]
- Fulda, S.; Jeremias, I.; Steiner, H.H.; Pietsch, T.; Debatin, K.M. Betulinic acid: A new cytotoxic agent against malignant brain-tumor cells. Int. J. Cancer 1999, 82, 435–441. [Google Scholar] [CrossRef]
- Raghuvar Gopal, D.V.; Narkar, A.A.; Badrinath, Y.; Mishra, K.P.; Joshi, D.S. Betulinic acid induces apoptosis in human chronic myelogenous leukemia (CML) cell line K-562 without altering the levels of Bcr-Abl. Toxicol. Lett. 2005, 155, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Ramaiah, S.K.; Safe, S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007, 67, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.R.; Kim, K.J.; Choi, C.H.; Lee, T.B.; Han, S.I.; Han, H.K.; Lim, S.C. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Puthli, A.; Balakrishnan, S.; Sapra, B.K.; Mishra, K.P. Betulinic acid-induced cytotoxicity in human breast tumor cell lines MCF-7 and T47D and its modification by tocopherol. Cancer Investig. 2014, 32, 402–408. [Google Scholar] [CrossRef]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic acid-mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor-kappa B (NF-κB) pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef]
- Eiznhamer, D.A.; Xu, Z.Q. Betulinic acid: A promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Lei, P.; Pathi, S.; Safe, S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer 2011, 11, 371. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Noratto, G.D.; Li, X.; Angel-Morales, G.; Bertoldi, M.C.; Safe, S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: Role of Sp transcription factors and microRNA-27a: ZBTB10. Mol. Carcinogen. 2013, 52, 591–602. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Mahmoud, N.; Sugimoto, Y.; Efferth, T.; Abdel-Aziz, H. Betulinic acid exerts cytotoxic activity against multidrug-resistant tumor cells via targeting autocrine motility factor receptor (AMFR). Front. Pharmacol. 2018, 9, 481. [Google Scholar] [CrossRef]
- Fulda, S.; Jeremias, I.; Debatin, K.M. Cooperation of betulinic acid and TRAIL to induce apoptosis in tumor cells. Oncogene 2004, 23, 7611–7620. [Google Scholar] [CrossRef] [Green Version]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.M.; David, J.M.; Sakukuma, M.C.K.; Costa-Lotufo, L.V.; Moura, A.F.; Araujo, A.J. Terpene esters from natural products: Synthesis and evaluation of cytotoxic activity. An. Acad. Bras. Cienc. 2017, 89, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Monção, N.B.; Araújo, B.Q.; Silva, J.N.; Lima, D.J.; Ferreira, P.M.; Airoldi, F.P.; Pessoa, C.; Citó, A.M. Assessing chemical constituents of Mimosa caesalpiniifolia stem bark: Possible bioactive components accountable for the cytotoxic effect of M. caesalpiniifolia on human tumour cell lines. Molecules 2015, 20, 4204–4224. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoon, S.K.; Ryu, S.Y. Cytotoxic triterpenes from stem bark of Physocarpus intermedius. Planta Med. 2000, 66, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, R.K.; Heller, L.; Csuk, R. Targeting mitochondria: Esters of rhodamine B with triterpenoids are mitocanic triggers of apoptosis. Eur. J. Med. Chem. 2018, 152, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Heller, L.; Perl, V.; Kluge, R.; Ströhl, D.; Csuk, R. Betulinic acid derived hydroxamates and betulin derived carbamates are interesting scaffolds for the synthesis of novel cytotoxic compounds. Eur. J. Med. Chem. 2015, 106, 194–210. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, J.B.; Dou, Y.C. Sequential treatment with betulinic acid followed by 5-fluorouracil shows synergistic cytotoxic activity in ovarian cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 252–259. [Google Scholar]
- Zhao, Z.; Wang, J.; Tang, J.; Liu, X.; Zhong, Q.; Wang, F.; Hu, W.; Yuan, Z.; Nie, C.; Wei, Y. JNK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem. J. 2012, 444, 291–301. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. https://doi.org/10.3390/biom9070257
Lee D, Lee SR, Kang KS, Ko Y, Pang C, Yamabe N, Kim KH. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules. 2019; 9(7):257. https://doi.org/10.3390/biom9070257
Chicago/Turabian StyleLee, Dahae, Seoung Rak Lee, Ki Sung Kang, Yuri Ko, Changhyun Pang, Noriko Yamabe, and Ki Hyun Kim. 2019. "Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis" Biomolecules 9, no. 7: 257. https://doi.org/10.3390/biom9070257
APA StyleLee, D., Lee, S. R., Kang, K. S., Ko, Y., Pang, C., Yamabe, N., & Kim, K. H. (2019). Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules, 9(7), 257. https://doi.org/10.3390/biom9070257