Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
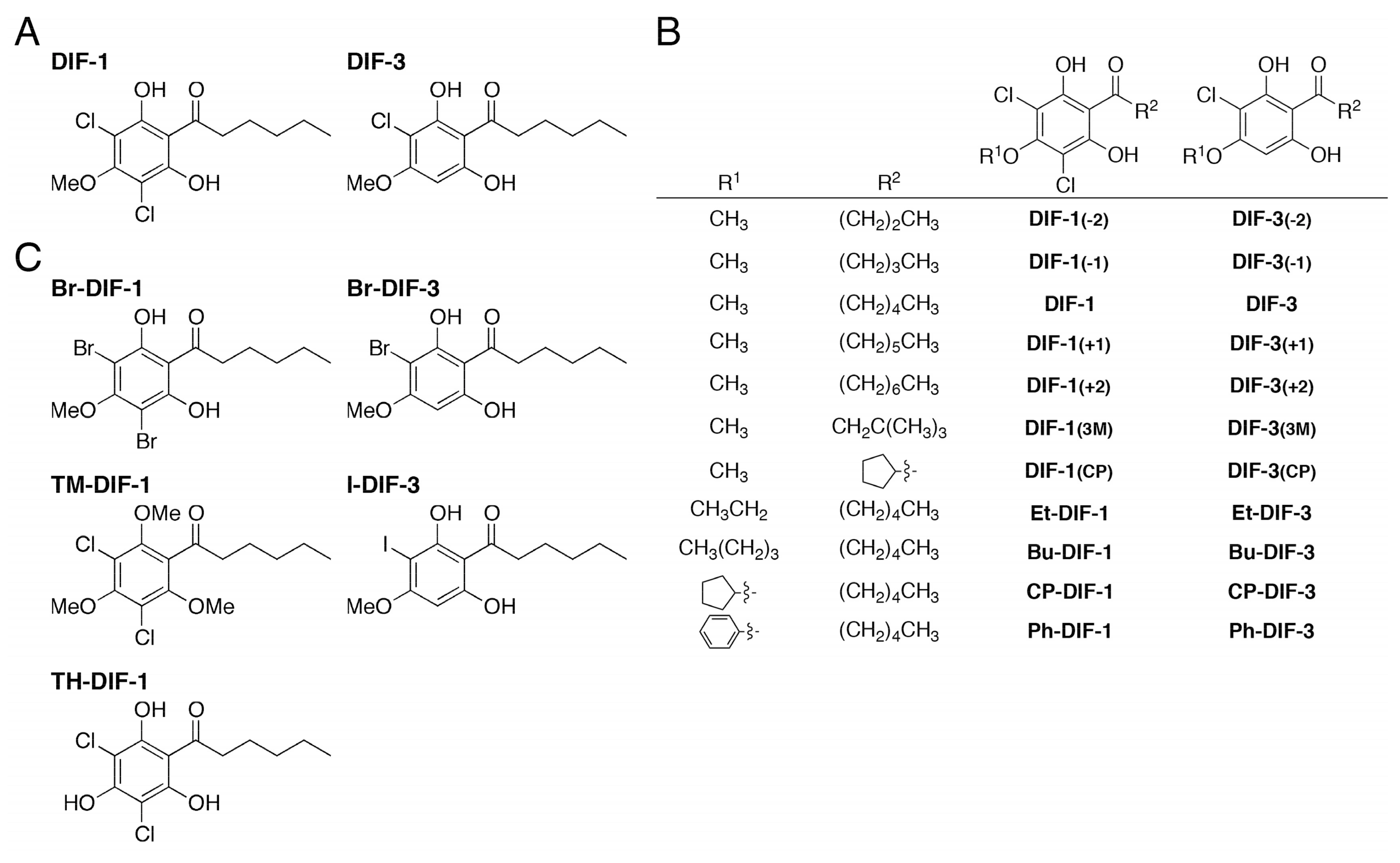
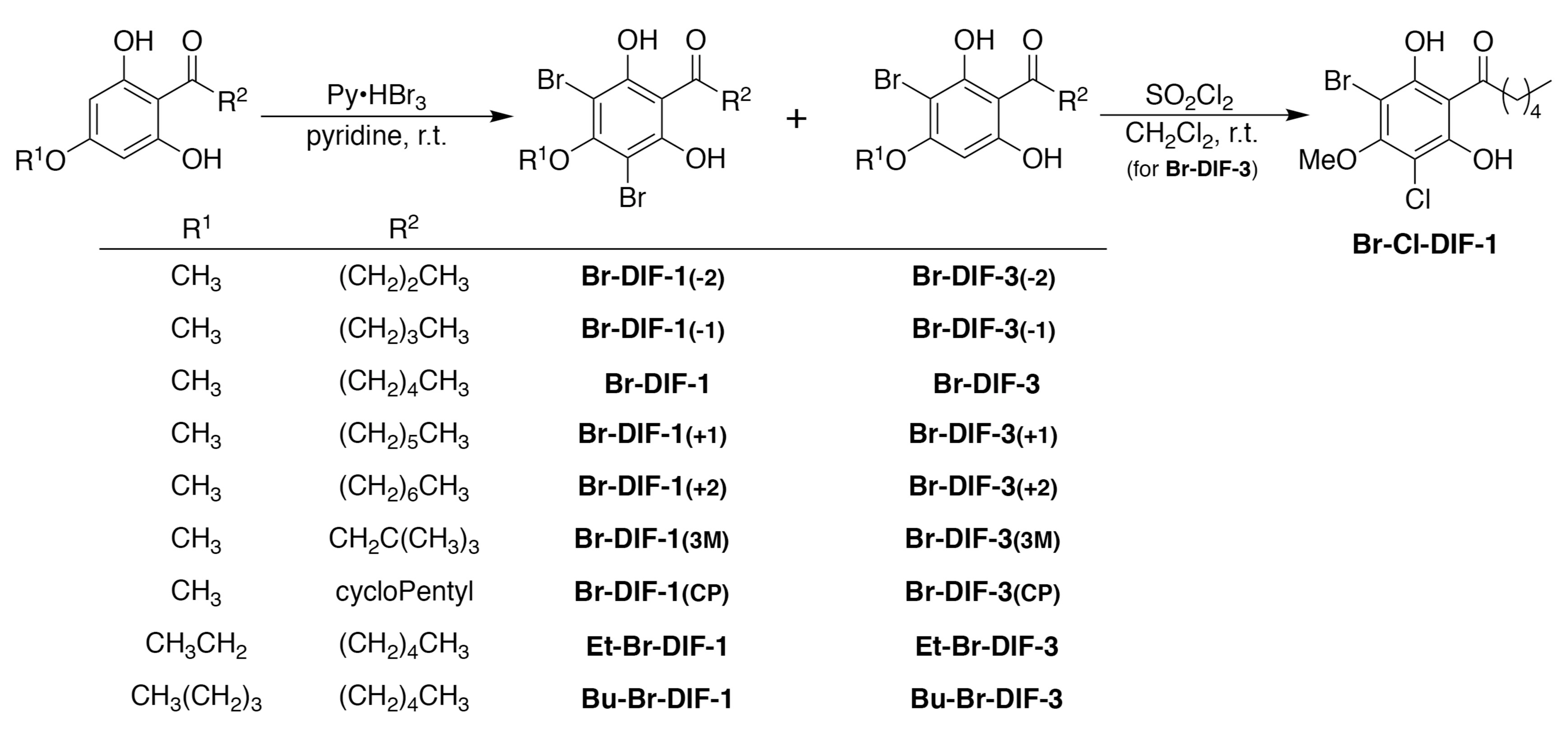
2.2. Synthesis of DIF Derivatives
2.3. Data for Br-DIF Derivatives
2.4. Cell migration Assay
2.5. Cell Proliferation Assay
2.6. Statistical Analysis
3. Results
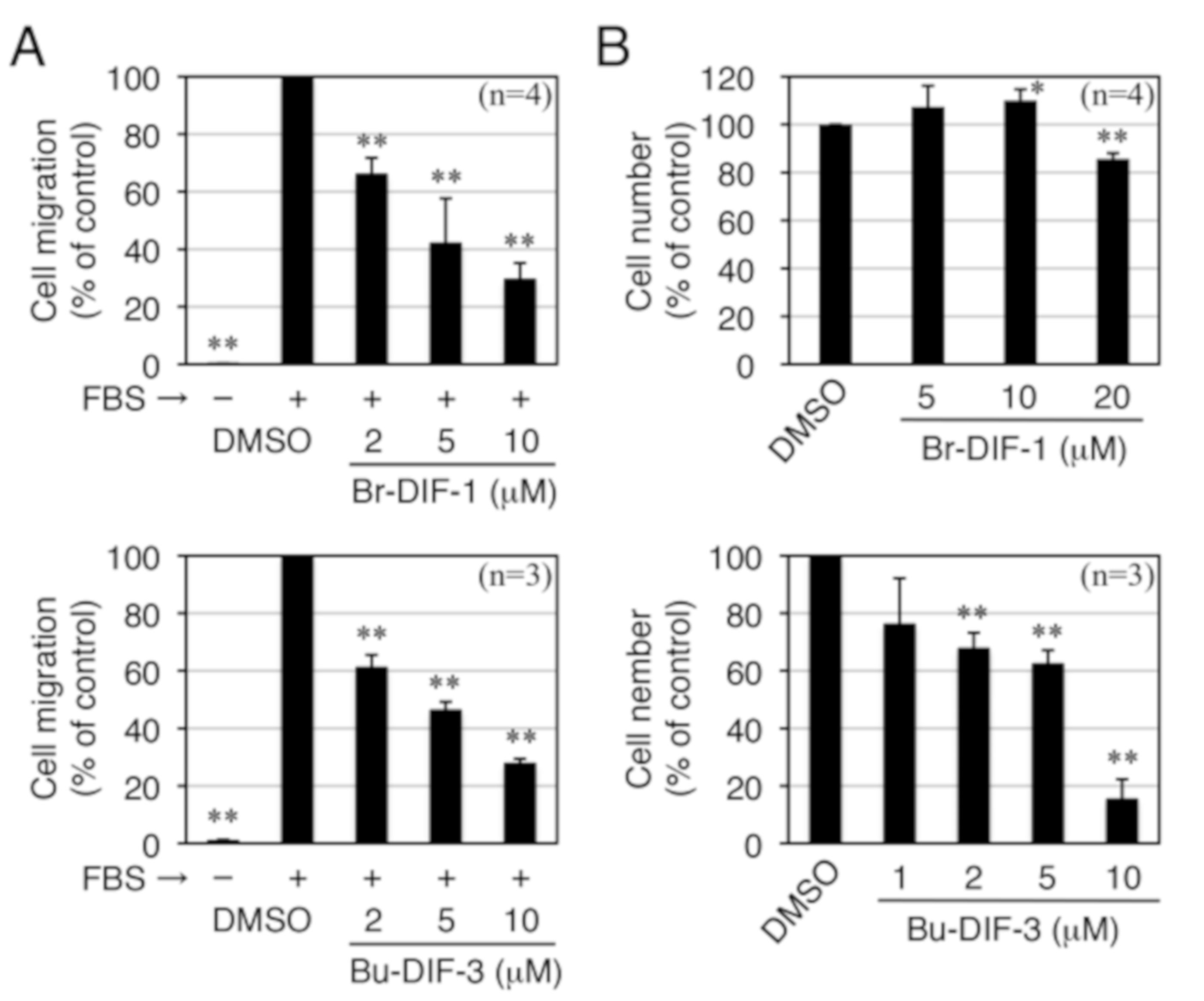
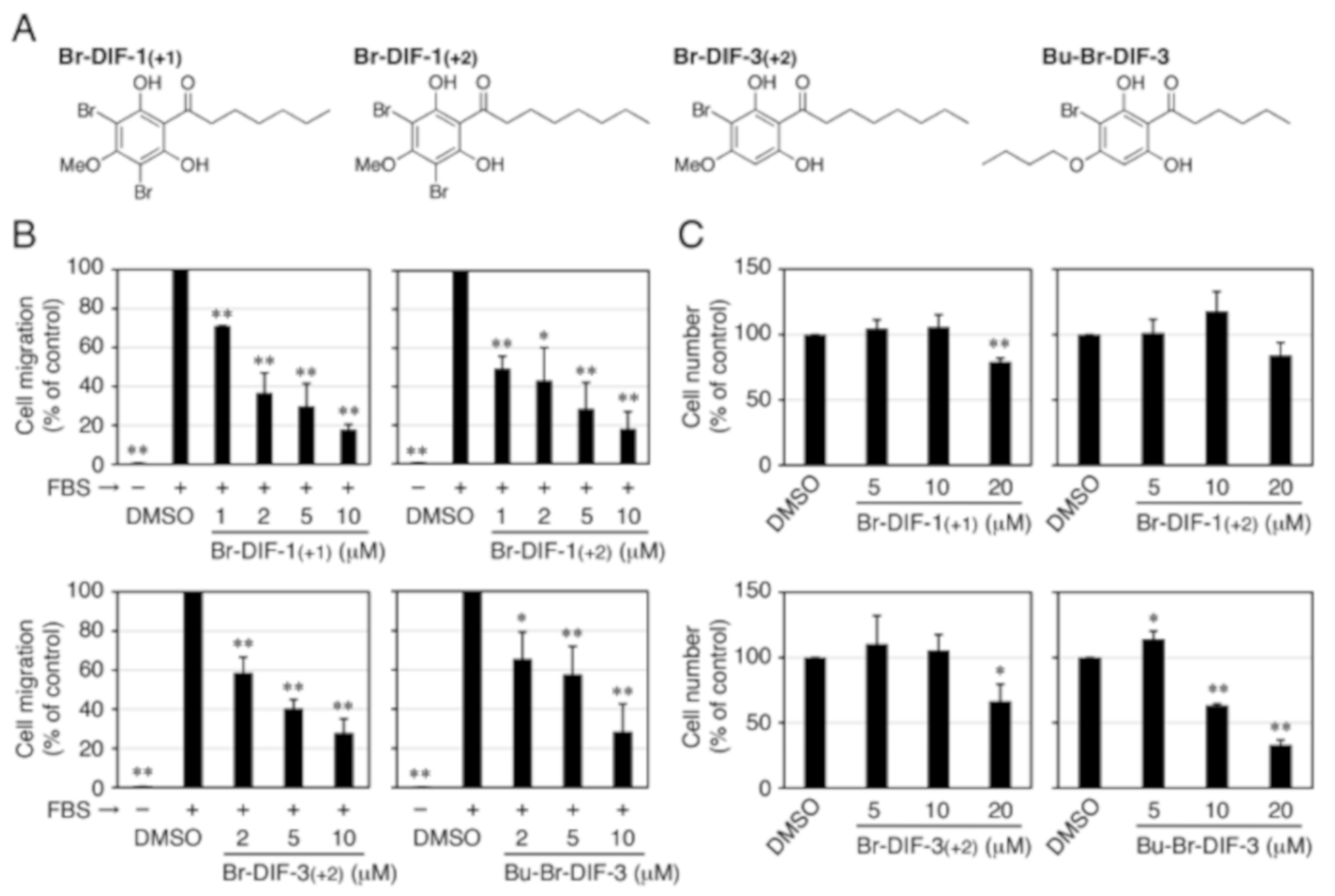
3.1. Effects of DIF Derivatives on Cell Migration and Cell Proliferation in MDA-MB-231 Cells
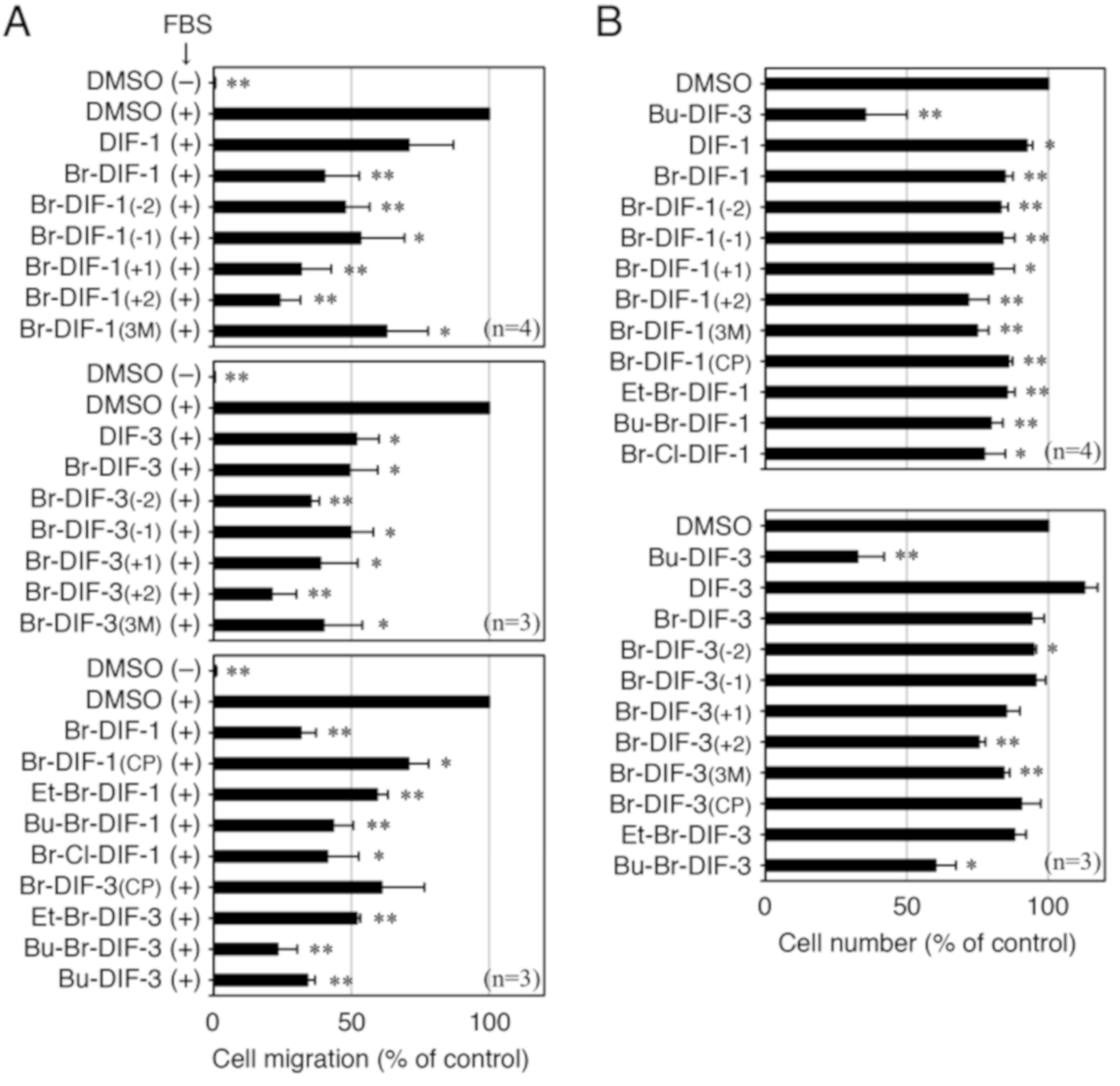
3.2. Comparison of the Biological Activities of Br-DIF-1 and Bu-DIF-3
3.3. Synthesis of Br-DIF-1 Derivatives and Structure–Activity Relationship Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Park, S.; Kwon, Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol. Ther. 2019, S0163–7258, 30020–30028. [Google Scholar] [CrossRef] [PubMed]
- Engebraaten, O.; Vollan, H.K.M.; Borresen-Dale, A.L. Triple-negative breast cancer and the need for new therapeutic targets. Am. J. Pathol. 2013, 183, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Wu, A.; Shao, G.; Peng, H.; Wang, M.; Ji, S.; Liu, P.; Zhang, J. The latest progress in research on triple negative breast cancer (TNBC): Risk factors, possible therapeutic targets and prognostic markers. J. Thorac. Dis. 2014, 6, 1329–1335. [Google Scholar] [PubMed]
- Abubakar, M.; Sung, H.; Bcr, D.; Guida, J.; Tang, T.S.; Pfeiffer, R.M.; Yang, X.R. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: Analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Johnsen, H.; Akslen, L.A.; Fluge, Ø.; Pergammenschlkov, A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, A. Triple-negative breast cancer clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Morris, H.R.; Taylor, G.W.; Masento, M.S.; Jermyn, K.A.; Kay, R.R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature 1987, 328, 811–814. [Google Scholar] [CrossRef]
- Morris, H.R.; Masento, M.S.; Taylor, G.W.; Jermyn, K.A.; Kay, R.R. Structure elucidation of two differentiation inducing factors (DIF-2 and DIF-3) from the cellular slime mould Dictyostelium discoideum. Biochem. J. 1988, 249, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.R.; Flatman, P.; Thompson, C.R.L. DIF signalling and cell fate. Semin. Cell Dev. Biol. 1999, 10, 577–585. [Google Scholar] [CrossRef]
- Asahi, K.; Sakurai, A.; Takahashi, N.; Kubohara, Y.; Okamoto, K.; Tanaka, Y. DIF-1, morphogen of Dictyostelium discoideum, induces the erythroid differentiation in murine and human leukemia cells. Biochem. Biophys. Res. Commun. 1995, 208, 1036–1039. [Google Scholar] [CrossRef]
- Kubohara, Y.; Saito, Y.; Tatemoto, K. Differentiation-inducing factor of D. discoideum raises intracellular calcium concentration and suppresses cell growth in rat pancreatic AR42J cells. FEBS Lett. 1995, 359, 119–122. [Google Scholar] [CrossRef]
- Kubohara, Y. DIF-1, putative morphogen of D. discoideum, suppresses cell growth and promotes retinoic acid-induced cell differentiation in HL-60. Biochem. Biophys. Res. Commun. 1997, 236, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y. Effects of differentiation-inducing factors (DIFs) of Dictyostelium discoideum on the human leukemia K562 cells: DIF-3 is the most potent anti-leukemic agent. Eur. J. Pharmacol. 1999, 381, 57–62. [Google Scholar] [CrossRef]
- Kanai, M.; Konda, Y.; Nakajima, T.; Izumi, Y.; Nanakin, A.; Kanda, N.; Kubohara, Y.; Chiba, T. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK dependent pathway. Oncogene 2003, 22, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Taba, Y.; Miwa, Y.; Kubohara, Y.; Watanabe, Y.; Hirata, M.; Morimoto, S.; Sasaguri, T. Dictyostelium differentiation-inducing factor-3 activates glycogen synthase kinase-3b and degrades cyclin D1 in mammalian cells. J. Biol. Chem. 2003, 278, 9663–9670. [Google Scholar] [CrossRef]
- Gokan, N.; Kikuchi, H.; Nakamura, K.; Oshima, Y.; Hosaka, K.; Kubohara, Y. Structural requirements of Dictyostelium differentiation-inducing factors for their stalk-cell-inducing activity in Dictyostelium cells and anti-proliferative activity in K562 human leukemic cells. Biochem. Pharmacol. 2005, 70, 676–685. [Google Scholar] [CrossRef]
- Kubohara, Y.; Kikuchi, H.; Matsuo, Y.; Oshima, Y.; Homma, Y. Mitochondria are the target organelle of differentiation-inducing factor-3, an anti-tumor agent isolated from Dictyostelium discoideum. PLoS ONE 2013, 8, e72118. [Google Scholar] [CrossRef]
- Kubohara, Y.; Komachi, M.; Homma, Y.; Kikuchi, H.; Oshima, Y. Derivatives of Dictyostelium differentiation-inducing factors inhibit lysophosphatidic acid–stimulated migration of murine osteosarcoma LM8 cells. Biochem. Biophys. Res. Commun. 2015, 463, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kubokura, N.; Takahashi-Yanaga, F.; Arioka, M.; Yoshihara, T.; Igawa, K.; Tomooka, K.; Morimoto, S.; Nakatsu, Y.; Tsuzuki, T.; Nakabeppu, Y.; et al. Differentiation-inducing factor-3 inhibits intestinal tumor growth in vitro and in vivo. J. Pharmacol. Sci. 2015, 127, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, P.; Kubohara, Y.; Kikuchi, H.; Oshima, Y.; Rusch, C.; Skerl, R.; Diakonova, M. A derivative of differentiation-inducing factor-3 inhibits PAK1 activity and breast cancer cell proliferation. Int. J. Cancer Clinic. Res. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Arioka, M.; Takahashi-Yanaga, F.; Kubo, M.; Igawa, K.; Tomooka, K.; Sasaguri, T. Anti-tumor effects of differentiation-inducing factor-1 in malignant melanoma: GSK-3-mediated inhibition of cell proliferation and GSK-3-independent suppression of cell migration and invasion. Biochem. Pharmacol. 2017, 138, 31–48. [Google Scholar] [CrossRef]
- Takahashi, K.; Kikuchi, H.; Nguyen, V.H.; Oshima, Y.; Ishigaki, H.; Nakajima-Shimada, J.; Kubohara, Y. Biological activities of novel derivatives of differentiation-inducing factor-3 from Dictyostelium discoideum. Biol. Pharm. Bull. 2017, 40, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y.; Kikuchi, H. Dictyostelium: An important source of structural and functional diversity in drug discovery. Cells 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef]
- Scully, O.J.; Bay, B.H.; Tip, G.; Yu, Y. Breast cancer metastasis. Cancer Genom. Proteom. 2012, 9, 311–320. [Google Scholar]
- Koztowski, J.; Koztowska, A.; Kochi, J. Breast cancer metastasis—Insight into selected molecular mechanisms the phenomenon. Postepy Hig. Med. Dosw. 2015, 69, 447–451. [Google Scholar] [CrossRef]
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia 2015, 17, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Kennoki, N.; Hori, S.; Yuki, T.; Hori, A. Transcatheter arterial chemoembolization with spherical embolic agent in patients with pulmonary or mediastinal metastases from breast cancer. J. Vasc. Interv. Radiol. 2017, 28, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, J.H.; Lee, E.; Jang, Y.J.; Son, J.E.; Kwon, J.Y.; Lim, T.G.; Kim, S.; Park, J.H.; Kim, J.E.; et al. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget 2016, 7, 67223–67234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, S.; Tseng, K.F.; Han, K.; Wang, Y.; Gan, Z.H.; Min, D.L.; Hu, H.Y. Selumetinib suppresses cell proliferation, migration and trigger apoptosis, G1 arrest in triple-negative breast cancer cells. BMC Cancer 2016, 16, 818. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y.; Kikuchi, H.; Oshima, Y. Derivatives of Dictyostelium differentiation-inducing factors inhibit serum-dependent cell migration of murine osteosarcoma LM8 cells. Juntendo Med. J. 2019, 65, 71–76. [Google Scholar] [CrossRef]

| IC50 (μM) vs. Cell Migration | IC50 (μM) vs. Cell Proliferation | ||||
|---|---|---|---|---|---|
| Compound | MDA-MB-231 1 | LM8 2 | MDA-MB-231 1 | LM8 2 | 3T3-L1 2 |
| DIF-1 | >10 | 8.5 | >20 | 18.2 | >20 |
| DIF-3 | >10 | 10.2 | >20 | 15.5 | >20 |
| Br-DIF-1 | 3.8 | 5.5 | >20 | 18.5 | >20 |
| Bu-DIF-3 | 3.9 | 4.2 | 6.0 | 2.0 | 4.3 |
| Compound | IC50 (μM) vs. Cell Migration | IC50 (μM) vs. Cell Proliferation |
|---|---|---|
| Br-DIF-1 | 3.8 | >20 |
| Br-DIF-1(+1) | 1.5 | >20 |
| Br-DIF-1(+2) | 1.0 | >20 |
| Br-DIF-3(+2) | 3.1 | >20 |
| Bu-Br-DIF-3 | 4.7 | 13.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totsuka, K.; Makioka, Y.; Iizumi, K.; Takahashi, K.; Oshima, Y.; Kikuchi, H.; Kubohara, Y. Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro. Biomolecules 2019, 9, 256. https://doi.org/10.3390/biom9070256
Totsuka K, Makioka Y, Iizumi K, Takahashi K, Oshima Y, Kikuchi H, Kubohara Y. Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro. Biomolecules. 2019; 9(7):256. https://doi.org/10.3390/biom9070256
Chicago/Turabian StyleTotsuka, Kyoko, Yuka Makioka, Kyoichi Iizumi, Katsunori Takahashi, Yoshiteru Oshima, Haruhisa Kikuchi, and Yuzuru Kubohara. 2019. "Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro" Biomolecules 9, no. 7: 256. https://doi.org/10.3390/biom9070256
APA StyleTotsuka, K., Makioka, Y., Iizumi, K., Takahashi, K., Oshima, Y., Kikuchi, H., & Kubohara, Y. (2019). Halogen-Substituted Derivatives of Dictyostelium Differentiation-Inducing Factor-1 Suppress Serum-Induced Cell Migration of Human Breast Cancer MDA-MB-231 Cells in Vitro. Biomolecules, 9(7), 256. https://doi.org/10.3390/biom9070256





