Mechanism of Cell Wall Polysaccharides Modification in Harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) Fruit Caused by Penicillium italicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Sample and Treatments
2.2. Cell Wall Component Analysis
2.3. ROS and Ascorbic Acid (AsA) Content Measurement
2.4. Enzyme Activities Assays
2.5. RNA Isolation and Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
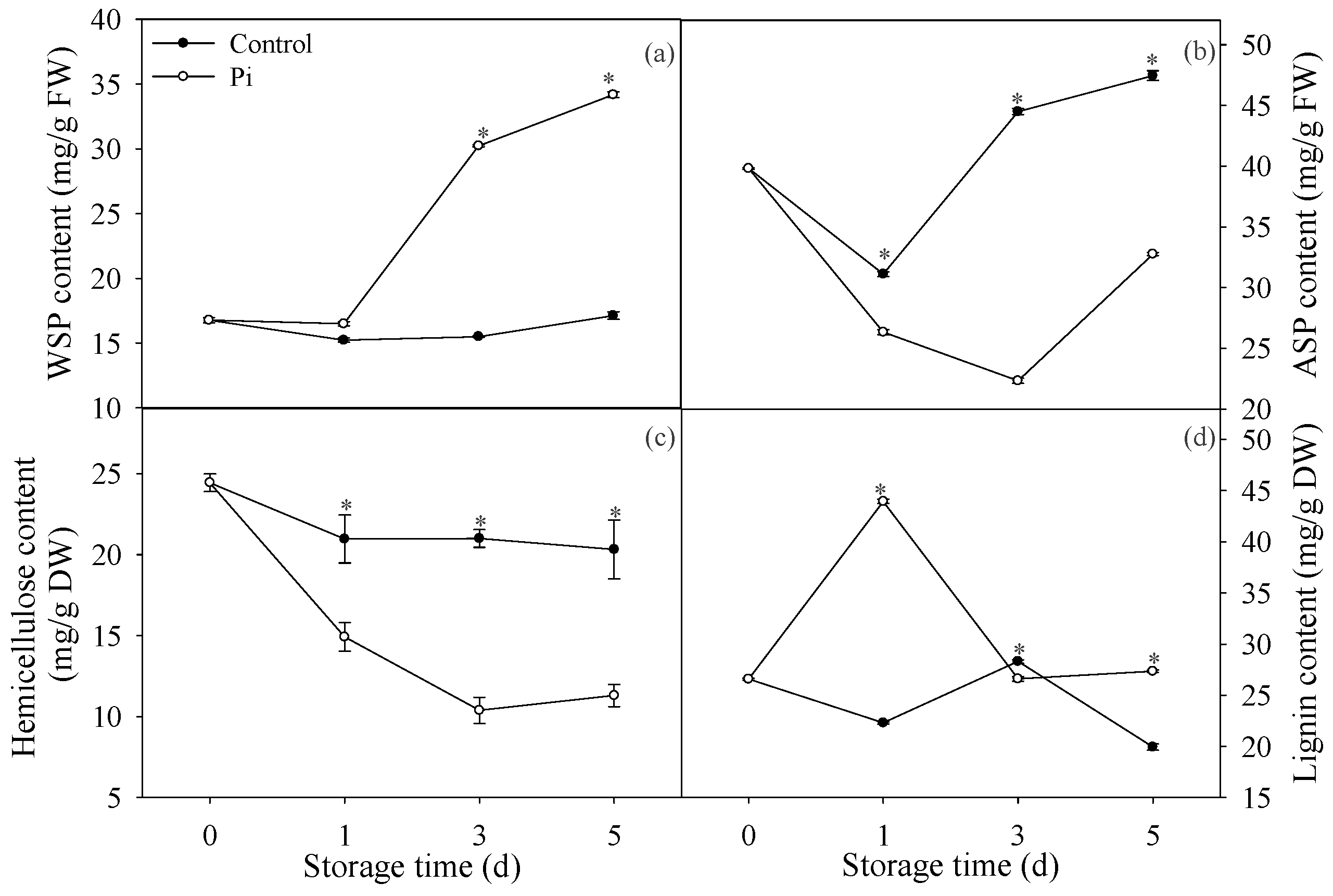
3.1. Effects of P. italicum Infection on Pectin and Lignin Contents of Mandarin Peel
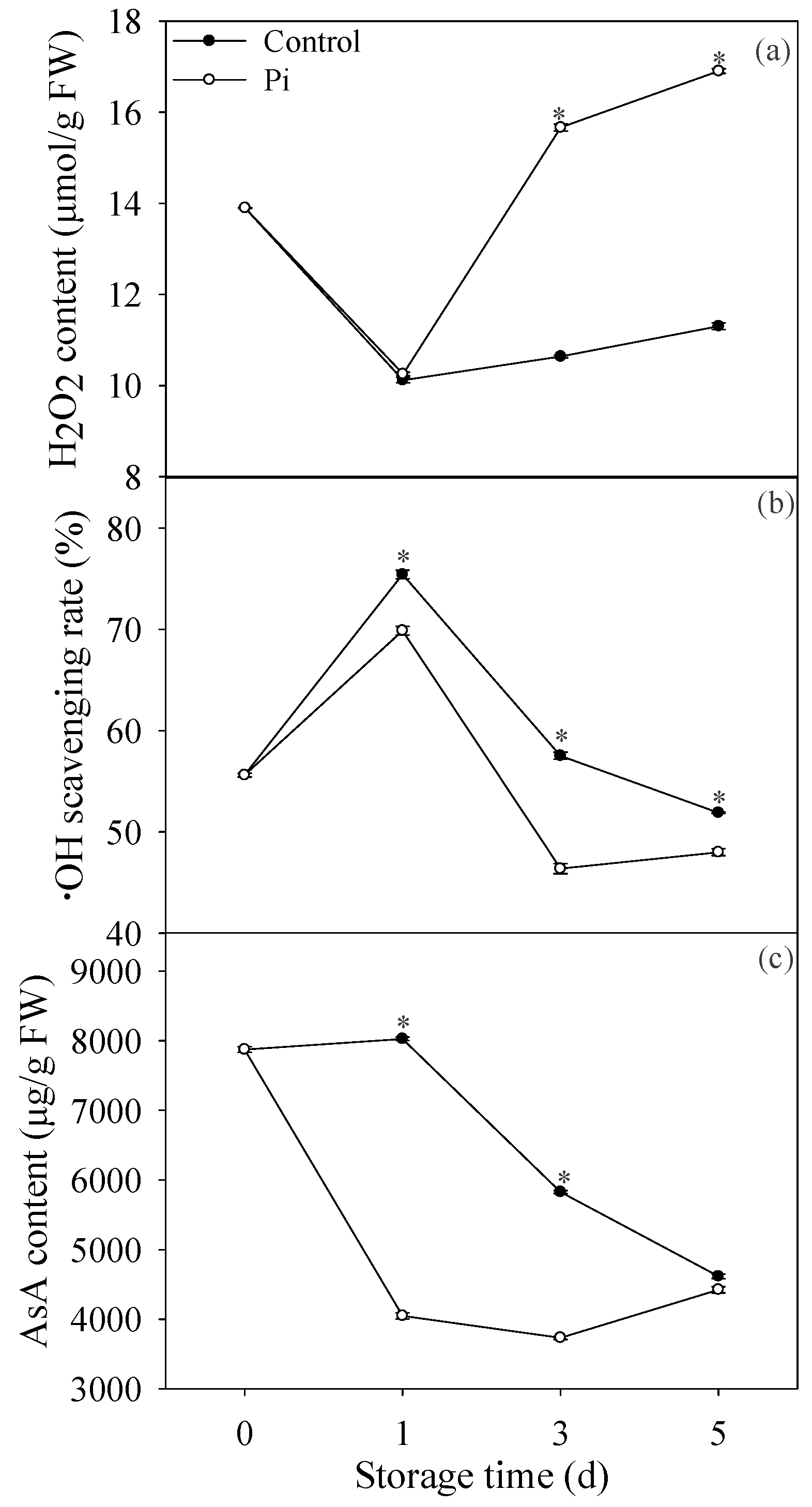
3.2. Effects of P. italicum Infection on H2O2 Content, ·OH Scavenging Rate, and AsA Content of Mandarin Peel
3.3. Effects of P. italicum Infection on SOD, CAT, and APX Activities of Mandarin Peel
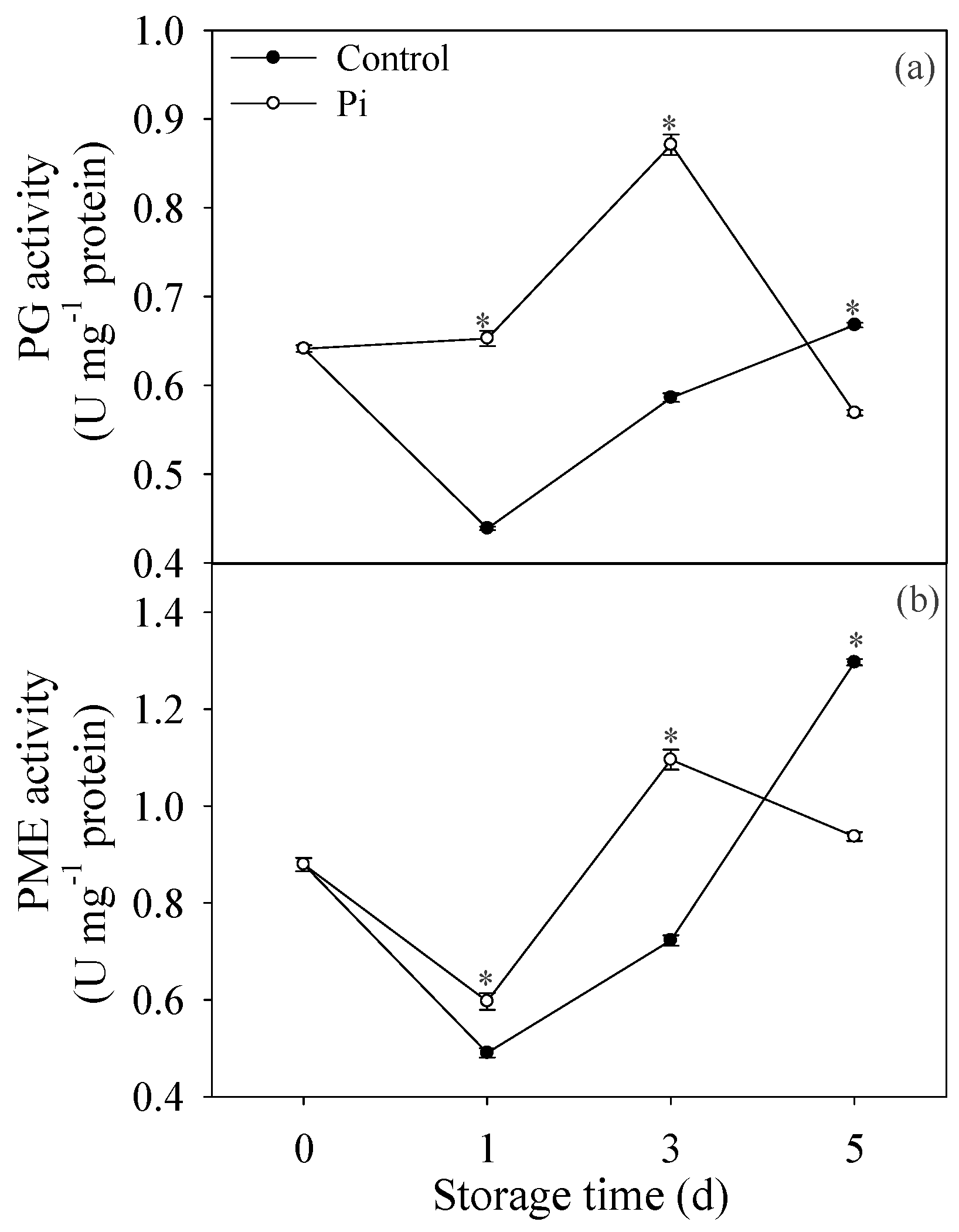
3.4. Effects of P. italicum Infection on PG and PME Activities of Mandarin Peel
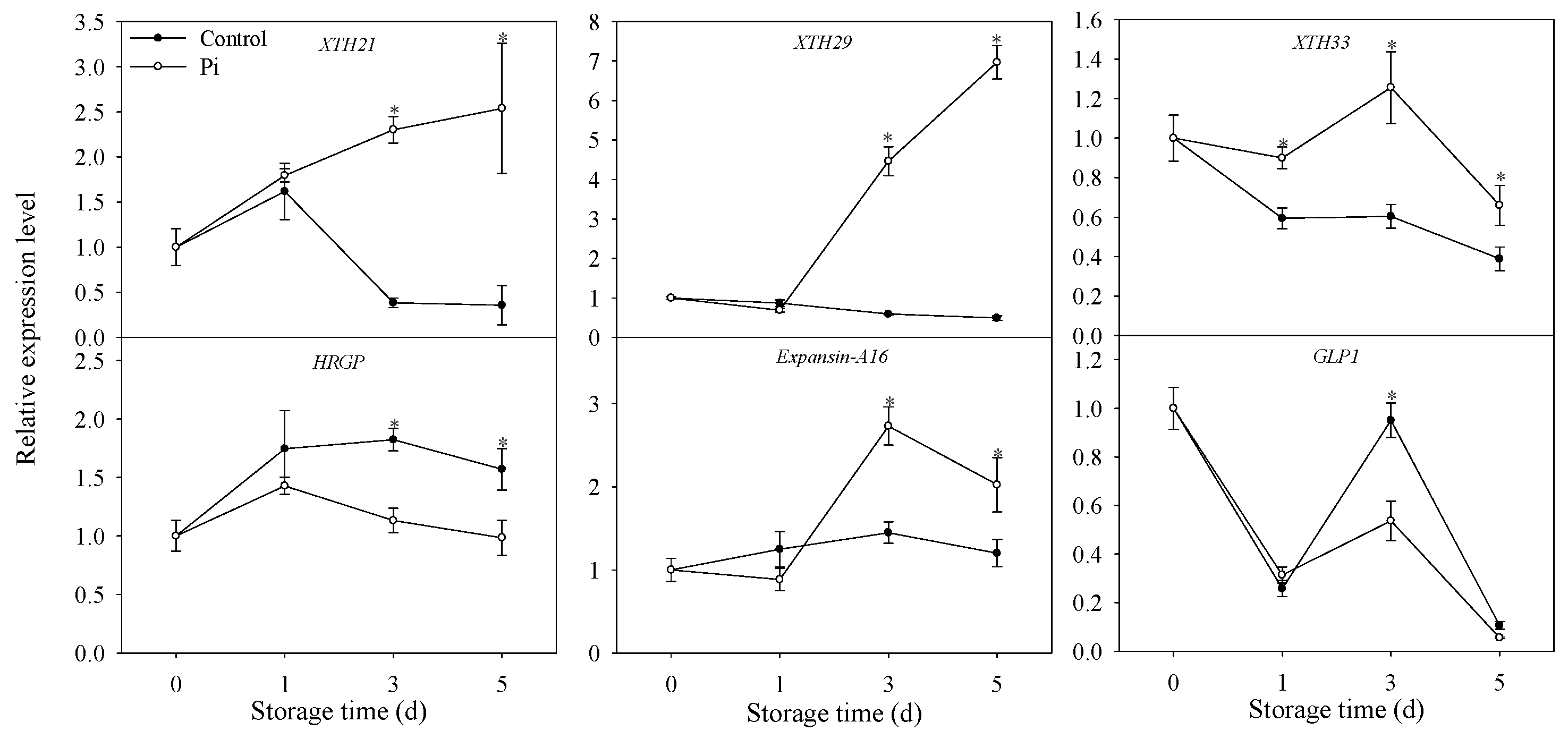
3.5. Effects of P. italicum Infection on Gene Expression Level of Mandarin Peel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhong, G.; Hu, M.; Luo, J.; Weng, Q.; Rizwan-ul-Haq, M. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Technol. 2010, 56, 39–43. [Google Scholar] [CrossRef]
- Yin, C.; Liu, H.; Shan, Y.; Gupta, V.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B as a biological preservative: purification, fungicidal activity and mechanism of action against Geotrichum citri-aurantii. Biomolecules 2019, 9, 125. [Google Scholar] [CrossRef]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Palou, L. Penicillium digitatum, Penicillium italicum (Green Mold, Blue Mold). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 45–102. [Google Scholar]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Teresa Lafuente, M.; Gonzalez-Candelas, L. Citrus phenylpropanoids and defence against pathogens. Part II: Gene expression and metabolite accumulation in the response of fruits to Penicillium digitatum infection. Food Chem. 2013, 136, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue molds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Hiickelhoven, R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 2007, 45, 101–127. [Google Scholar] [CrossRef]
- Vorwerk, S.; Somerville, S.; Somerville, C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004, 9, 203–209. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, H.; Jiao, J.; Wang, F.; Lu, Y.; Deng, J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chem. 2019, 283, 92–100. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Zhu, H.; Qu, H.; You, S.; Duan, X.; Jiang, Y. Proteomic analysis of differentially expressed proteins involved in peel senescence in harvested mandarin fruit. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Miedes, E.; Lorences, E.P. Changes in cell wall pectin and pectinase activity in apple and tomato fruits during Penicillium expansum infection. J. Sci. Food Agric. 2006, 86, 1359–1364. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.P.; Duan, X.W.; Shi, J.; Lu, W.J.; Luo, Y.B.; Jiang, W.B.; Jiang, Y.M. Effects of reactive oxygen species on cellular wall disassembly of banana fruit during ripening. Food Chem. 2008, 109, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Fry, S.C. Solubilisation of tomato fruit pectins by ascorbate: a possible non-enzymic mechanism of fruit softening. Planta 2003, 217, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.M.; Werder, J.; Nystroem, L. Reactive oxygen species responsible for beta-glucan degradation. Food Chem. 2013, 141, 589–596. [Google Scholar] [CrossRef]
- Muller, K.; Linkies, A.; Vreeburg, R.A.M.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef]
- Schopfer, P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: Implications for the control of elongation growth. Plant J. 2001, 28, 679–688. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. Engl. 2019, 6, 17. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Brecht, J.K.; Jiang, T.; Zheng, X. Pre-harvest application of oxalic acid increases quality and resistance to Penicillium expansum in kiwifruit during postharvest storage. Food Chem. 2016, 190, 537–543. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, H.; Zhang, D.; Sheng, J.; Lin, H.; Jiang, Y. Role of hydroxyl radical in modification of cell wall polysaccharides and aril breakdown during senescence of harvested longan fruit. Food Chem. 2011, 128, 203–207. [Google Scholar] [CrossRef]
- Duan, X.W.; Cheng, G.P.; Yang, E.; Yi, C.; Ruenroengklin, N.; Lu, W.J.; Luo, Y.B.; Jiang, Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008, 111, 144–149. [Google Scholar] [CrossRef]
- Ding, Y.; Chang, J.; Ma, Q.; Chen, L.; Liu, S.; Jin, S.; Han, J.; Xu, R.; Zhu, A.; Guo, J.; et al. Network analysis of postharvest senescence process in citrus fruits revealed by transcriptomic and metabolomic profiling. Plant Physiol. 2015, 168, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, F.; Li, T.; Su, X.; Jiang, G.; Qu, H.; Jiang, Y.; Duan, X. 1-Methylcyclopropene extends the shelf-life of ‘Shatangju’ mandarin (Citrus reticulate Blanco) fruit with attached leaves. Postharvest Biol. Technol. 2012, 67, 92–95. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Liu, X.; Huai, B.; Chen, J.; Yao, Q.; Qin, Y.; Liu, Z.; Luo, X. Effect of Zn and NAA co-treatment on the occurrence of creasing fruit and the peel development of ‘Shatangju’ mandarin. Sci. Hortic. Amst. 2016, 201, 230–237. [Google Scholar] [CrossRef]
- Deng, B.; Wang, W.; Deng, L.; Yao, S.; Ming, J.; Zeng, K. Comparative RNA-seq analysis of citrus fruit in response to infection with three major postharvest fungi. Postharvest Biol. Technol. 2018, 146, 134–146. [Google Scholar] [CrossRef]
- Cheng, G.; Duan, X.; Yang, B.; Jiang, Y.; Lu, W.; Luo, Y.; Jiang, W. Effect of hydroxyl radical on the scission of cellular wall polysaccharides in vitro of banana fruit at various ripening stages. Acta Physiol. Plant. 2008, 30, 257–263. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, L.; Zhou, Y.; Ming, J.; Yao, S.; Zeng, K. Wound healing in citrus fruit is promoted by chitosan and Pichia membranaefaciens as a resistance mechanism against Colletotrichum gloeosporioides. Postharvest Biol. Technol. 2018, 145, 134–143. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Wang, J.; Jiang, L.; Shan, T.; Zheng, Y. Effect of beta-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20 degrees C. Food Chem. 2015, 175, 471–477. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, K.; Zhou, Y.; Huang, Y. Effects of postharvest oligochitosan treatment on anthracnose disease in citrus (Citrus sinensis L. Osbeck) fruit. Eur. Food Res. Technol. 2015, 240, 795–804. [Google Scholar] [CrossRef]
- Li, T.T.; Shi, D.D.; Wu, Q.X.; Zhang, Z.K.; Qu, H.X.; Jiang, Y.M. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef]
- Sun, J.Z.; Lin, H.T.; Zhang, S.; Lin, Y.F.; Wang, H.; Lin, M.S.; Hung, Y.C.; Chen, Y.H. The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem. 2018, 247, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, L.; Zhou, Y.; Yao, S.; Zeng, K. Chitosan and Pichia membranaefaciens control anthracnose by maintaining cell structural integrity of citrus fruit. Biol. Control 2018, 124, 92–99. [Google Scholar] [CrossRef]
- Su, D.-L.; Li, P.-J.; Quek, S.Y.; Huang, Z.-Q.; Yuan, Y.-J.; Li, G.-Y.; Shan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Van Buggenhout, S.; Verlinden, B.E.; Christiaens, S.; Shpigelman, A.; Vicent, V.; Kermani, Z.J.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of Jonagold apples. Food Chem. 2014, 158, 283–291. [Google Scholar] [CrossRef]
- Cheng, G.P.; Duan, X.W.; Jiang, Y.M.; Sun, J.; Yang, S.Y.; Yang, B.; He, S.G.; Liang, H.; Luo, Y.B. Modification of hemicellulose polysaccharides during ripening of postharvest banana fruit. Food Chem. 2009, 115, 43–47. [Google Scholar] [CrossRef]
- Wakabayashi, K. Changes in cell wall polysaccharides during fruit ripening. J. Plant Res. 2000, 113, 231–237. [Google Scholar] [CrossRef]
- Cao, S.F.; Zheng, Y.H.; Wang, K.T.; Rui, H.J.; Tang, S.S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.J.; Fu, G.F.; Tao, L.X. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef]
- Gomez-Ros, L.V.; Gabaldon, C.; Nunez-Flores, M.J.L.; Gutierrez, J.; Herrero, J.; Zapata, J.M.; Sottomayor, M.; Cuello, J.; Barcelo, A.R. The promoter region of the Zinnia elegans basic peroxidase isoenzyme gene contains cis-elements responsive to nitric oxide and hydrogen peroxide. Planta 2012, 236, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Dangol, S.; Chen, Y.F.; Hwang, B.K.; Jwa, N.S. Iron- and reactive oxygen species-dependent ferroptotic cell death in rice-Magnaporthe oryzae interactions. Plant Cell 2019, 31, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; MaClean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, N.; Dey, P.; Maiti, M.K. Transgenically expressed rice germin-like protein1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components. Biochem. Biophys. Res. Commun. 2010, 402, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]

 Represents negative effects. AsA: ascorbic acid; ROS: reactive oxygen species.
Represents negative effects. AsA: ascorbic acid; ROS: reactive oxygen species.
 Represents negative effects. AsA: ascorbic acid; ROS: reactive oxygen species.
Represents negative effects. AsA: ascorbic acid; ROS: reactive oxygen species.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Shi, D.; Wu, Q.; Yin, C.; Li, F.; Shan, Y.; Duan, X.; Jiang, Y. Mechanism of Cell Wall Polysaccharides Modification in Harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) Fruit Caused by Penicillium italicum. Biomolecules 2019, 9, 160. https://doi.org/10.3390/biom9040160
Li T, Shi D, Wu Q, Yin C, Li F, Shan Y, Duan X, Jiang Y. Mechanism of Cell Wall Polysaccharides Modification in Harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) Fruit Caused by Penicillium italicum. Biomolecules. 2019; 9(4):160. https://doi.org/10.3390/biom9040160
Chicago/Turabian StyleLi, Taotao, Dingding Shi, Qixian Wu, Chunxiao Yin, Fengjun Li, Youxia Shan, Xuewu Duan, and Yueming Jiang. 2019. "Mechanism of Cell Wall Polysaccharides Modification in Harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) Fruit Caused by Penicillium italicum" Biomolecules 9, no. 4: 160. https://doi.org/10.3390/biom9040160
APA StyleLi, T., Shi, D., Wu, Q., Yin, C., Li, F., Shan, Y., Duan, X., & Jiang, Y. (2019). Mechanism of Cell Wall Polysaccharides Modification in Harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) Fruit Caused by Penicillium italicum. Biomolecules, 9(4), 160. https://doi.org/10.3390/biom9040160







