Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds, Bacteria, and Animals
2.2. Preparation of the Extracts
2.3. Gas Chromatography–Mass Spectrometry Analysis
2.4. DPPH Radical Scavenging Assay
2.5. Cytotoxicity and Cell Viability Assay
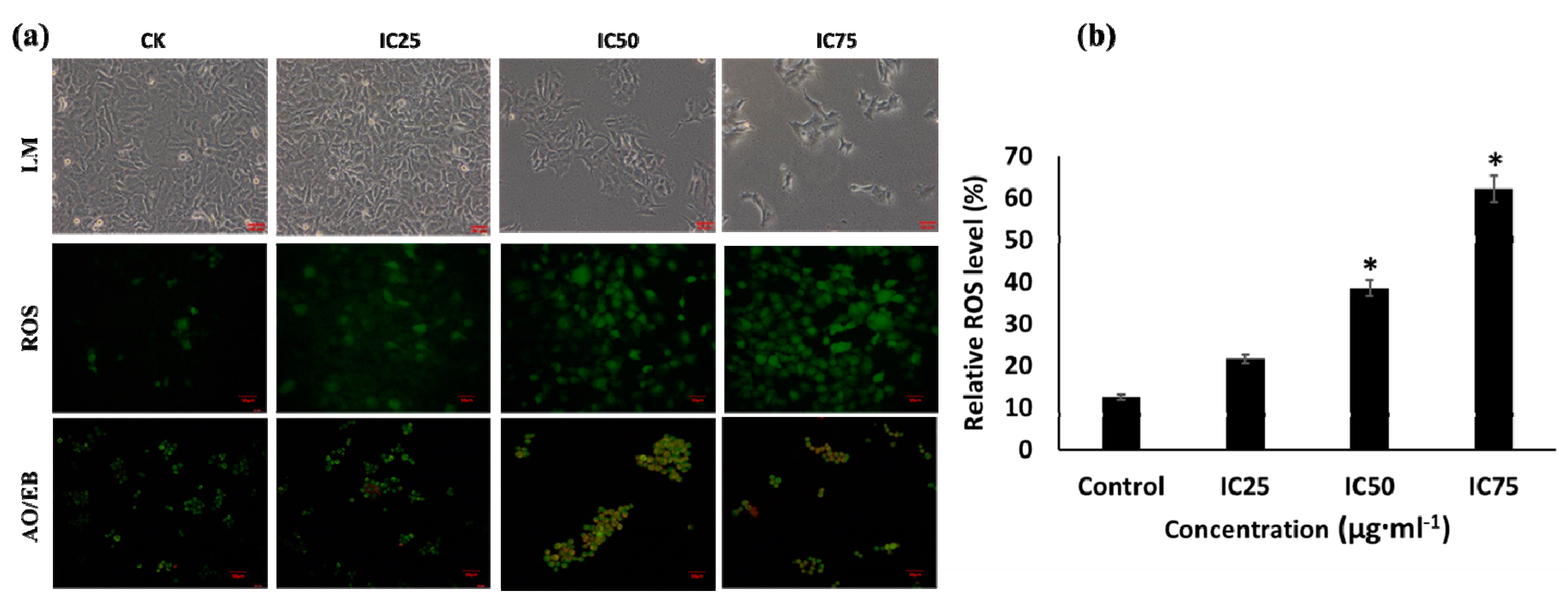
2.6. Reactive Oxygen Species
2.7. Acridine Orange (AO)/Ethidium Bromide (EB) Staining Assay
2.8. Cell Cycle Assay
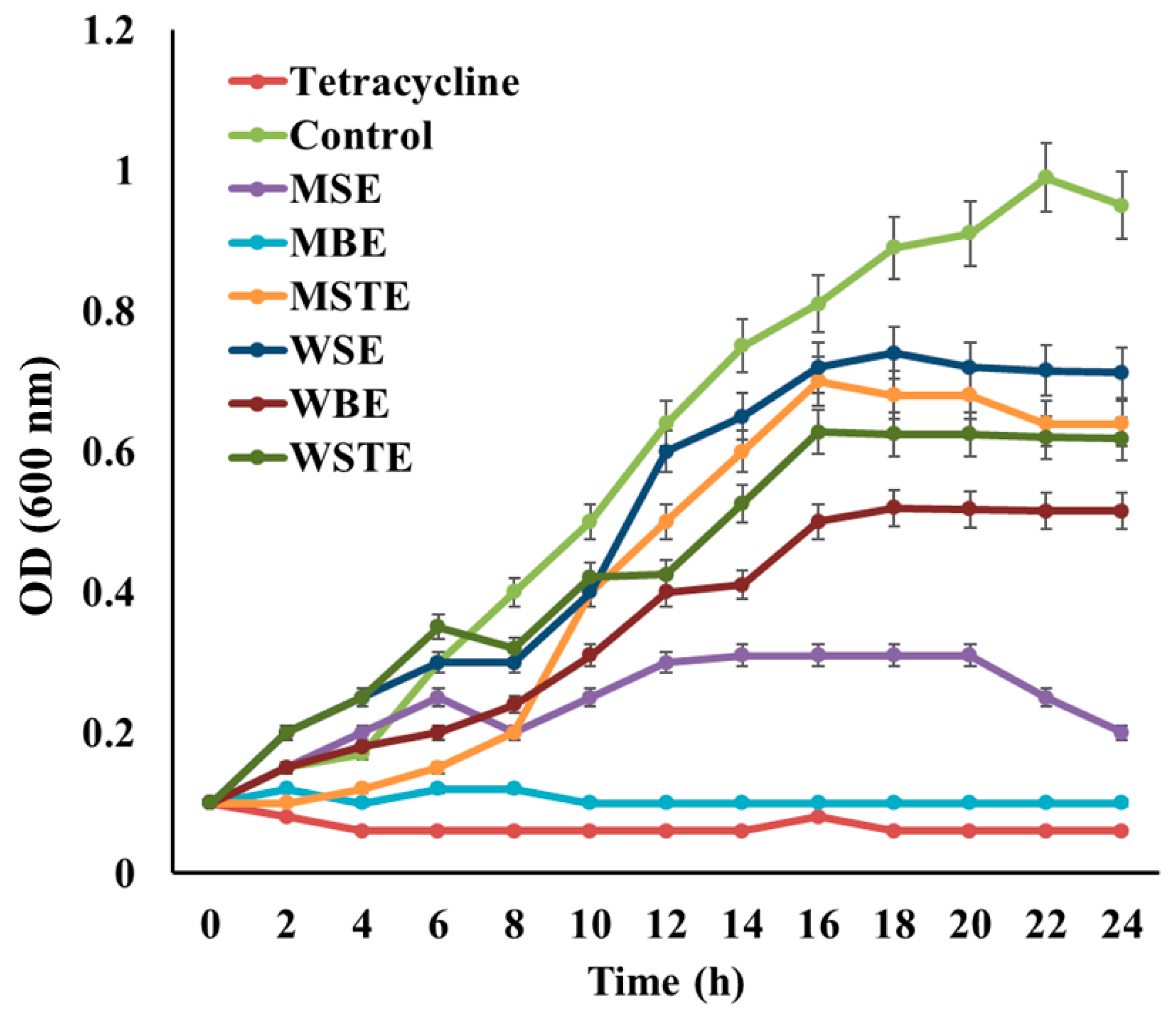
2.9. In Vitro Antibacterial Assay
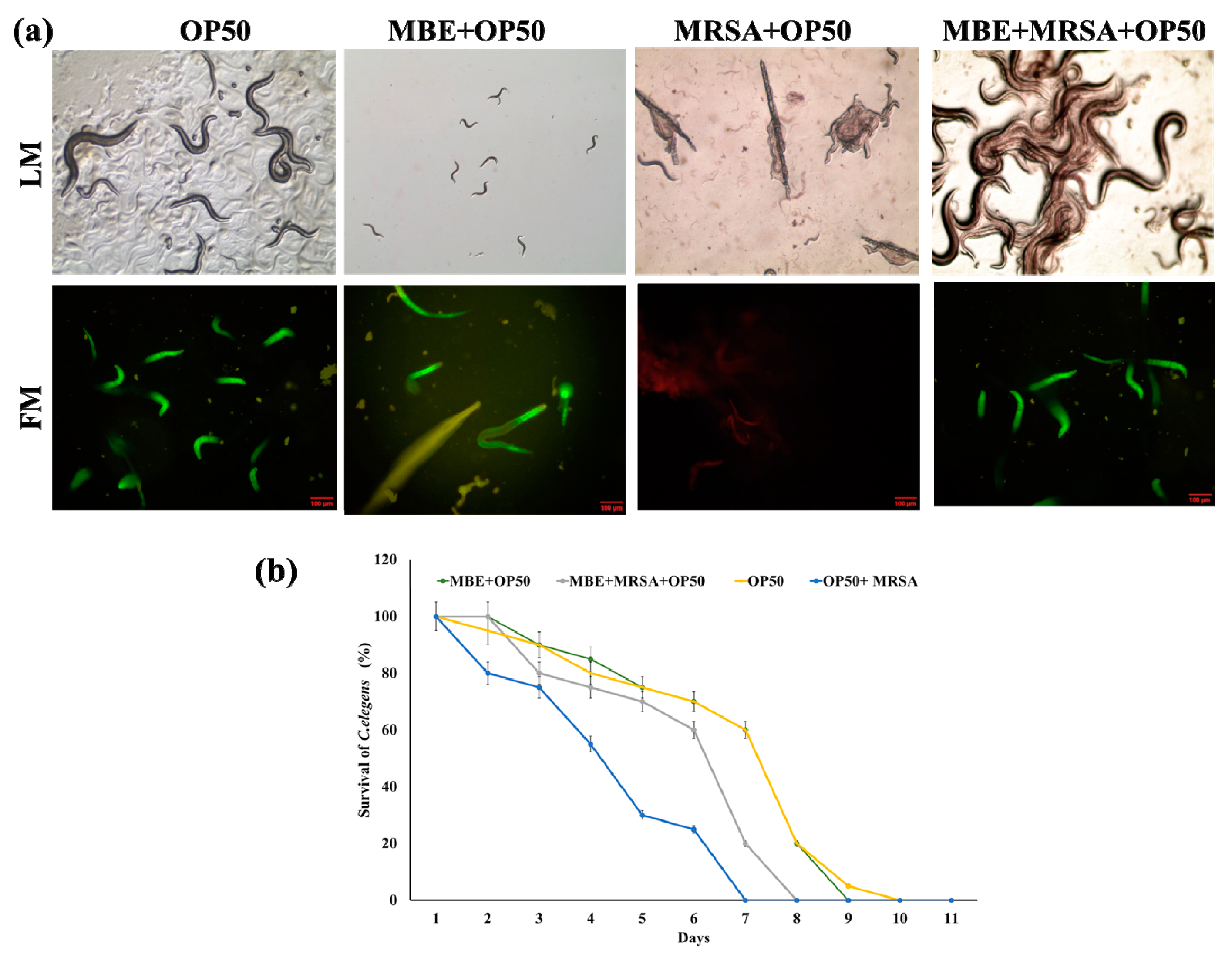
2.10. In Vivo Antibacterial Assay
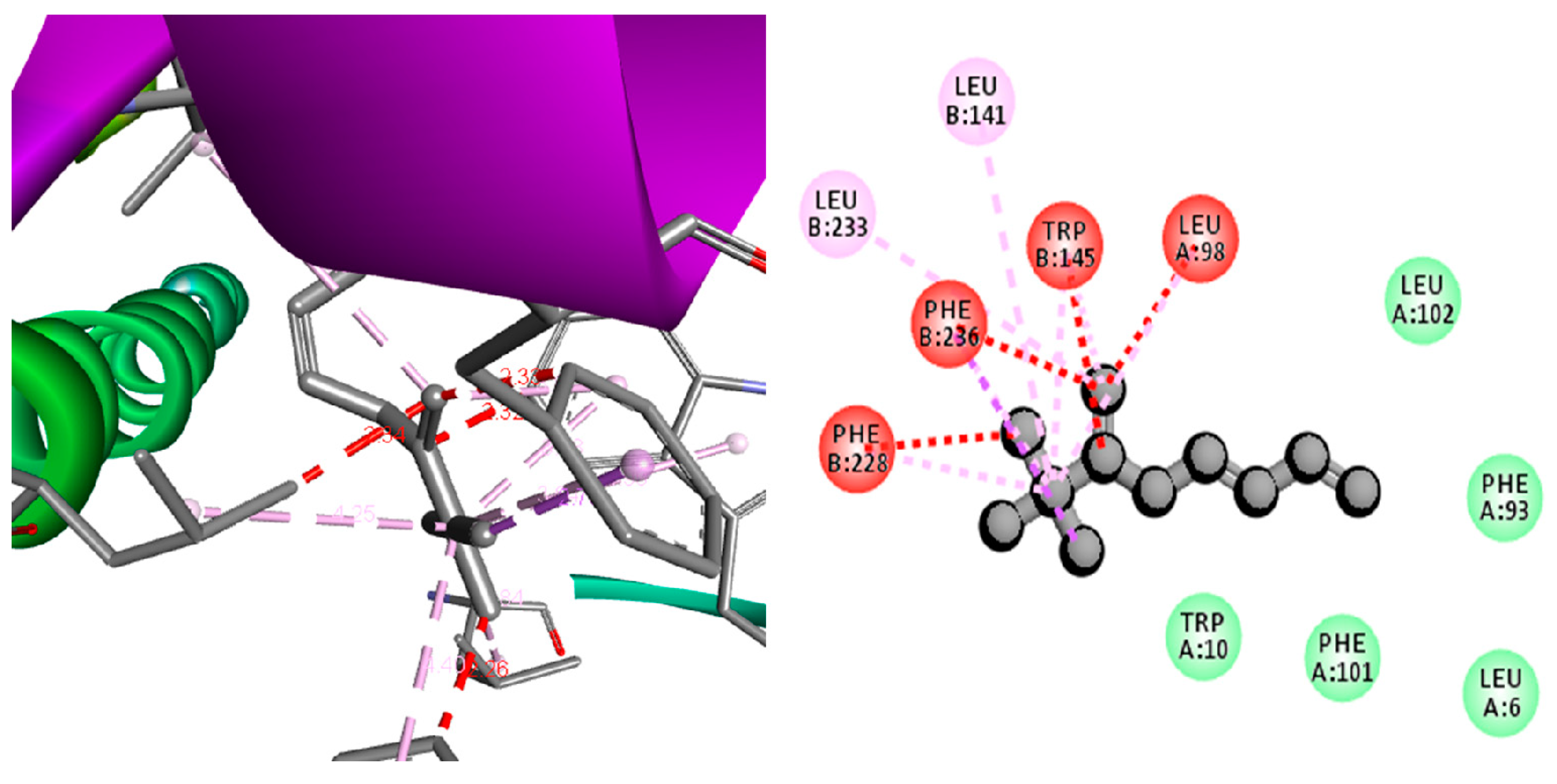
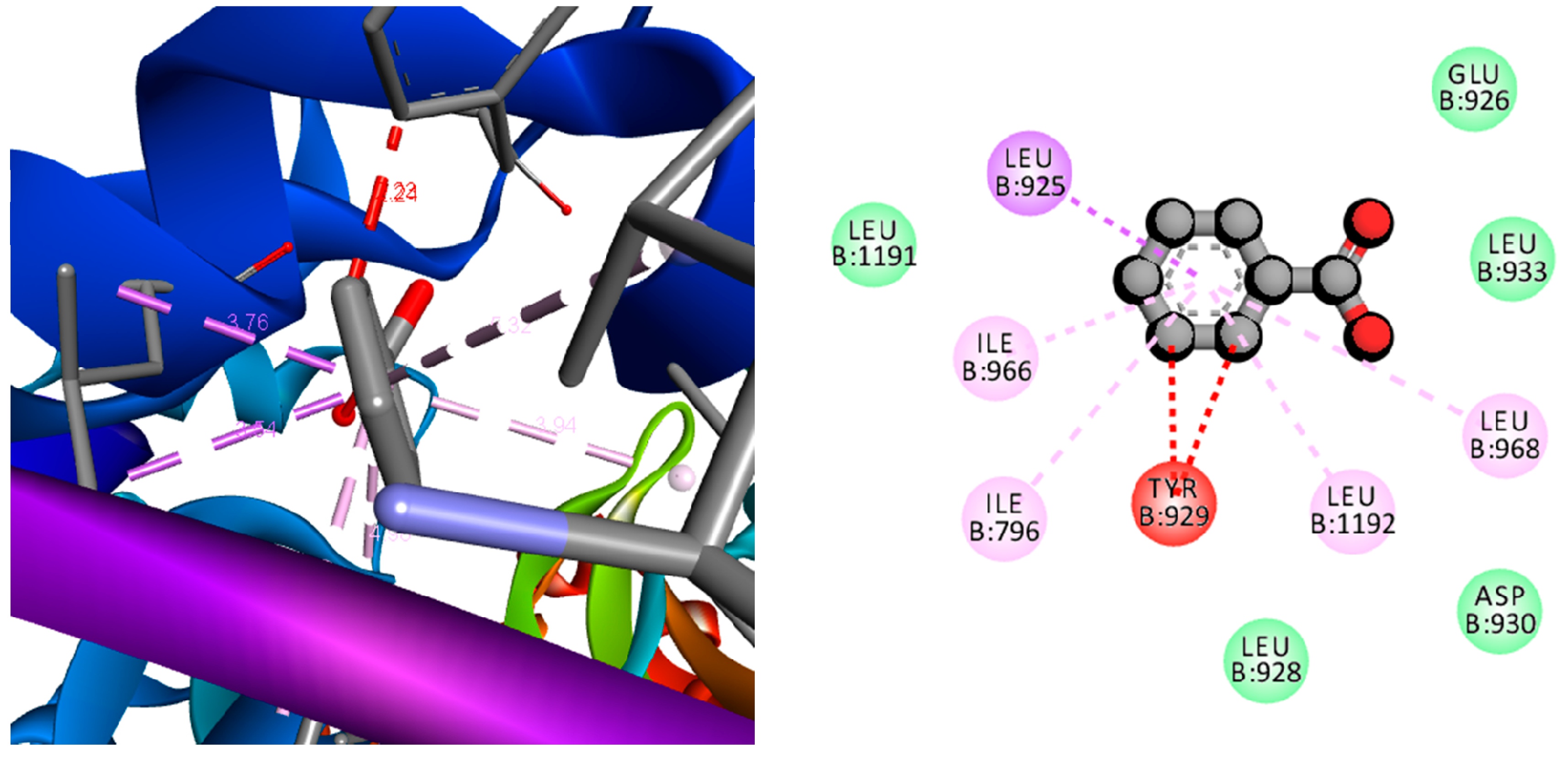
2.11. Computational Screening of the Compounds
3. Results and Discussion
3.1. Gas Chromatography–Mass Spectrometry (GC-MS) Based Identification Metabolites
3.2. Antioxidant Assay
3.3. Anti-Lung Cancer Activity
3.4. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborty, A.; Pal, N.; Sarkar, S.; Gupta, M. Antibiotic resistance pattern of Enterococci isolates from nosocomial infections in a tertiary care hospital in Eastern India. J. Nat. Sci. Biol. Med. 2015, 6, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Gashaw, M.; Berhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Ahmed, Y.; Fentahun, N.; Assefa, H.; Wieser, A.; et al. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: A cross sectional study. Antimicrob. Resist. Infect. Control 2018, 7, 138. [Google Scholar] [CrossRef]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Prasanna, G.; Ujwal, A.; Diliprajudominic, S.; Marimuthu, T.; Saraswathi, N.T. A new pipeline to discover antimycotics by inhibiting ergosterol and riboflavin synthesis: The inspirations of Siddha medicine. Med. Chem. Res. 2014, 23, 2651–2658. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry 2013, 92, 113–121. [Google Scholar] [CrossRef]
- Petlevski, R.; Flajs, D.; Kalođera, Z.; Zovko Končić, M. Composition and antioxidant activity of aqueous and ethanolic Pelargonium radula extracts. S. Afr. J. Bot. 2013, 85, 17–22. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Cesa, S.; Sisto, F.; Zengin, G.; Scaccabarozzi, D.; Kokolakis, A.K.; Scaltrito, M.M.; Grande, R.; Locatelli, M.; Cacciagrano, F.; Angiolella, L.; et al. Phytochemical analyses and pharmacological screening of Neem oil. S. Afr. J. Bot. 2018, 120, 331–337. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2018, 120, 253–260. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, E.; Mudili, V.; Kalagatur, N.K.; Anthuvan, A.J.; Krishna, K.; Thangaraj, P.; Natarajan, G. Antioxidant and anticancer activities of Plectranthus stocksii Hook. f. leaf and stem extracts. Agric. Nat. Resour. 2017, 51, 63–73. [Google Scholar] [CrossRef]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2018, 8. [Google Scholar] [CrossRef]
- Jang, J.Y.; Shin, H.; Lim, J.W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.J.; Kang, S.Y.; Lee, M.K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar] [CrossRef]
- Bosma-den Boer, M.M.; van Wetten, M.L.; Pruimboom, L. Chronic inflammatory diseases are stimulated by current lifestyle: How diet, stress levels and medication prevent our body from recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, Y.C.; Ko, S.G. Integrating traditional medicine into modern inflammatory diseases care: Multitargeting by Rhus verniciflua Stokes. Mediat. Inflamm. 2014, 2014, 154561. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Schimmer, A.D. Inhibitor of Apoptosis Proteins: Translating Basic Knowledge into Clinical Practice. Cancer Res. 2004, 64, 7183–7190. [Google Scholar] [CrossRef] [Green Version]
- Mavroeidis, L.; Sheldon, H.; Briasoulis, E.; Marselos, M.; Pappas, P.; Harris, A.L. Metronomic vinorelbine: Anti-angiogenic activity in vitro in normoxic and severe hypoxic conditions, and severe hypoxia-induced resistance to its anti-proliferative effect with reversal by Akt inhibition. Int. J. Oncol. 2015, 47, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Altieri, D.C. Characterization of Locus and Transcriptional Requirements of Basal and Cell Cycle-dependent Expression. Cancer Res. 1999, 59, 3143–3151. [Google Scholar]
- Shakeel, E.; Akhtar, S.; Khan, M.K.A.; Lohani, M.; Arif, J.M.; Siddiqui, M.H. Molecular docking analysis of aplysin analogs targeting survivin protein. Bioinformation 2017, 13, 293–300. [Google Scholar] [CrossRef]
- Zhu, N.; Gu, L.; Li, F.; Zhou, M. Inhibition of the Akt/survivin pathway synergizes the antileukemia effect of nutlin-3 in acute lymphoblastic leukemia cells. Mol. Cancer Ther. 2008, 7, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendram, M.; Hurley, K.A.; Foss, M.H.; Thornton, K.M.; Moore, J.T.; Shaw, J.T.; Weibel, D.B. Gyramides prevent bacterial growth by inhibiting DNA gyrase and altering chromosome topology. ACS Chem. Boil. 2014, 9, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Franco-Ulloa, S.; La Sala, G.; Miscione, G.P.; De Vivo, M. Novel Bacterial Topoisomerase Inhibitors Exploit Asp83 and the Intrinsic Flexibility of the DNA Gyrase Binding Site. Int. J. Mol. Sci. 2018, 19, 453. [Google Scholar] [CrossRef] [PubMed]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting bacterial DNA gyrase as a drug target: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Charrier, C.; Salisbury, A.M.; Savage, V.J.; Duffy, T.; Moyo, E.; Chaffer-Malam, N.; Ooi, N.; Newman, R.; Cheung, J.; Metzger, R.; et al. Novel Bacterial Topoisomerase Inhibitors with Potent Broad-Spectrum Activity against Drug-Resistant Bacteria. Antimicrob. Agents Chemother. 2017, 61, e02100–e02116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Bradley, P.; Lu, J.; et al. Oxabicyclooctane-Linked Novel Bacterial Topoisomerase Inhibitors as Broad Spectrum Antibacterial Agents. ACS Med. Chem. Lett. 2014, 5, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.M.; Gill, C.J.; Wu, J.; Toussaint, N.; Yin, J.; Tsuchiya, T.; Garlisi, C.G.; Kaelin, D.; Meinke, P.T.; Miesel, L.; et al. In Vitro and In Vivo Characterization of the Novel Oxabicyclooctane-Linked Bacterial Topoisomerase Inhibitor AM-8722, a Selective, Potent Inhibitor of Bacterial DNA Gyrase. Antimicrob. Agents Chemother. 2016, 60, 4830–4839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Um, E.S.; Jung, B.B.; Choi, E.S.; Kim, E.Y.; Lee, S.; Jang, E.; Lee, J.H.; Kim, Y. Rhus verniciflua Stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol. Rep. 2018, 39, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, G.O.; Lee, K.T.; Choi, J.; Choi, M.Y.; Kim, G.T.; Jung, H.J.; Park, H.J. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004, 90, 73–79. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Chun, W.J.; Kim, T.Y.; Sun, J.; Yu, C.Y.; Kim, M.J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.J.; Lee, H.J.; Choi, W.C.; Yoon, S.W.; Ko, S.G.; Ahn, K.S.; Choi, S.H.; Ahn, K.S.; Lieske, J.C.; et al. Rhus verniciflua Stokes prevents cisplatin-induced cytotoxicity and reactive oxygen species production in MDCK-I renal cells and intact mice. Phytomedicine 2009, 16, 188–197. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chellia, R.; Hu, X.; Kathiresan, K.; Oh, D.H.; Wang, M.H. Eradication of Helicobacter pylori through the inhibition of urease and peptide deformylase: Computational and biological studies. Microb. Pathog. 2019, 128, 236–244. [Google Scholar] [CrossRef]
- Saravanan, M.; Senthilkumar, P.; Kalimuthu, K.; Chinnadurai, V.; Vasantharaj, S.; Pugazhendhi, A. Phytochemical and pharmacological profiling of Turnera subulata Sm., a vital medicinal herb. Ind. Crop. Prod. 2018, 124, 822–833. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Vivek, R.; Sithranga Boopathy, N.; Yaqian, L.; Kathiresan, K.; Chen, J. Anticancer potential of bioactive 16-methylheptadecanoic acid methyl ester derived from marine Trichoderma. J. Appl. Biomed. 2015, 13, 199–212. [Google Scholar] [CrossRef]
- Mason, P. Central mechanisms of pain modulation. Curr. Opin. Neurobiol. 1999, 9, 436–441. [Google Scholar] [CrossRef]
- Jiang, G.Z.; Li, J.C. Protective Effects of Ginsenoside Rg1 Against Colistin Sulfate-Induced Neurotoxicity in PC12 Cells. Cell. Mol. Neurobiol. 2014, 34, 167–172. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; Shanmugam, S.; Varukattu, N.B.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J. Photochem. Photobiol. B Biol. 2018, 185, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Fujita, T.; Stankovic, K.M.; Welling, D.B.; Moon, I.S.; Choi, J.Y.; Yun, J.; Kang, J.S.; Lee, J.D. Sulforaphane, a natural component of broccoli, inhibits vestibular schwannoma growth in vitro and in vivo. Sci. Rep. 2016, 6, 36215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, K.; Wang, M.H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microb. Pathog. 2018, 114, 269–273. [Google Scholar] [CrossRef]
- Brumfitt, W.; Hamilton-Miller, J.M.; Franklin, I. Antibiotic activity of natural products: 1. Propolis. Microbios 1990, 62, 19–22. [Google Scholar] [PubMed]
- Rubab, M.; Chellia, R.; Saravanakumar, K.; Mandava, S.; Khan, I.; Tango, C.N.; Hussain, M.S.; Daliri, E.B.M.; Kim, S.H.; Ramakrishnan, S.R.; et al. Preservative effect of Chinese cabbage (Brassica rapa subsp. pekinensis) extract on their molecular docking, antioxidant and antimicrobial properties. PLoS ONE 2018, 13, e0203306. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO Pathway Mediates Longevity Induced by a Novel Method of Dietary Restriction in C. elegans. Curr. Biol. 2007, 17, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Negi, B.; Kumar, D.; Kumbukgolla, W.; Jayaweera, S.; Ponnan, P.; Singh, R.; Agarwal, S.; Rawat, D.S. Anti-methicillin resistant Staphylococcus aureus activity, synergism with oxacillin and molecular docking studies of metronidazole-triazole hybrids. Eur. J. Med. Chem. 2016, 115, 426–437. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Jung, H.S.; Eo, W.K.; Lee, S.K.; Lee, S.Y.; Kim, S.H.; Shim, B.S. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: Report of two cases. Ann. Oncol. 2010, 21, 1383–1385. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, M.J. Anti-oxidant activity of Rhus verniciflua stokes by extract conditions. J. Med. Chem. Plants Res. 2011, 5, 2617–2623. [Google Scholar]
- Choi, H.S.; Kim, M.K.; Park, H.S.; Yun, S.E.; Mun, S.P.; Kim, J.; Sapkota, K.; Kim, S.; Kim, T.Y.; Kim, S.J. Biological detoxification of lacquer tree (Rhus verniciflua Stokes) stem bark by mushroom species. Food Sci. Biotechnol. 2007, 16, 935–942. [Google Scholar]
- Hema, R.; Kumaravel, S.; Alagusundaram, K. GC/MS determination of bioactive components of Murraya koenigii. J. Am. Sci. 2011, 7, 80–83. [Google Scholar]
- Belakhdar, G.; Benjouad, A.; Abdennebi, E.H. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015, 6, 2778–2783. [Google Scholar]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, J.H.; Hong, M.H.; Seog, H.M.; Oh, S.H.; Lee, P.J.; Kim, G.J.; Kim, H.M.; Um, J.Y.; Ko, S.G. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-κB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2007, 110, 490–497. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, D.S.; Song, M.Y.; Park, B.H.; Kang, D.G.; Lee, H.S.; Kwon, K.B.; Kim, Y.C. Butein from Rhus verniciflua Protects Pancreatic & beta; Cells against Cytokine-Induced Toxicity Mediated by Inhibition of Nitric Oxide Formation. Biol. Pharm. Bull. 2011, 34, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kim, S.H.; Kim, I.S.; Lee, D.; Dong, M.S.; Na, C.S.; Nhiem, N.X.; Yoo, H.H. Simultaneous determination of bioactive phenolic compounds in the stem extract of Rhus verniciflua stokes by high performance liquid chromatography. Food Chem. 2013, 141, 3813–3819. [Google Scholar] [CrossRef]
- Lee, J.C.; Lim, K.T.; Jang, Y.S. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim. Biophys. Acta General Subj. 2002, 1570, 181–191. [Google Scholar] [CrossRef]
- Lim, K.T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef]
- Kitts, D.D.; Lim, K.T. Antitumorigenic and cytotoxic properties of an ethanol extract derived from Rhus verniciflua Stokes (RVS). J. Toxicol. Environ. Health A 2001, 64, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Lee, S.J.; Lim, K.T. 36kDa Glycoprotein isolated from Rhus verniciflua Stokes fruit has a protective activity to glucose/glucose oxidase-induced apoptosis in NIH/3T3 cells. Toxicol. In Vitro 2005, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res./Fundam. Mol. Mech. Mutag. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, A.; Pipelzadeh, M.H.; Aghel, N.; Esmaeilian, M.; Zali, I. A Comparative Study upon the Therapeutic Indices of Some Natural and Synthetic Anti-inflammatory Agents. Iran. J. Basic Med. Sci. 2011, 14, 340–348. [Google Scholar] [PubMed]
- Tuama, A.A.; Mohammed, A.A. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi J. Biol. Sci. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Invest. 2015, 125, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Chiaradia, L.D.; Silva, A.H.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B. Involvement of extrinsic and intrinsic apoptotic pathways together with endoplasmic reticulum stress in cell death induced by naphthylchalcones in a leukemic cell line: Advantages of multi-target action. Toxicol. In Vitro 2014, 28, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501. [Google Scholar] [CrossRef]
- Murad, H.; Hawat, M.; Ekhtiar, A.; AlJapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I. Cyclin-Dependent Kinase Pathways as Targets for Cancer Treatment. J. Clin. Oncol. 2006, 24, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.G.; Walker, C.L. Cyclins and cell cycle checkpoints. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, K.Y.; Kim, J.; Na, C.S.; Jung, N.C.; Chung, G.H.; Jang, Y.S. Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem. Toxicol. 2004, 42, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Choi, J.H.; Yang, H.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kook, S.H.; Son, Y.O.; Kim, J.G.; Jeon, Y.M.; Jang, Y.S.; Choi, K.C.; Kim, J.; Han, S.K.; Lee, K.Y.; et al. Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim. Biophys. Acta General Subj. 2005, 1726, 309–316. [Google Scholar] [CrossRef] [PubMed]





| Extracts | IUPAC Name of Compound | Retention Time | Area (%) | Molecular Weight (g/mol) | Antibacterial Protein | Anti-Pro Apoptotic Protein | |
|---|---|---|---|---|---|---|---|
| 4PLB | 3ZFZ | 1F3H | |||||
| MSTE | |||||||
| 1 | Pyrazolo[1,5-a]pyridine | 21.73 | 2.56 | 118.14 | −7.34 | −7.51 | −8.02 |
| 2 | N,N-dimethylquinolin-2-amine | 20.957 | 28.19 | 172.231 | −8.50 | −8.95 | −10.61 |
| 3 | Benzaldehyde | 22.721 | 5.88 | 106.124 | −8.41 | −9.46 | −10.15 |
| 4 | methyl-bis(trimethylsilyloxy)silicon | 23.063 | 4.13 | 221.498 | −7.98 | −8.98 | −10.44 |
| 5 | Tetrasiloxane | 24.158 | 7.45 | 170.417 | −3.56 | −3.57 | −3.51 |
| MBE | |||||||
| 1 | Hexamethylcyclotrisiloxane | 3.455 | 17.59 | 222.46 | −7.40 | −8.29 | −9.34 |
| 2 | Octamethylcyclotetrasiloxane | 5.562 | 1.15 | 296.62 | −7.76 | −7.92 | −10.82 |
| 3 | 2,3,3-trimethyl-Octane | 15.809 | 1.56 | 156.308 | −13.74 | −11.25 | −14.9 |
| 4 | 2,5-Bis-(4-bromophenyl)pyridine | 18.404 | 0.92 | 389.084 | −10.12 | −11.04 | −14.01 |
| 5 | 1-Ethenyl-4-methylbenzene | 20.915 | 4.09 | 117.171 | −9.77 | −9.01 | −12.87 |
| 6 | Benzoic acid | 20.915 | 16.38 | 122.123 | −8.39 | −12.72 | −9.88 |
| 7 | Benzene, 1-ethoxy-2-(2-nitro-1-propenyl)-, (E)- (9CI) | 22.721 | 1.63 | 207.226 | −9.45 | −9.34 | −11.52 |
| 8 | Pentadioic acid, dihydrazide N2,N2’-bis(2-furfurylideno) | 23.022 | 1.13 | 316.312 | −9.36 | −9.3 | −9.51 |
| Antibiotic | |||||||
| Oxacillin | - | - | - | −11.02 | −10.25 | - | |
| Cancer Drug | |||||||
| Celecoxib | - | - | −11.49 | ||||
| Extract Code | CC50 in NIH3T3 (µg·mL−1) | IC50 A549 (µg·mL−1) | Therapeutic Index (CC50/IC50) (µg·mL−1) |
|---|---|---|---|
| MSE | 500 | 218.75 | 2.28 |
| MBE | 130.25 | 5.85 | 22.26 |
| MSTE | 62.5 | 156.25 | 0.40 |
| WSE | ND | 1000 | 0 |
| WBE | 187.5 | 46.8 | 4.00 |
| WSTE | 15.6 | 23.4 | 0.66 |
| Pathogens | Concentration (µg·mL−1) | |||||
|---|---|---|---|---|---|---|
| MSE | MBE | MSTE | WSE | WBE | WSTE | |
| S. aureus | 10.24 ± 1.1 | 7.12 ± 0.8 | 9.25 ± 0.2 | 8.75 ± 0.2 | 9.21 ± 0.8 | 9.45 ± 1.5 |
| S. enteria subp.enterica | 15.24 ± 2.1 | 11.58 ± 1.2 | 15.24 ± 2.1 | 15.75 ± 1.5 | 20.52 ± 0.6 | 16.52 ± 1.5 |
| P. aeruginosa | 8.95 ± 0.8 | 8.52 ± 1.5 | 11.54 ± 1.9 | 14.52 ± 1.6 | 17.24 ± 0.8 | 14.65 ± 1.4 |
| E. coli | 15.2 ± 1.9 | 9.45 ± 1.6 | 9.85 ± 1.2 | 18.65 ± 1.8 | 15.45 ± 0.4 | 21.45 ± 1.8 |
| B. cereus | 8.56 ± 0.5 | 5.86 ± 1.4 | 12.15 ± 1.2 | 18.45 ± 1.4 | 10.25 ± 1.2 | 19.45 ± 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravanakumar, K.; Chelliah, R.; Hu, X.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum. Biomolecules 2019, 9, 127. https://doi.org/10.3390/biom9040127
Saravanakumar K, Chelliah R, Hu X, Oh D-H, Kathiresan K, Wang M-H. Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum. Biomolecules. 2019; 9(4):127. https://doi.org/10.3390/biom9040127
Chicago/Turabian StyleSaravanakumar, Kandasamy, Ramachandran Chelliah, Xiaowen Hu, Deog-Hwan Oh, Kandasamy Kathiresan, and Myeong-Hyeon Wang. 2019. "Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum" Biomolecules 9, no. 4: 127. https://doi.org/10.3390/biom9040127
APA StyleSaravanakumar, K., Chelliah, R., Hu, X., Oh, D.-H., Kathiresan, K., & Wang, M.-H. (2019). Antioxidant, Anti-Lung Cancer, and Anti-Bacterial Activities of Toxicodendron vernicifluum. Biomolecules, 9(4), 127. https://doi.org/10.3390/biom9040127








