Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor’s Intrinsically Disordered Domain
Abstract
1. Introduction
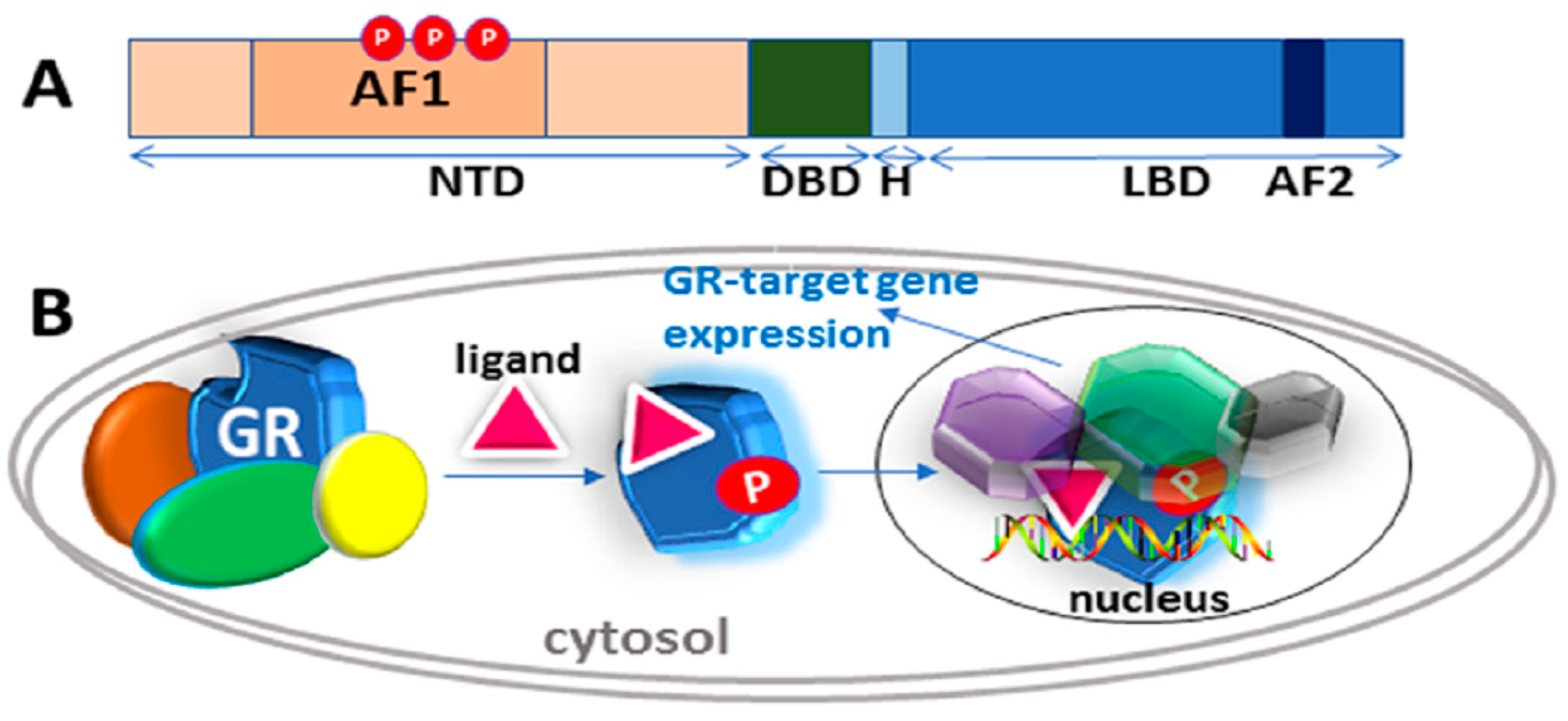
2. The Structure of the Glucocorticoid Receptor and its Gene
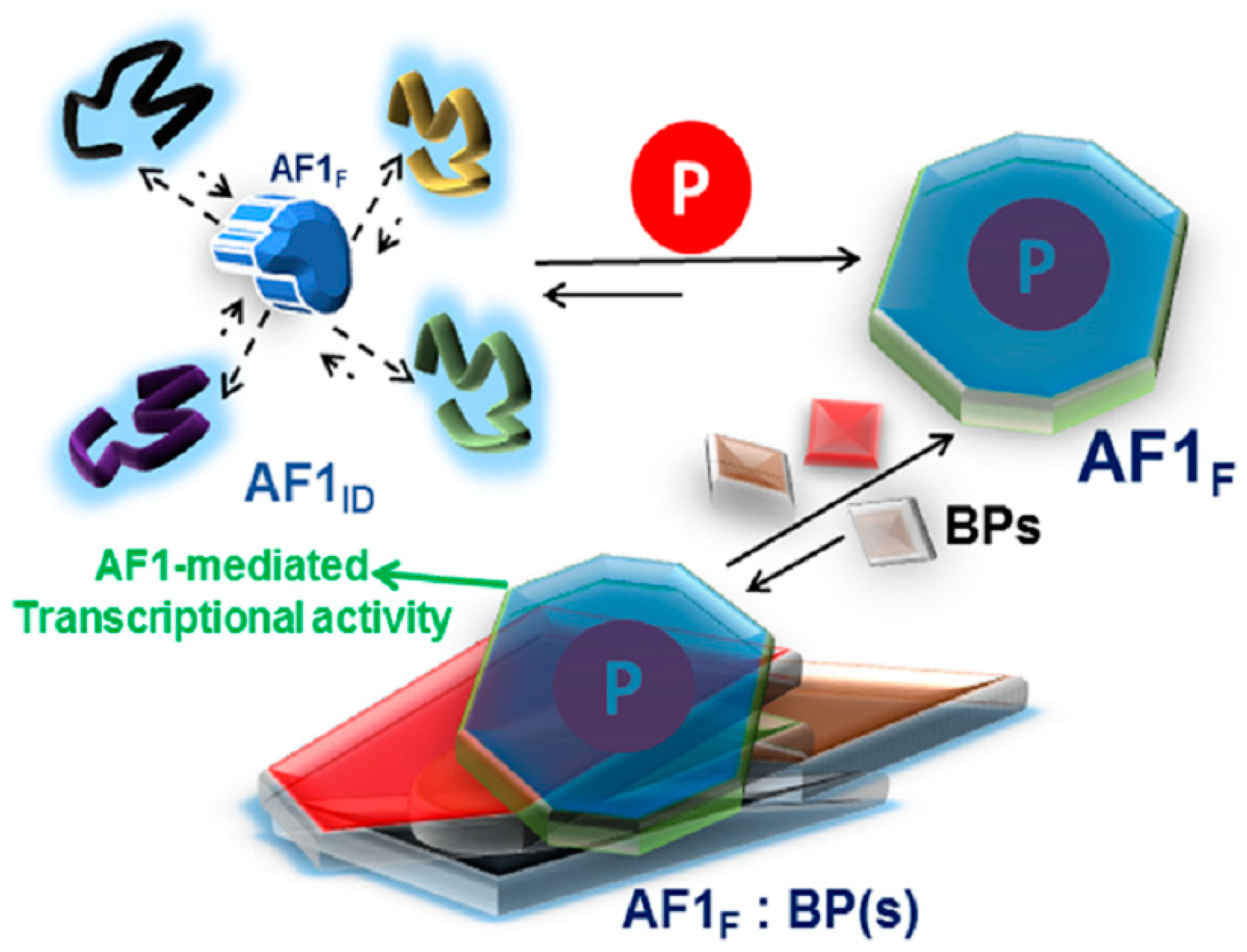
3. The IDP Nature of the GR NTD/AF1 Means that it Can be Thought of as a Large Ensemble of Rapidly Interchanging Conformations
4. Role of Phosphorylation in the Regulation of Intrinsically Disordered AF1 Structure and Functions
5. Discussion
6. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Caratti, G.; Matthews, L.; Poolman, T.; Kershaw, S.; Baxter, M.; Ray, D. Glucocorticoid receptor function in health and disease. Clin. Endocrinol. 2015, 83, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.N.; Hong, M.H.; Negro-Vilar, A. New and improved glucocorticoid receptor ligands. Expert Opin. Investig. Drugs 2005, 14, 1527–1545. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.S.; Kumar, R. Steroid resistance in leukemia. World J. Exp. Med. 2013, 3, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.B.; Morris, S.A.; Hager, G.L. Complex genomic interactions in the dynamic regulation of transcription by the glucocorticoid receptor. Mol. Cell. Endocrinol. 2013, 380, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure and functions relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Johnson, B.H.; Thompson, E.B. Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essay Biochem. 2004, 40, 27–39. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Transactivation functions of the N-terminal domains of nuclear receptors: Protein folding and coactivator interactions. Mol. Endocrinol. 2003, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Thompson, E.B. The structure of the nuclear hormone receptors. Steroids 1999, 64, 310–319. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Weinberger, C.; Ong, E.S.; Cerelli, G.; Oro, A.; Lebo, R.; Thompson, E.B.; Rosenfeld, M.G.; Evans, R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985, 318, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997, 18, 306–360. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Morishima, Y.; Osawa, Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 2008, 283, 22885–22889. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Murphy, P.J.; Chen, J.; Brown, L.; Pratt, W.B.; Simons, S.S., Jr. Mutations at positions 547–553 of rat glucocorticoid receptors reveal that hsp90 binding requires the presence, but not defined composition, of a seven-amino acid sequence at the amino terminus of the ligand binding domain. J. Biol. Chem. 2002, 277, 36223–36232. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Khursheed, B.; Garabedian, M.J.; Fortin, M.G.; Lindquist, S.; Yamamoto, K.R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 1990, 348, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.C.; Yamamoto, K.R. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 2002, 296, 2232–2235. [Google Scholar] [CrossRef] [PubMed]
- Yudt, M.R.; Jewell, C.M.; Bienstock, R.J.; Cidlowski, J.A. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol. Cell. Biol. 2003, 23, 4319–4330. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Z.; Cidlowski, J.A. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 2005, 18, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Luisi, B.F.; Xu, W.X.; Otwinowski, Z.; Freedman, L.P.; Yamamoto, K.R.; Sigler, P.B. Crystallographic Analysis of the Interaction of The Glucocorticoid Receptor with DNA. Nature 1991, 352, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, R.B.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; Mckee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal Structure of the Glucocorticoid Receptor Ligand Binding Domain Reveals a Novel Mode of Receptor Dimerization and Coactivator Recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef]
- Khan, S.H.; Awasthi, S.; Guo, C.; Goswami, D.; Ling, J.; Griffin, P.R.; Simons, S.S., Jr.; Kumar, R. Binding of the N-terminal region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations. J. Biol. Chem. 2012, 287, 44546–44560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lee, J.C.; Bolen, D.W.; Thompson, E.B. The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J. Biol. Chem. 2001, 276, 18146–18152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Moure, C.M.; Khan, S.H.; Callaway, C.; Grimm, S.L.; Goswami, D.; Griffin, P.R.; Edwards, D.P. Regulation of the structurally dynamic disordered amino-terminal domain of progesterone receptor by protein induced folding. J. Biol. Chem. 2013, 288, 30285–30299. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Arnott, J.A.; Kumar, R. Naturally occurring osmolyte, trehalose induces functional conformation in an intrinsically disordered region of the glucocorticoid receptor. PLoS ONE 2011, 6, e19689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; McEwan, I.J. Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation. Endocr. Rev. 2012, 33, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Dahlman-Wright, K.; Almlöf, T.; McEwan, I.J.; Gustafsson, J.A.; Wright, A.P. Delineation of a small region within the major transactivation domain of the human glucocorticoid receptor that mediates transactivation of gene expression. Proc. Natl. Acad. Sci. USA 1994, 91, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Dahlman-Wright, K.; Baumann, H.; McEwan, I.J.; Almlöf, T.; Wright, A.P.; Gustafsson, J.A.; Härd, T. Structural characterization of a minimal functional transactivation domain from the human glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1994, 92, 1699–1703. [Google Scholar] [CrossRef]
- Li, J.; White, J.T.; Saavedra, H.; Wrabl, J.O.; Motlagh, H.N.; Liu, K.; Sowers, J.; Schroer, T.A.; Thompson, E.B.; Hilser, V.J. Genetically tunable frustration controls allostery in an intrinsically disordered transcription factor. Elife 2017, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Pascal, B.; Kumar, R.; Edwards, D.P.; Griffin, P.R. Influence of Domain Interactions on Conformational Mobility of the Progesterone Receptor Detected by Hydrogen/Deuterium Exchange Mass Spectrometry. Structure 2014, 22, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.S.; Edwards, D.P.; Kumar, R. Dynamic Structures of Nuclear Hormone Receptors: New Promises and Challenges. Mol. Endocrinol. 2014, 28, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Betney, R.; Li, J.; Thompson, E.B.; McEwan, I.J. Induced alpha-helix structure in AF1 of the androgen receptor upon binding transcription factor TFIIF. Biochemistry 2004, 43, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically Disordered Proteins in Cellular Signaling and Regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Jolly, M.K.; Jia, D.; Mooney, S.M.; Bhargava, A.; Kagohara, L.T.; Chen, Y.; Hao, P.; He, Y.; Veltri, R.W.; et al. Phosphorylation-induced conformational dynamics in an intrinsically disordered protein and potential role in phenotypic heterogeneity. Proc. Natl. Acad. Sci. USA 2017, 114, E2644–E2653. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Uversky, V.N. Signal transduction via unstructured protein conduits. Nat. Chem. Biol. 2008, 4, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Ling, J.; Kumar, R. TBP binding-induced folding of the glucocorticoid receptor AF1 domain facilitates its interaction with steroid receptor coactivator-1. PLos ONE 2011, 6, e21939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Volk, D.E.; Li, J.; Gorenstein, D.G.; Lee, J.C.; Thompson, E.B. TBP binding induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc. Natl. Acad. Sci. USA 2004, 101, 16425–16430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Perumal, N.B.; Oldfield, C.J.; Su, E.W.; Uversky, V.N.; Dunker, A.K. Intrinsic disorder in transcription factors. Biochemistry 2006, 45, 6873–6888. [Google Scholar] [CrossRef] [PubMed]
- Iakoucheva, L.M.; Brown, C.J.; Lawson, J.D.; Obradovic, Z.; Dunker, A.K. Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol. 2002, 323, 573–584. [Google Scholar] [CrossRef]
- Ward, J.J.; Sodhi, J.S.; McGuffin, L.J.; Buxton, B.F.; Jones, D.T. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 2004, 337, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 2002, 12, 54–60. [Google Scholar] [CrossRef]
- Namba, K. Roles of partly unfolded conformations in macromolecular self-assembly. Genes Cells 2001, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crivici, A.; Ikura, M. Molecular and structural basis of target recognition by calmodulin. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Obradovic, Z.; Dunker, A.K. Natively disordered proteins: Functions and predictions. Appl. Bioinformat. 2004, 3, 105–113. [Google Scholar] [CrossRef]
- Ostertag, M.S.; Messias, A.C.; Sattler, M.; Popowicz, G.M. The Structure of the SPOP-Pdx1 Interface Reveals Insights into the Phosphorylation-Dependent Binding Regulation. Structure 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, T.; Tan, M.H.; Dearsley, O.; Cloosterman, D.; Hii, C.S.; Gécz, J.; Shoubridge, C. Regulating transcriptional activity by phosphorylation: A new mechanism for the ARX homeodomain transcription factor. PLoS One 2018, 13, e0206914. [Google Scholar] [CrossRef] [PubMed]
- Orea-Soufi, A.; Dávila, D.; Salazar-Roa, M.; de Mar Lorente, M.; Velasco, G. Phosphorylation of FOXO Proteins as a Key Mechanism to Regulate Their Activity. Methods Mol. Biol. 2019, 1890, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Ferguson, S.M.; Brugarolas, J.; Ballabio, A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 2018, 37, e98804. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Liu, M.X.; Kreis, W.; Budman, D.R. Phosphorylation/dephosphorylation of androgen as a determinant of androgen agonistic or antagonistic activity. Biochem. Biophys. Res. Commun. 1999, 259, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Weigel, N.L. Phosphorylation and steroid hormone action. Vit. Horm. 1995, 51, 289–313. [Google Scholar]
- Gioeli, D.; Ficarro, S.B.; Kwiek, J.J.; Aaronson, D.; Hancock, M.; Catling, A.D.; White, F.M.; Christian, R.E.; Settlage, R.E.; Shabanowitz, J.; et al. Androgen receptor phosphorylation. J. Biol. Chem. 2002, 32, 29304–29314. [Google Scholar] [CrossRef] [PubMed]
- Rogatsky, I.; Waase, C.L.; Garabedian, M.J. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J. Biol. Chem. 1998, 273, 14315–14321. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.D.; Rogatsky, I.; Yamamoto, K.R.; Garabedian, M.J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 1997, 17, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Bodwell, J.E.; Webster, J.C.; Jewell, C.M.; Cidlowski, J.A.; Hu, J.M.; Munck, A. Glucocorticoid receptor phosphorylation: Overview, function and cell cycle-dependence. J. Steroid Biochem. Mol. Biol. 1998, 65, 91–99. [Google Scholar] [CrossRef]
- Bodwell, J.E.; Orti, E.; Coull, J.M.; Pappin, D.J.C.; Smith, L.I.; Swift, F. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J. Biol. Chem. 1991, 266, 7549–7555. [Google Scholar] [PubMed]
- Ismaili, N.; Garabedian, M.J. Modulation of glucocorticoid receptor function via phosphorylation. Ann. N. Y. Acad. Sci. 2004, 1024, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Housley, P.R. Site-directed mutagenesis of the phosphorylation sites in the mouse glucocorticoid receptor. J. Biol. Chem. 1993, 268, 21501–21504. [Google Scholar] [PubMed]
- Duma, D.; Jewell, C.M.; Cidlowski, J.A. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J. Steroid Biochem. Mol. Biol. 2006, 102, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.M.; Khan, S.H.; Kumar, R. Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Mol. Cell. Biol. 2010, 30, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Webb, M.S.; Copik, A.J.; Wang, Y.; Johnson, B.H.; Kumar, R.; Thompson, E.B. p38 mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: Correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol. Endocrinol. 2005, 19, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; McLaughlin, W.A.; Kumar, R. Site-specific phosphorylation regulates the structure and function of an intrinsically disordered domain of the glucocorticoid receptor. Sci. Rep. 2017, 7, 15440. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.S.; Miller, A.L.; Johnson, B.H.; Thompson, E.B. Converting cell lines representing hematological malignancies from glucocorticoid-resistant to glucocorticoid-sensitive: Signaling pathway interactions. Leuk. Res. 2009, 33, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cidlowski, J.A. The human glucocorticoid receptor: One gene, multiple proteins and diverse responses. Steroids 2005, 70, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.; Kono, E.; Dang, T.; Garabedian, M.J. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol. Endocrinol. 2007, 21, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, N.; Blind, R.; Garabedian, M.J. Stabilization of the unliganded glucocorticoid receptor by TSG101. J. Biol. Chem. 2005, 280, 11120–11126. [Google Scholar] [CrossRef] [PubMed]
- Iakoucheva, L.M.; Radivojac, P.; Brown, C.J.; O’Connor, T.R.; Sikes, J.G.; Obradovic, Z.; Dunker, A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004, 32, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Bah, A.; Forman-Kay, J.D. Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications. J. Biol. Chem. 2016, 291, 6696–6705. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Kumar, R. Role of an intrinsically disordered conformation in AMPK-mediated phosphorylation of ULK1 and regulation of autophagy. Mol. Biosyst. 2012, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Calhoun, W.J. Differential regulation of the transcriptional activity of the glucocorticoid receptor through site-specific phosphorylation. Biol. Targets Ther. 2008, 2, 845–854. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Influence of flanking sequences on signaling between the activation function AF1 and DNA-binding domain of glucocorticoid receptor. Arch. Biochem. Biophys. 2010, 496, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Hudson, R.P.; Kanelis, V.; Choy, W.Y.; Thibodeau, P.H.; Thomas, P.J.; Forman-Kay, J.D. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat. Struct. Mol. Biol. 2007, 14, 738–745. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Y.; Mooney, S.M.; Rajagopalan, K.; Bhargava, A.; Sacho, E.; Weninger, K.; Bryan, P.N.; Kulkarni, P.; Orban, J. Phosphorylation-induced conformational ensemble switching in an intrinsically disordered cancer/testis antigen. J. Biol. Chem. 2015, 290, 25090–25102. [Google Scholar] [CrossRef] [PubMed]
- Bah, A.; Vernon, R.M.; Siddiqui, Z.; Krzeminski, M.; Muhandiram, R.; Zhao, C.; Sonenberg, N.; Kay, L.E.; Forman-Kay, J.D. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 2015, 519, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Mittag, T.; Pawson, T.; Tyres, M.; Forman-Kay, J.D.; Chan, H.S. Polyelectrostatic interactions of disordered ligands suggest a physical basis for ultrasensitivity. Proc. Natl. Acad. Sci. USA 2007, 104, 9650–9655. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Thompson, E.B. Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor’s Intrinsically Disordered Domain. Biomolecules 2019, 9, 95. https://doi.org/10.3390/biom9030095
Kumar R, Thompson EB. Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor’s Intrinsically Disordered Domain. Biomolecules. 2019; 9(3):95. https://doi.org/10.3390/biom9030095
Chicago/Turabian StyleKumar, Raj, and E. Brad Thompson. 2019. "Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor’s Intrinsically Disordered Domain" Biomolecules 9, no. 3: 95. https://doi.org/10.3390/biom9030095
APA StyleKumar, R., & Thompson, E. B. (2019). Role of Phosphorylation in the Modulation of the Glucocorticoid Receptor’s Intrinsically Disordered Domain. Biomolecules, 9(3), 95. https://doi.org/10.3390/biom9030095



