Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties
Abstract
1. Introduction
2. Research Methodology
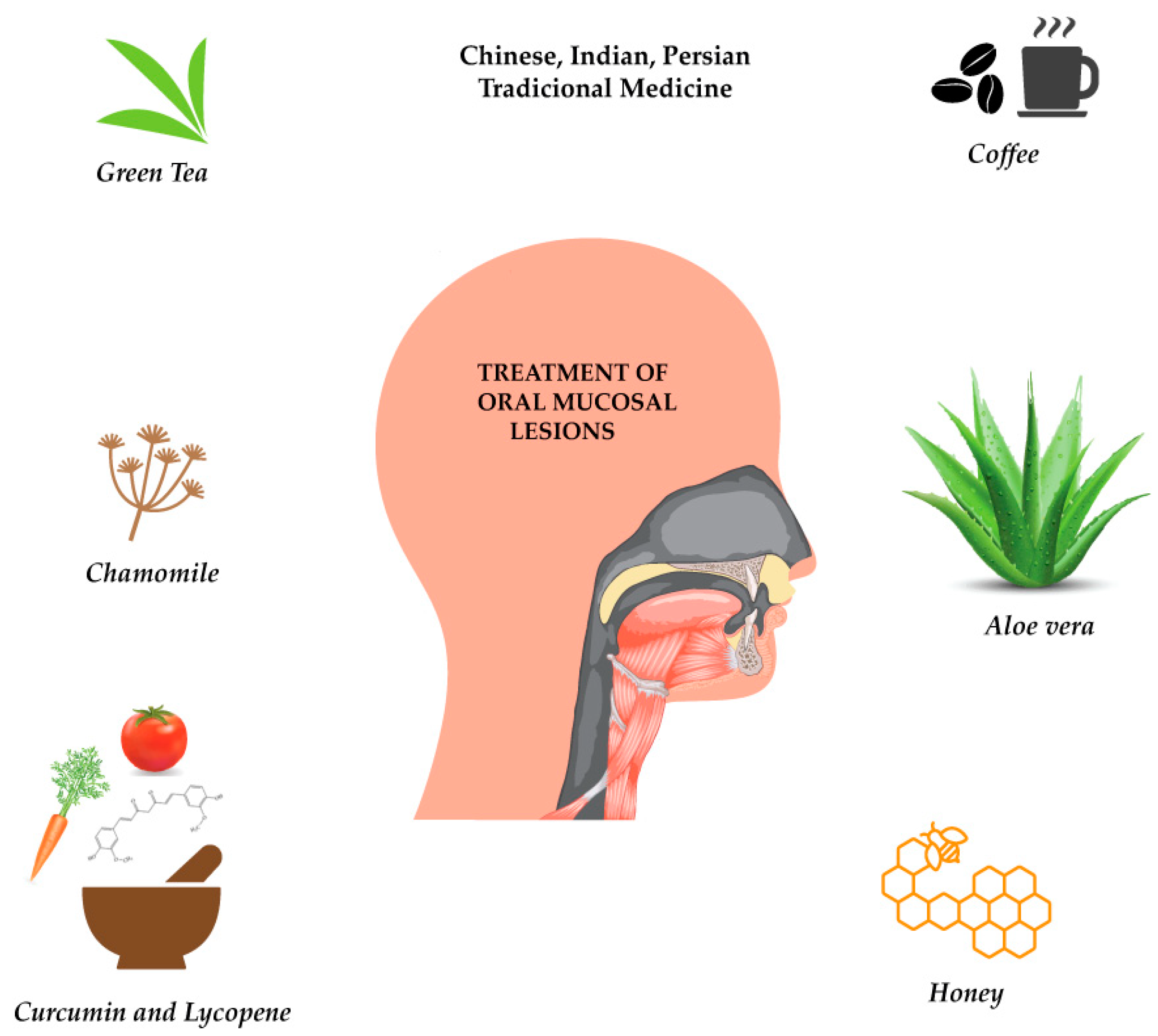
3. Traditional Knowledge on Plants’ Use against Oral Mucosal Lesions
3.1. Traditional Chinese Medicine
3.2. Ayurveda, Indian Traditional System of Medicine
3.3. Traditional Persian Medicine
4. Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee in Oral Mucosal Lesions: A Brief Overview
5. In Vitro and In Vivo Anti-Oral Mucosal Lesions Effects
5.1. Curcumin: Effects on Oral Mucosal Carcinogenesis and Anti-Inflammatory Properties
5.1.1. Effects on Oral Mucosal Carcinogenesis
5.1.2. Anti-Inflammatory Properties
5.2. Lycopene: Antioxidant and Chemopreventive Properties
5.2.1. Antioxidant Properties
5.2.2. Chemopreventive Properties
5.3. Chamomile: Antioxidant, Antimicrobial, Oral Mucosal Protector and Anti-Carcinogenic Effects
5.3.1. Antioxidant Properties
5.3.2. Antimicrobial Properties
5.3.3. Oral Mucosal Protector
5.3.4. Anti-Carcinogenic Properties
5.4. Aloe vera: Oral Lesions Healing and Tissue Regeneration, Immunomodulatory, Anti-Inflammatory, Antioxidant, Antibacterial, Antifungal, Antiviral, and Anti-Carcinogenic Effects
5.4.1. Oral Lesions Healing and Tissue Regeneration
5.4.2. Immunomodulatory Potential
5.4.3. Anti-Inflammatory Potential
5.4.4. Antioxidant Potential
5.4.5. Antibacterial, Antifungal, and Antiviral Potential
5.4.6. Anticarcinogenic Potential
6. Clinical Effects of Phytochemicals in Oral Mucosal Lesions in Humans
6.1. Curcumin
6.2. Lycopene
6.3. Green Tea and Coffee
6.4. Chamomile
6.5. Aloe Vera
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espinoza, I.; Rojas, R.; Aranda, W.; Gamonal, J. Prevalence of oral mucosal lesions in elderly people in Santiago, Chile. J. Oral Pathol. Med. 2003, 32, 571–575. [Google Scholar] [CrossRef]
- Reichart, P.A. Oral mucosal lesions in a representative cross-sectional study of aging germans. Community Dent. Oral Epidemiol. 2000, 28, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. The world oral health report 2003: Continuous improvement of oral health in the 21st century—The approach of the who global oral health programme. Community Dent. Oral Epidemiol. 2003, 31 (Suppl. 1), 3–23. [Google Scholar] [CrossRef] [PubMed]
- Bork, K. Diseases of the lips and mouth. In Braun-Falcoґs Dermatology, 3rd ed.; Burgdorf, W.H.C., Plewig, G., Wolff, H.H., Landthaler, M., Eds.; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Pindborg, J.J. Atlas of Diseases of the Oral Mucosa, 5th ed.; Munksgaard: Copenhagen, Denmark, 1992; 258p. [Google Scholar]
- Al-Maskari, A.Y.; Al-Maskari, M.Y.; Al-Sudairy, S. Oral manifestations and complications of diabetes mellitus: A review. Sultan Qaboos Univ. Med. J. 2011, 11, 179–186. [Google Scholar] [PubMed]
- Yakubov, G.E.; Gibbins, H.; Proctor, G.B.; Carpenter, G.H. Oral Mucosa: Physiological and Physicochemical Aspects. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; John Wiley & Sons: West Sussex, UK, 2014. [Google Scholar]
- Nair, G.R.; Naidu, G.S.; Jain, S.; Nagi, R.; Makkad, R.S.; Jha, A. Clinical effectiveness of aloe vera in the management of oral mucosal diseases—A systematic review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE07. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.; Sharopov, F.; Martorell, M.; Ademiluyi, A.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Leal, A.L.A.B.; et al. Salvia spp. Plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Zahidin, N.S.; Saidin, S.; Zulkifli, R.M.; Muhamad, I.I.; Ya’akob, H.; Nur, H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J. Ethnopharmacol. 2017, 207, 146–173. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Prakash, M.A.; Sharifi-Rad, M.; Shariati, M.; Mabkhot, Y.; Al-Showiman, S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. 2018, 64, 27. [Google Scholar] [CrossRef]
- Mishra, A.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Kawsud, P.; Pahumunto, N.; Puripattanavong, J. Screening for antibacterial and antibiofilm activity in thai medicinal plant extracts against oral microorganisms. J. Tradit. Complement. Med. 2017, 7, 172–177. [Google Scholar] [CrossRef]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An in vitro investigation of indigenous south african medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Hua, H.; Cheung, L.K. Traditional chinese medicine and oral diseases: Today and tomorrow. Oral Dis. 2011, 17, 7–12. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Qiu, Y.L.; An, Z.G.; Yang, F.Y. Role of the chinese herbal medicine xianhuayin on the reversal of premalignant mucosal lesions in the golden hamster buccal pouch. Int. J. Oral Sci. 2010, 2, 53–58. [Google Scholar] [CrossRef]
- Xing, L.; Tan, Z.R.; Cheng, J.L.; Huang, W.H.; Zhang, W.; Deng, W.; Yuan, C.S.; Zhou, H.H. Bioavailability and pharmacokinetic comparison of tanshinones between two formulations of Salvia miltiorrhiza in healthy volunteers. Sci. Rep. 2017, 7, 4709. [Google Scholar] [CrossRef]
- Liu, X.S.; Gao, Y.; Zheng, L.W.; Hua, H. New alternative therapy for orofacial localized scleroderma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, E15–E19. [Google Scholar] [CrossRef]
- He, X.R.; Wang, X.X.; Fang, J.C.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.H.; Huang, X.Q. The genus achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017, 203, 260–278. [Google Scholar] [CrossRef]
- Meyer-Hamme, G.; Beckmann, K.; Radtke, J.; Efferth, T.; Greten, H.J.; Rostock, M.; Schroder, S. A survey of chinese medicinal herbal treatment for chemotherapy-induced oral mucositis. Evid.-Based Complement. Altern. Med. 2013, 2013, 284959. [Google Scholar] [CrossRef]
- Saini, S.; Dhiman, A.; Nanda, S. Traditional indian medicinal plants with potential wound healing activity: A review. Int. J. Pharm. Sci. Res. 2016, 7, 1809–1819. [Google Scholar]
- Bhalang, K.; Thunyakitpisal, P.; Rungsirisatean, N. Acemannan, a polysaccharide extracted from Aloe vera, is effective in the treatment of oral aphthous ulceration. J. Altern. Complement. Med. 2013, 19, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chakraborty, B.; Nandy, S. Aloe vera plant: Review with significant pharmacological activities. Mintage J. Pharm. Med. Sci. 2013, 1, 21–24. [Google Scholar]
- Jamil, S.; Nizami, Q.; Salam, M. Centella asiatica (linn.) urban—A review. Nat. Prod. Radiance 2007, 6, 158–170. [Google Scholar]
- Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P. Stimulation of collagen-synthesis in fibroblast-cultures by a triterpene extracted from centella-asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.B.; Huang, F.; Gong, Z.N.; Meng, Q.Y. Madecassoside isolated from centello asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008, 74, 809–815. [Google Scholar] [CrossRef]
- Pistorius, A.; Willershausen, B.; Steinmeier, E.M.; Kreisler, M. Efficacy of subgingival irrigation using herbal extracts on gingival inflammation. J. Periodontol. 2003, 74, 616–622. [Google Scholar] [CrossRef]
- Naz, S.; Jabeen, S.; Ilyas, S.; Manzoor, F.; Aslam, F.; Ali, A. Antibacterial activity of curcuma longa varieties against different strains of bacteria. Pak. J. Bot. 2010, 42, 455–462. [Google Scholar]
- Nadkarni, K. Indian Materia Medica with Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic and Home Remedies, 3rd ed.; Popular Prakashan: Bombay, India, 1999; Volume 1. [Google Scholar]
- Amruthesh, S.; Pramod, K.; Venkatesh, B.; Ramesh, C. Evaluation of radio protective effects of tinospor acordifolia in patients on radiotherapy for squamous cell carcinoma of head and neck-pilot study. Int. J. Contemp. Dent. 2010, 1, 24–30. [Google Scholar]
- Mandawgade, S.; Patil, K. Wound healing potential of some active principles of Lawsonia alba lam. Leaves. Ind. J. Pharm. Sci. 2003, 65, 390–394. [Google Scholar]
- Hosein Farzaei, M.; Abbasabadi, Z.; Reza Shams-Ardekani, M.; Abdollahi, M.; Rahimi, R. A comprehensive review of plants and their active constituents with wound healing activity in traditional iranian medicine. Wounds 2014, 26, 197–206. [Google Scholar]
- Vasconcelos, L.C.; Sampaio, M.C.; Sampaio, F.C.; Higino, J.S. Use of punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 2003, 46, 192–196. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Aghel, N.; Rashidi, I.; Gholampur-Aghdami, A. Topical grape (Vitis vinifera) seed extract promotes repair of full thickness wound in rabbit. Int. Wound. J. 2011, 8, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, M. The pharmacologic management of common lesions of the oral cavity. Dent. Clin. N. Am. 2016, 60, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Nagi, R.; Patil, D.J.; Rakesh, N.; Jain, S.; Sahu, S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J. Oral Biol. Craniofac. Res. 2018, 8, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Varoni, E.M.; Sharifi-Rad, M.; Rajabi, S.; Zucca, P.; Iriti, M.; Sharifi-Rad, J. Epithelial-mesenchymal transition as a target for botanicals in cancer metastasis. Phytomedicine 2019, 55, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al Zohairy, M.A.; Aly, S.M.; Khan, M.A. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. BioMed Res. Int. 2014, 2014, 761608. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.A.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. Pharmacological profile of apigenin, a flavonoid isolated from matricaria chamomilla. Biochem. Pharmacol. 2000, 59, 1387–1394. [Google Scholar] [CrossRef]
- Pino, J.A.; Bayat, F.; Marbot, R.; Aguero, J. Essential oil of chamomile Chamomilla recutita (L.) rausch from Iran. J. Essent. Oil Res. 2002, 14, 407–408. [Google Scholar] [CrossRef]
- Pirzad, A.; Alyari, H.; Shakiba, M.; Zehtab-Salmasi, S.; Mohammadi, A. Essential oil content and composition of german chamomile (Matricaria chamomilla L.) at different irrigation regimes. J. Agron. 2006, 5, 451–455. [Google Scholar]
- Hamman, J. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, K.; Khatkar, B. Processing, food applications and safety of Aloe vera products: A review. J. Food Sci. Technol. 2011, 48, 525–533. [Google Scholar] [CrossRef]
- Bawankar, R.; Deepti, V.; Singh, P.; Subashkumar, R.; Vivekanandhan, G.; Babu, S. Evaluation of bioactive potential of an Aloe vera sterol extract. Phytother. Res. 2013, 27, 864–868. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Beland, F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 103–154. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.; Perrin, C.; Austin, S.; Vera, F.A. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Misir, J.; Brishti, F.H.; Hoque, M. Aloe vera gel as a novel edible coating for fresh fruits: A review. Am. J. Food Sci. Technol. 2014, 2, 93–97. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Basu, A.; Masek, E.; Ebersole, J.L. Dietary polyphenols and periodontitis—A mini-review of literature. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Nutraceuticals as new treatment approaches for oral cancer—I: Curcumin. Oral Oncol. 2013, 49, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Hirose, Y.; Wang, A.J.; Mori, H.; Satoh, K.; Hara, A.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary curcumin and hesperidin—Comparison with the protective effect of β-carotene. Cancer Res. 1994, 54, 4653–4659. [Google Scholar] [PubMed]
- Ushida, J.; Sugie, S.; Kawabata, K.; Pham, Q.V.; Tanaka, T.; Fujii, K.; Takeuchi, H.; Ito, Y.; Mori, H. Chemopreventive effect of curcumin on N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn. J. Cancer Res. 2000, 91, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Azuine, M.A.; Bhide, S.V. Adjuvant chemoprevention of experimental cancer—Catechin and dietary turmeric in forestomach and oral-cancer models. J. Ethnopharmacol. 1994, 44, 211–217. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.X.; Liao, J.; Yang, G.Y.; Wang, S.; Josephson, Y.; Han, C.; Chen, J.S.; Huang, M.T.; Yang, C.S. Inhibition of 7,12-dimethylbenz a anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 2002, 23, 1307–1313. [Google Scholar] [CrossRef]
- Manoharan, S.; Balakrishnan, S.; Menon, V.P.; Alias, L.M.; Reena, A.R. Chemopreventive efficacy of curcumin and piperine during 7,12-dimethylbenz a anthracene-induced hamster buccal pouch carcinogenesis. Singap. Med. J. 2009, 50, 139–146. [Google Scholar]
- Lin, Y.C.; Chen, H.W.; Kuo, Y.C.; Chang, Y.F.; Lee, Y.J.; Hwang, J.J. Therapeutic efficacy evaluation of curcumin on human oral squamous cell carcinoma xenograft using multimodalities of molecular imaging. Am. J. Chin. Med. 2010, 38, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Patel, V.; Shyur, L.F.; Lee, W.L. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine 2016, 23, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Gomez-Garcia, F. Effect of curcumin and irradiation in PE/CA-PJ15 oral squamous cell carcinoma. Acta Odontol. Scand. 2011, 69, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Javvadi, P.; Hertan, L.; Kosoff, R.; Datta, T.; Kolev, J.; Mick, R.; Tuttle, S.W.; Koumenis, C. Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res. 2010, 70, 1941–1950. [Google Scholar] [CrossRef]
- Tuttle, S.; Hertan, L.; Daurio, N.; Porter, S.; Kaushick, C.; Li, D.; Myamoto, S.; Lin, A.; O’Malley, B.W.; Koumenis, C. The chemopreventive and clinically used agent curcumin sensitizes HPV (−) but not HPV (+) hnscc to ionizing radiation, in vitro and in a mouse orthotopic model. Cancer Biol. Ther. 2012, 13, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Lev-Ari, S.; Vexler, A.; Barnea, I.; Starr, A.; Karaush, V.; Haif, S.; Ben-Yosef, R. Curcumin: A potential radio-enhancer in head and neck cancer. Laryngoscope 2009, 119, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef]
- Nagpal, M.; Sood, S. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3–7. [Google Scholar]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.V.; Prabhuji, M.L.; Roopa, D.A.; Ravirajan, S.; Kishore, H.C. Efficacy of lycopene in the treatment of gingivitis: A randomised, placebo-controlled clinical trial. Oral Health Prev. Dent. 2007, 5, 327–336. [Google Scholar] [PubMed]
- Qin, X.; Qiao, H.; Wu, S.; Cheng, J.; Wan, Q.; Liu, R. Curcumin inhibits monocyte chemoattractant protein-1 expression in TNF-α induced astrocytes through ampk pathway. Neurochem. Res. 2018, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
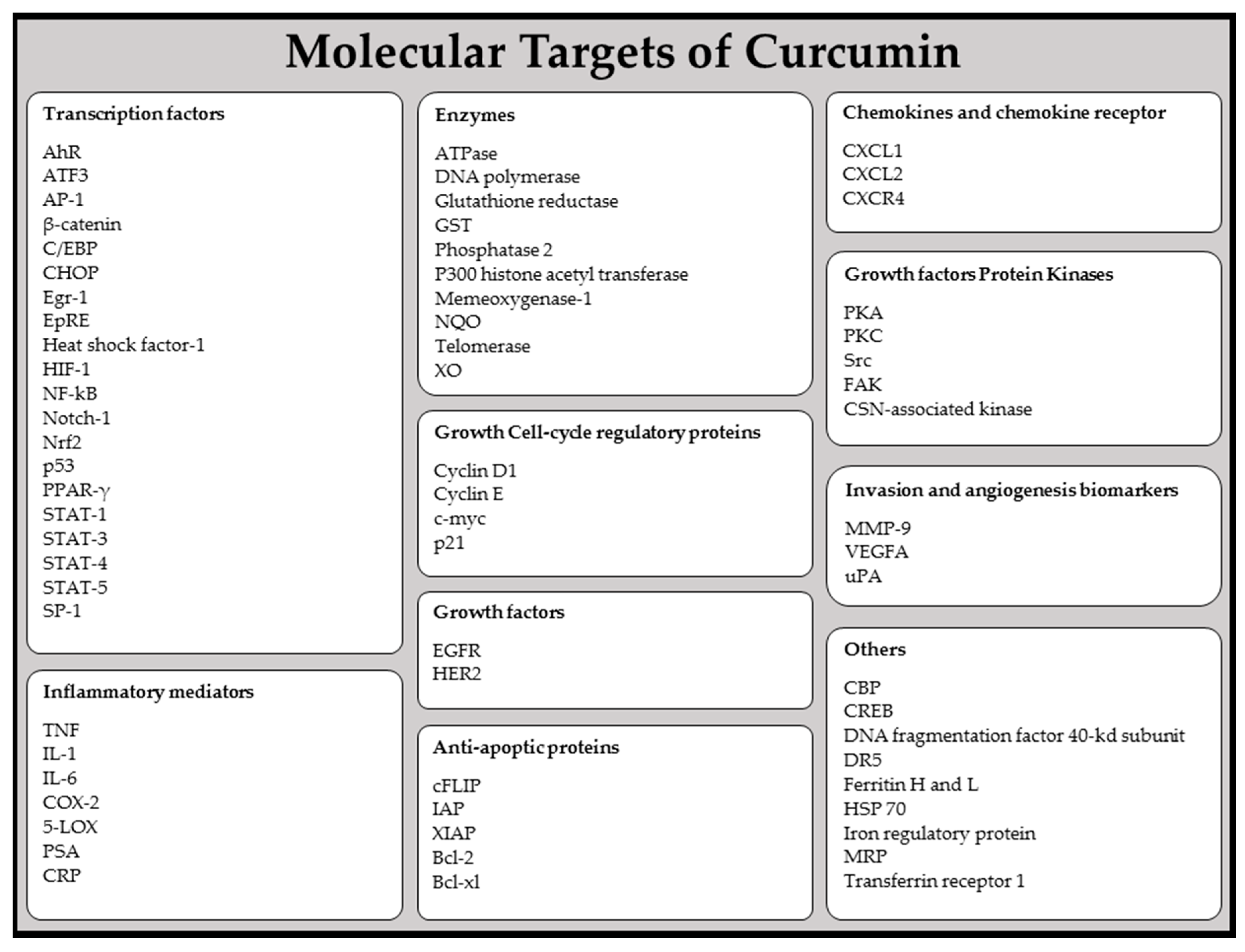
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef]
- Roy, M.; Mukherjee, S. Reversal of resistance towards cisplatin by curcumin in cervical cancer cells. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 1403–1410. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1). Cell Cycle 2007, 6, 2953–2961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099–1105. [Google Scholar] [CrossRef]
- Lee, H.P.; Li, T.M.; Tsao, J.Y.; Fong, Y.C.; Tang, C.H. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012, 13, 163–169. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Eckert, R.L. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715. [Google Scholar] [CrossRef]
- Di Donato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, Y.-Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through janus kinase-stat3 signalling pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, H.; Huang, C.; Lin, J. Cycle arrest and apoptosis in MDA-MB-231/HER2 cells induced by curcumin. Eur. J. Pharmacol. 2012, 690, 22. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, S103. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Shen, H.L. Effect of low dose lycopene intake on lycopene bioavailability and oxidative stress. Nutr. Res. 2002, 22, 1125–1131. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Rao, A.V.; Ray, M.R.; Rao, L.G. Lycopene. Adv. Food Nutr. Res. 2006, 51, 99–164. [Google Scholar]
- Waliszewski, K.N.; Blasco, G. Nutraceutical properties of lycopene. Salud Publica Mex. 2010, 52, 254–265. [Google Scholar] [CrossRef]
- Cruz Bojórquez, R.M.; González Gallego, J.; Sánchez Collado, P. Functional properties and health benefits of lycopene. Nutr. Hosp. 2013, 28, 6–15. [Google Scholar]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M. Is lycopene beneficial to human health? Phytochemistry 2000, 54, 233–236. [Google Scholar] [CrossRef]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takeshima, M.; Nakano, S. Mechanism of the anticancer effect of lycopene (tetraterpenoids). Enzymes 2015, 37, 139–166. [Google Scholar]
- Livny, O.; Kaplan, I.; Reifen, R.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J. Nutr. 2002, 132, 3754–3759. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Zavras, A.I.; Lefatzis, D.; Dessypris, N.; Laskaris, G.; Dokianakis, G.; Segas, J.; Douglas, C.W.; Diehl, S.R.; Trichopoulos, D. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer 2002, 94, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Dan, H.; Wu, R.; Meng, W.; Liu, N.; Jin, X.; Zhou, M.; Zeng, X.; Zhou, G.; Chen, Q. Lycopene: Features and potential significance in the oral cancer and precancerous lesions. J. Oral Pathol. Med. 2011, 40, 361–368. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef]
- Leoncini, E.; Edefonti, V.; Hashibe, M.; Parpinel, M.; Cadoni, G.; Ferraroni, M.; Serraino, D.; Matsuo, K.; Olshan, A.F.; Zevallos, J.P.; et al. Carotenoid intake and head and neck cancer: A pooled analysis in the international head and neck cancer epidemiology consortium. Eur. J. Epidemiol. 2016, 31, 369–383. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Velmurugan, B.; Nagini, S. Dose-response effect of tomato paste on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. J. Exp. Clin. Cancer Res. 2004, 23, 241–249. [Google Scholar] [PubMed]
- Bhuvaneswari, V.; Nagini, S. Lycopene: A review of its potential as an anticancer agent. Curr. Med. Chem. Anticancer Agents 2005, 5, 627–635. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Velmurugan, B.; Balasenthil, S.; Ramachandran, C.R.; Nagini, S. Chemopreventive efficacy of lycopene on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Fitoterapia 2001, 72, 865–874. [Google Scholar] [CrossRef]
- Cheng, H.C.; Chien, H.; Liao, C.H.; Yang, Y.Y.; Huang, S.Y. Carotenoids suppress proliferating cell nuclear antigen and cyclin D1 expression in oral carcinogenic models. J. Nutr. Biochem. 2007, 18, 667–675. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Kosman, V.M.; Selezneva, A.I.; Urakova, I.N.; Makarova, M.N.; Makarov, V.G. Immunomodulatory and antioxidants properties of fixed combination of fish oil with plant extracts. Synergy 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.; Bezerra, F.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Munir, N.; Iqbal, A.S.; Altaf, I.; Bashir, R.; Sharif, N.; Saleem, F.; Naz, S. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa Belladonna and Matricaria chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 111–117. [Google Scholar] [CrossRef]
- Svehliková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-o-glucoside from chamomile (Chamomilla recutita [L.] rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar]
- Al-Hindawi, M.K.; Al-Deen, I.H.; Nabi, M.H.; Ismail, M.A. Anti-inflammatory activity of some Iraqi plants using intact rats. J. Ethnopharmacol. 1989, 26, 163–168. [Google Scholar] [CrossRef]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71, S117–S123. [Google Scholar] [CrossRef]
- Curra, M.; Martins, M.A.; Lauxen, I.S.; Pellicioli, A.C.; Sant’Ana Filho, M.; Pavesi, V.C.; Carrard, V.C.; Martins, M.D. Effect of topical chamomile on immunohistochemical levels of IL-1β and TNF-α in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother. Pharm. 2013, 71, 293–299. [Google Scholar] [CrossRef]
- Pavesi, V.C.; Lopez, T.C.; Martins, M.A.; Sant’Ana Filho, M.; Bussadori, S.K.; Fernandes, K.P.; Mesquita-Ferrari, R.A.; Martins, M.D. Healing action of topical chamomile on 5-fluoracil induced oral mucositis in hamster. Support Care Cancer 2011, 19, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.; Pavesi, V.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P. Comparative analysis between chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Quirino, M.R.; Patrocínio, M.C.; Anbinder, A.L. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e716–e721. [Google Scholar]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrest and apoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef]
- Ketkaew, Y.; Osathanon, T.; Pavasant, P.; Sooampon, S. Apigenin inhibited hypoxia induced stem cell marker expression in a head and neck squamous cell carcinoma cell line. Arch. Oral Biol. 2017, 74, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.M. Aloe vera. Its chemical and therapeutic properties. Int. J. Dermatol. 1991, 30, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Vogler, B.K.; Ernst, E. Aloe vera: A systematic review of its clinical effectiveness. Br. J. Gen. Pract. 1999, 49, 823–828. [Google Scholar] [PubMed]
- Choi, S.W.; Son, B.W.; Son, Y.S.; Park, Y.I.; Lee, S.K.; Chung, M.H. The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. Br. J. Dermatol. 2001, 145, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Khorasani, G.; Azadbakht, M.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol. Croat. Adc 2010, 18, 2–7. [Google Scholar]
- Kim, J.; Lee, I.; Park, S.; Choue, R. Effects of scutellariae radix and Aloe vera gel extracts on immunoglobulin E and cytokine levels in atopic dermatitis nc/nga mice. J. Ethnopharmacol. 2010, 132, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of aloe ferox miller and aloe arborescens miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The review on properties of Aloe vera in healing of cutaneous wounds. BioMed Res. Int. 2015, 2015, 714216. [Google Scholar] [CrossRef] [PubMed]
- Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J. Periodontal Res. 2014, 49, 164–178. [Google Scholar] [CrossRef]
- Lakhanpal, G.; Bhalerao, S.; Sharma, S.; Patil, H. To study the efficacy of different formulations of Aloe vera (spp. Aloe barbadensis) on wound healing in rats. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 432–440. [Google Scholar]
- Tabandeh, M.R.; Oryan, A.; Mohammadalipour, A. Polysaccharides of aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int. J. Biol. Macromol. 2014, 65, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kitamoto, D.; Asikin, Y.; Takara, K.; Wada, K. Liposomes encapsulating Aloe vera leaf gel extract significantly enhance proliferation and collagen synthesis in human skin cell lines. J. Oleo Sci. 2009, 58, 643–650. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Cruz-Flores, P.; Mariscal-Domínguez, M.F.; de la Mora-López, G.S.; Campas-Baypoli, O.N. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. J. Appl. Res. Med. Aromat. Plants 2018, 10, 93–98. [Google Scholar] [CrossRef]
- Cataldi, V.; Di Bartolomeo, S.; Di Campli, E.; Nostro, A.; Cellini, L.; Di Giulio, M. In vitro activity of aloe vera inner gel against microorganisms grown in planktonic and sessile phases. Int. J. Immunopathol. Pharmacol. 2015, 28, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mishra, B.; Gill, K.; Ashraf, M.S.; Singh, A.K.; Sinha, M.; Sharma, S.; Xess, I.; Dalal, K.; Singh, T.P.; et al. Isolation and characterization of novel protein with anti-fungal and anti-inflammatory properties from Aloe vera leaf gel. Int. J. Biol. Macromol. 2011, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773. [Google Scholar]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Ma, Y.S.; Liao, C.L.; Weng, S.W.; Lai, T.Y.; Chung, J.G. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int. J. Oncol. 2010, 36, 1113–1120. [Google Scholar] [PubMed]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Yang, J.S.; Ma, Y.S.; Liao, C.L.; Lai, T.Y.; Tang, N.Y.; Chung, J.G. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010, 30, 945–951. [Google Scholar] [PubMed]
- Lin, M.L.; Lu, Y.C.; Chung, J.G.; Wang, S.G.; Lin, H.T.; Kang, S.E.; Tang, C.H.; Ko, J.L.; Chen, S.S. Down-regulation of MMP-2 through the p38 MAPK-NF-κB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol. Carcinog. 2010, 49, 783–797. [Google Scholar] [CrossRef]
- Xiao, B.; Guo, J.; Liu, D.; Zhang, S. Aloe-emodin induces in vitro G2/M arrest and alkaline phosphatase activation in human oral cancer KB cells. Oral Oncol. 2007, 43, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Madden, E.; Lozada-Nur, F.; Silverman, S. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 2012, 66, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N.; Silverman, S.; Reingold, A.; Bostrom, A.; Lozada-Nur, F.; Weintraub, J. Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Solanki, S.; Chinmaya, B.R.; Tandon, S.; Ashwini, B. The magic of herbal curcumin therapy in recurrent oral lichen planus. Am. J. Ethnomed. 2014, 1, 96–101. [Google Scholar]
- Dos Santos, E.X.; Arantes, D.A.C.; Leite, A.F.O.; Batista, A.C.; de Mendonca, E.F.; Marreto, R.N.; Naves, L.N.; Lima, E.M.; Valadares, M.C. Randomized clinical trial of a mucoadhesive formulation containing curcuminoids (zingiberaceae) and Bidens pilosa linn (asteraceae) extract (fitoprot) for prevention and treatment of oral mucositis—Phase I study. Chem. Biol. Interact. 2018, 291, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Reddy, P.S. Micronuclei assay of exfoliated oral buccal cells: Means to assess the nuclear abnormalities in different diseases. J. Cancer Res. Ther. 2012, 8, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Belludi, S.A.; Verma, S.; Banthia, R.; Bhusari, P.; Parwani, S.; Kedia, S.; Saiprasad, S.V. Effect of lycopene in the treatment of periodontal disease: A clinical study. J. Contemp. Dent. Pract. 2013, 14, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Ikeda, N.; Warnakulasuriya, S.; Fukano, H.; Yuasa, H.; Yano, M.; Miyazaki, H.; Ito, Y. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol. 2000, 36, 466–470. [Google Scholar] [CrossRef]
- Nagao, T.; Warnakulasuriya, S.; Ikeda, N.; Fukano, H.; Yamamoto, S.; Yano, M.; Miyazaki, H.; Ito, Y. Serum antioxidant micronutrient levels in oral Lichen planus. J. Oral Pathol. Med. 2001, 30, 264–267. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K.; Makimura, M.; Otake, S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J. Periodontal Res. 2002, 37, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Hrishi, T.S.; Kundapur, P.P.; Naha, A.; Thomas, B.S.; Kamath, S.; Bhat, G.S. Effect of adjunctive use of green tea dentifrice in periodontitis patients—A randomized controlled pilot study. Int. J. Dent. Hyg. 2016, 14, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Tavani, A.; Pelucchi, C.; Turati, F.; Winn, D.M.; Levi, F.; Yu, G.P.; Morgenstern, H.; Kelsey, K.; Dal Maso, L.; et al. Coffee and tea intake and risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: A randomised controlled trial. BMC Complement. Altern. Med. 2014, 14, 293. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Vrentzos, G.E.; Papadakis, J.A.; Babalis, D.E.; Ganotakis, E.S. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomedicine 2005, 12, 25–27. [Google Scholar] [CrossRef]
- Dos Reis, P.E.; Ciol, M.A.; de Melo, N.S.; Figueiredo, P.T.; Leite, A.F.; Manzi, N.E.M. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: A pilot study. Support Care Cancer 2016, 24, 4393–4398. [Google Scholar] [CrossRef]
- Su, C.K.; Mehta, V.; Ravikumar, L.; Shah, R.; Pinto, H.; Halpern, J.; Koong, A.; Goffinet, D.; Le, Q.T. Phase II double-blind randomized study comparing oral Aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A. Potential prevention: Aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin. J. Integr. Med. 2012, 18, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, A.; Momen-Heravi, F.; Saheb-Jamee, M.; Esfehani, M.; Khalilzadeh, O.; Momen-Beitollahi, J. Comparison of Aloe vera mouthwash with triamcinolone acetonide 0.1% on oral lichen planus: A randomized double-blinded clinical trial. Am. J. Med Sci. 2011, 342, 447–451. [Google Scholar] [CrossRef]
- Choonhakarn, C.; Busaracome, P.; Sripanidkulchai, B.; Sarakarn, P. The efficacy of Aloe vera gel in the treatment of oral Lichen planus: A randomized controlled trial. Br. J. Dermatol. 2008, 158, 573–577. [Google Scholar] [CrossRef]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Furtado, K.S.; Prado, M.G.; Aguiar e Silva, M.A.; Dias, M.C.; Rivelli, D.P.; Rodrigues, M.A.M.; Barbisan, L.F. Coffee and caffeine protect against liver injury induced by thioacetamide in male wistar rats. Basic Clin. Pharmacol. Toxicol. 2012, 111, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Daneshyar, F.; Khamverdi, Z.; Toliat, T.; Alikhani, M. Effect of green tea varnish on depth of root caries. J. Contemp. Dent. Pract. 2018, 19, 137. [Google Scholar] [CrossRef]
- Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.; Sardella, A.; Kerr, A.; Carrassi, A.; MacDonald, L.; Worthington, H. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst. Rev. 2016, 7, CD001829. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Sadrzadeh, A.; Habibi, E.; Dadgar, K.; Akbari, J.; Moosazadeh, M.; Hossein, B.; Ahangarkani, F.; Vaezi, A. Efficacy of Camellia sinensis extract against candida species in patients with denture stomatitis. Curr. Med. Mycol. 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Rodrigues, M.; Henriques, M. Promising alternative therapeutics for oral candidiasis. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Ling, J.; Wu, C. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral candida species. Arch. Oral Biol. 2015, 60, 1565. [Google Scholar] [CrossRef]
- Yan, L.; Chen, F.; Liu, D.; Huang, J.; Liu, F.; Wu, J.; Liu, F.; Ye, J.; Qiu, Y.; Lin, L. Tea, coffee intakes and risk of oral squamous cell carcinoma: A case-control study. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2016, 37, 1531–1535. [Google Scholar]
- Song, I.-S.; Han, K.; Ryu, J.-J.; Choi, Y.-J.; Park, J.-B. Coffee intake as a risk indicator for tooth loss in Korean adults. Sci. Rep. 2018, 8, 2392. [Google Scholar] [CrossRef]
- Song, I.-S.; Han, K.; Ko, Y.; Park, Y.-G.; Ryu, J.-J.; Park, J.-B. Associations between the consumption of carbonated beverages and periodontal disease: The 2008–2010 Korea national health and nutrition examination survey. Medicine 2016, 95, e4253. [Google Scholar] [CrossRef]
- Miranda, J.; Monteiro, L.; Albuquerque, R.; Pacheco, J.-J.; Khan, Z.; Lopez-Lopez, J.; Warnakulasuryia, S. Coffee is protective against oral and pharyngeal cancer: A systematic review and meta-analysis. Med. Oralpatol. Oral Y Cir. Bucal 2017, 22, e554. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, S.; Zhu, C.; Huang, H.; Wu, L.; Wan, X.; Yang, X.; Zhang, H.; Miao, R.; He, L. Coffee and cancer risk: A meta-analysis of prospective observational studies. Sci. Rep. 2016, 6, 33711. [Google Scholar] [CrossRef] [PubMed]
- Moura Rocha, N.F.; Venâncio, E.T.; Moura, B.A.; Gomes Silva, M.I.; Aquino Neto, M.R.; Vasconcelos Rios, E.R.; De Sousa, D.P.; Mendes Vasconcelos, S.M.; De França Fonteles, M.M.; De Sousa, F.C.F. Gastroprotection of (−)-α-bisabolol on acute gastric mucosal lesions in mice: The possible involved pharmacological mechanisms. Fundam. Clin. Pharmacol. 2010, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sendlbeck, M.; Araujo, E.G.; Schett, G.; Englbrecht, M. Psychometric properties of three single-item pain scales in patients with rheumatoid arthritis seen during routine clinical care: A comparative perspective on construct validity, reproducibility and internal responsiveness. Rmd Open 2015, 1, e000140. [Google Scholar] [CrossRef] [PubMed]
- WHO. Handbook for Reporting Results of Cancer Treatment; WHO Offset Publication No. 48: Geneva, Switzerland, 1979. [Google Scholar]
- Varoni, E.M.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Plant polyphenols and oral health: Old phytochemicals for new fields. Curr. Med. Chem. 2012, 19, 1706–1720. [Google Scholar] [CrossRef] [PubMed]
| Anti-inflammatory | |
| Anti-neoplastic via cell-cycle arrest | |
| Induction of apoptotic signals |
| Action | Treatment | Reference |
|---|---|---|
| A. vera’s glycoproteins fraction accelerates lesion healing and cell proliferation in rats | Ointment 10 mg/g/day for eight days, topically applied in an affected area | [129] |
| A. vera cream reduces hot water-induced lesion size in rat skin and increases re-epithelialization | A cream containing 0.5% A. vera in powder form applied 24 h after damage for 25 days | [130] |
| A. vera gel reduces inflammation and increases immunoglobulin E in a dermatitis mouse model | 50 mg/kg/day for six weeks orally | [131] |
| A. ferox and A. vera prevent bacterial and fungal growth in rat and rabbit lesions. Preparations do not present secondary effects and help lesion healing | A. vera or A. ferox juice Rat model: 2 mL/8 h/2 days applied topically to lesions Rabbit model: 3 mL/6 h/4 days topically applied to lesions | [132] |
| Phytochemicals/Plants | Diseases | Observations | Ref. |
|---|---|---|---|
| Curcumin | Leukoplakia, lichen planus, and oral submucous fibrosis | Anti-tumor activity increasing vitamins C and E levels and preventing lipid peroxidation and DNA damage | Rai et al. [146] |
| Oral lichen planus | Reduction of symptoms and signs | Chainani-Wu et al. [147] | |
| Reduction in signs (erythema and ulceration level) | Chainani-Wu et al. [148] | ||
| Signs disappeared with no adverse effects | Sumanth et al. [149] | ||
| Curcumin, Bidens pilosa | Chemoradiotherapy-induced oral mucosal lesion | Prevention and treatment without associated inflammatory process | dos Santos et al. [150] Kashyap et al. [151] |
| Lycopene | Moderate periodontitis, moderate gingivitis | Effect as an adjunct to oral prophylaxis | Belludi et al. [152] |
| Gingivitis | Reduced gingivitis, bleeding index and non-invasive measures of plaque | Chandra et al. [76] | |
| Lycopene, β-carotene | Leukoplakia | Association between leukoplakia and low serum lycopene and β-carotene levels | Nagao et al. [153] |
| Atrophic/erosive oral lichen planus | Association between oral lichen planus and low serum lycopene levels | Nagao et al. [154] | |
| Green tea | Periodontal disease | Bactericidal effect | Hirasawa et al. [155] |
| Chronic periodontitis | Green tea dentifrice improved probing depth, gingival index, and clinical attachment level | Hrishi et al. [156] | |
| Coffee | Cancer of the oral cavity and pharynx | Caffeinated coffee intake was inversely related to a cancer risk of the oral cavity and pharynx | Galeone et al. [157] |
| Coffee, honey | Oral mucositis | Oral mucositis can be successfully treated by a combination of honey and coffee | Raeessi et al. [158] |
| Chamomile | Methotrexate-induced oral mucositis | Successfully reduced with mouthwash treatment | Mazokopakis et al. [159] |
| Oral mucositis | Lower mouth pain score and fewer ulcerations | dos Reis et al. [160] | |
| Aloe vera | Radiation-induced mucositis | No beneficial effect reported as an adjunct to head-and-neck radiotherapy | Su et al. [161] |
| Oral candidiasis | Reduced oral candidiasis in patients with head and neck radiotherapy | Ahmadi et al. [162] | |
| Oral lichen planus | Reduced pain and burning sensation score, size and clinical characteristics of the lesions | Mansourian et al. [163] | |
| Induced clinical and symptom improvement | Choonhakarn et al. [164] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Lopez-Jornet, P.; Pons-Fuster López, E.; Calina, D.; Sharifi-Rad, M.; Ramírez-Alarcón, K.; Forman, K.; Fernández, M.; Martorell, M.; Setzer, W.N.; et al. Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties. Biomolecules 2019, 9, 106. https://doi.org/10.3390/biom9030106
Salehi B, Lopez-Jornet P, Pons-Fuster López E, Calina D, Sharifi-Rad M, Ramírez-Alarcón K, Forman K, Fernández M, Martorell M, Setzer WN, et al. Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties. Biomolecules. 2019; 9(3):106. https://doi.org/10.3390/biom9030106
Chicago/Turabian StyleSalehi, Bahare, Pia Lopez-Jornet, Eduardo Pons-Fuster López, Daniela Calina, Mehdi Sharifi-Rad, Karina Ramírez-Alarcón, Katherine Forman, Marcos Fernández, Miquel Martorell, William N. Setzer, and et al. 2019. "Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties" Biomolecules 9, no. 3: 106. https://doi.org/10.3390/biom9030106
APA StyleSalehi, B., Lopez-Jornet, P., Pons-Fuster López, E., Calina, D., Sharifi-Rad, M., Ramírez-Alarcón, K., Forman, K., Fernández, M., Martorell, M., Setzer, W. N., Martins, N., Rodrigues, C. F., & Sharifi-Rad, J. (2019). Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties. Biomolecules, 9(3), 106. https://doi.org/10.3390/biom9030106











