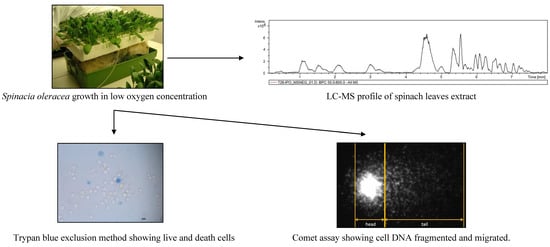
Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Spinach Juice
2.4. Liquid Cromatography-Mass Spectrometry Analysis
2.5. Trypan Blue Exclusion Cell Viability Assay
2.6. Comet Test
3. Results and Discussion
3.1. Oxygen Availability Effect on Spinach Leaf Juice Composition
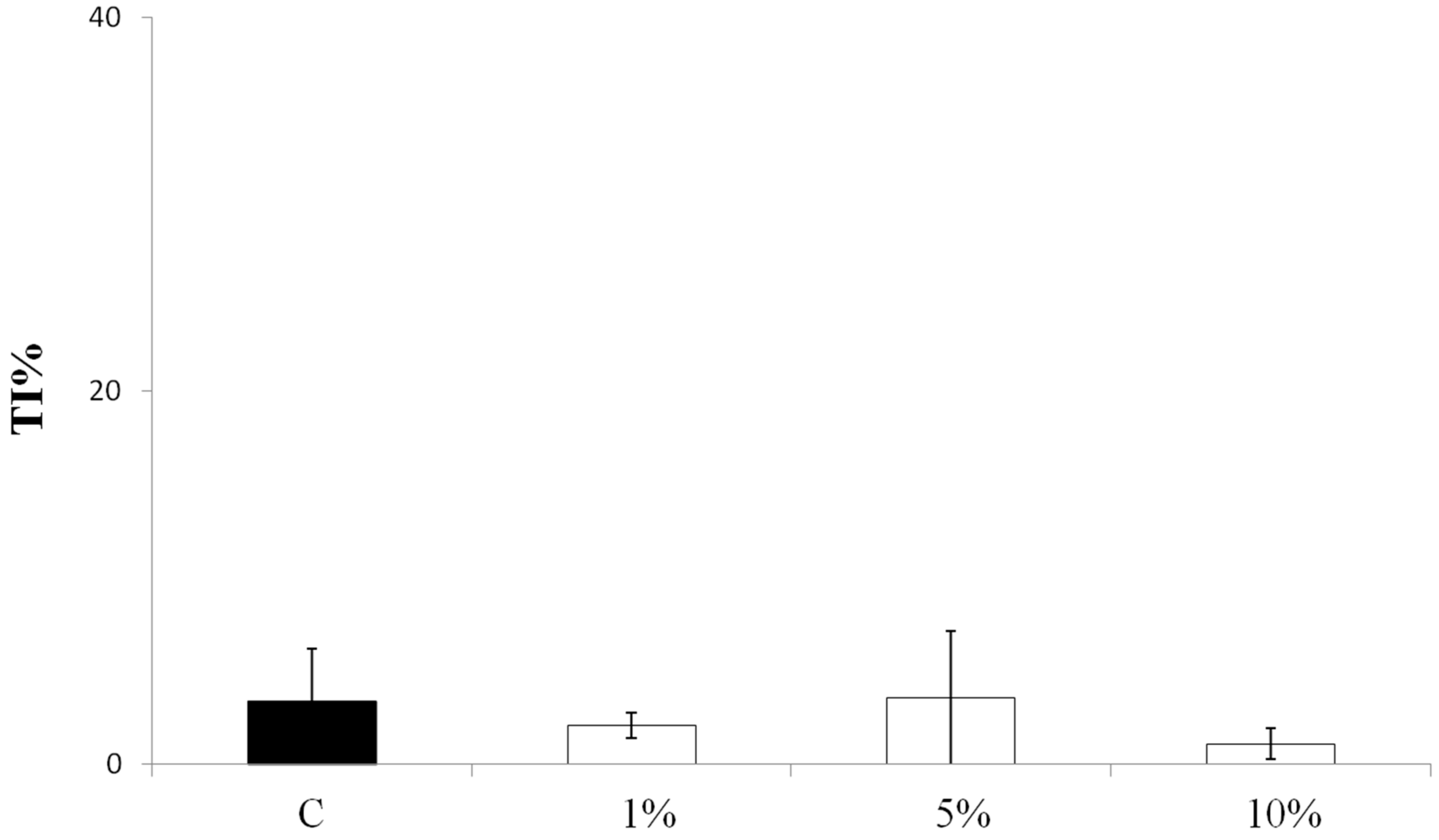
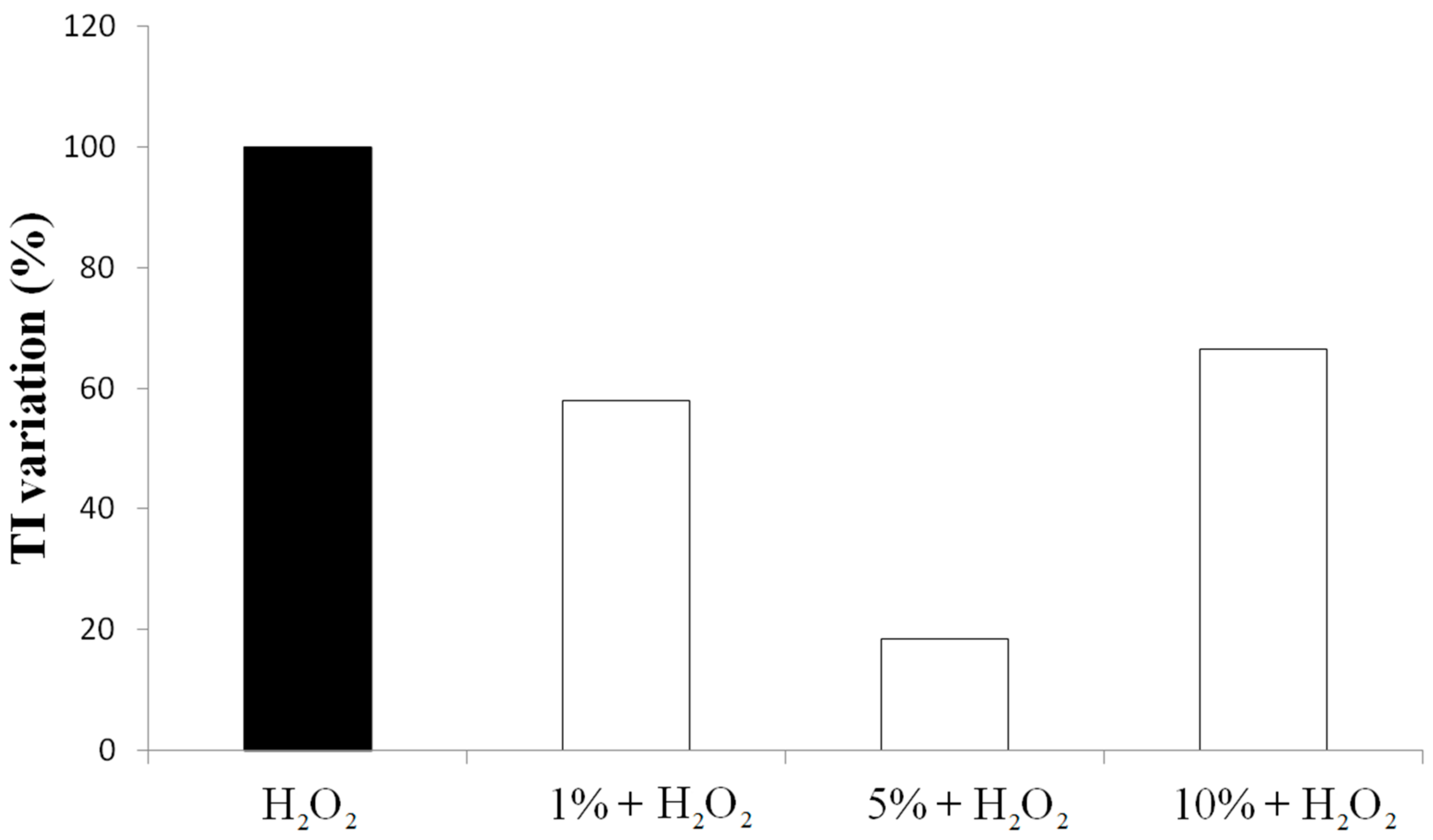
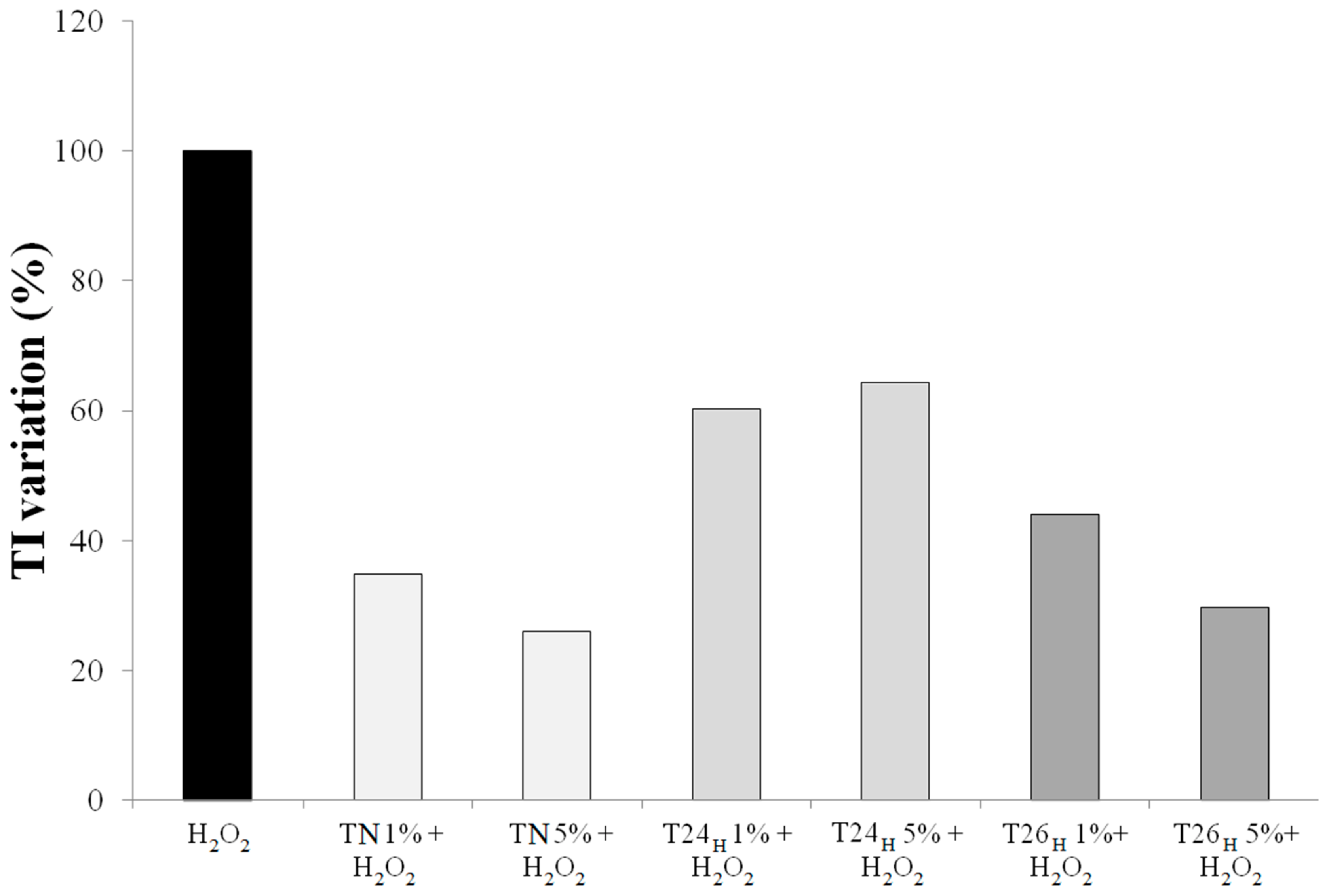
3.2. Effect on Viability and Antioxidant Activity on HT29 Human Colon Cancer Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Beneteau, A.; Baret, P.; Payet, B.; Lefebvre d’hellencourt, C.; Gonthier, M.P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Rad. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; van Dongen, J.T.; Giuntoli, B.; Novi, G.; Santaniello, A.; Geigenberger, P.; Perata, P. HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 2010, 62, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Arru, L.; Fornaciari, S.; Mancuso, S. Oxygen deficiency-induced root-to-shoot communication. In Long-Distance Systemic Signaling and Communication in Plants; Baluška, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 125–147. ISBN 978-3-642-36470-9. [Google Scholar]
- Banti, V.; Giuntoli, B.; Gonzali, S.; Loreti, E.; Magneschi, L.; Novi, G.; Paparelli, E.; Parlanti, S.; Pucciariello, C.; Santaniello, A.; et al. Low oxygen response mechanisms in green organisms. Int. J. Mol. Sci. 2013, 14, 4734–4761. [Google Scholar] [CrossRef] [PubMed]
- Monk, L.S.; Fagerstedt, K.V.; Crawford, R.M. Superoxide Dismutase as an Anaerobic Polypeptide: A Key Factor in Recovery from Oxygen Deprivation in Iris pseudacorus? Plant Physiol. 1987, 85, 1016–1020. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation. Physiol. Plant. 1998, 104, 747–752. [Google Scholar] [CrossRef]
- Chool Boo, Y.; Jung, J. Water Deficit—Induced Oxidative Stress and Antioxidative Defenses in Rice Plants. J. Plant Physiol. 1999, 155, 255–261. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Bhattacharjee, S. The language of reactive oxygen species signaling in plants. J. Bot. 2012, 1–22. [Google Scholar] [CrossRef]
- Hsu, A.; Wong, C.P.; Yu, Z.; Williams, D.E.; Dashwood, R.H.; Ho, E. Promoter de-methylation of Cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics 2011, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Fornaciari, S.; Milano, F.; Mussi, F.; Pinto-Sanchez, L.; Forti, L.; Buschini, A.; Arru, L. Assessment of antioxidant and antiproliferative properties of spinach plants grown under low oxygen availability. J. Sci. Food Agric. 2015, 95, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Liao, C.M.; Wu, S.C. Influence of Maillard reaction products on DNA damage in human lymphocytes. J. Agric. Food Chem. 2002, 50, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Damien Dorman, H.J.; Shikov, A.N.; Pozharitskaya, O.N.; Hiltunen, R. Antioxidant and pro-oxidant evaluation of a Potentilla alba L. rhizome extract. Chem. Biodivers. 2011, 8, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Ferreres, F.; Tomas-Barberan, F.A. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Hait-Darshan, R.; Grossman, S.; Bergman, M.; Deutsch, M.; Zurgil, N. Synergistic activity between a spinach-derived natural antioxidant (NAO) and commercial antioxidants in a variety of oxidation systems. Food Res. Int. 2009, 42, 246–253. [Google Scholar] [CrossRef]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, efficacy and safety of spinach extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lomnitski, L.; Carbonatto, M.; Ben-Shaul, V.; Peano, S.; Conz, A.; Corradin, L.; Maronpot, R.R.; Grossman, S.; Nyska, A. The prophylactic effects of natural water-soluble antioxidant from spinach and apocynin in a rabbit model of lipopolysaccharide-induced endotoxemia. Toxicol. Pathol. 2000, 28, 588–600. [Google Scholar] [CrossRef]
- Toledo, M.E.A.; Ueda, Y.; Imahori, Y.; Ayaki, M. L-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biol. Technol. 2003, 28, 47–57. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; Maclean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Gibeaut, D.M.; Hulett, J.; Cramer, G.R.; Seemann, J.R. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997, 115, 317–319. [Google Scholar] [CrossRef]
- Huttner, D.; Bar-Zvi, D. An improved, simple, hydroponic method for growing Arabidopsis thaliana. Plant. Mol. Biol. Rep. 2003, 21, 59–63. [Google Scholar] [CrossRef]
- Ferrarini, L.; Pellegrini, N.; Mazzeo, T.; Miglio, C.; Galati, S.; Milano, F.; Rossi, C.; Buschini, A. Anti-proliferative activity and chemoprotective effects towards DNA oxidative damage of fresh and cooked Brassicaceae. Br. J. Nutr. 2012, 107, 1324–1332. [Google Scholar] [CrossRef]
- Bergquist, S.A.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in baby spinach (Spinacia oleracea L.): Changes during plant growth and storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Jic, X.; Lockec, S.J.; Critchleye, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Stobiecki, M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry 2000, 54, 237–256. [Google Scholar] [CrossRef]
- Destreza, B.; Pinel, G.; Monteaua, F.; Lafont, R.; Le Bizec, B. Detection and identification of 20-hydroxyecdysone metabolites in calf urine by liquid chromatography-high resolution or tandem mass spectrometry measurements and establishment of their kinetics of elimination after 20-hydroxyecdysone administration. Anal. Chim. Acta 2009, 637, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Giusepponi, D.; Torquato, P.; Bartolini, D.; Piroddi, M.; Birringer, M.; Lorkowski, S.; Libetta, C.; Cruciani, G.; Moretti, S.; Saluti, G.; et al. Determination of tocopherols and their metabolites by liquid chromatography coupled with tandem mass spectrometry in human plasma and serum. Talanta 2017, 170, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Fagerstedt, K.V. Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol. Biochem. 2010, 48, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: Its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Lee, D.G.; Lee, S.H.; Lee, K.W.; Bahk, J.D.; Lee, B.H. A proteomic screen and identification of waterlogging-regulated proteins in tomato roots. Plant Soil 2007, 295, 37–51. [Google Scholar] [CrossRef]
- Gout, E.; Boisson, A.M.; Aubert, S.; Douce, R.; Bligny, R. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and phosphorus-31 nuclear magnetic resonance studies. Plant Physiol. 2001, 125, 912–925. [Google Scholar] [CrossRef]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and maturation. J. Agric. Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Kuz’menko, A.I.; Morozova, R.P.; Nikolenko, I.A.; Donchenko, G.V. Antioxidant effect of 20-hydroxyecdysone in a model system. Ukr. Biokhim. Zh. 1999, 71, 35–38. [Google Scholar]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; El-Readi, M.Z.; Janibekov, A.A.; Tahrani, A.; Wink, M. Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant activity. Z. Naturforsch. 2011, 66C, 215–224. [Google Scholar] [CrossRef]
- Nair, S.; Li, W.; Kong, A.N. Natural dietary anti-cancer chemopreventive compounds: Redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol. Sin. 2007, 28, 459–472. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sungrae, C.; Jin Sung, C.; Hocheol, S.; Yujeong, S.; Haeun, S.; Youngwook, K.; Byong Chul, Y.; Kangsan, R.; Seungchan, C.; Eui-joon, K.; Hee-seong, B.; et al. Hormetic dose response to L-ascorbic acid as an anti-cancer drug in colorectal cancer cell lines according to SVCT-2 expression. Sci. Rep. 2018, 8, 11372. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
| Peak Nr. | Ret Time (min) | [M-H]− m/z | [M+H]+ m/z | Other Related m/z Peaks | Relative Abundance | Putative Identity | Ref. | |
|---|---|---|---|---|---|---|---|---|
| T24/TN | T26/TN | |||||||
| 1 | 0.5 | 175.0 | 115 (M-C2H4O2) 215 (M+K) 199 (M+Na) | 4.46 | 2.62 | Ascorbic acid b | ||
| 2 | 4.4 | 193.0 | 195.0 | 177 (M-OH) | 1.59 | 0.99 | Ferulic acid b | |
| 3 | 4.9 | 787.2 | 655.1 (M-apiose-H) 331 (patuletin -H) | 3.00 | 1.14 | Patuletin-3-glucosyl-(1→6) [apiosyl(1→2)] glucoside | [32,33] | |
| 4 | 5.2 | 639.2 | 641.2 | 611.2 (M- 2H2O) 663.2 (M+Na) | 4.24 | 2.78 | Flavonol diglycoside | [34] |
| 5 | 5.5 | 481.3 | 463.3 (M-OH) 445.3 (M-2OH) 427.3 (M-3OH) | 2.48 | 0.89 | 20-hydroxyecdysone | [35] | |
| 6 | 6 | 429.3 | 431.3 | 453.3 (M+Na) | 2.34 | 1.32 | α-tocopherol | [36] |
| 7 | 6.1 | 535.4 | 537.4 | 2.85 | 0.87 | Jaceidin glucuronide | [33] | |
| 8 | 6.2 | 521.1 | 523.1 | 2.38 | 1.40 | Spinatoside | [32,33] | |
| 9 | 6.4 | 519.1 | 521.1 | 309 (M-glucuronide) | 2.77 | 1.15 | 5,3′,4′-Trihydroxy-3-methoxy-6:7-methylendioxyflavone-4′-glucuronide | [32,33] |
| 10 | 6.5 | 533.1 | 535.1 | 557.1 (M+Na) 505.1 (M-MeO) | 2.59 | 0.94 | 5,4′-dihydroxy-3,3′-dimethoxy-6:7-methylendioxyflavone-4′-glucuronide | [32,33] |
| Juice Concentration (%) | Mortality (%) | |
|---|---|---|
| C | 0 | 3 |
| Normoxic Condition | ||
| T24N | 10 | 1 |
| 25 | 2 | |
| 50 | 39 | |
| T26N | 10 | 11 |
| 25 | 13 | |
| 50 | 17 | |
| Stress Condition | ||
| T24H | 10 | 3 |
| 25 | 2 | |
| 50 | 71 | |
| T26H | 10 | 28 |
| 25 | 15 | |
| 50 | 17 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milano, F.; Mussi, F.; Fornaciari, S.; Altunoz, M.; Forti, L.; Arru, L.; Buschini, A. Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice. Biomolecules 2019, 9, 53. https://doi.org/10.3390/biom9020053
Milano F, Mussi F, Fornaciari S, Altunoz M, Forti L, Arru L, Buschini A. Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice. Biomolecules. 2019; 9(2):53. https://doi.org/10.3390/biom9020053
Chicago/Turabian StyleMilano, Francesco, Francesca Mussi, Silvia Fornaciari, Meltem Altunoz, Luca Forti, Laura Arru, and Annamaria Buschini. 2019. "Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice" Biomolecules 9, no. 2: 53. https://doi.org/10.3390/biom9020053
APA StyleMilano, F., Mussi, F., Fornaciari, S., Altunoz, M., Forti, L., Arru, L., & Buschini, A. (2019). Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice. Biomolecules, 9(2), 53. https://doi.org/10.3390/biom9020053