Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52
Abstract
:1. Introduction
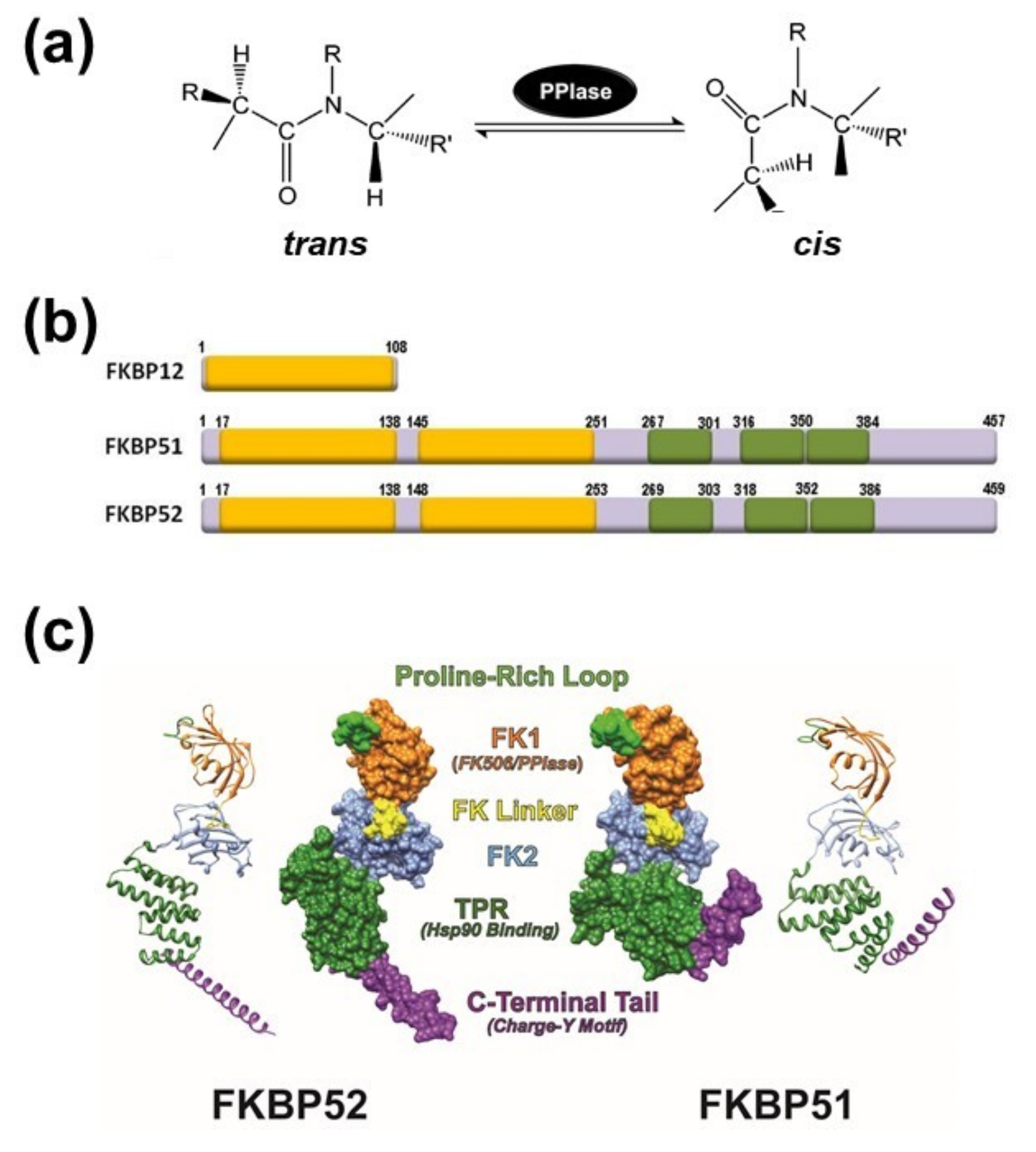
2. The PPIase Activity Affects Protein Conformation
3. General Aspects of FKPB51 and FKBP52
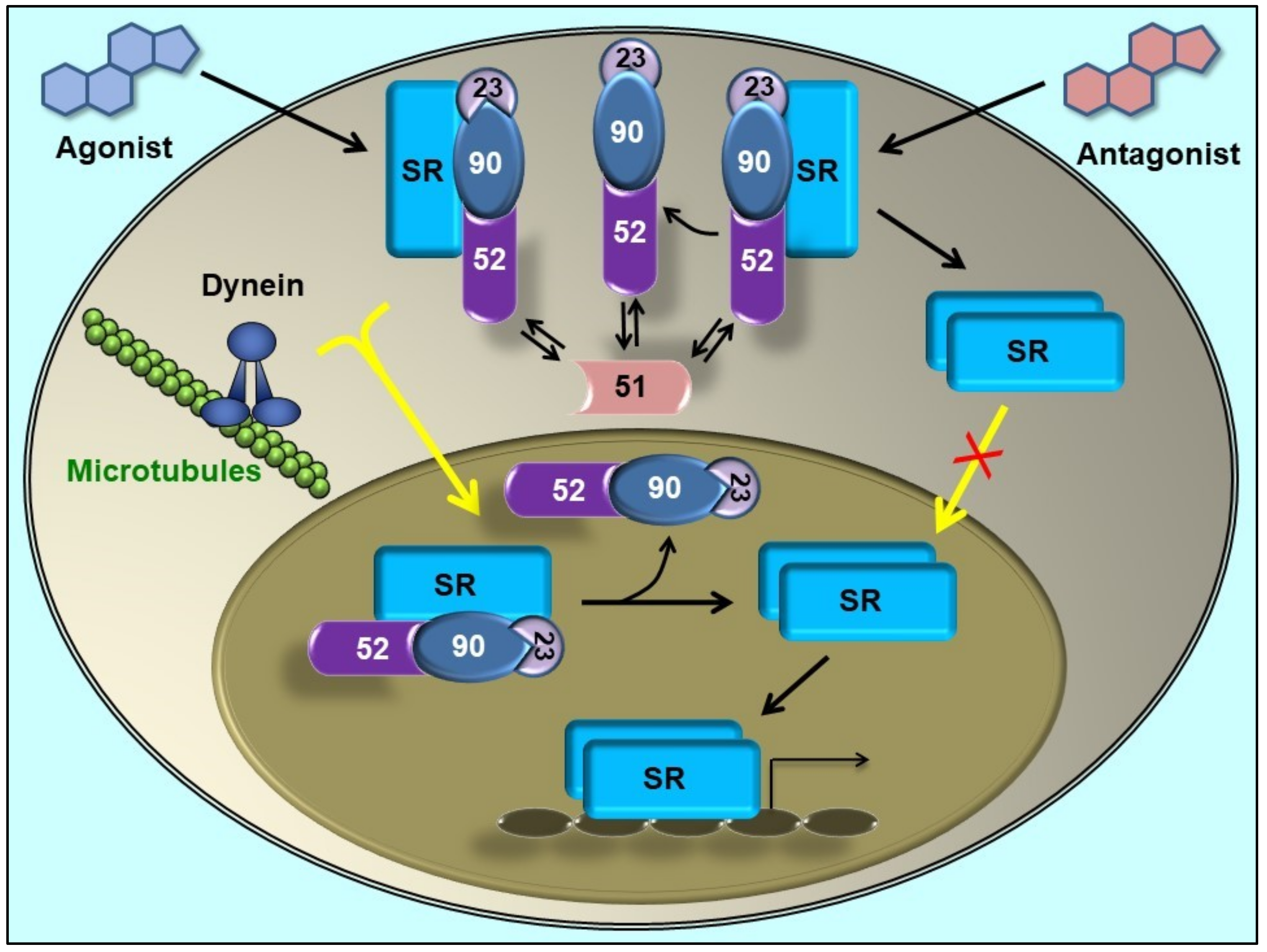
4. Immunophilins Play a Key Role in Protein Trafficking
5. Immunophilins in Steroid Receptor-Related Cancer
6. Immunophilins Regulate NF-κB Activity
7. AKT/mTOR Signalling Cascade
8. The hTERT•Hsp90•FKBP51/FKB52 Complex
9. Role of Immunophilins in Malignancies
10. Immunophilins and Cell Differentiation
11. Stress-Related Neurologic Disorders
12. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Schiene-Fischer, C. Multidomain Peptidyl Prolyl cis/trans Isomerases. Biochim. Biophys. Acta 2015, 1850, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.B.; Hong, Y.; Dhe-Paganon, S.; Yoon, H.S. FKBP family proteins: Immunophilins with versatile biological functions. Neurosignals 2008, 16, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1987, 40, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Ruegger, A.; Kuhn, M.; Lichti, H.; Loosli, H.R.; Huguenin, R.; Quiquerez, C.; von Wartburg, A. [Cyclosporin A, a Peptide Metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity]. Helv. Chim. Acta 1976, 59, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- John Wiley & Sons. Ciclosporin. In Drug Discovery—A History; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Hanes, S.D. Prolyl isomerases in gene transcription. Biochim. Biophys. Acta 2015, 1850, 2017–2034. [Google Scholar] [CrossRef]
- Matena, A.; Rehic, E.; Honig, D.; Kamba, B.; Bayer, P. Structure and function of the human parvulins Pin1 and Par14/17. Biol. Chem. 2018, 399, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Dual-Family Peptidylprolyl Isomerases (Immunophilins) of Select Monocellular Organisms. Biomolecules 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Netzer, W.J.; Hartl, F.U. Recombination of protein domains facilitated by co-translational folding in eukaryotes. Nature 1997, 388, 343–349. [Google Scholar] [CrossRef]
- Helbig, S.; Patzer, S.I.; Schiene-Fischer, C.; Zeth, K.; Braun, V. Activation of colicin M by the FkpA prolyl cis-trans isomerase/chaperone. J. Biol. Chem. 2011, 286, 6280–6290. [Google Scholar] [CrossRef]
- Theuerkorn, M.; Fischer, G.; Schiene-Fischer, C. Prolyl cis/trans isomerase signalling pathways in cancer. Curr. Opin. Pharmacol. 2011, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Zhang, J.; Ouyang, C.H.; Li, C.Y.; Zhao, F.B.; Liu, Y.W.; Ai, Y.X.; Hu, W.P. Potentiation by WIN 55,212-2 of GABA-activated currents in rat trigeminal ganglion neurones. Br. J. Pharmacol. 2009, 158, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rao, A.; Hogan, P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011, 21, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, I.; Renoir, J.M.; Lebeau, M.C.; Massol, N.; Burny, A.; Baulieu, E.E.; Mornon, J.P. An immunophilin that binds M(r) 90,000 heat shock protein: Main structural features of a mammalian p59 protein. Proc. Natl. Acad. Sci. USA 1992, 89, 6270–6274. [Google Scholar] [CrossRef]
- Smith, D.F. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 2004, 9, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Chambraud, B.; Radanyi, C.; Leclerc, J.; Lebeau, M.C.; Renoir, J.M.; Shirai, R.; Catelli, M.G.; Yahara, I.; Baulieu, E.E. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: Regulation of HSP90-binding activity of FKBP52. Proc. Natl. Acad. Sci. USA 1997, 94, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- Riggs, D.L.; Cox, M.B.; Cheung-Flynn, J.; Prapapanich, V.; Carrigan, P.E.; Smith, D.F. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 279–295. [Google Scholar] [CrossRef]
- Fries, G.R.; Gassen, N.C.; Rein, T. The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2017, 18, 2614. [Google Scholar] [CrossRef]
- Cauerrhff, A.A.; Galigniana, M.D. Structural characteristics of the TPR protein-Hsp90 interaction: A new target in biotechnology. In Role of Molecular Chaperones in Structural Folding, Biological Actions, and Drug Interactions of Client Proteins; Galigniana, M.D., Ed.; Bentham Science Publishers: Emirate of Sharjah, United Arab Emirates, 2018; Volume 1, pp. 73–173. [Google Scholar]
- LeMaster, D.M.; Mustafi, S.M.; Brecher, M.; Zhang, J.; Heroux, A.; Li, H.; Hernandez, G. Coupling of Conformational Transitions in the N-terminal Domain of the 51-kDa FK506-binding Protein (FKBP51) Near Its Site of Interaction with the Steroid Receptor Proteins. J. Biol. Chem. 2015, 290, 15746–15757. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Storer, C.L.; Dickey, C.A.; Galigniana, M.D.; Rein, T.; Cox, M.B. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 2011, 22, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Sinars, C.R.; Cheung-Flynn, J.; Rimerman, R.A.; Scammell, J.G.; Smith, D.F.; Clardy, J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc. Natl. Acad. Sci. USA 2003, 100, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, P.; Liu, Y.; Lou, Z.; Ding, Y.; Shu, C.; Ye, S.; Bartlam, M.; Shen, B.; Rao, Z. 3D structure of human FK506-binding protein 52: Implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc. Natl. Acad. Sci. USA 2004, 101, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Guy, N.C.; Garcia, Y.A.; Sivils, J.C.; Galigniana, M.D.; Cox, M.B. Functions of the Hsp90-binding FKBP immunophilins. Subcell. Biochem. 2015, 78, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Sivils, J.C.; Storer, C.L.; Galigniana, M.D.; Cox, M.B. Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52). Curr. Opin. Pharmacol. 2011, 11, 314–319. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.T.; Iwai, A.; Feau, C.; Garcia, Y.; Balsiger, H.A.; Storer, C.L.; Suro, R.M.; Garza, K.M.; Lee, S.; Kim, Y.S.; et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11878–11883. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Daneri-Becerra, C.; Zgajnar, N.R.; Lotufo, C.M.; Galigniana, M.D. Gene expression regulation by heat-shock proteins: The cardinal roles of HSF1 and Hsp90. Biochem. Soc. Trans. 2018, 46, 51–65. [Google Scholar] [CrossRef]
- Pirkl, F.; Buchner, J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol. 2001, 308, 795–806. [Google Scholar] [CrossRef]
- Pratt, W.B. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu. Rev. Pharm. Toxicol. 1997, 37, 297–326. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, M.D. The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl. Recept. Res. 2018, 5, 101320. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997, 18, 306–360. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, F. Phosphorylation of steroid receptors. J. Steroid Biochem. 1989, 32, 613–622. [Google Scholar] [CrossRef]
- Galigniana, M.D. Native rat kidney mineralocorticoid receptor is a phosphoprotein whose transformation to a DNA-binding form is induced by phosphatases. Biochem. J. 1998, 333, 555–563. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, D.; McEwan, I.J. Posttranslational Modifications of Steroid Receptors: Phosphorylation. Methods Mol. Biol. 2016, 1443, 105–117. [Google Scholar] [CrossRef]
- Harrell, J.M.; Kurek, I.; Breiman, A.; Radanyi, C.; Renoir, J.M.; Pratt, W.B.; Galigniana, M.D. All of the protein interactions that link steroid receptor.hsp90.immunophilin heterocomplexes to cytoplasmic dynein are common to plant and animal cells. Biochemistry 2002, 41, 5581–5587. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Krishna, P.; Olsen, L.J. Hsp90-binding immunophilins in plants: The protein movers. Trends Plant Sci. 2001, 6, 54–58. [Google Scholar] [CrossRef]
- Erlejman, A.G.; Lagadari, M.; Toneatto, J.; Piwien-Pilipuk, G.; Galigniana, M.D. Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim. Biophys. Acta 2014, 1839, 71–87. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Radanyi, C.; Renoir, J.M.; Housley, P.R.; Pratt, W.B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 2001, 276, 14884–14889. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Erlejman, A.G.; Monte, M.; Gomez-Sanchez, C.; Piwien-Pilipuk, G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol. Cell. Biol. 2010, 30, 1285–1298. [Google Scholar] [CrossRef]
- Darshan, M.S.; Loftus, M.S.; Thadani-Mulero, M.; Levy, B.P.; Escuin, D.; Zhou, X.K.; Gjyrezi, A.; Chanel-Vos, C.; Shen, R.; Tagawa, S.T.; et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011, 71, 6019–6029. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Galigniana, M.D.; Harrell, J.M.; DeFranco, D.B. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004, 16, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, J.; DeFranco, D.B. Subnuclear trafficking of glucocorticoid receptors in vitro: Chromatin recycling and nuclear export. J. Cell Biol. 1997, 137, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liang, C.; Li, F.; Wang, L.; Wu, X.; Lu, A.; Xiao, G.; Zhang, G. The Rules and Functions of Nucleocytoplasmic Shuttling Proteins. Int. J. Mol. Sci. 2018, 19, 1445. [Google Scholar] [CrossRef] [PubMed]
- Mazaira, G.I.; Lagadari, M.; Erlejman, A.G.; Galigniana, M.D. The Emerging Role of TPR-Domain Immunophilins in the Mechanism of Action of Steroid Receptors. Nucl. Recept. Res. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Echeverria, P.C.; Mazaira, G.; Erlejman, A.; Gomez-Sanchez, C.; Piwien Pilipuk, G.; Galigniana, M.D. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol. Cell. Biol. 2009, 29, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Presman, D.M.; Alvarez, L.D.; Levi, V.; Eduardo, S.; Digman, M.A.; Marti, M.A.; Veleiro, A.S.; Burton, G.; Pecci, A. Insights on glucocorticoid receptor activity modulation through the binding of rigid steroids. PLoS ONE 2010, 5, e13279. [Google Scholar] [CrossRef]
- Grossmann, C.; Ruhs, S.; Langenbruch, L.; Mildenberger, S.; Stratz, N.; Schumann, K.; Gekle, M. Nuclear shuttling precedes dimerization in mineralocorticoid receptor signaling. Chem. Biol. 2012, 19, 742–751. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Galigniana, M.D.; Chen, M.S.; Owens-Grillo, J.K.; Chinkers, M.; Pratt, W.B. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J. Biol. Chem. 1997, 272, 16224–16230. [Google Scholar] [CrossRef]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef]
- Madan, A.P.; DeFranco, D.B. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc. Natl. Acad. Sci. USA 1993, 90, 3588–3592. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Echeverria, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D. Steroid receptor coupling becomes nuclear. Chem. Biol. 2012, 19, 662–663. [Google Scholar] [CrossRef]
- Gallo, L.I.; Ghini, A.A.; Piwien Pilipuk, G.; Galigniana, M.D. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 2007, 46, 14044–14057. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Lagadari, M.; Harris, D.C.; Cox, M.B.; Galigniana, M.D. Molecular chaperone activity and biological regulatory actions of the TPR-domain immunophilins FKBP51 and FKBP52. Curr. Protein Pept. Sci. 2014, 15, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, T.; Cluning, C.; Ward, B.K. Steroid Receptor-Associated Immunophilins: A Gateway to Steroid Signalling. Clin. Biochem. Rev. 2015, 36, 31–52. [Google Scholar] [PubMed]
- Oroz, J.; Chang, B.J.; Wysoczanski, P.; Lee, C.T.; Perez-Lara, A.; Chakraborty, P.; Hofele, R.V.; Baker, J.D.; Blair, L.J.; Biernat, J.; et al. Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex. Nat. Commun. 2018, 9, 4532. [Google Scholar] [CrossRef] [PubMed]
- Cluning, C.; Ward, B.K.; Rea, S.L.; Arulpragasam, A.; Fuller, P.J.; Ratajczak, T. The helix 1-3 loop in the glucocorticoid receptor LBD is a regulatory element for FKBP cochaperones. Mol. Endocrinol. 2013, 27, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, J.J.; Cordova, R.A.; Zheng, D.; Criado-Marrero, M.; Lemus, A.; Li, P.; Baker, J.D.; Nordhues, B.A.; Darling, A.L.; Martinez-Licha, C.; et al. Targeting the FKBP51/GR/Hsp90 Complex to Identify Functionally Relevant Treatments for Depression and PTSD. ACS Chem. Biol. 2018, 13, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.C.; Picard, D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 2010, 1803, 641–649. [Google Scholar] [CrossRef]
- Ebong, I.O.; Beilsten-Edmands, V.; Patel, N.A.; Morgner, N.; Robinson, C.V. The interchange of immunophilins leads to parallel pathways and different intermediates in the assembly of Hsp90 glucocorticoid receptor complexes. Cell Discov. 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. On the trail of the glucocorticoid receptor: Into the nucleus and back. Traffic 2012, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Her, J.; Oh, S.Y.; Chung, I.K. Hsp90-binding immunophilin FKBP52 modulates telomerase activity by promoting the cytoplasmic retrotransport of hTERT. Biochem. J. 2016, 473, 3517–3532. [Google Scholar] [CrossRef] [PubMed]
- Vafopoulou, X.; Steel, C.G. Cytoplasmic travels of the ecdysteroid receptor in target cells: Pathways for both genomic and non-genomic actions. Front. Endocrinol. 2012, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Schnell, L.; Feigl, P.; Birkhofer, C.; Mohr, K.; Roeder, M.; Carle, S.; Langer, S.; Tippel, F.; Buchner, J.; et al. The Hsp90 machinery facilitates the transport of diphtheria toxin into human cells. Sci. Rep. 2017, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; De Leo, S.A.; Mazaira, G.I.; Molinari, A.M.; Camisay, M.F.; Fontana, V.; Cox, M.B.; Piwien-Pilipuk, G.; Galigniana, M.D. NF-κB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: A role for peptidyl-prolyl isomerase activity. J. Biol. Chem. 2014, 289, 26263–26276. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D.; Harrell, J.M.; O'Hagen, H.M.; Ljungman, M.; Pratt, W.B. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J. Biol. Chem. 2004, 279, 22483–22489. [Google Scholar] [CrossRef]
- Colo, G.P.; Rubio, M.F.; Nojek, I.M.; Werbajh, S.E.; Echeverria, P.C.; Alvarado, C.V.; Nahmod, V.E.; Galigniana, M.D.; Costas, M.A. The p160 nuclear receptor co-activator RAC3 exerts an anti-apoptotic role through a cytoplasmatic action. Oncogene 2008, 27, 2430–2444. [Google Scholar] [CrossRef]
- Lagadari, M.; Zgajnar, N.R.; Gallo, L.I.; Galigniana, M.D. Hsp90-binding immunophilin FKBP51 forms complexes with hTERT enhancing telomerase activity. Mol. Oncol. 2016, 10, 1086–1098. [Google Scholar] [CrossRef]
- McKeen, H.D.; McAlpine, K.; Valentine, A.; Quinn, D.J.; McClelland, K.; Byrne, C.; O'Rourke, M.; Young, S.; Scott, C.J.; McCarthy, H.O.; et al. A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology 2008, 149, 5724–5734. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Rimerman, R.A.; Toran, E.J.; Chen, S.; Prapapanich, V.; Butts, R.N.; Smith, D.F. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol. Cell. Biol. 1997, 17, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Barent, R.L.; Nair, S.C.; Carr, D.C.; Ruan, Y.; Rimerman, R.A.; Fulton, J.; Zhang, Y.; Smith, D.F. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol. 1998, 12, 342–354. [Google Scholar] [CrossRef]
- Ratajczak, T.; Hlaing, J.; Brockway, M.J.; Hahnel, R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J. Steroid Biochem. 1990, 35, 543–553. [Google Scholar] [CrossRef]
- Thadani-Mulero, M.; Portella, L.; Sun, S.; Sung, M.; Matov, A.; Vessella, R.L.; Corey, E.; Nanus, D.M.; Plymate, S.R.; Giannakakou, P. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 2014, 74, 2270–2282. [Google Scholar] [CrossRef]
- Pratt, W.B.; Czar, M.J.; Stancato, L.F.; Owens, J.K. The hsp56 immunophilin component of steroid receptor heterocomplexes: Could this be the elusive nuclear localization signal-binding protein? J. Steroid Biochem. Mol. Biol. 1993, 46, 269–279. [Google Scholar] [CrossRef]
- Jhaveri, K.; Ochiana, S.O.; Dunphy, M.P.; Gerecitano, J.F.; Corben, A.D.; Peter, R.I.; Janjigian, Y.Y.; Gomes-DaGama, E.M.; Koren, J., 3rd; Modi, S.; et al. Heat shock protein 90 inhibitors in the treatment of cancer: Current status and future directions. Expert Opin. Investig. Drugs 2014, 23, 611–628. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Inda, C.; Bolaender, A.; Wang, T.; Gandu, S.R.; Koren, J., 3rd. Stressing Out Hsp90 in Neurotoxic Proteinopathies. Curr. Top. Med. Chem. 2016, 16, 2829–2838. [Google Scholar] [CrossRef]
- Reynolds, P.D.; Ruan, Y.; Smith, D.F.; Scammell, J.G. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab. 1999, 84, 663–669. [Google Scholar] [CrossRef]
- Denny, W.B.; Valentine, D.L.; Reynolds, P.D.; Smith, D.F.; Scammell, J.G. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 2000, 141, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Westberry, J.M.; Sadosky, P.W.; Hubler, T.R.; Gross, K.L.; Scammell, J.G. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J. Steroid Biochem. Mol. Biol. 2006, 100, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Putz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Roberts, P.J.; Chirillo, S.C.; Cheung-Flynn, J.; Prapapanich, V.; Ratajczak, T.; Gaber, R.; Picard, D.; Smith, D.F. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003, 22, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.K.; Mark, P.J.; Ingram, D.M.; Minchin, R.F.; Ratajczak, T. Expression of the estrogen receptor-associated immunophilins, cyclophilin 40 and FKBP52, in breast cancer. Breast Cancer Res. Treat. 1999, 58, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, T. Steroid Receptor-Associated Immunophilins: Candidates for Diverse Drug-Targeting Approaches in Disease. Curr. Mol. Pharmacol. 2015, 9, 66–95. [Google Scholar] [CrossRef]
- Gougelet, A.; Bouclier, C.; Marsaud, V.; Maillard, S.; Mueller, S.O.; Korach, K.S.; Renoir, J.M. Estrogen receptor α and beta subtype expression and transactivation capacity are differentially affected by receptor-, hsp90- and immunophilin-ligands in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 71–81. [Google Scholar] [CrossRef]
- Periyasamy, S.; Warrier, M.; Tillekeratne, M.P.; Shou, W.; Sanchez, E.R. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology 2007, 148, 4716–4726. [Google Scholar] [CrossRef]
- Lin, J.F.; Xu, J.; Tian, H.Y.; Gao, X.; Chen, Q.X.; Gu, Q.; Xu, G.J.; Song, J.D.; Zhao, F.K. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int. J. Cancer 2007, 121, 2596–2605. [Google Scholar] [CrossRef]
- Mostaghel, E.A.; Page, S.T.; Lin, D.W.; Fazli, L.; Coleman, I.M.; True, L.D.; Knudsen, B.; Hess, D.L.; Nelson, C.C.; Matsumoto, A.M.; et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007, 67, 5033–5041. [Google Scholar] [CrossRef]
- Periyasamy, S.; Hinds, T., Jr.; Shemshedini, L.; Shou, W.; Sanchez, E.R. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene 2010, 29, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yang, C.S.; Gioeli, D.; Frierson, H.; Toft, D.O.; Paschal, B.M. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol. Cell. Biol. 2010, 30, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tao, X.; Chen, Z.P.; Han, J.T.; Jia, W.J.; Zhu, N.; Li, X.; Wang, Z.; He, Y.X. The environmental endocrine disruptor p-nitrophenol interacts with FKBP51, a positive regulator of androgen receptor and inhibits androgen receptor signaling in human cells. J. Hazard. Mater. 2016, 307, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.B.; Patel, D.; Morton, D.J.; Sharma, P.; Zou, J.; Hewa Bostanthirige, D.; Gorantla, Y.; Nagappan, P.; Komaragiri, S.K.; Sivils, J.C.; et al. Inactivation of ID4 promotes a CRPC phenotype with constitutive AR activation through FKBP52. Mol. Oncol. 2017, 11, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Laakso, M.; Pihlajamaa, P.; Ovaska, K.; Sinielnikov, I.; Hautaniemi, S.; Janne, O.A. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013, 73, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Kach, J.; Conzen, S.D.; Szmulewitz, R.Z. Targeting the glucocorticoid receptor in breast and prostate cancers. Sci. Transl. Med. 2015, 7, 305ps319. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, A.; Czwornog, J.; Chebotaev, D.; Karseladze, A.; Kulevitch, E.; Yang, X.; Budunova, I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene 2007, 26, 1885–1896. [Google Scholar] [CrossRef]
- Leach, D.A.; Trotta, A.P.; Need, E.F.; Risbridger, G.P.; Taylor, R.A.; Buchanan, G. The prognostic value of stromal FK506-binding protein 1 and androgen receptor in prostate cancer outcome. Prostate 2017, 77, 185–195. [Google Scholar] [CrossRef]
- Cheung-Flynn, J.; Prapapanich, V.; Cox, M.B.; Riggs, D.L.; Suarez-Quian, C.; Smith, D.F. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol. Endocrinol. 2005, 19, 1654–1666. [Google Scholar] [CrossRef]
- Yong, W.; Yang, Z.; Periyasamy, S.; Chen, H.; Yucel, S.; Li, W.; Lin, L.Y.; Wolf, I.M.; Cohn, M.J.; Baskin, L.S.; et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J. Biol. Chem. 2007, 282, 5026–5036. [Google Scholar] [CrossRef]
- Yeh, S.; Tsai, M.Y.; Xu, Q.; Mu, X.M.; Lardy, H.; Huang, K.E.; Lin, H.; Yeh, S.D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.R. Chaperoning steroidal physiology: Lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim. Biophys. Acta 2012, 1823, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Cox, M.B.; Tardif, H.L.; Hessling, M.; Buchner, J.; Smith, D.F. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol. Cell. Biol. 2007, 27, 8658–8669. [Google Scholar] [CrossRef] [PubMed]
- Solassol, J.; Mange, A.; Maudelonde, T. FKBP family proteins as promising new biomarkers for cancer. Curr. Opin. Pharmacol. 2011, 11, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Merolla, F.; Mascolo, M.; Ilardi, G.; Romano, S.; Varricchio, S.; Napolitano, V.; Celetti, A.; Postiglione, L.; Di Lorenzo, P.P.; et al. FKBP51 Immunohistochemical Expression: A New Prognostic Biomarker for OSCC? Int. J. Mol. Sci. 2017, 18, 443. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.M.; Boulianne, G.L. Diverse structures, functions and uses of FK506 binding proteins. Cell Signal. 2017, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Chao, C.C. Silencing of Taxol-Sensitizer Genes in Cancer Cells: Lack of Sensitization Effects. Cancers 2015, 7, 1052–1071. [Google Scholar] [CrossRef]
- Rotoli, D.; Morales, M.; Del Carmen Maeso, M.; Del Pino Garcia, M.; Morales, A.; Avila, J.; Martin-Vasallo, P. Expression and localization of the immunophilin FKBP51 in colorectal carcinomas and primary metastases, and alterations following oxaliplatin-based chemotherapy. Oncol. Lett. 2016, 12, 1315–1322. [Google Scholar] [CrossRef]
- Rotoli, D.; Morales, M.; Avila, J.; Maeso, M.D.C.; Garcia, M.D.P.; Mobasheri, A.; Martin-Vasallo, P. Commitment of Scaffold Proteins in the Onco-Biology of Human Colorectal Cancer and Liver Metastases after Oxaliplatin-Based Chemotherapy. Int. J. Mol. Sci. 2017, 18, 891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Xing, Z.; Yuan, X.; Wu, Y.; Xu, M.; Tu, K.; Li, Q.; Wu, C.; Zhao, M.; et al. Proteomic mining in the dysplastic liver of WHV/c-myc mice--insights and indicators for early hepatocarcinogenesis. FEBS J. 2010, 277, 4039–4053. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, H.; Li, G.; Sun, Y.; Chen, J.; Shi, L.; Cai, X.; Chang, C. The miR-367-3p Increases Sorafenib Chemotherapy Efficacy to Suppress Hepatocellular Carcinoma Metastasis through Altering the Androgen Receptor Signals. EBioMedicine 2016, 12, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.G.; Keen, C.L.; Oteiza, P.I. Microtubules are required for NF-κB nuclear translocation in neuroblastoma IMR-32 cells: Modulation by zinc. J. Neurochem. 2006, 99, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Karin, M. Missing pieces in the NF-κB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Gojoubori, T.; Ota, H.; Kusunoki, M.; Nishio, Y.; Nishio, K.; Iwasa, S.; Kaneko, Y.; Asano, M. Electrolytically generated acid functional water inhibits NF-κB activity by attenuating nuclear-cytoplasmic shuttling of p65 and p50 subunits. J. Recept. Signal Transduct. Res. 2016, 36, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Ryo, A.; Suizu, F.; Yoshida, Y.; Perrem, K.; Liou, Y.C.; Wulf, G.; Rottapel, R.; Yamaoka, S.; Lu, K.P. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [Google Scholar] [CrossRef]
- Wulf, G.; Ryo, A.; Liou, Y.C.; Lu, K.P. The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res. 2003, 5, 76–82. [Google Scholar] [CrossRef]
- Wulf, G.M.; Ryo, A.; Wulf, G.G.; Lee, S.W.; Niu, T.; Petkova, V.; Lu, K.P. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 2001, 20, 3459–3472. [Google Scholar] [CrossRef]
- Bao, L.; Kimzey, A.; Sauter, G.; Sowadski, J.M.; Lu, K.P.; Wang, D.G. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 2004, 164, 1727–1737. [Google Scholar] [CrossRef]
- Yoshimura, A.; Mori, H.; Ohishi, M.; Aki, D.; Hanada, T. Negative regulation of cytokine signaling influences inflammation. Curr. Opin. Immunol. 2003, 15, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Xiao, Y.; Nakaya, M.; D'Angelillo, A.; Chang, M.; Jin, J.; Hausch, F.; Masullo, M.; Feng, X.; Romano, M.F.; et al. FKBP51 employs both scaffold and isomerase functions to promote NF-κB activation in melanoma. Nucleic Acids Res. 2015, 43, 6983–6993. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; D'Angelillo, A.; Pacelli, R.; Staibano, S.; De Luna, E.; Bisogni, R.; Eskelinen, E.L.; Mascolo, M.; Cali, G.; Arra, C.; et al. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010, 17, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Li, Y.; Yin, Y.; Li, L.; Wu, C.; Chen, Y.; Nowsheen, S.; Hu, Q.; Zhang, L.; Lou, Z.; et al. USP49 negatively regulates tumorigenesis and chemoresistance through FKBP51-AKT signaling. EMBO J. 2017, 36, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. FKBP51 regulation of AKT/protein kinase B phosphorylation. Curr. Opin. Pharmacol. 2011, 11, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Dogan, F.; Biray Avci, C. Correlation between telomerase and mTOR pathway in cancer stem cells. Gene 2018, 641, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Hausch, F.; Kozany, C.; Theodoropoulou, M.; Fabian, A.K. FKBPs and the Akt/mTOR pathway. Cell Cycle 2013, 12, 2366–2370. [Google Scholar] [CrossRef]
- Baretic, D.; Williams, R.L. The structural basis for mTOR function. Semin. Cell Dev. Biol. 2014, 36, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Sorrentino, A.; Di Pace, A.L.; Nappo, G.; Mercogliano, C.; Romano, M.F. The emerging role of large immunophilin FK506 binding protein 51 in cancer. Curr. Med. Chem. 2011, 18, 5424–5429. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Valentino, J.D.; Gulhati, P.; Evers, B.M. mTOR inhibitors in cancer therapy. Cancer Lett. 2012, 319, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, C.; Ji, J.; Zhang, T.; Yuan, X.; Zhang, Y.; Ma, W.; Yang, J.; Yang, L.; Jiang, Z.; et al. Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol. Rep. 2017, 38, 271–278. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Verusio, C.; Ciccarese, M.; Fumagalli, A.; Sartori, D.; Ancona, C.; Airoldi, M.; Moretti, G.; Ficorella, C.; Arcangeli, V.; et al. Everolimus (EVE) and exemestane (EXE) in patients with advanced breast cancer aged >/= 65 years: New lessons for clinical practice from the EVA study. Oncotarget 2018, 9, 31877–31887. [Google Scholar] [CrossRef] [PubMed]
- Hasskarl, J. Everolimus. Recent Results Cancer Res. 2018, 211, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 2011, 286, 30152–30160. [Google Scholar] [CrossRef]
- Akiyama, T.; Shiraishi, T.; Qin, J.; Konno, H.; Akiyama, N.; Shinzawa, M.; Miyauchi, M.; Takizawa, N.; Yanai, H.; Ohashi, H.; et al. Mitochondria-nucleus shuttling FK506-binding protein 51 interacts with TRAF proteins and facilitates the RIG-I-like receptor-mediated expression of type I IFN. PLOS ONE 2014, 9, e95992. [Google Scholar] [CrossRef]
- Toneatto, J.; Guber, S.; Charo, N.L.; Susperreguy, S.; Schwartz, J.; Galigniana, M.D.; Piwien-Pilipuk, G. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J. Cell Sci. 2013, 126, 5357–5368. [Google Scholar] [CrossRef]
- Fan, A.C.; Young, J.C. Function of cytosolic chaperones in Tom70-mediated mitochondrial import. Protein Pept. Lett. 2011, 18, 122–131. [Google Scholar] [CrossRef]
- Eisenstein, M. Telomeres: All's well that ends well. Nature 2011, 478, S13–S15. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Shay, J.W.; Wright, W.E. Telomere biology in Metazoa. FEBS Lett. 2010, 584, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Shore, D.; Bianchi, A. Telomere length regulation: Coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009, 28, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Young, N.S. Telomere diseases. New Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Kaneko, S.; Hayashi, N.; Yamashita, T.; Shirota, Y.; Kobayashi, K.; Murakami, S. Telomerase activity reconstituted in vitro with purified human telomerase reverse transcriptase and human telomerase RNA component. J. Biol. Chem. 2000, 275, 22568–22573. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, H.L.; Jarvis, J.L.; Turner, J.W.; Elmore, L.W.; Holt, S.E. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J. Biol. Chem. 2001, 276, 15571–15574. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.E.; Aisner, D.L.; Baur, J.; Tesmer, V.M.; Dy, M.; Ouellette, M.; Trager, J.B.; Morin, G.B.; Toft, D.O.; Shay, J.W.; et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999, 13, 817–826. [Google Scholar] [CrossRef]
- Gaali, S.; Kirschner, A.; Cuboni, S.; Hartmann, J.; Kozany, C.; Balsevich, G.; Namendorf, C.; Fernandez-Vizarra, P.; Sippel, C.; Zannas, A.S.; et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 2015, 11, 33–37. [Google Scholar] [CrossRef]
- Yao, Y.L.; Liang, Y.C.; Huang, H.H.; Yang, W.M. FKBPs in chromatin modification and cancer. Curr. Opin. Pharmacol. 2011, 11, 301–307. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Sanchez, E.R. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr. Opin. Pharmacol. 2011, 11, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Buchanan, G. Stromal Androgen Receptor in Prostate Cancer Development and Progression. Cancers 2017, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, Y.J.; Lee, J.M.; Kim, E.K.; Park, Y.J.; Choe, S.K.; Ko, H.J.; Kang, C.Y. Functional changes in myeloid-derived suppressor cells (MDSCs) during tumor growth: FKBP51 contributes to the regulation of the immunosuppressive function of MDSCs. J. Immunol. 2012, 188, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lou, Z.; Wang, L. The role of FKBP5 in cancer aetiology and chemoresistance. Br. J. Cancer 2011, 104, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Mukaide, H.; Adachi, Y.; Taketani, S.; Iwasaki, M.; Koike-Kiriyama, N.; Shigematsu, A.; Shi, M.; Yanai, S.; Yoshioka, K.; Kamiyama, Y.; et al. FKBP51 expressed by both normal epithelial cells and adenocarcinoma of colon suppresses proliferation of colorectal adenocarcinoma. Cancer Investig. 2008, 26, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Noei, F.; Feroz, T.; Lee, J.M. Geldanamycin anisimycins activate Rho and stimulate Rho- and ROCK-dependent actin stress fiber formation. Mol. Cancer Res. 2007, 5, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Le Boeuf, F.; Houle, F.; Sussman, M.; Huot, J. Phosphorylation of focal adhesion kinase (FAK) on Ser732 is induced by rho-dependent kinase and is essential for proline-rich tyrosine kinase-2-mediated phosphorylation of FAK on Tyr407 in response to vascular endothelial growth factor. Mol. Biol. Cell 2006, 17, 3508–3520. [Google Scholar] [CrossRef]
- Takaoka, M.; Ito, S.; Miki, Y.; Nakanishi, A. FKBP51 regulates cell motility and invasion via RhoA signaling. Cancer Sci. 2017, 108, 380–389. [Google Scholar] [CrossRef]
- Ostrow, K.L.; Park, H.L.; Hoque, M.O.; Kim, M.S.; Liu, J.; Argani, P.; Westra, W.; Van Criekinge, W.; Sidransky, D. Pharmacologic unmasking of epigenetically silenced genes in breast cancer. Clin. Cancer Res. 2009, 15, 1184–1191. [Google Scholar] [CrossRef]
- Pearson, J.D.; Mohammed, Z.; Bacani, J.T.; Lai, R.; Ingham, R.J. The heat shock protein-90 co-chaperone, Cyclophilin 40, promotes ALK-positive, anaplastic large cell lymphoma viability and its expression is regulated by the NPM-ALK oncoprotein. BMC Cancer 2012, 12, 229. [Google Scholar] [CrossRef]
- D'Arrigo, P.; Russo, M.; Rea, A.; Tufano, M.; Guadagno, E.; Del Basso De Caro, M.L.; Pacelli, R.; Hausch, F.; Staibano, S.; Ilardi, G.; et al. A regulatory role for the co-chaperone FKBP51s in PD-L1 expression in glioma. Oncotarget 2017, 8, 68291–68304. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gundelach, J.H.; Bram, R.J. Cycloheximide promotes paraptosis induced by inhibition of cyclophilins in glioblastoma multiforme. Cell Death Dis. 2017, 8, e2807. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Boerner, J.E.; TiongYip, C.; Weidmann, B.; Ryder, N.S.; Cooreman, M.P.; Lin, K. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with α interferon. Antimicrob. Agents Chemother. 2006, 50, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Duzgun, Z.; Eroglu, Z.; Biray Avci, C. Role of mTOR in glioblastoma. Gene 2016, 575, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Donley, C.; McClelland, K.; McKeen, H.D.; Nelson, L.; Yakkundi, A.; Jithesh, P.V.; Burrows, J.; McClements, L.; Valentine, A.; Prise, K.M.; et al. Identification of RBCK1 as a novel regulator of FKBPL: Implications for tumor growth and response to tamoxifen. Oncogene 2014, 33, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.; McKeen, H.D.; Marshall, A.; Mulrane, L.; Starczynski, J.; Storr, S.J.; Lanigan, F.; Byrne, C.; Arthur, K.; Hegarty, S.; et al. FKBPL: A marker of good prognosis in breast cancer. Oncotarget 2015, 6, 12209–12223. [Google Scholar] [CrossRef]
- Renoir, J.M. Estradiol receptors in breast cancer cells: Associated co-factors as targets for new therapeutic approaches. Steroids 2012, 77, 1249–1261. [Google Scholar] [CrossRef]
- Renoir, J.M.; Marsaud, V.; Lazennec, G. Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem. Pharmacol. 2013, 85, 449–465. [Google Scholar] [CrossRef]
- Desmetz, C.; Bascoul-Mollevi, C.; Rochaix, P.; Lamy, P.J.; Kramar, A.; Rouanet, P.; Maudelonde, T.; Mange, A.; Solassol, J. Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin. Cancer Res. 2009, 15, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Dhamad, A.E.; Zhou, Z.; Zhou, J.; Du, Y. Systematic Proteomic Identification of the Heat Shock Proteins (Hsp) that Interact with Estrogen Receptor α (ERα) and Biochemical Characterization of the ERα-Hsp70 Interaction. PLoS ONE 2016, 11, e0160312. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000004478-FKBP4/pathology (accessed on 20 October 2018).
- Garifulin, O.M.; Kykot, V.O.; Gridina, N.Y.; Kiyamova, R.G.; Gout, I.T.; Filonenko, V.V. Application of serex-analysis for identification of human colon cancer antigens. Exp. Oncol. 2015, 37, 173–180. [Google Scholar] [CrossRef]
- Duthie, K.A.; Osborne, L.C.; Foster, L.J.; Abraham, N. Proteomics analysis of interleukin (IL)-7-induced signaling effectors shows selective changes in IL-7Rα449F knock-in T cell progenitors. Mol. Cell. Proteom. 2007, 6, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Quinta, H.R.; Galigniana, N.M.; Erlejman, A.G.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. Management of cytoskeleton architecture by molecular chaperones and immunophilins. Cell Signal. 2011, 23, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Quinta, H.R.; Galigniana, M.D. The neuroregenerative mechanism mediated by the Hsp90-binding immunophilin FKBP52 resembles the early steps of neuronal differentiation. Br. J. Pharmacol. 2012, 166, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Litzenburger, U.M.; Rauschenbach, K.J.; Bunse, L.; Ochs, K.; Sahm, F.; Pusch, S.; Opitz, C.A.; Blaes, J.; von Deimling, A.; et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia 2015, 63, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cazacu, S.; Xiang, C.; Zenklusen, J.C.; Fine, H.A.; Berens, M.; Armstrong, B.; Brodie, C.; Mikkelsen, T. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-κB signaling pathway. Neoplasia 2008, 10, 235–243. [Google Scholar] [CrossRef]
- Lim, S.O.; Park, S.J.; Kim, W.; Park, S.G.; Kim, H.J.; Kim, Y.I.; Sohn, T.S.; Noh, J.H.; Jung, G. Proteome analysis of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2002, 291, 1031–1037. [Google Scholar] [CrossRef]
- Ruiz-Estevez, M.; Staats, J.; Paatela, E.; Munson, D.; Katoku-Kikyo, N.; Yuan, C.; Asakura, Y.; Hostager, R.; Kobayashi, H.; Asakura, A.; et al. Promotion of Myoblast Differentiation by Fkbp5 via Cdk4 Isomerization. Cell Rep. 2018, 25, 2537–2551.e8. [Google Scholar] [CrossRef]
- Schulke, J.P.; Wochnik, G.M.; Lang-Rollin, I.; Gassen, N.C.; Knapp, R.T.; Berning, B.; Yassouridis, A.; Rein, T. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS ONE 2010, 5, e11717. [Google Scholar] [CrossRef] [PubMed]
- Tranguch, S.; Smith, D.F.; Dey, S.K. Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation. Reprod. Biomed. Online 2006, 13, 651–660. [Google Scholar] [CrossRef]
- Hubler, T.R.; Denny, W.B.; Valentine, D.L.; Cheung-Flynn, J.; Smith, D.F.; Scammell, J.G. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 2003, 144, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Febbo, P.G.; Lowenberg, M.; Thorner, A.R.; Brown, M.; Loda, M.; Golub, T.R. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J. Urol. 2005, 173, 1772–1777. [Google Scholar] [CrossRef]
- Lagadari, M.; De Leo, S.A.; Camisay, M.F.; Galigniana, M.D.; Erlejman, A.G. Regulation of NF-κB signalling cascade by immunophilins. Curr. Mol. Pharmacol. 2016, 9, 99–108. [Google Scholar] [CrossRef]
- Quintá, H.R.; Maschi, D.; Gomez-Sanchez, C.; Piwien-Pilipuk, G.; Galigniana, M.D. Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth. J. Neurochem. 2010, 115, 716–734. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Koren, J., 3rd; Borysov, S.I.; Schmid, A.B.; Abisambra, J.F.; Blair, L.J.; Johnson, A.G.; Jones, J.R.; Shults, C.L.; O'Leary, J.C., 3rd; et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J. Neurosci. 2010, 30, 591–599. [Google Scholar] [CrossRef]
- Chambraud, B.; Sardin, E.; Giustiniani, J.; Dounane, O.; Schumacher, M.; Goedert, M.; Baulieu, E.E. A role for FKBP52 in Tau protein function. Proc. Natl. Acad. Sci. USA 2010, 107, 2658–2663. [Google Scholar] [CrossRef]
- Sanokawa-Akakura, R.; Dai, H.; Akakura, S.; Weinstein, D.; Fajardo, J.E.; Lang, S.E.; Wadsworth, S.; Siekierka, J.; Birge, R.B. A novel role for the immunophilin FKBP52 in copper transport. J. Biol. Chem. 2004, 279, 27845–27848. [Google Scholar] [CrossRef]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef]
- Mills, E.; Dong, X.P.; Wang, F.; Xu, H. Mechanisms of brain iron transport: Insight into neurodegeneration and CNS disorders. Future Med. Chem. 2010, 2, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Warrier, M.; Hinds, T.D., Jr.; Ledford, K.J.; Cash, H.A.; Patel, P.R.; Bowman, T.A.; Stechschulte, L.A.; Yong, W.; Shou, W.; Najjar, S.M.; et al. Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology 2010, 151, 3225–3236. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Csermely, P.; Soti, C. Hsp90 chaperones PPARgamma and regulates differentiation and survival of 3T3-L1 adipocytes. Cell Death Differ. 2013, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Desarzens, S.; Liao, W.H.; Mammi, C.; Caprio, M.; Faresse, N. Hsp90 blockers inhibit adipocyte differentiation and fat mass accumulation. PLoS ONE 2014, 9, e94127. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, V.L.; Renes, J.; Ramaekers, F.C.; Kamps, M.; Kuijpers, H.J.; Verheyen, F.; Wabitsch, M.; Steijlen, P.M.; van Steensel, M.A.; Broers, J.L. Reorganization of the nuclear lamina and cytoskeleton in adipogenesis. Histochem. Cell Biol. 2011, 135, 251–261. [Google Scholar] [CrossRef] [PubMed]
- D'Angelo, M.A.; Hetzer, M.W. The role of the nuclear envelope in cellular organization. Cell. Mol. Life Sci. 2006, 63, 316–332. [Google Scholar] [CrossRef]
- Stuurman, N. Identification of a conserved phosphorylation site modulating nuclear lamin polymerization. FEBS Lett. 1997, 401, 171–174. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Hinds, T.D., Jr.; Ghanem, S.S.; Shou, W.; Najjar, S.M.; Sanchez, E.R. FKBP51 reciprocally regulates GRα and PPARgamma activation via the Akt-p38 pathway. Mol. Endocrinol. 2014, 28, 1254–1264. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Hinds, T.D., Jr.; Khuder, S.S.; Shou, W.; Najjar, S.M.; Sanchez, E.R. FKBP51 controls cellular adipogenesis through p38 kinase-mediated phosphorylation of GRα and PPARgamma. Mol. Endocrinol. 2014, 28, 1265–1275. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- O'Leary, J.C., 3rd; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A new anti-depressive strategy for the elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6, e24840. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Gassen, N.C.; Herrmann, L.; Cheung-Flynn, J.; Bull, D.R.; Ionescu, I.A.; Heinzmann, J.M.; Knapman, A.; Siebertz, A.; Depping, A.M.; et al. FK506 binding protein 5 shapes stress responsiveness: Modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 2011, 70, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Wagner, K.V.; Liebl, C.; Scharf, S.H.; Wang, X.D.; Wolf, M.; Hausch, F.; Rein, T.; Schmidt, U.; Touma, C.; et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 2012, 62, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Stechschulte, L.A.; Qiu, B.; Warrier, M.; Hinds, T.D., Jr.; Zhang, M.; Gu, H.; Xu, Y.; Khuder, S.S.; Russo, L.; Najjar, S.M.; et al. FKBP51 Null Mice Are Resistant to Diet-Induced Obesity and the PPARgamma Agonist Rosiglitazone. Endocrinology 2016, 157, 3888–3900. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, L.; Leng, X.; Parker, S.B.; Harper, J.W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996, 10, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Verba, K.A.; Wang, R.Y.; Arakawa, A.; Liu, Y.; Shirouzu, M.; Yokoyama, S.; Agard, D.A. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 2016, 352, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, L.J.A.; Azzarelli, R.; Philpott, A. Cell cycle-dependent phosphorylation and regulation of cellular differentiation. Biochem. Soc. Trans. 2018, 46, 1083–1091. [Google Scholar] [CrossRef]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Muller, M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef]
- Fries, G.R.; Gassen, N.C.; Schmidt, U.; Rein, T. The FKBP51-Glucocorticoid Receptor Balance in Stress-Related Mental Disorders. Curr. Mol. Pharmacol. 2015, 9, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Lester, B.M.; Koestler, D.C.; Lesseur, C.; Armstrong, D.A.; Marsit, C.J. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS ONE 2014, 9, e104913. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Rein, T.; Binder, E.B.; Porter, J.T.; Koren, J., 3rd; Blair, L.J. Hsp90 and FKBP51: Complex regulators of psychiatric diseases. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 2018, 373, 20160532. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Wagner, K.V.; Gaali, S.; Kirschner, A.; Kozany, C.; Ruhter, G.; Dedic, N.; Hausl, A.S.; Hoeijmakers, L.; Westerholz, S.; et al. Pharmacological Inhibition of the Psychiatric Risk Factor FKBP51 Has Anxiolytic Properties. J. Neurosci. 2015, 35, 9007–9016. [Google Scholar] [CrossRef]
- Ising, M.; Depping, A.M.; Siebertz, A.; Lucae, S.; Unschuld, P.G.; Kloiber, S.; Horstmann, S.; Uhr, M.; Muller-Myhsok, B.; Holsboer, F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008, 28, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.B.; Zandi, P.P.; Potash, J.B.; Nestadt, G.; Wand, G.S. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology 2013, 227, 231–241. [Google Scholar] [CrossRef]
- Shibuya, N.; Suzuki, A.; Sadahiro, R.; Kamata, M.; Matsumoto, Y.; Goto, K.; Hozumi, Y.; Otani, K. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neurosci. Lett. 2010, 485, 194–197. [Google Scholar] [CrossRef]
- Fani, N.; King, T.Z.; Reiser, E.; Binder, E.B.; Jovanovic, T.; Bradley, B.; Ressler, K.J. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology 2014, 39, 1206–1213. [Google Scholar] [CrossRef]
- Fani, N.; Gutman, D.; Tone, E.B.; Almli, L.; Mercer, K.B.; Davis, J.; Glover, E.; Jovanovic, T.; Bradley, B.; Dinov, I.D.; et al. FKBP5 and attention bias for threat: Associations with hippocampal function and shape. JAMA Psychiatry 2013, 70, 392–400. [Google Scholar] [CrossRef]
- Fujii, T.; Ota, M.; Hori, H.; Hattori, K.; Teraishi, T.; Sasayama, D.; Higuchi, T.; Kunugi, H. Association between the common functional FKBP5 variant (rs1360780) and brain structure in a non-clinical population. J. Psychiatr. Res. 2014, 58, 96–101. [Google Scholar] [CrossRef]
- Levy-Gigi, E.; Szabo, C.; Kelemen, O.; Keri, S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiatry 2013, 74, 793–800. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Bogdan, R.; Fisher, P.M.; Munoz, K.E.; Williamson, D.E.; Hariri, A.R. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genesbrainand Behav. 2012, 11, 869–878. [Google Scholar] [CrossRef]
- Klengel, T.; Binder, E.B. Allele-specific epigenetic modification: A molecular mechanism for gene-environment interactions in stress-related psychiatric disorders? Epigenomics 2013, 5, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, M.L.; Hausl, A.S.; Harbich, D.; Balsevich, G.; Engelhardt, C.; Feng, X.; Breitsamer, M.; Hausch, F.; Winter, G.; Schmidt, M.V. Pharmacological Modulation of the Psychiatric Risk Factor FKBP51 Alters Efficiency of Common Antidepressant Drugs. Front. Behav. Neurosci. 2018, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Fries, G.R.; Zannas, A.S.; Hartmann, J.; Zschocke, J.; Hafner, K.; Carrillo-Roa, T.; Steinbacher, J.; Preissinger, S.N.; Hoeijmakers, L.; et al. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 2015, 8, ra119. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Zannas, A.S.; Kretzschmar, A.; Zschocke, J.; Maccarrone, G.; Hafner, K.; Zellner, A.; Kollmannsberger, L.K.; Wagner, K.V.; et al. FKBP51 inhibits GSK3beta and augments the effects of distinct psychotropic medications. Mol. Psychiatry 2016, 21, 277–289. [Google Scholar] [CrossRef] [PubMed]
| FKBP51 | FKBP52 | Ref. | |
|---|---|---|---|
| Malignancies | |||
| Breast | ↑ | ↑ | [173,174] |
| Prostate | ↑ | ↑ | [90,96] |
| Melanoma | ↑ | N | [125,175] |
| Pancreas | ↓ | N | [126,175] |
| Oral squamous cell carcinoma | ↑ | (n.d.) | [107] |
| Hepatocarcinoma | ↓ | ↑ | [112,113] |
| Colorectal carcinoma | ↑ | ↑ | [110,176] |
| Lymphoma | ↑ | ↑ | [162,177] |
| Nervous System | |||
| Neurodifferentiation | ↓ | ↑ | [178] |
| Neuroregeneration | ↓ | ↑ | [179] |
| Astrocytoma | ↑ | ↑ | [180,181,182] |
| Myoblast differentiation | ↑(*) | ↓ | [183] |
| Adipogenesis | ↑ | ↓ | [141] |
| Steroid receptors | |||
| GR | ↓ | ↑ | [18,86] |
| MR | ↓ | N | [56,184] |
| PR | ↓ | ↑ | [185,186] |
| AR | ↑ | ↑ | [93,95,187] |
| NF-κB signaling | |||
| Melanoma cells | ↑ | ↑ | [124,175] |
| Kidney fibroblasts/Placenta cells | ↓ | ↑ | [69,188] |
| mTOR signaling | ↑ | ↑ | [131] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgajnar, N.R.; De Leo, S.A.; Lotufo, C.M.; Erlejman, A.G.; Piwien-Pilipuk, G.; Galigniana, M.D. Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules 2019, 9, 52. https://doi.org/10.3390/biom9020052
Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G, Galigniana MD. Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules. 2019; 9(2):52. https://doi.org/10.3390/biom9020052
Chicago/Turabian StyleZgajnar, Nadia R., Sonia A. De Leo, Cecilia M. Lotufo, Alejandra G. Erlejman, Graciela Piwien-Pilipuk, and Mario D. Galigniana. 2019. "Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52" Biomolecules 9, no. 2: 52. https://doi.org/10.3390/biom9020052
APA StyleZgajnar, N. R., De Leo, S. A., Lotufo, C. M., Erlejman, A. G., Piwien-Pilipuk, G., & Galigniana, M. D. (2019). Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52. Biomolecules, 9(2), 52. https://doi.org/10.3390/biom9020052






