Electrocardiographic and Seasonal Patterns Allow Accurate Differentiation of Tako-Tsubo Cardiomyopathy from Acute Anterior Myocardial Infarction: Results of a Multicenter Study and Systematic Overview of Available Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Electrocardiographic Pattern
2.1.2. Seasonal Variation
2.2. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features
3.2. Triggering Life Events
3.3. Laboratory Values
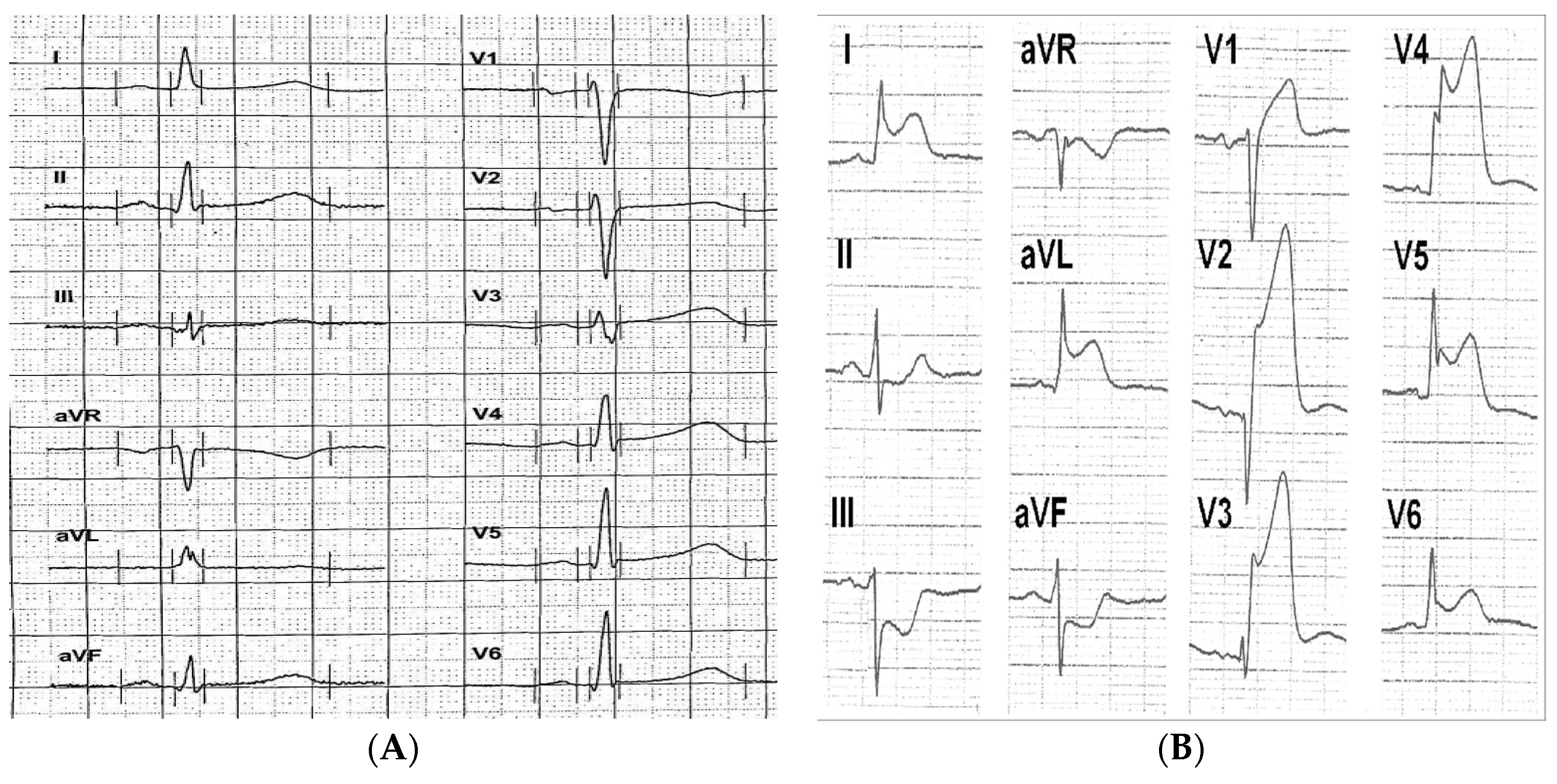
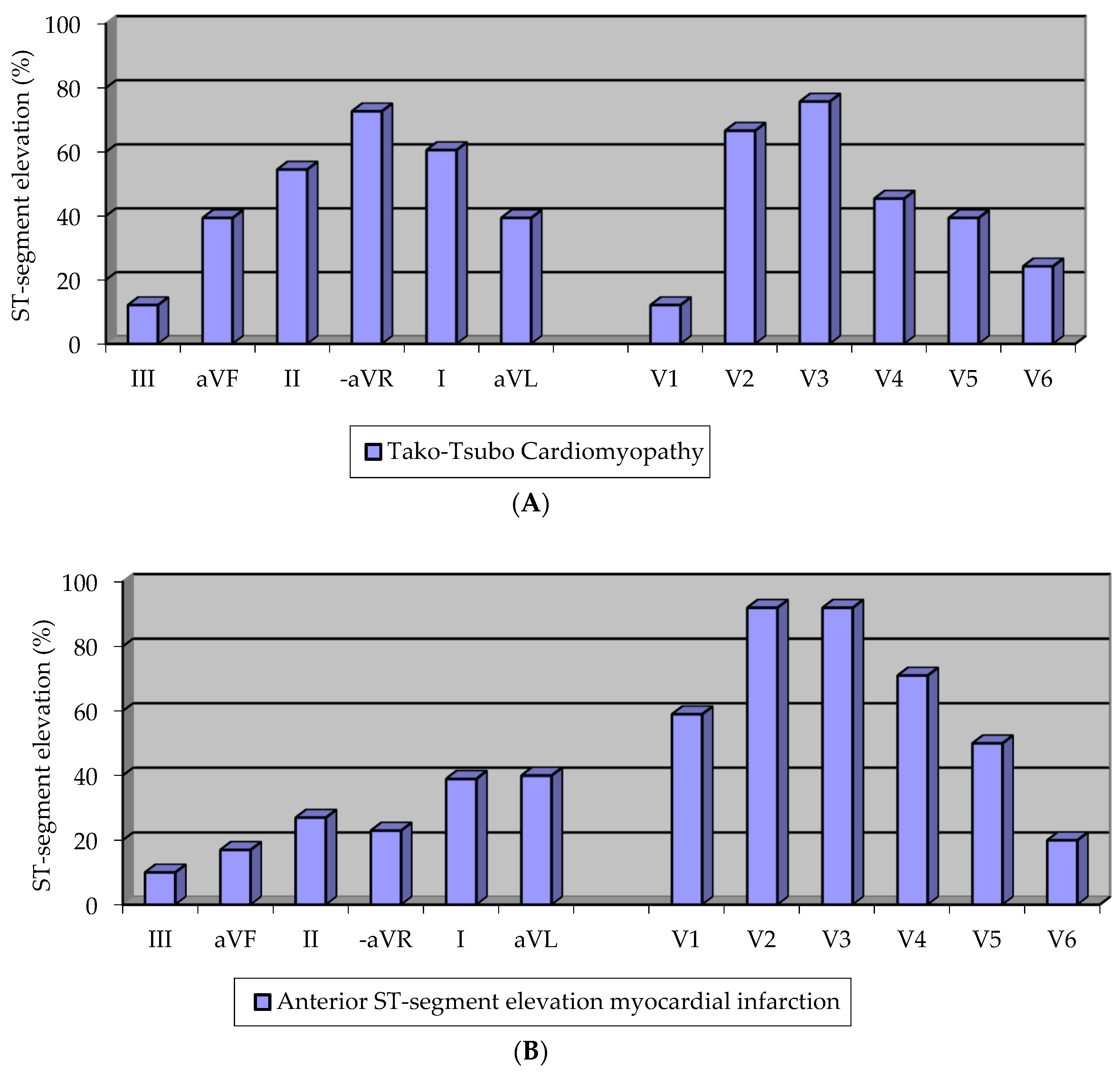
3.4. Electrocardiographic Pattern
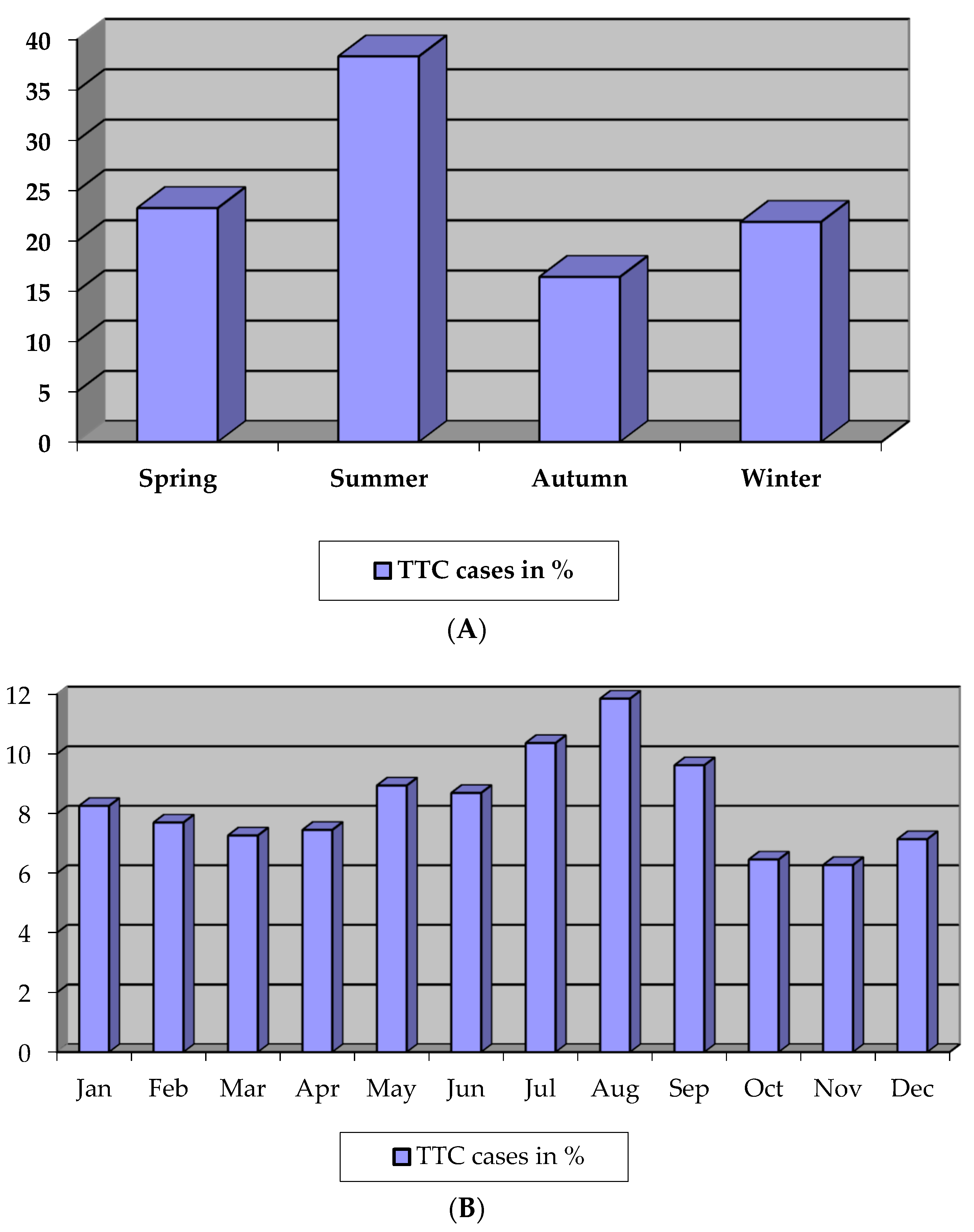
3.5. Seasonal Variation
4. Discussion
4.1. Baseline Characteristics
4.2. Electrocardiographic Pattern
4.3. Seasonal Variation
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Dote, K.; Satoh, H.; Tateishi, H.; Uchida, T.; Ishihara, M. Myocardial stunning due to simultaneous multivessel coronary spasm: A review of 5 cases. J. Cardiol. 1991, 21, 203–214. [Google Scholar] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart Association; et al. Contemporary Definitions and Classification of the Cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [PubMed]
- Kosuge, M.; Ebina, T.; Hibi, K.; Morita, S.; Okuda, J.; Iwahashi, N.; Tsukahara, K.; Nakachi, T.; Kiyokuni, M.; Ishikawa, T.; et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Previtali, M.; Bovelli, D.; Vriz, O.; Astarita, C.; Patella, M.M.; Provenza, G.; Armentano, C.; Ciampi, Q.; Gregorio, G.; et al. Chronobiological patterns of onset of tako-tsubocardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Mansencal, N.; El Mahmoud, R.; Dubourg, O. Occurrence of Tako-tsubo cardiomyopathy and chronobiological variation. J. Am. Coll. Cardiol. 2010, 55, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, I.; Kay, S.; Mussap, C.; Nelson, G.I.; Rasmussen, H.H.; Hansen, P.S.; Ward, M.R. Apical sparing in tako-tsubo cardiomyopathy. Int. Med. J. 2006, 36, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.R.; Dib, C.; Prasad, A. Chronobiology of tako-tsubo cardiomyopathy (apical ballooning syndrome). J. Am. Geriatr. Soc. 2010, 58, 805–806. [Google Scholar] [CrossRef]
- Regnante, R.A.; Zuzek, R.W.; Weinsier, S.B.; Latif, S.R.; Linsky, R.A.; Ahmed, H.N.; Sadiq, I. Clinical characteristics and four-year outcomes of patients in the Rhode Island takotsubo cardiomyopathy registry. Am. J. Cardiol. 2009, 103, 1015–1019. [Google Scholar] [CrossRef]
- Hertting, K.; Krause, K.; Härle, T.; Boczor, S.; Reimers, J.; Kuck, K.H. Transient left ventricular apical ballooning in a community hospital in Germany. Int. J. Cardiol. 2006, 112, 282–288. [Google Scholar] [CrossRef]
- Wagner, G.S.; Macfarlane, P.; Wellens, H.; Josephson, M.; Gorgels, A.; Mirvis, D.M.; Pahlm, O.; Surawicz, B.; Kligfield, P.; Childers, R.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part VI: Acute ischemia/infarction. J. Am. Coll. Cardiol. 2009, 53, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kondo, M.; Matsuoka, R.; Araki, M.; Dohyama, K.; Tanio, H. Assesment of clinical features in transient left ventricular apical ballooning. J. Am. Coll. Cardiol. 2003, 41, 737–742. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Nakazawa, K.; Sakakibara, M.; Miyake, F.; Koike, H.; Sasaka, K. The clinical features of takotsubo cardiomyopathy. QJM 2003, 96, 563–573. [Google Scholar] [CrossRef]
- Bybee, K.A.; Kara, T.; Prasad, A.; Lerman, A.; Barsness, G.W.; Wright, R.S.; Rihal, C.S. Transient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction. Ann. Int. Med. 2004, 141, 858–865. [Google Scholar] [CrossRef]
- Kurisu, S.; Sato, H.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nishioka, K.; Kono, Y.; Umemura, T.; Nakamura, S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: A novel cardiac syndrome mimicking acute myocardial infarction. Am. Heart J. 2002, 143, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K.; Ueshima, K.; Uchida, T.; Oh-mura, N.; Kimura, K.; Owa, M.; Yoshiyama, M.; Miyazaki, S.; Haze, K.; Ogawa, H.; et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. InterTAK Co-investigators: Happy heart syndrome: Role of positive emotional stress in takotsubo syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Lesser, J.R.; Zenovich, A.G.; Maron, M.S.; Lindberg, J.; Longe, T.F.; Maron, B.J. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005, 111, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Nef, H.M.; Möllmann, H.; Troidl, C.; Kostin, S.; Böttger, T.; Voss, S.; Hilpert, P.; Krause, N.; Weber, M.; Rolf, A.; et al. Expression profiling of cardiac genes in tako-tsubo cardiomyopathy: Insight into a new cardiac entity. J. Mol. Cell. Cardiol. 2008, 44, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Bartel, S.; Kirchhefer, W.; Kokott, A.; Krause, E.G.; Neumann, J.; Schmitz, W.; Scholz, H. Relation between contractile function and regulatory cardiac proteins in hypertrophied hearts. Am. J. Physiol. 1996, 270, 2021–2028. [Google Scholar] [CrossRef]
- Asahi, M.; Otsu, K.; Nakayama, H.; Hikoso, S.; Takeda, T.; Gramolini, A.O.; Trivieri, M.G.; Oudit, G.Y.; Morita, T.; Kusakari, Y.; et al. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 9199–9204. [Google Scholar] [CrossRef]
- Eshtehardi, P.; Koestner, S.C.; Adorjan, P.; Windecker, S.; Meier, B.; Hess, O.M.; Wahl, A.; Cook, S. Transient apical ballooning syndrome—Clinical characteristics, ballooning pattern, and long-term follow-up in a Swiss population. Int. J. Cardiol. 2009, 135, 370–375. [Google Scholar] [CrossRef]
- Madhavan, M.; Borlaug, B.A.; Lerman, A.; Rihal, C.S.; Prasad, A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): Inights into the clinical signifikance of B-type natriuretic peptide and troponin levels. Heart 2009, 95, 1436–1441. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima Joao, A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Barriales Villa, R.; Bilbao Quesada, R.; Iglesias Rio, E.; Bayón Meleiro, N.; Mantilla González, R.; Penas Lado, M. Transient left ventricular apical ballooning without coronary stenoses syndrome: Importance of the intraventricular pressure gradient. Rev. Esp. Cardiol. 2004, 57, 85–88. [Google Scholar] [CrossRef]
- Elesber, A.A.; Prasad, A.; Bybee, K.A; Valeti, U.; Motiei, A.; Lerman, A.; Chandrasekaran, K.; Rihal, C.S. Transient cardiac apical ballooning syndrome: Prevalence and clinical implications of right ventricular involvement. J. Am. Cardiol. 2006, 47, 1082–1083. [Google Scholar] [CrossRef]
- Inoue, M.; Shimizu, M.; Ino, H.; Yamaguchi, M.; Terai, H.; Fujino, N.; Sakata, K.; Funada, A.; Tatami, R.; Ishise, S.; et al. Differentiation Between Patients with Takotsubo Cardiomyopathy and Those with Anterior Acute Myocardial Infarction. Circ. J. 2005, 69, 89–94. [Google Scholar] [CrossRef]
- Bybee, K.A.; Motiei, A.; Syed, I.S; Kara, T.; Prasad, A.; Lennon, R.J.; Murphy, J.G.; Hammill, S.C.; Rihal, C.S.; Wright, R.S. Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction. J. Electrocardiol. 2007, 40, 1–6. [Google Scholar] [CrossRef]
- Jim, M.H.; Chan, A.O.O.; Tsui, P.T.; Lau, S.T.; Siu, C.W.; Chow, W.H.; Lau, C.P. A new ECG criterion to identify takotsubo cardiomyopathy from anterior myocardial infarction: Role of inferior leads. Heart Vessels 2009, 24, 124–130. [Google Scholar] [CrossRef]
- Karakas, M.; Januzzi, J.L., Jr.; Meyer, J.; Lee, H.; Schlett, C.L.; Truong, Q.A.; Rottbauer, W.; Bamberg, F.; Dasdemir, S.; Hoffmann, U.; Koenig, W. Copeptin does not add diagnostic information to high-sensitivity troponin T in low-to intermediate-risk patients with acute chest pain: Results from the rule out myocardial infarction by computed tomography (ROMICAT) study. Clin. Chem. 2011, 57, 1137–1145. [Google Scholar] [CrossRef]
- Sclarovsky, S.; Nikus, K. The electrocardiographic paradox of tako-tsubo cardiomyopathy-comparison with acute ischemic syndromes and consideration of molecular biology and electrical-mechanical mismatching. J. Electrocardiol. 2010, 43, 173–176. [Google Scholar] [CrossRef]
- Ranki, H.J.; Budas, G.R.; Crawford, R.M.; Jovanovic, A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol. 2001, 38, 906–915. [Google Scholar] [CrossRef]
- Spencer, F.; Goldberg, R.J.; Becker, R.C.; Gore, J.M. Seasonal distribution of acute myocardial infarction in the second national registry of myocardial infarction. J. Am. Coll. Cardiol. 1998, 32, 1226–1233. [Google Scholar] [CrossRef]
- Hansen, A.M.; Garde, A.H.; Skovgaard, L.T.; Christensen, J.M. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clin. Chim. Acta 2001, 309, 25–35. [Google Scholar] [CrossRef]
- Sherwood, A.; Park, S.B.; Hughes, J.W.; Blumenthal, J.A.; Hinderliter, A.; Trivedi, R.; McFetridge-Durdle, J. Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause 2010, 17, 403–409. [Google Scholar] [CrossRef]
- Sherwood, A.; Hughes, J.W.; Kuhn, C.; Hinderliter, A.L. Hostility is related to blunted beta-adrenergic receptor responsiveness among middle-aged women. Psychosom. Med. 2004, 66, 507–513. [Google Scholar] [CrossRef]
- Ueyama, T.; Kasamatsu, K.; Hano, T.; Tsuruo, Y.; Ishikura, F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann. N. Y. Acad. Sci. 2008, 1148, 479–485. [Google Scholar] [CrossRef]
| Clinical Characteristics of Patients with Tako-Tsubo Cardiomyopathy | Number of Patients (%) |
|---|---|
| Symptoms at presentation | |
| Chest pain | 86 (74.8%) |
| Dyspnoea | 51 (44.3%) |
| Palpitation | 11 (9.6%) |
| Vertigo | 7 (6.1%) |
| Vegetative accompanying symptoms | 24 (20.9%) |
| Syncope | 4 (3.5%) |
| Preceding stressors | |
| Emotional | 53 (46.0%) |
| Clinical | 42 (36.5%) |
| Physical | 12 (10.4%) |
| Unknown trigger | 8 (7.1%) |
| Arterial hypertension | 72 (62.6%) |
| Hyperlipoproteinemia | 48 (41.7%) |
| Diabetes mellitus | 14 (12.2%) |
| Familiar disposition for CVE | 26 (22.6%) |
| Nicotine consumption | 19 (16.5%) |
| Chronic alcohol consumption | 3 (2.6%) |
| Body-Mass-Index female (kg/m²) | Median: 25.5; IQR: 22.6–30.3 |
| Body-Mass-Index male (kg/m²) | Median: 26.6; IQR: 25.0–30.3 |
| Pre-existing obstructive coronary heart disease | 0 (0%) |
| Pre-existing coronary artery plaque | 2 (1.7%) |
| Reduced left ventricular ejection fraction (<55%) | 72 (62.6%) |
| Apical left ventricular thrombus | 2 (1.7%) |
| Increased end-diastolic left ventricular pressure | |
| LVEDP > 11 mmHg | 68 (74.7%) |
| Right ventricular participation | 5 (4.3%) |
| Obstructive Coronary artery disease (stenosis > 50%) | 5 (4.3%) |
| In-hospital outcomes | |
| TIA/apoplexy | 3 (2.6%) |
| Cardiogenic shock | 5 (4.3%) |
| Death | 3 (2.6%), all female patients |
| Long-term follow-up | |
| Death from any cause | 7 (6.1% per patient-year); Median: 34 months; IQR: 13.5–63 |
| Recurrence | 10 (8.7% per patient-year); Median: 22 months; IQR: 9–33 |
| Clinical Stressor, Number of Patients n = 42 (36.5%) | Emotional Stressor, Number of Patients n = 53 (46%) | Physical Stressor, Number of Patients n = 12 (10.4%) |
|---|---|---|
| Perioperative status (e.g., emergency caesarean section with atonic postpartum hemorrhage) | Death in the family | Agricultural work |
| Malignant disease | Family disputes | Heavy gardening |
| Febrile/protracted infection | Stress at work | Hiking |
| Acute abdomen | Excessive celebration | |
| Acute cholecystitis | Pronounced euphoria after | |
| Sepsis | grandchild’s birth | |
| Advanced ALS with aspiration- | ||
| pneumonia | ||
| Apoplexy | ||
| Laryngitis | ||
| Tracheomalacia | ||
| Hypertensive crisis | ||
| Cardiac decompensation | ||
| Fall with rhabdomyolysis | ||
| Vitreous hemorrhage | ||
| Migraine |
| Biomarker | Number of Patients (%) | Laboratory Value (Median; IQR) |
|---|---|---|
| Troponin Ion admission | 57 (49.6%) | 0.42 µg/L (ng/mL); IQR: 0.11–1.52 |
| Troponin Imaximum | 22 (19.1%) | 2.84 µg/L (ng/mL); IQR: 0.9–7.65 |
| Troponin Ton admission | 49 (42.6%) | 0.2 ng/mL (µg/L); IQR: 0.09–0.66 |
| Troponin Tmaximum | 11 (9.6%) | 0.43 ng/mL (µg/L); IQR: 0.16–1.12 |
| hsTroponinon admission | 5 (4.3%) | 32 ng/L; IQR: 14–756.5 |
| CKon admission | 101 (87.8%) | 137 U/L; IQR: 83.5–241.5 |
| CKmaximum | 43 (37.4%) | 232 U/L; IQR: 119–407 |
| CK-MBon admission | 83 (72.2%) | 19 U/L; IQR: 7–33 U/L |
| CK-MBmaximum | 38 (33.0%) | 18 U/L; IQR: 7.6–39.9U/L |
| NT-proBNPon admission | 9 (7.8%) | 2771 ng/L; IQR: 452–6147 |
| hsCRPon admission | 83 (72.2%) | 6.6 mg/L; IQR: 2.53–36.5 |
| Biomarker | Number of Patients (%) | Laboratory Value (Median; IQR) |
|---|---|---|
| Troponin I | 95 (95%) | 15.8 µg/L; IQR: 2.8–81.9 |
| CK | 99 (99%) | 1826 U/L; IQR: 671–3849 |
| CK-MB | 96 (96%) | 165 U/L; IQR: 36.5–482.6 |
| NT-proBNP | 17 (17%) | 2404 ng/L; IQR: 1346–3789 |
| hsCRP | 93 (93%) | 60.9 mg/L; IQR: 13.15–167.3 |
| Diagnostic Accuracy | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Prevalence of ST-segment elevation in -aVR (e.g., ST-segment depression in aVR) and absence of ST elevation in V1 | 73% | 84% | 63% | 89% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çatalkaya Demir, S.; Demir, E.; Çatalkaya, S. Electrocardiographic and Seasonal Patterns Allow Accurate Differentiation of Tako-Tsubo Cardiomyopathy from Acute Anterior Myocardial Infarction: Results of a Multicenter Study and Systematic Overview of Available Studies. Biomolecules 2019, 9, 51. https://doi.org/10.3390/biom9020051
Çatalkaya Demir S, Demir E, Çatalkaya S. Electrocardiographic and Seasonal Patterns Allow Accurate Differentiation of Tako-Tsubo Cardiomyopathy from Acute Anterior Myocardial Infarction: Results of a Multicenter Study and Systematic Overview of Available Studies. Biomolecules. 2019; 9(2):51. https://doi.org/10.3390/biom9020051
Chicago/Turabian StyleÇatalkaya Demir, Seher, Erdem Demir, and Sibel Çatalkaya. 2019. "Electrocardiographic and Seasonal Patterns Allow Accurate Differentiation of Tako-Tsubo Cardiomyopathy from Acute Anterior Myocardial Infarction: Results of a Multicenter Study and Systematic Overview of Available Studies" Biomolecules 9, no. 2: 51. https://doi.org/10.3390/biom9020051
APA StyleÇatalkaya Demir, S., Demir, E., & Çatalkaya, S. (2019). Electrocardiographic and Seasonal Patterns Allow Accurate Differentiation of Tako-Tsubo Cardiomyopathy from Acute Anterior Myocardial Infarction: Results of a Multicenter Study and Systematic Overview of Available Studies. Biomolecules, 9(2), 51. https://doi.org/10.3390/biom9020051




