Long-Lasting Effects of GSPE on Ileal GLP-1R Gene Expression Are Associated with a Hypomethylation of the GLP-1R Promoter in Female Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Proanthocyanidin Extract
2.2. Animal Experiments
2.3. Quantitative Real-Time RT-PCR Analysis
2.4. Analysis of DNA Methylation
2.5. Statistical Analysis
3. Results
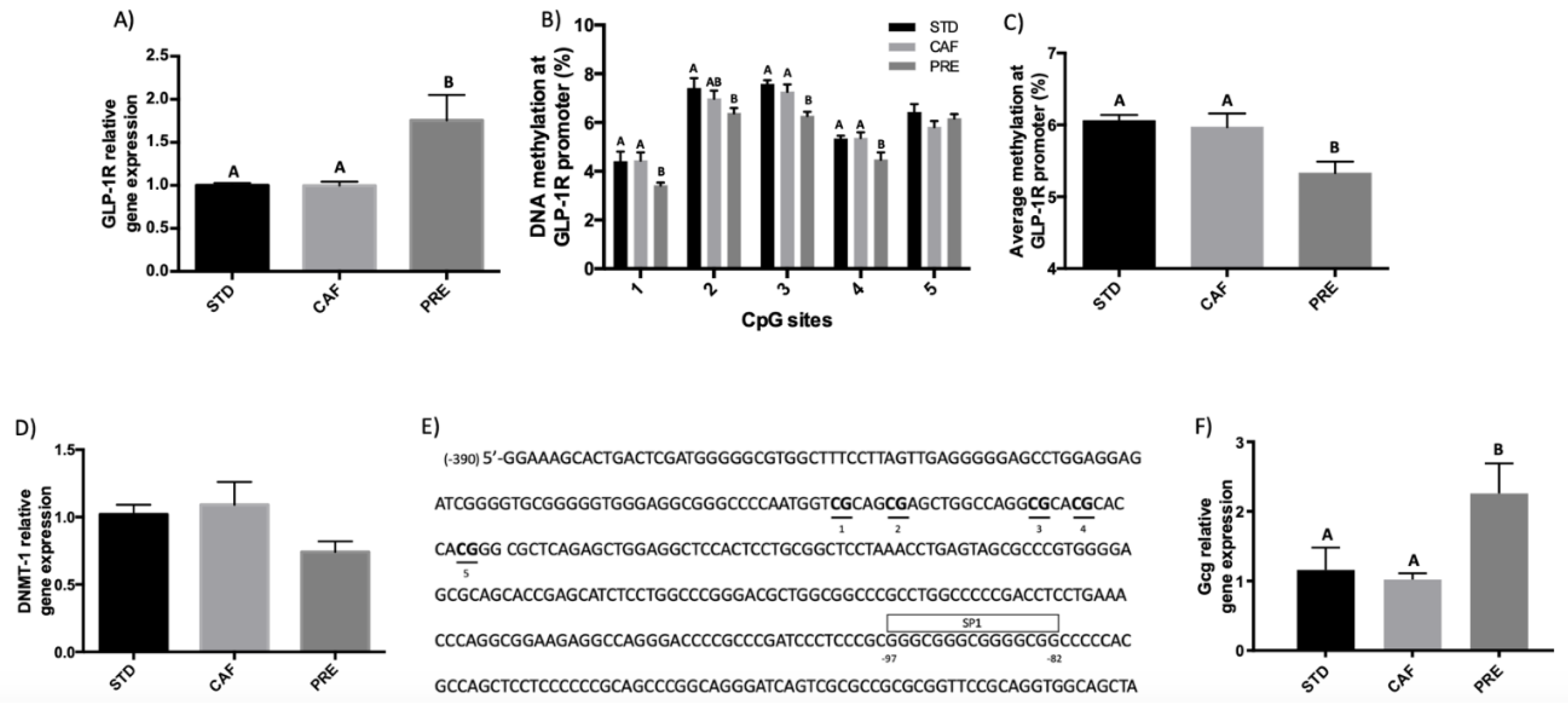
3.1. GSPE Has Long-Lasting Effects on GLP-1R Gene Expression in the Ileum in Rats under a Cafeteria Diet
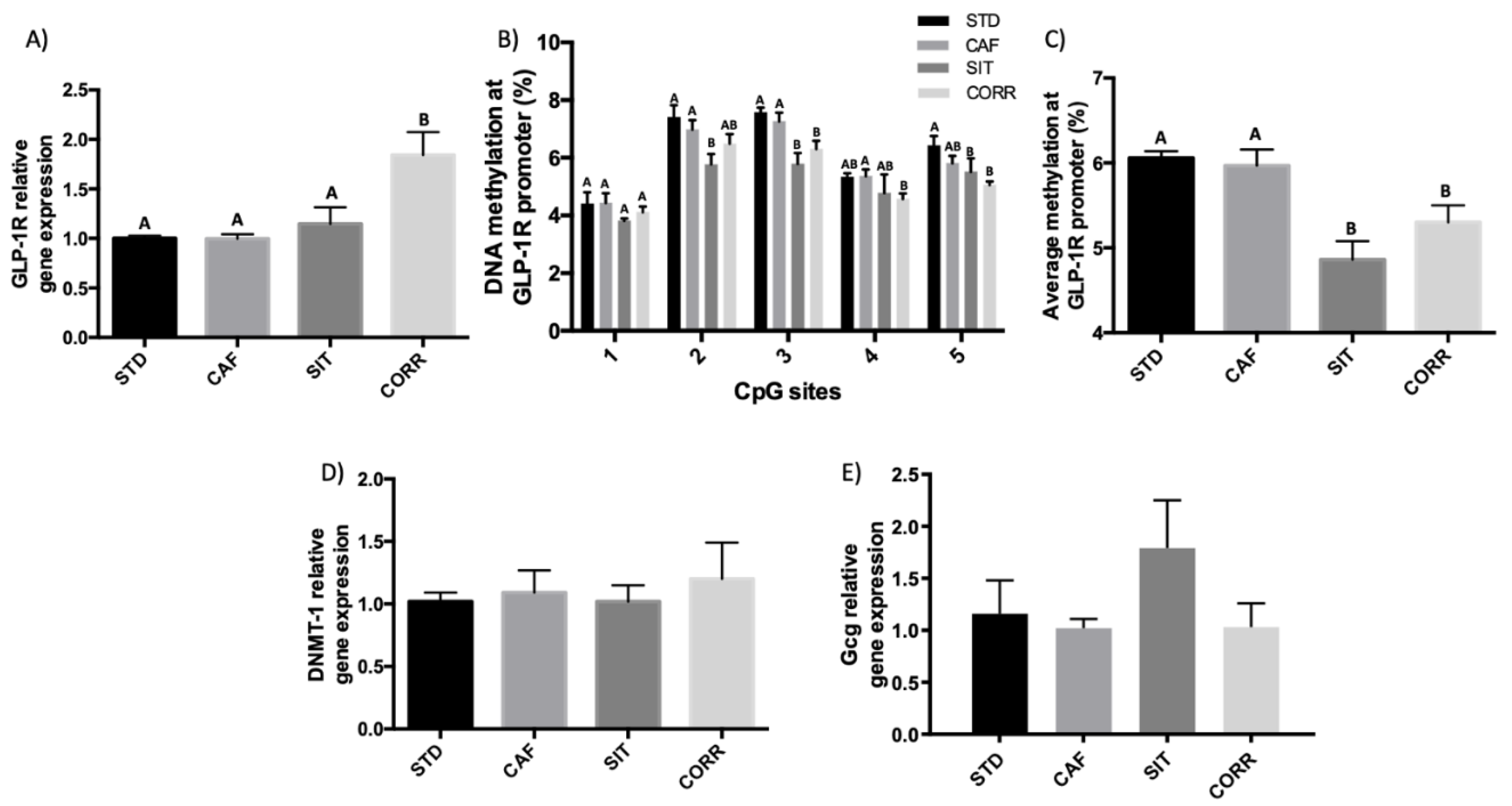
3.2. Hypomethylation of GLP-1R Promoter Participates in the Regulation of GLP-1R Gene Expression after Short-Term Corrective GSPE Treatment in the Ileum
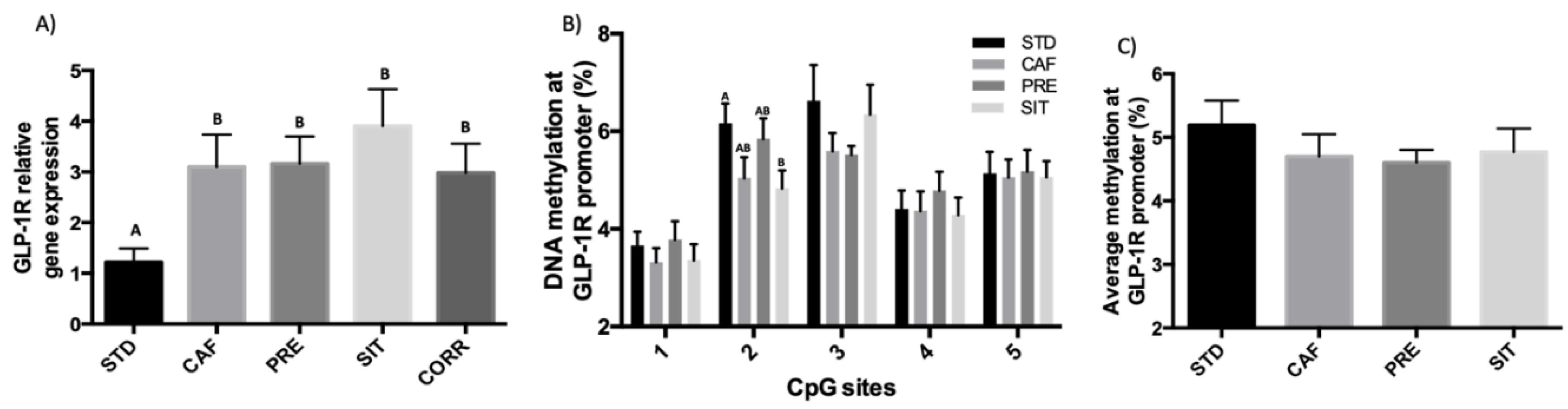
3.3. GSPE Effects in the Ileum Do not Extend to the Colon
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dijk, S.J.; Tellam, R.L.; Morrison, J.L.; Muhlhausler, B.S.; Molloy, P.L. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin. Epigenetics 2015, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Cedó, L.; Montagut, G.; Blay, M.; Ardévol, A. Procyanidins improve some disrupted glucose homoeostatic situations: An analysis of doses and treatments according to different animal models. Crit. Rev. Food Sci. Nutr. 2012, 52, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Pinent-Armengol, M.; Blade, M.C.; Salvado, M.J.; Arola, L.; Hackl, H.; Quackenbush, J.; Trajanoski, Z.; Ardevol, A. Grape-seed derived procyanidins interfere with adipogenesis of 3T3-L1 cells at the onset of differentiation. Int. J. Obes. Relat. Metab. Disord. 2005, 29, 934–941. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; De Mejia, E.G. Antioxidant capacity and in Vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Pajuelo, D.; Fernández-Iglesias, A.; Díaz, S.; Quesada, H.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Improvement of mitochondrial function in muscle of genetically obese rats after chronic supplementation with proanthocyanidins. J. Agric. Food Chem. 2011, 59, 8491–8498. [Google Scholar] [CrossRef]
- Pajuelo, D.; Quesada, H.; Díaz, S.; Fernández-Iglesias, A.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Chronic dietary supplementation of proanthocyanidins corrects the mitochondrial dysfunction of brown adipose tissue caused by diet-induced obesity in Wistar rats. Br. J. Nutr. 2012, 107, 170–178. [Google Scholar] [CrossRef]
- Gil-cardoso, K.; Ginés, I.; Ardévol, A.; Blay, M.; Terra, X. Effects of fl avonoids on intestinal in flammation barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2018, 29, 234–248. [Google Scholar]
- Villa-Rodriguez, J.A.; Ifie, I.; Gonzalez-Aguilar, G.A.; Roopchand, D.E. The Gastrointestinal Tract as Prime Site for Cardiometabolic Protection by Dietary Polyphenols. Adv. Nutr. 2019, 10, 999–1011. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ginés, I.; Gil-Cardoso, K.; Serrano, J.; Casanova-Martí, À.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X.; Gin, I.; Gil-Cardoso, K.; et al. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, Ó.; Soria, A.C.; Rodríguez-Sánchez, S.; Martínez, J.A.; Campión, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharm. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.-Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharm. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Choi, K.-C.; Jung, M.G.; Lee, Y.-H.; Yoon, J.C.; Kwon, S.H.; Kang, H.-B.; Kim, M.-J.; Cha, J.-H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-Gallate, a Histone Acetyltransferase Inhibitor, Inhibits EBV-Induced B Lymphocyte Transformation via Suppression of RelA Acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.-C.; Dai, X.-Z.; Yang, Z.; Song, Q.; Hu, W.-S.; Cao, D.-Q.; Zhang, X. Effects of Proanthocyanidins on Intestinal Motility Disturbance Following Intestinal Ischemia/Reperfusion. J. Investig. Surg. 2016, 29, 335–342. [Google Scholar] [CrossRef]
- Ko, J.-L.; Tsai, C.-H.; Liu, T.-C.; Lin, M.-Y.; Lin, H.-L.; Ou, C.-C. Differential effects of grape juice on gastric emptying and renal function from cisplatin-induced acute adverse toxicity. Hum. Exp. Toxicol. 2016, 35, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Marti, A.; Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients 2016, 8, 652. [Google Scholar] [CrossRef]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar] [CrossRef]
- Yang, M.; Fukui, H.; Eda, H.; Xu, X.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J.; et al. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G367–G373. [Google Scholar] [CrossRef]
- May, A.T.; Crowe, M.S.; Blakeney, B.A.; Mahavadi, S.; Wang, H.; Grider, J.R.; Murthy, K.S. Identification of expression and function of the glucagon-like peptide-1 receptor in colonic smooth muscle. Peptides 2019, 112, 48–55. [Google Scholar] [CrossRef]
- Halim, M.A.; Degerblad, M.; Sundbom, M.; Karlbom, U.; Holst, J.J.; Webb, D.-L.; Hellström, P.M. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J. Clin. Endocrinol. Metab. 2018, 103, 575–585. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Gender-related similarities and differences in the body distribution of grape seed flavanols in rats. Mol. Nutr. Food Res. 2016, 60, 760–772. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Terra, X.; Blay, M.; Pérez-Vendrell, A.M.; Pinent, M.; Ardévol, A. Grape Seed Proanthocyanidins Target the Enteroendocrine System in Cafeteria-Diet-Fed Rats. Mol. Nutr. Food Res. 2019, 63, e1800912. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M.; Smithson, A.; Stowell, G.; Greene, S.; Kenny, E.; Damico, C.; Leone-Bay, A.; Baughman, R.; Grant, M.; Richardson, P. Receptor-mediated inhibition of small bowel migrating complex by GLP-1 analog ROSE-010 delivered via pulmonary and systemic routes in the conscious rat. Regul. Pept. 2012, 179, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, e315. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Prasad, R.; Singh, T.; Jones, V.; Katiyar, S.K. Grape seed proanthocyanidins reactivate silenced tumor suppressor genes in human skin cancer cells by targeting epigenetic regulators. Toxicol. Appl. Pharm. 2012, 263, 122–130. [Google Scholar] [CrossRef]
- Downing, L.E.; Ferguson, B.S.; Rodriguez, K.; Ricketts, M.-L. A grape seed procyanidin extract inhibits HDAC activity leading to increased Pparα phosphorylation and target-gene expression. Mol. Nutr. Food Res. 2017, 61, 1600347. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Arola-Arnal, A.; Bladé, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345. [Google Scholar] [CrossRef]
- Castell-Auvi, A.; Cedo, L.; Movassat, J.; Portha, B.; Sanchez-Cabo, F.; Pallares, V.; Blay, M.; Pinent, M.; Ardevol, A.; Castell-Auví, A.; et al. Procyanidins Modulate MicroRNA Expression in Pancreatic Islets. J. Agric. Food Chem. 2013, 61, 355–363. [Google Scholar] [CrossRef]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. Biofactors 2016, 42, 5–12. [Google Scholar]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I.; et al. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Del Bas, J.M.; Ricketts, M.-L.; Vaqué, M.; Sala, E.; Quesada, H.; Ardevol, A.; Salvadó, M.J.; Blay, M.; Arola, L.; Moore, D.D.; et al. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol. Nutr. Food Res. 2009, 53, 805–814. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Dietary proanthocyanidins modulate the rhythm of BMAL1 expression and induce RORα transactivation in HepG2 cells. J. Funct. Foods 2015, 13, 336–344. [Google Scholar] [CrossRef]
- Yiannakopoulou, E.C. Targeting DNA Methylation with Green Tea Catechins. Pharmacology 2015, 95, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wildhage, I.; Trusheim, H.; Göke, B.; Lankat-Buttgereit, B. Gene Expression of the Human Glucagon-Like Peptide-1 Receptor Is Regulated by Sp1 and Sp3 1. Endocrinology 1999, 140, 624–631. [Google Scholar] [CrossRef][Green Version]
- Li, L.; He, S.; Sun, J.-M.; Davie, J.R. Gene regulation by Sp1 and Sp3. Biochem. Cell Biol. 2004, 82, 460–471. [Google Scholar] [CrossRef]
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmcol. Ther. 2015, 152, 111–124. [Google Scholar] [CrossRef]
- Hall, E.; Dayeh, T.; Kirkpatrick, C.L.; Wollheim, C.B.; Dekker Nitert, M.; Ling, C. DNA methylation of the glucagon-like peptide 1 receptor (GLP1R) in human pancreatic islets. BMC Med. Genet. 2013, 14, 76. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Blay, M.; Ardevol, A.; Pinent, M. Grape-seed procyanidins prevent the cafeteria diet-induced decrease of glucagon-like peptide-1 production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef]
- Peiris, M.; Aktar, R.; Raynel, S.; Hao, Z.; Mumphrey, M.B.; Berthoud, H.-R.; Blackshaw, L.A. Effects of Obesity and Gastric Bypass Surgery on Nutrient Sensors, Endocrine Cells, and Mucosal Innervation of the Mouse Colon. Nutrients 2018, 10, 1529. [Google Scholar] [CrossRef]
- Kappe, C.; Zhang, Q.; Nyström, T.; Sjöholm, Å. Effects of high-fat diet and the anti-diabetic drug metformin on circulating GLP-1 and the relative number of intestinal L-cells. Diabetol. Metab. Syndr. 2014, 6, 70. [Google Scholar] [CrossRef]
- Howell, K.J.; Kraiczy, J.; Nayak, K.M.; Gasparetto, M.; Ross, A.; Lee, C.; Mak, T.N.; Koo, B.-K.; Kumar, N.; Lawley, T.; et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 2018, 154, 585–598. [Google Scholar] [CrossRef]
- Kraiczy, J.; Nayak, K.; Ross, A.; Raine, T.; Mak, T.N.; Gasparetto, M.; Cario, E.; Rakyan, V.; Heuschkel, R.; Zilbauer, M. Assessing DNA methylation in the developing human intestinal epithelium: Potential link to inflammatory bowel disease. Mucosal Immunol. 2016, 9, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J. Funct. Foods 2015, 12, 478–488. [Google Scholar] [CrossRef]
- Wentzel, J.F.; Lewies, A.; Bronkhorst, A.J.; van Dyk, E.; du Plessis, L.H.; Pretorius, P.J. Exposure to high levels of fumarate and succinate leads to apoptotic cytotoxicity and altered global DNA methylation profiles in vitro. Biochimie 2017, 135, 28–34. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Liang, A.; Fan, X.; Wang, R.; Li, P.; Qi, K. Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice. Br. J. Nutr. 2018, 120, 385–392. [Google Scholar] [CrossRef]
| Rat | GLP-1R | Forward | 5′-GTTGAGGGGGAGTTTGGA-3′ |
| Reverse | 5′-ACCCCAAAAATAAAACCTCCAACTCTA-3′ | ||
| Sequencing | 5′-GGGAGGAGGGTTTTAATG-3′ |
| Variables | Formic | Acetic | Propionic | Butyric | Valeric | Succinic |
|---|---|---|---|---|---|---|
| Pos. 1 | −0.081 | 0.033 | 0.192 | 0.162 | 0.030 | 0.284 |
| Pos. 2 | −0.064 | 0.023 | 0.024 | 0.229 | −0.035 | 0.381 * |
| Pos. 3 | −0.013 | 0.098 | −0.221 | 0.346 # | 0.096 | 0.197 |
| Pos. 4 | 0.113 | 0.115 | −0.093 | 0.451 * | 0.386 * | 0.598 * |
| Pos. 5 | −0.028 | 0.054 | −0.222 | 0.418 * | 0.261 | 0.557 * |
| Average of Positions | −0.063 | 0.014 | −0.079 | 0.331 # | 0.142 | 0.487 * |
| Variables | Formic | Acetic | Propionic | Butyric | Valeric | Succinic |
|---|---|---|---|---|---|---|
| Pos. 1 | −0.410 # | −0.448 # | −0.464 # | −0.163 | −0.370 | −0.379 |
| Pos. 2 | −0.170 | −0.327 | −0.392 | 0.026 | −0.244 | −0.525 * |
| Pos. 3 | −0.300 | −0.405 # | −0.224 | −0.238 | −0.370 | −0.517 * |
| Pos. 4 | −0.505 * | −0.617 * | −0.380 | −0.349 | −0.449 # | −0.369 |
| Pos. 5 | −0.450 # | −0.550 * | −0.318 | −0.416 # | −0.465 * | −0.474 * |
| Average of Positions | −0.352 | −0.486 * | −0.346 | −0.216 | −0.399 # | −0.424 # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginés, I.; Gil-Cardoso, K.; D’Addario, C.; Falconi, A.; Bellia, F.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M.; Beltrán-Debón, R. Long-Lasting Effects of GSPE on Ileal GLP-1R Gene Expression Are Associated with a Hypomethylation of the GLP-1R Promoter in Female Wistar Rats. Biomolecules 2019, 9, 865. https://doi.org/10.3390/biom9120865
Ginés I, Gil-Cardoso K, D’Addario C, Falconi A, Bellia F, Blay MT, Terra X, Ardévol A, Pinent M, Beltrán-Debón R. Long-Lasting Effects of GSPE on Ileal GLP-1R Gene Expression Are Associated with a Hypomethylation of the GLP-1R Promoter in Female Wistar Rats. Biomolecules. 2019; 9(12):865. https://doi.org/10.3390/biom9120865
Chicago/Turabian StyleGinés, Iris, Katherine Gil-Cardoso, Claudio D’Addario, Anastasia Falconi, Fabio Bellia, M Teresa Blay, Ximena Terra, Anna Ardévol, Montserrat Pinent, and Raúl Beltrán-Debón. 2019. "Long-Lasting Effects of GSPE on Ileal GLP-1R Gene Expression Are Associated with a Hypomethylation of the GLP-1R Promoter in Female Wistar Rats" Biomolecules 9, no. 12: 865. https://doi.org/10.3390/biom9120865
APA StyleGinés, I., Gil-Cardoso, K., D’Addario, C., Falconi, A., Bellia, F., Blay, M. T., Terra, X., Ardévol, A., Pinent, M., & Beltrán-Debón, R. (2019). Long-Lasting Effects of GSPE on Ileal GLP-1R Gene Expression Are Associated with a Hypomethylation of the GLP-1R Promoter in Female Wistar Rats. Biomolecules, 9(12), 865. https://doi.org/10.3390/biom9120865










