Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential
Abstract
1. Introduction
2. Importance of Essential Polyunsaturated Fatty Acids for Human Health
3. Primary Producers of Polyunsaturated Fatty Acids in Marine Ecosystems
3.1. Microalgae
3.2. Heterotrophic Protists
4. Biochemical Markers for Identification of Mollusk Feeding Patterns
5. Fatty Acids of Marine Mollusks
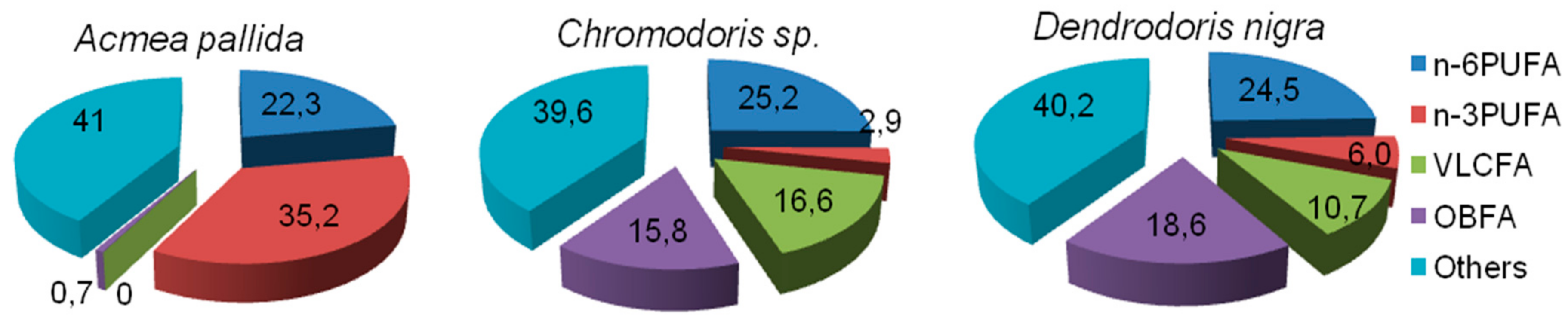
5.1. Gastropoda
5.2. Bivalvia
5.3. Cephalopoda
6. Contribution of Symbionts to the Fatty Acid Pool of Mollusks
7. Biosynthesis of Fatty Acids in Mollusks
8. Dietary Source of PUFAs Versus Own Biosynthetic Capability of Mollusks
9. Variations in Fatty Acids in Response to Environmental Factors
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Parrish, C.C. Essential fatty acids in aquatic food webs. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: New York, NY, USA, 2009; pp. 309–326. ISBN 978-0-387-88607-7. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Kalachova, G.S.; Makhutova, O.N. Stable isotope composition of fatty acids in organisms of different trophic levels in the Yenisei River. PLoS ONE 2012, 7, e34059. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.M.; Wacker, A.; Parrish, C.C.; Kainz, M.J.; Arts, M.T. A fundamental dichotomy in long-chain polyunsaturated fatty acid abundance between and within marine and terrestrial ecosystems. Environ. Rev. 2017, 25, 163–174. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.H.; Whittle, K.J. Lipids and hydrocarbons in the marine food web. In Analysis of Marine Ecosystems; Longhurst, A., Ed.; Academic Press: New York, NY, USA, 1981; pp. 491–533. [Google Scholar]
- Dalsgaard, J.; John, M.S.; Kattner, G.; Muller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 241–251. [Google Scholar]
- Reis, D.B.; Rodríguez, C.; Acosta, N.G.; Almansa, E.; Tocher, D.R.; Andrade, J.P.; Sykes, A.V. In vivo metabolism of unsaturated fatty acids in Sepia officinalis hatchlings. Aquaculture 2016, 450, 67–73. [Google Scholar] [CrossRef]
- Kabeya, N.; Fonseca, M.M.; Ferrier, D.E.K.; Navarro, J.C.; Bay, L.K.; Francis, D.S.; Tocher, D.R.; Castro, L.F.C.; Monroig, Ó. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 2018, 4, eaar6849. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Monroig, Ó.; Kabeya, N. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: A comprehensive review. Fish. Sci. 2018, 84, 911–928. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Surm, J.M.; Prentis, P.J.; Pavasovic, A. Comparative analysis and distribution of omega-3 LCPUFA biosynthesis genes in marine molluscs. PLoS ONE 2015, 10, e0136301. [Google Scholar] [CrossRef]
- Khan, B.M.; Liu, Y. Marine mollusks: Food with benefits. Compr. Rev. Food Sci. Food Saf. 2019, 18, 548–564. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid-related modulations of membrane fluidity in cells: Detection and implications. Free Radic. Res. 2016, 50, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Das, U.N.; Ramos, E.J.; Meguid, M.M. Metabolic alterations during inflammation and its modulation by central actions of omega-3 fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 413–419. [Google Scholar] [CrossRef]
- Klingenberg, R.; Hansson, G.K. Treating inflammation in atherosclerotic cardiovascular disease: Emerging therapies. Eur. Heart J. 2009, 30, 2838–2844. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients 2017, 21, 784. [Google Scholar] [CrossRef]
- Schubert, R.; Kitz, R.; Beermann, C.; Rose, M.A.; Baer, P.C.; Zielen, S.; Boehles, H. Influence of low-dose polyunsaturated fatty acids supplementation on the inflammatory response of healthy adults. Nutrition 2007, 23, 724–730. [Google Scholar] [CrossRef]
- Külzow, N.; Witte, A.V.; Kerti, L.; Grittner, U.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheimers Dis. 2016, 51, 713–725. [Google Scholar] [CrossRef]
- Yelland, L.N.; Gajewski, B.J.; Colombo, J.; Gibson, R.A.; Makrides, M.; Carlson, S.E. Predicting the effect of maternal docosahexaenoic acid (DHA) supplementation to reduce early preterm birth in Australia and the United States using results of within country randomized controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 2016, 112, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V.; Novgorodtseva, T.P. Lipid composition of erythrocytes at cardiovascular and hepatobiliary diseases. In Lipids: Categories, Biological Functions and Metabolism, Nutrition and Health; Gilmore, P.L., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; pp. 1–45. ISBN 978-1-61668-464-8. [Google Scholar]
- Novgorodtseva, T.P.; Denisenko, Y.K.; Zhukova, N.V.; Antonyuk, M.V.; Knyshova, V.V.; Gvozdenko, T.A. Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. Lipids Health Dis. 2013, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Novgorodtseva, T.H.; Karaman, Y.K.; Zhukova, V.V.; Lobanova, T.G.; Antonyuk, M.V.; Kantur, T.A. Composition of fatty acids in plasma and erythrocytes and eicosanoids level in patients with metabolic syndrome. Lipids Health Dis. 2011, 10, 82. [Google Scholar] [CrossRef]
- Echeverria, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fatty Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gil, A. Omega 3 fatty acids in cardiovascular disease risk factors: An updated systematic review of randomised clinical trials. Clin Nutr. 2018, 37, 72–77. [Google Scholar] [CrossRef]
- Reimers, A.; Ljung, H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther. Adv. Psychopharm. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef]
- Campoy, C.; Escolano-Margarit, V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Role of diets rich in omega-3 and omega-6 in the development of cancer. Bol. Med. Hosp. Infant Mex. 2016, 73, 446–456. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar]
- Dunstan, G.A.; Volkman, J.K.; Barrtt, S.M.; Leroi, J.-M.; Jeffrey, S.W. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae). Phytochemistry 1994, 35, 155–161. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Hruzova, K.; Rova, U.; Christakopoulos, P. Biosynthesis of nutraceutical fatty acids by the oleaginous marine microalgae Phaeodactylum tricornutum utilizing hydrolysates from organosolv-pretreated birch and spruce biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef]
- Nichols, P.D.; Jones, G.J.; De Leeuw, J.W.; Johns, R.B. The fatty acid and sterol composition of two marine dinoflagellates. Phytochemistry 1984, 23, 1043–1047. [Google Scholar] [CrossRef]
- Mansour, M.P.; Volkman, J.K.; Jackson, A.E.; Blackburn, S.I. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 1999, 35, 710–720. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, G.A.; Volkman, J.K.; Jeffrey, S.W.; Barrtt, S.M. Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J. Exp. Mar. Biol. Ecol. 1992, 161, 115–134. [Google Scholar] [CrossRef]
- Volkman, J.K.; Brown, M.R.; Dunstan, G.A.; Jeffrey, S.W. The biochemical composition of marine microalgae from the class Eustigmatophyceae. J. Phycol. 1993, 29, 69–78. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Brown, M.R.; Volkman, J.K. Cryptophyceaea and Rodophyceae; chemotaxonomy, phylogeny, and application. Phytochemistry 2005, 66, 2557–2570. [Google Scholar] [CrossRef]
- Peltomaa, E.; Johnson, M.D.; Taipale, S.J. Marine cryptophytes are great sources of EPA and DHA. Mar. Drugs 2018, 16, 3. [Google Scholar] [CrossRef]
- Volkman, J.K.; Dunstan, G.A.; Jeffrey, S.W.; Kearney, P.S. Fatty acids from microalgae of the genus Pavlova. Phytochemistry 1991, 30, 1855–1859. [Google Scholar] [CrossRef]
- Alonzo, L.; Grima, E.M.; Pérez, J.A.S.; Sánchez, J.L.G.; Camacho, F.G. Fatty acid variation among different isolates of a single strain of Isochrysis galbana. Phytochemistry 1992, 31, 3901–3904. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of Protozoa; Science Technical Publishers: Madison, WL, USA, 1987; pp. 1–197. ISBN 978-3-662-06819-9. [Google Scholar] [CrossRef]
- Sul, D.; Erwin, J.A. The membrane lipids of the marine ciliated protozoan Parauronema acutum. Biochim. Biophys. Acta 1997, 1345, 162–171. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Kharlamenko, V.I. Sources of essential fatty acids in the marine microbial loop. Aquat. Microb. Ecol. 1999, 17, 153–157. [Google Scholar] [CrossRef]
- Bec, A.; Martin-Creuzburg, D.; Von Elert, E. Fatty acid composition of the heterotrophic nanoflagellate Paraphysomonas sp.: Influence of diet and de novo biosynthesis. Aquat. Biol. 2010, 9, 107–112. [Google Scholar] [CrossRef][Green Version]
- Zhukova, N.V.; Aizdaicher, N.A. Fatty acid composition of 15 species of marine microalgae. Phytochemistry 1995, 39, 351–356. [Google Scholar] [CrossRef]
- Desvilettes, C.; Bec, A. Formation and transfer of fatty acids in aquatic microbial food webs—role of heterotrophic protists. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M., Kainz, M., Eds.; Springer: New York, NY, USA, 2009; pp. 25–42. [Google Scholar]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary traces in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Makhutova, O.N.; Sushchik, N.N.; Gladyshev, M.I.; Ageev, A.V.; Pryanichnikova, E.G.; Kalachova, G.S. Is the fatty acid composition of freshwater zoobenthic invertebrates controlled by phylogenetic or trophic factors? Lipids 2011, 46, 709–721. [Google Scholar] [CrossRef]
- Van der Heijden, L.H.; Graeve, M.; Asmus, R.; Rzeznik-Orignac, J.; Niquil, N.; Bernier, Q.; Guillou, G.; Asmus, H.; Lebreton, B. Trophic importance of microphytobenthos and bacteria to meiofauna in soft-bottom intertidal habitats: A combined trophic marker approach. Mar. Environ. Res. 2019, 149, 50–66. [Google Scholar] [CrossRef]
- Graeve, M.; Kattner, G.; Piepenburg, D. Lipids in Arctic benthos: Does the fatty acid and alcohol composition reflect feeding and trophic interactions? Polar Biol. 1997, 18, 53–61. [Google Scholar] [CrossRef]
- Perry, G.J.; Volkman, J.K.; Johns, R.B.; Bavor, H.J. Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta 1979, 43, 1715–1725. [Google Scholar] [CrossRef]
- Gillan, F.T.; Hogg, R.W. A method for the estimation of bacterial biomass and community structure in mangrove-associated sediments. J. Microbiol. Methods 1984, 2, 275–293. [Google Scholar] [CrossRef]
- Findlay, R.H.; Trexler, M.B.; Guckert, J.B.; White, D.C. Laboratory study of disturbance in marine sediments: Response of a microbial community. Mar. Ecol. Prog. Ser. 1990, 62, 121–133. [Google Scholar] [CrossRef]
- Kaneda, T. Iso-fatty and anteiso-fatty acids in bacteria—biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [PubMed]
- Suhr, S.B.; Pond, D.W.; Gooday, A.J.; Smith, C.R. Selective feeding by benthic foraminifera on phytodetritus on the western Antarctic Peninsula shelf: Evidence from fatty acid biomarker analysis. Mar. Ecol. Prog. Ser. 2003, 262, 153–162. [Google Scholar] [CrossRef]
- Topping, J.N.; Murray, J.W.; Pond, D.W. Sewage effects on the food sources and diets of benthic foraminifera living in oxic sediment: A microcosm experiment. J. Exp. Mar. Biol. Ecol. 2006, 329, 239–250. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the Pacific coast of north California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Lawson, M.P.; Lavis, A.; Cambie, R.C. Fatty acid composition and the classification of the Porifera. Biochem. Syst. Ecol. 1984, 12, 63–84. [Google Scholar] [CrossRef]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.-M.; Barnathan, G. Demospongic acids revisited. Mar. Drugs 2010, 8, 2569–2577. [Google Scholar] [CrossRef]
- Imbs, A.B. High level of tetracosapolyenoic fatty acids in the cold-water mollusk Tochuina tetraquetra is a result of the nudibranch feeding on soft corals. Polar Biol. 2016, 39, 1511–1514. [Google Scholar] [CrossRef]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates. Part II: Mollusca. Prog. Lipid Res. 1982, 21, 109–153. [Google Scholar] [CrossRef]
- Tsikhon-Lukanina, E.A. Trofologiya Vodnykh Mollyuskov (Trophology of Aquatic Mollusks); Nauka: Moscow, Russia, 1987; pp. 1–177. [Google Scholar]
- Zhukova, N.V.; Svetashev, V.I. Non-methylene-interrupted dienoic fatty acids in mollusks from the Sea of Japan. Comp. Biochem. Physiol. 1986, 83, 643–646. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipids and fatty acids of nudibranch mollusks: Potential sources of bioactive compounds. Mar. Drugs 2014, 12, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Kattner, G.; Hagen, W.; Graeve, M.; Albers, C. Exceptional lipids and fatty acids in the pteropod Clione limacine Gastropoda from both polar oceans. Mar. Chem. 1998, 61, 219–228. [Google Scholar] [CrossRef]
- Fernandes, I.; Fernandes, T.; Cordeiro, N. Nutritional value and fatty acid profile of two wild edible limpets from the Madeira Archipelago. Eur. Food Res. Technol. 2019, 245, 895–905. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chromodoris sp. and Phyllidia Coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Fernandes, F.; Madureira-Carvalho, Á.; Valentão, P.; Lobo-da-Cunha, A.; Calado, G.; Andrade, P.B. Profiling of heterobranchia sea slugs from Portuguese coastal waters as producers of anti-cancer and anti-inflammatory agents. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef]
- Lavaud, R.; Artigaud, S.; Le Grand, F.; Donval, A.; Soudant, P.; Flye-Sainte-Marie, J.; Strohmeier, T.; Strand, O.; Leynaert, A.; Beker, B.; et al. New insights into the seasonal feeding ecology of Pecten maximus using pigments, fatty acids and sterols analyses. Mar. Ecol. Prog. Ser. 2018, 590, 109–129. [Google Scholar] [CrossRef]
- Mathieu-Resuge, M.; Kraffe, E.; Le Grand, F.; Boens, A.; Bideau, A.; Lluch-Cota, S.E.; Racotta, I.S.; Schaal, G. Trophic ecology of suspension-feeding bivalves inhabiting a north-eastern Pacific coastal lagoon: Comparison of different biomarkers. Mar. Environ. Res. 2019, 145, 155–163. [Google Scholar] [CrossRef]
- Silina, A.V.; Zhukova, N.V. Growth variability and feeding of scallop Patinopecten yessoensis on different bottom sediments: Evidence from fatty acid analysis. J. Exp. Mar. Biol. Ecol. 2007, 348, 46–59. [Google Scholar] [CrossRef]
- Purroya, A.; Najdekb, M.; Islac, E.; Župand, I.; Thébaulte, J.; Peharda, M. Bivalve trophic ecology in the Mediterranean: Spatio-temporal variations and feeding behavior. Mar. Environ. Res. 2018, 142, 234–249. [Google Scholar] [CrossRef]
- Ruano, F.; Ramos, P.; Quaresma, M.; Bandarra, N.M.; Fonseca, I.P. Evolution of fatty acid profile and Condition Index in mollusc bivalves submitted to different depuration periods. Rev. Port. Cienc. Vet. 2012, 107, 75–84. [Google Scholar]
- Chu, F.L.; Webb, K.L.; Chen, J. Seasonal changes of lipids and fatty acids in oyster tissues (Crassostrea virginica) an estuarine particulate matter. Comp. Biochem. Physiol. A 1990, 95, 385–391. [Google Scholar] [CrossRef]
- Napolitano, G.E.; Ackman, R.G. Fatty acid dynamics in sea scallops Placopecten magellanicus (Gmelin 1791) from Georges Bank, Nova Scotia. J. Shellfish. Res. 1993, 12, 267–277. [Google Scholar]
- Baptista, M.; Repolho, T.; Maulvault, A.L.; Lopes, V.M.; Narciso, L.; Marques, A.; Bandarra, N.; Rosa, R. Temporal dynamics of amino and fatty acid composition in the razor clam Ensis siliqua (Mollusca: Bivalvia). Helgol. Mar. Res. 2014, 68, 465–482. [Google Scholar] [CrossRef]
- Hu, X.P.; Da An, Q.; Zhou, D.; Lu, T.; Yin, F.W.; Song, L.; Zhao, Q.; Zhang, J.H.; Qin, L.; Zhu, B.W.; et al. Lipid profiles in different parts of two species of scallops (Chlamys farreri and Patinopecten yessoensis). Food Chem. 2018, 243, 319–327. [Google Scholar] [CrossRef]
- Nerot, C.; Meziane, T.; Schaal, G.; Grall, J.; Lorrain, A.; Paulet, Y.M.; Kraffe, E. Spatial changes in fatty acids signatures of the great scallop Pecten maximus across the Bay of Biscay continental shelf. Cont. Shelf Res. 2015, 109, 1–9. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.-J.; Garrido, J.L.; Irisarri, J. Fatty acid composition in Mytilus galloprovincialis organs: Trophic interactions, sexual differences and differential anatomical distribution. Mar. Ecol. Prog. Ser. 2015, 528, 221–234. [Google Scholar] [CrossRef]
- Najdek, M.; Blazina, M.; Ezgeta-Balic, D.; Peharda, M. Diets of fan shells (Pinna nobilis) of different sizes: Fatty acid profiling of digestive gland and adductor muscle. Mar. Biol. 2013, 160, 921–930. [Google Scholar] [CrossRef]
- Phillips, K.L.; Nichols, P.D.; Jackson, G.D. Lipid and fatty acid composition of the mantle and digestive gland of four Southern Ocean squid species: Implications for food-web studies. Antarct. Sci. 2002, 14, 212–220. [Google Scholar] [CrossRef]
- Stowasser, G.; Pierce, G.J.; Moffat, C.F.; Collins, M.A.; Forsythe, J.W. Experimental study on the effect of diet on fatty acid and stable isotope profiles of the squid Lolliguncula brevis. J. Exp. Mar. Biol. Ecol. 2006, 333, 97–114. [Google Scholar] [CrossRef]
- Villanueva, R.; Perricone, V.; Fiorito, G. Cephalopods as predators: A short journey among behavioral flexibilities, adaptions, and feeding habits. Front. Physiol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Ozogul, Y.; Duysak, O.; Ozogul, F.; Ozkutuk, A.S.; Tureli, C. Seasonal effects in the nutritional quality of the body structural tissue of cephalopods. Food Chem. 2008, 108, 847–852. [Google Scholar] [CrossRef]
- Pethybridge, H.; Virtue, P.; Casper, R.; Yoshida, T.; Green, C.P.; Jackson, G.; Nichols, P.D. Seasonal variations in diet of arrow squid (Nototodarus gouldi): Stomach content and signature fatty acid analysis. J. Mar. Biol. Assoc. UK 2012, 92, 187–196. [Google Scholar] [CrossRef]
- Phillips, K.L.; Nichols, P.D.; Jackson, G.D. Size-related dietary changes observed in the squid Moroteuthis ingens at the Falkland Islands: Stomach contents and fatty-acid analyses. Polar Biol. 2003, 26, 474–485. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Chen, X.; Chen, L. Potential use of stable isotope and fatty acid analyses for traceability of geographic origins of jumbo squid (Dosidicus gigas). Rapid Commun. Mass Spectrom. 2018, 32, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Han, F.; Xuan, S.; Chen, X. Fatty acid composition and the evidence for mixed income–capital breeding in female Argentinean short-fin squid Illex argentines. Mar. Biol. 2019, 166, 90. [Google Scholar] [CrossRef]
- Monroig, Ó.; Navarro, J.C.; Dick, J.R.; Alemany, F.; Tocher, D.R. Identification of a Δ5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar. Biotechnol. 2012, 14, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, M.; Jackson, G.D.; Nichols, P.D.; Wotherspoon, S. An experimental study of the effect of diet on the fatty acid profiles of the European Cuttlefish (Sepia offcinalis). Mar. Biol. 2008, 154, 363–372. [Google Scholar] [CrossRef]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef]
- Rumpho, M.E.; Pelletreau, K.N.; Moustafa, A.; Bhattacharya, D. The making of a photosynthetic animal. J. Exp. Biol. 2011, 214, 303–311. [Google Scholar] [CrossRef]
- Venn, A.A.; Loram, J.E.; Douglas, A.E. Photosynthetic symbioses in animals. J. Exp. Bot. 2008, 59, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.M.; Fung, J.M.; Sharp, K.H.; Benner, J.S.; McClung, C.; Cushing, S.; Lamkin, E.R.; Fomenkov, A.I.; Henrissat, B.; Londer, Y.Y.; et al. Gill bacteria enable a novel digestive strategy in a wood-feeding mollusk. Proc. Natl. Acad. Sci. USA 2014, 111, E5096–E5104. [Google Scholar] [CrossRef] [PubMed]
- Visik, K.; Ruby, E.G. Vibrio fischeri and its host: It takes two to tango. Curr. Opin. Microbiol. 2007, 9, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Fricke, W.F.; Hamann, M.T.; Esquenazi, E.; Dorrestein, P.C.; Hill, R.T. Characterization of the bacterial community of the chemically defended hawaiian Sacoglossan Elysia rufescens. Appl. Environ. Microbiol. 2013, 79, 7073–7081. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Thomas, J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009, 459, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Conway, N.; McDowell Capuzzo, J. Incorporation and utilization of bacterial lipids in the Solemya velum symbiosis. Mar. Biol. 1991, 108, 277–291. [Google Scholar] [CrossRef]
- Saito, H. Unusual novel n-4 polyunsaturated fatty acids in cold-seep mussels (Bathymodiolus japonicus and Bathymodiolus platifrons), originating from symbiotic methanotrophic bacteria. J. Chtomatogr. 2008, 1200, 242–254. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Kharlamenko, V.I.; Svetashev, V.I.; Rodionov, I.A. Fatty-acids as markers of bacterial symbionts of marine bivalve mollusks. J. Exp. Mar. Biol. Ecol. 1992, 162, 253–263. [Google Scholar] [CrossRef]
- Kharlamenko, V.I.; Zhukova, N.V.; Khotimchenko, S.V.; Svetashev, V.I.; Kamenev, G.M. Fatty acids as markers of food sources in a shallow-water hydrothermal ecosystem (Kraternaya Bight, Yankich Island, Kurile Islands). Mar. Ecol. Prog. Ser. 1995, 120, 231–241. [Google Scholar] [CrossRef]
- Saito, H. Identification of novel n-4 series polyunsaturated fatty acids in a deep-sea clam, Calyptogena phaseoliformis. J. Chromatogr. A 2007, 1163, 247–259. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Eliseikina, M.G. Symbiotic bacteria in the nudibranch mollusk Dendrodoris nigra: Fatty acid composition and ultrastructure analysis. Mar. Biol. 2012, 159, 1783–1794. [Google Scholar] [CrossRef]
- Saito, H.; Hashimoto, J. Characteristics of the fatty acid composition of a deep-sea vent gastropod, Ifremeria Naut. Lipids 2010, 45, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kharlamenko, V.I.; Kiyashko, S.I.; Sharina, S.N.; Ivin, V.V.; Krylova, E.M. An ecological study of two species of chemosymbiotrophic bivalve molluscs (Bivalvia: Vesicomyidae: Pliocardiinae) from the Deryugin Basin of the Sea of Okhotsk using analyses of the stable isotope ratios and fatty acid compositions. Deep Sea Res. Part I 2019, 150, 103058. [Google Scholar] [CrossRef]
- Imbs, A.B.; Yakovleva, I.M.; Dautova, T.N.; Bui, L.H.; Jones, P. Diversity of fatty acid composition of symbiotic dinoflagellates in corals: Evidence for the transfer of host PUFAs to the symbionts. Phytochemistry 2014, 101, 76–82. [Google Scholar] [CrossRef]
- Dubousquet, V.; Gros, E.; Berteaux-Lecellier, V.; Viguier, B.; Raharivelomanana, P.; Bertrand, C.; Lecellier, G.J. Changes in fatty acid composition in the giant clam Tridacna maxima in response to thermal stress. Biol. Open 2016, 5, 1400–1407. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Titlyanov, E.A. Fatty acid variations in symbiotic dinoflagellates from Okinawan corals. Phytochemistry 2003, 62, 191–195. [Google Scholar] [CrossRef]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef]
- De Moreno, J.E.A.; Moreno, V.J.; Brenner, R.R. Lipid metabolism of the yellow clam, Mesodesma macroides: 2—Polyunsaturated fatty acid metabolism. Lipids 1976, 11, 561–566. [Google Scholar] [CrossRef]
- De Moreno, J.E.A.; Moreno, V.J.; Brenner, R.R. Lipid metabolism of the yellow clam, Mesodesma macroides: 3—Saturated fatty acids and acetate metabolism. Lipids 1977, 12, 804–808. [Google Scholar] [CrossRef]
- Waldock, M.J.; Holland, D.L. Fatty acid metabolism in young oyster, Crassostrea gigas: Polyunsaturated fatty acids. Lipids 1984, 19, 332–336. [Google Scholar] [CrossRef]
- Reis, D.B.; Acosta, N.G.; Almansa, E.; Navarro, J.C.; Tocher, D.R.; Monroig, Ó.; Andrade, J.P.; Sykes, A.V.; Rodríguez, C. In vivo metabolism of unsaturated fatty acids in Octopus vulgaris hatchlings determined by incubation with 14C-labelled fatty acids added directly to seawater as protein complexes. Aquaculture 2014, 431, 28–33. [Google Scholar] [CrossRef]
- Zhukova, N.V. The pathway of the biosynthesis of non-methylene-interrupted dienoic fatty acids in mollusks. Comp. Biochem. Physiol. B 1991, 100, 801–804. [Google Scholar] [CrossRef]
- Zhukova, N.V. Biosynthesis of non-methylene-interrupted dienoic fatty acids for [C-14] acetate in molluscs. Biochim. Et Biophys. Acta 1986, 878, 131–133. [Google Scholar] [CrossRef]
- Li, M.; Mai, K.; He, G.; Ai, Q.; Zhang, W.; Xu, W.; Wang, J.F.; Liufu, Z.; Zhang, Y.; Zhou, H. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannai Ino). Aquaculture 2013, 416–417, 48–56. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Zheng, H.; Wang, S.; Wang, Y.; Liu, W.; Zhang, G. Functional characterization of a Δ5-likefatty acyl desaturase and its expression during early embryogenesis in the noble scallop Chlamys nobilis Reeve. Mol. Biol. Rep. 2014, 41, 7437–7445. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Xu, J.; Liao, K.; Monroig, O.; Navarro, J.C.; Oboh, A.; Jin, M.; Zhou, Q.; Zhou, C.; Douglas, R.; et al. Biosynthesis of long-chain polyunsaturated fatty acids in the razor clam Sinonovacula constricta: Characterization of four fatty acyl elongases and a novel desaturase capacity. Biochim. Et Biophys. Acta 2019, 1864, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef]
- Garrido, D.; Kabeya, N.; Hontoria, F.; Navarro, J.C.; Reis, D.B.; Martín, M.V.; Rodríguez, C.; Almansa, E.; Monroig, Ó. Methyl-end desaturases with ∆12 and ω3 regioselectivities enable the de novo PUFA biosynthesis in the cephalopod Octopus vulgaris. Biochim. Et Biophys. Acta 2019, 1864, 1134–1144. [Google Scholar] [CrossRef]
- Ben-Mlih, F.; Marty, J.C.; Fiala-Medioni, A. Fatty acid composition in deep hydrothermal vent symbiotic bivalves. J. Lipid Res. 1992, 33, 1797–1806. [Google Scholar]
- Zhang, H.; Liu, H.; Cheng, D.; Liu, H.; Zheng, H. Molecular cloning and functional characterisation of a polyunsaturated fatty acid elongase in a marine bivalve Crassostrea angulata. J. Food Nutr. Res. 2018, 6, 89–95. [Google Scholar] [CrossRef]
- Allen, C.E.; Tyler, P.A.; Van Dover, C.L. Lipid composition of the hydrothermal vent clam Calyptogena pacifica (Mollusca: Bivalvia) as a trophic indicator. J. Mar. Biol. Assoc. UK 2001, 81, 817–821. [Google Scholar] [CrossRef]
- Howell, K.L.; Pond, D.W.; Billett, D.S.M.; Tyler, P.A. Feeding ecology of deep- sea seastars (Echinodermata: Asteroidea): A fatty acid biomarker approach. Mar. Ecol. Prog. Ser. 2003, 255, 193–206. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Groce, A.K.; Cary, S.C.; Coyne, K.J.; Nichols, P.D. Lipid composition of deep-sea hydrothermal vent tubeworm Riftia pachyptila, crabs Munidopsis subsquamosa and Bythograea thermydron, mussels Bathymodiolus sp. and limpets Lepetodrilus spp. Comp. Biochem. Physiol. B 2005, 141, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Klingensmith, J.S. Distribution of methylene and nonmethylene-interrupted dienoic fatty acids in polar lipids and triacylglycerols of selected tissues of the hardshell clam (Mercinaria mercenaria). Lipids 1982, 17, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Pranal, V.; Fiala-Medioni, A.; Guezennec, J. Fatty acid characteristics in two symbiotic gastropods from a deep hydrothermal vent of the West Pacific. Mar. Ecol. Prog. Ser. 1996, 142, 175–184. [Google Scholar] [CrossRef]
- Hurtado, M.; Racotta, I.; Arcos, F.; Morales-Bojorquez, E.; Moal, J.; Soudant, P.; Palacios, E. Seasonal variations of biochemical, pigment, fatty acid, and sterol compositions in female Crassostrea corteziensis oysters in relation to the reproductive cycle. Comp. Biochem. Physiol. B 2012, 163, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ezgeta-Balic, D.; Najdek, M.; Peharda, M.; Blazina, M. Seasonal fatty acid profile analysis to trace origin of food sources of four commercially important bivalves. Aquaculture 2012, 334, 89–100. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Comeau, L.; Guderley, H. Temperature adaptation in two bivalve species from different thermal habitats: Energetics and remodelling of membrane lipids. J. Exp. Biol. 2007, 210, 2999–3014. [Google Scholar] [CrossRef]
- Pernet, F.; Tremblay, R.; Redjah, I.; Sevigny, J.-M.; Gionet, C. Physiological and biochemical traits correlate with differences in growth rate and temperature adaptation among groups of the eastern oyster Crassostrea virginica. J. Exp. Biol. 2008, 211, 969–977. [Google Scholar] [CrossRef]
- Shimeta, J.; Amos, C.L.; Beaulien, S.E.; Katz, S.L. Resuspension of benthic protists at subtidal coastal sites with differing sediment composition. Mar. Ecol. Prog. Ser. 2003, 259, 103–115. [Google Scholar] [CrossRef]
- Gonçalves, A.M.M.; Borroso, D.V.; Serafim, T.L.; Verdelhos, T.; Marques, J.C.; Gonçalves, F. The biochemical response of two commercial bivalve species to exposure to strong salinity changes illustrated by selected biomarkers. Ecol. Indic. 2017, 77, 59–66. [Google Scholar] [CrossRef]
- Nemova, N.N.; Fokina, N.N.; Nefedova, Z.A. Modifications of gill lipid composition in littoral and cultured blue mussels Mytilus edulis L. under the influence of ambient salinity. Polar Rec. 2013, 154, 217–225. [Google Scholar] [CrossRef]
- Fokina, N.N.; Ruokolainen, T.R.; Nemova, N.N.; Bakhmet, I.N. Changes of blue mussels Mytilus edulis L. lipid composition under cadmium and copper toxic effect. Biol. Trace Elem. Res. 2013, 154, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chelomin, V.P.; Belcheva, N.N. Alterations of microsomal lipid-synthesis in gill cells of bivalve mollusk Mizuhopecten-yessoensis in response to cadmium accumulation. Comp. Biochem. Phys. C 1991, 99, 1–5. [Google Scholar] [CrossRef]
- Gonçalves, F.; Mesquita, A.F.; Verdelhos, T.; Coutinho, J.A.P.; Marques, J.C.; Gonçalves, A.M.M. Fatty acids’ profiles as indicators of stress induced by of a common herbicide on two marine bivalves species: Cerastoderma edule (Linnaeus, 1758) and Scrobicularia plana (da Costa, 1778). Ecol. Indic. 2016, 63, 209–218. [Google Scholar] [CrossRef]
- Signa, G.; Di Leonardo, R.; Vaccaro, A.; Tramati, C.D.; Mazzola, A.; Vizzini, S. Lipid and fatty acid biomarkers as proxies for environmental contamination in caged mussels Mytilus Galloprovincialis. Ecol. Idic. 2015, 57, 384–394. [Google Scholar] [CrossRef]
- Mesquita, A.F.; Gonçalves, F.; Verdelhos, T.; Marques, J.C.; Gonçalves, A.M.M. Fatty acids profiles modifications in the bivalves Cerastoderma edule and Scrobicularia plana in response to copper sulphate. Ecol. Indic. 2018, 85, 318–328. [Google Scholar] [CrossRef]
- Perrat, E.; Couzinet-Mossion, A.; FossiTankoua, O.; Amiard-Triquet, C.; Wielgosz-Collinn, G. Variation of content of lipid classes, sterols and fatty acids in gonads and digestive glands of Scrobicularia plana in relation to environment pollution levels. Ecotoxicol. Environ. Saf. 2013, 90, 112–120. [Google Scholar] [CrossRef]
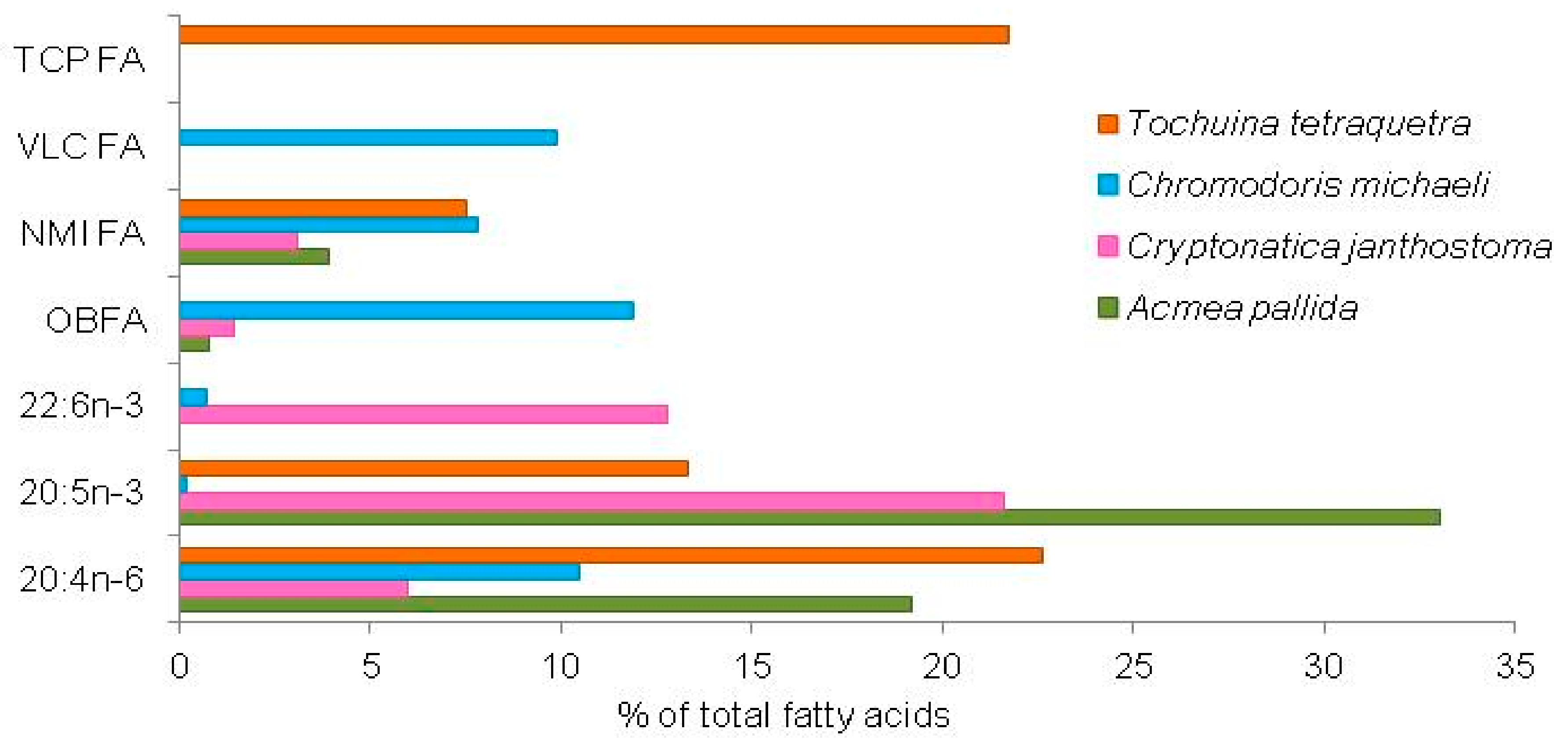
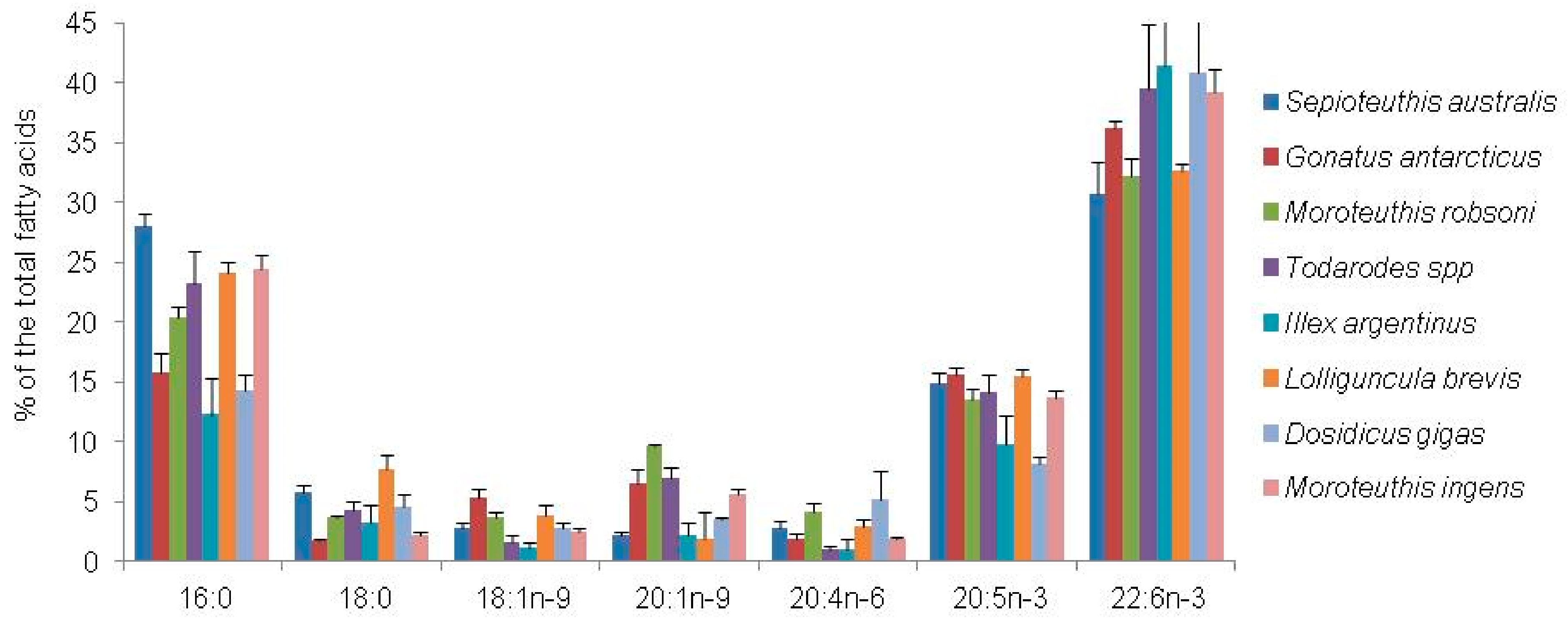
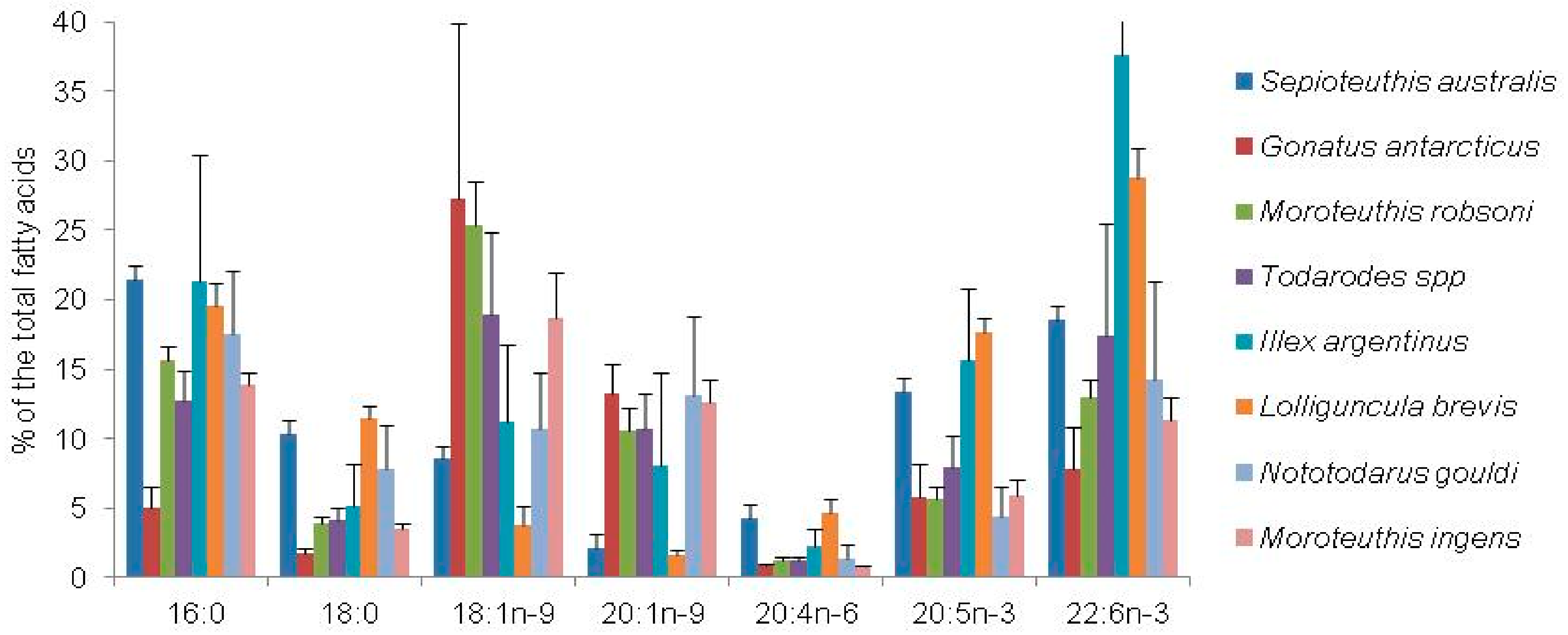
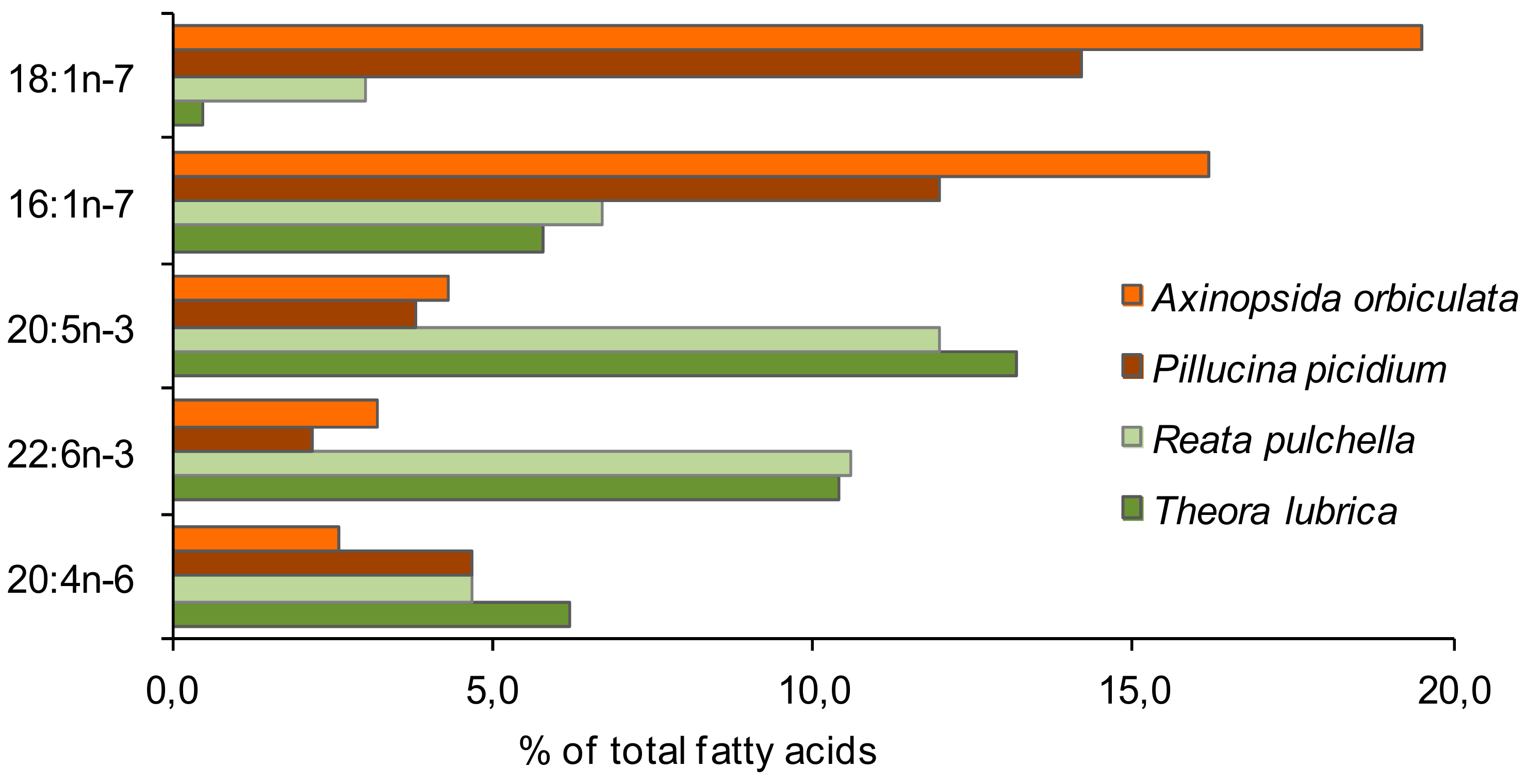
| Fatty Acid Markers | Food Source | References |
|---|---|---|
| 20:5n-3, 16:1n-7/16:0 > 1, 14:0, 16:2n-4, 16:3n-4, 16:4n-1 | Diatoms | [41,42] |
| 18:4n-3, 22:6n-3 | Dinoflagellates | [46] |
| 18:2n-6, 20:4n-6, 22:6n-3 | Heterotrophic flagellates | [56,57] |
| 22:6n-3, 18:1n-9 | Animal material Meiobenthos | [59,62] |
| 15:0, 15:1, iso-15:0, anteiso-15:0, iso-16:0, 17:0, iso-17:0, anteiso-17:0 | Heterotrophic bacteria | [63,64,65,66] |
| 16:0, 18:0, 22:0 | Detritus | [67,68] |
| 18:1n-9, 18:2n-6, 18:3n-3, 18:4n-3, 20:4n-6, 20:5n-3 | Brown algae | [69] |
| Very long-chain FAs: iso-5,9-25:2; 25:2Δ5,9; 26:2Δ5,9; 27:2Δ5,9; 26:3Δ5,9,19; 26:3Δ5,9,17; 27:3Δ5,9,19 | Sponges | [70,71,72] |
| Tetracosapolyenoic acids: 24:5n-6, 24:6n-3 | Soft corals | [73] |
| Fatty Acids | Acmea pallida | Lottia dorsuosa | Ischnochiton hakodadensis | Cryptonatica janthostoma |
|---|---|---|---|---|
| 14:0 | 0.4 | 4.6 | 4.5 | 3.4 |
| 15:0 | 0.3 | 1.0 | 0.5 | 0.6 |
| 16:0 | 5.9 | 13.9 | 13.2 | 6.4 |
| 16:1 | 0.8 | 5.8 | 3.8 | 2.8 |
| 17:0 | – | 0.5 | – | – |
| 16:3n-4 | 0.8 | 2.9 | 0.4 | 1.7 |
| 17:1n-8 | 0.4 | – | 0.4 | 0.8 |
| 18:0 | 6.3 | 4.5 | – | 8.8 |
| 18:1 | 13.2 | 15.9 | 15.7 | 3.6 |
| 18:2n-6 | – | 4.9 | 2.0 | 2.6 |
| 18:3n-6 | – | – | 0.5 | 0.2 |
| 18:3n-3 | 1.1 | – | 4.4 | 0.6 |
| 20:1 | 10.5 | 9.2 | 3.9 | 7.3 |
| 18:4n-3 | 1.1 | 2.2 | 1.7 | 0.5 |
| 20:2NMI | 1.5 | 1.2 | – | 8.1 |
| 20:3n-6 | 1.6 | 0.9 | 3.5 | 4.2 |
| 20:4n-6 | 19.2 | 15.5 | 6.8 | 6.0 |
| 22:2NMI | 3.9 | 4.2 | 3.6 | 3.1 |
| 20:5n-3 | 33.0 | 11.8 | 13.3 | 21.6 |
| 22:4n-6 | – | 0.7 | 4.2 | 1.1 |
| 22:5n-6 | – | – | 0.8 | 0.5 |
| 22:5n-3 | – | 0.9 | 4.4 | 2.6 |
| 22:6n-3 | – | – | 0.8 | 12.8 |
| Fatty Acids | Arcidae | Mytilidae | Ostreidae | Cardiidae | Veneridae | Mactridae | Pectinidae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scapharca broughtoni | Arca boucardi | Anadara maculosa | Mytilus edulis | Crenomitilus grayanus | Modiolus difficilus | Crassostrea gigas | Clinocardium californiense | Callista brevisiphonata | Saxidomus purpuratus | Protothaca jedoensis | Mercenaria stimpsoni | Spisula voyi | Mactra chinensis | Patinopecten yessoensis | Chlamys swifti | |
| 14:0 | 0.6 | 0.6 | 2.7 | 2.1 | 3.9 | 2.7 | 3.0 | 2.0 | 0.6 | 1.8 | 2.8 | 7.8 | 6.9 | 3.1 | 3.0 | 4.2 |
| 15:0 | 1.1 | 0.4 | 0.3 | 0.7 | 0.5 | 0.5 | 0.8 | 0.9 | 0.5 | 0.4 | 0.4 | 0.6 | 0.6 | 0.6 | 0.5 | 0.4 |
| 16:0 | 10.2 | 9.2 | 13.3 | 14.8 | 16.6 | 14.9 | 14.9 | 12.9 | 13.5 | 11.0 | 10.9 | 14.8 | 12.4 | 15.0 | 11.2 | 13.7 |
| 16:1n-7 | 1.5 | 3.2 | 2.4 | 5.0 | 8.3 | 5.5 | 4.9 | 4.9 | 2.4 | 3.3 | 6.6 | 9.2 | 5.6 | 9.6 | 5.0 | 4.9 |
| 16:3n-4 | 3.1 | 1.2 | 4.2 | 0.8 | 1.5 | 2.0 | 2.1 | 1.8 | 1.0 | 0.1 | 2.4 | 1.4 | 1.9 | 1.8 | 1.1 | 0.3 |
| 17:1n-8 | 2.2 | 1.3 | 0.2 | 0.2 | 0.6 | 1.4 | 1.0 | 2.4 | 2.0 | 0.6 | 0.8 | 0.6 | 0.8 | 0.8 | 08 | 0.9 |
| 18:0 | 10.6 | 6.6 | 13.5 | 3.5 | 2.8 | 5.8 | 3.6 | 5.5 | 0.5 | 5.8 | 4.0 | 6.4 | 6.8 | 4.0 | 6.1 | 5.3 |
| 18:1n-7 | 5.7 | 2.8 | 4.7 | 3.6 | 5.7 | 6.6 | 12.1 | 7.1 | 0.3 | 4.2 | 6.8 | 3.2 | 4.9 | 6.0 | 6.1 | 8.2 |
| 18:2n-6 | 2.2 | 1.6 | 3.8 | 1.7 | 2.2 | 2.1 | 2.2 | 1.2 | 0.5 | 0.8 | 1.3 | 1.4 | 1.3 | 0.4 | 9.0 | 1.6 |
| 18:3n-6 | 0.1 | – | 0.5 | 0.1 | – | – | 0.3 | 0.6 | 0.4 | 0.1 | 0.2 | 0.6 | 0.2 | 0.3 | 0.2 | 0.5 |
| 18:3n-3 | 1.0 | 2.1 | 1.5 | 1.4 | 1.5 | 1.5 | 1.8 | 1.5 | 0.2 | 0.7 | 0.3 | 0.7 | 0.8 | 0.4 | 0.4 | 0.9 |
| 20:1 | 10.8 | 12.5 | 8.9 | 12.3 | 5.6 | 7.4 | 6.8 | 3.2 | 12.5 | 17.3 | 5.8 | 3.6 | 6.5 | 6.8 | 3.9 | 5.5 |
| 18:4n-3 | 0.6 | 1.4 | 0.8 | 1.8 | 2.7 | 2.1 | 3.1 | 1.2 | 1.3 | 2.7 | 1.0 | 4.5 | 3.7 | 2.7 | 2.6 | 4.5 |
| 20:2NMI | 0.1 | 0.7 | 0.1 | 0.8 | 3.9 | 1.2 | 1.3 | 3.4 | 0.2 | 0.3 | 0.2 | 1.9 | 1.0 | – | 0.9 | 0.2 |
| 20:3n-6 | 0.1 | – | 0.8 | 1.7 | – | – | 1.2 | 0.5 | 0.7 | – | 1.1 | 1.5 | 1.4 | 0.1 | 0.7 | |
| 20:4n-6 | 5.8 | 6.4 | 7.8 | 3.9 | 2.5 | 3.3 | 2.1 | 4.3 | 3.6 | 2.9 | 3.5 | 1.5 | 2.7 | 2.1 | 3.0 | 3.8 |
| 22:2NMI | 20.7 | 12.8 | 12.7 | 4.6 | 4.6 | 3.8 | 4.7 | 6.1 | 6.0 | 0.7 | 6.5 | 1.9 | 1.7 | 0.6 | 0.7 | 0.6 |
| 20:5n-3 | 6.1 | 10.3 | 4.0 | 14.5 | 16.2 | 22.9 | 16.7 | 13.4 | 18.3 | 22.3 | 14.4 | 24.5 | 17.3 | 21.2 | 19.7 | 20.2 |
| 22:3n-6 | 1.2 | 1.3 | 0.4 | 1.5 | 0.8 | 1.3 | 1.0 | 1.4 | 1.6 | 1.5 | 1.6 | 1.6 | 1.4 | 1.4 | 0.9 | 1.1 |
| 22:4n-6 | 0.9 | 0.8 | 1.3 | 0.1 | 0.2 | 0.4 | 0.1 | 2.5 | 1.1 | 2.2 | 1.4 | 0.4 | 0.8 | 0.8 | 0.1 | 0.2 |
| 22:5n-6 | 1.3 | 1.4 | 2.3 | 0.7 | 0.4 | 0.6 | 0.4 | 1.1 | 1.6 | 1.1 | 1.1 | 0.3 | 0.9 | 0.9 | 0.6 | 0.6 |
| 22:5n-3 | 1.0 | 1.3 | 0.7 | 1.1 | 0.8 | 1.1 | 0.1 | 3.0 | 2.0 | 3.3 | 1.5 | 0.1 | 2.0 | 2.0 | 0.5 | 0.9 |
| 22:6n-3 | 14.2 | 22.4 | 13.1 | 23.0 | 15.3 | 12.0 | 16.0 | 16.7 | 19.5 | 15.2 | 24.2 | 12.3 | 17.4 | 17.4 | 18.5 | 21.2 |
| Type of Nutrition | Symbionts | Function | Host | References |
|---|---|---|---|---|
| Chemotrophic | Bacteria | Nutritional | Bivalves and gastropods | [104] |
| Phototrophic | Zooxanthellae | Nutritional | Giant clam Tridacna squamosa, Gastropod Strombus gigas | [105] |
| Algal chloroplasts | Nutritional | Sea slug Elysia chlorotica | [106] | |
| Chlorella | Nutritional | Clams, e.g., Anodonta | [107] | |
| Heterotrophic | Bacteria | Nutritional | Bivalve shipworm Bankia setacea | [108] |
| Light production | Squid Euprymna scolopes | [109] | ||
| Chemical defense | Sacoglossan Elysia rufescens | [110] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, N.V. Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential. Biomolecules 2019, 9, 857. https://doi.org/10.3390/biom9120857
Zhukova NV. Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential. Biomolecules. 2019; 9(12):857. https://doi.org/10.3390/biom9120857
Chicago/Turabian StyleZhukova, Natalia V. 2019. "Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential" Biomolecules 9, no. 12: 857. https://doi.org/10.3390/biom9120857
APA StyleZhukova, N. V. (2019). Fatty Acids of Marine Mollusks: Impact of Diet, Bacterial Symbiosis and Biosynthetic Potential. Biomolecules, 9(12), 857. https://doi.org/10.3390/biom9120857





