Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Pulmonary Function Testing
2.3. Blood Samples
2.4. Isolation of Serum microRNA
2.5. Differential Expression of miRNA-22 in Serum by PCR Arrays
2.6. Validation of Samples by TaqMan RT-qPCR
2.7. Protein Quantification
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Women in the Study
3.2. miRNA-22 was Upregulated in COPD by Biomass Related to COPD by Smoking
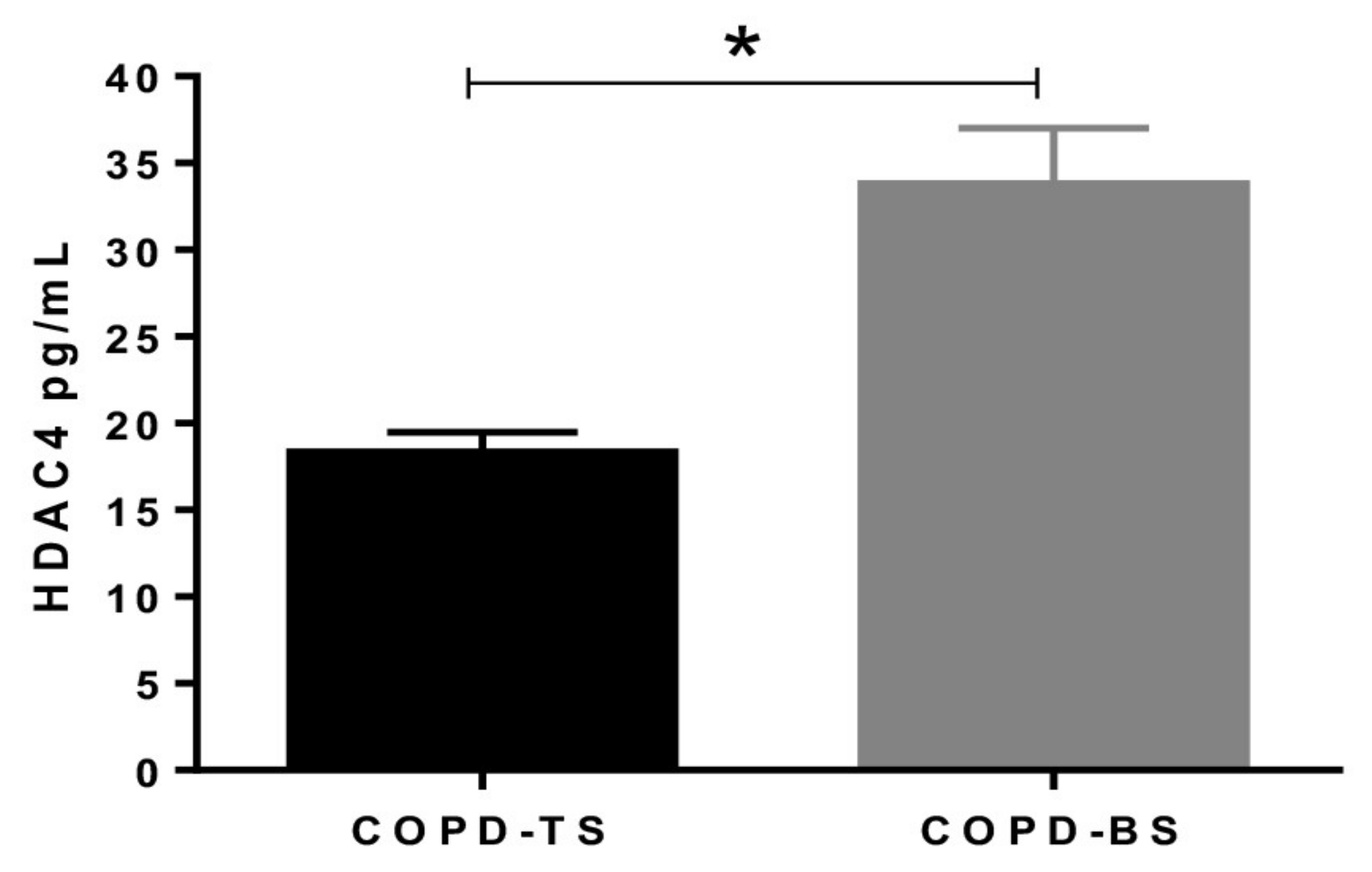
3.3. Serum HDAC4 is Higher in COPD by Biomass that COPD by Smoking
3.4. HDAC4 Positively Correlated with DLCOsb %P in COPD
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019. Available online: GOLD-2019-POCKET-GUIDE-FINAL_WMS.pdf (accessed on 6 December 2019).
- Perez-Padilla, R.; Ramirez-Venegas, A.; Sansores-Martinez, R. Clinical characteristics of patients with biomass smoke-associated COPD and chronic bronchitis, 2004–2014. Chronic. Obstr. Pulm. Dis. 2014, 1, 23–32. [Google Scholar] [CrossRef][Green Version]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Paré, P.D.; Müller, N.L.; Silva, I.S.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef]
- Shah, A.; Shah, U.; Jayalakshmi, T.K.; Mirchandani, L.; Iyer, A.; Nair, G. COPD phenotypes according to high resolution CT scan findings. Eur. Respir. J. 2014, 44 Suppl 58, 3001. [Google Scholar]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am. J. Respir. Crit. Care. Med. 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Ramirez, A.; Sansores, R.; Velazquez, M.; Perez, O. Nonsmoker and Biomass Exposure, in Controversies in COPD; Anzueto, A., Heijdra, Y., Hurst, J.R., Eds.; European Respiratory Society monograph: Sheffield, UK, 2015; pp. 35–45. Available online: https://books.ersjournals.com/content/controversies-in-copd (accessed on 5 December 2019).
- Rivera, R.; Cosio, M.; Ghezzo, H.; Salazar, M.; Pérez-Padilla, R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tuberc. Lung Dis. 2008, 12, 972–977. [Google Scholar]
- Tan, W.; Shen, H.; Wong, W. Dysregulated autophagy in COPD: A pathogenic process to be deciphered. Pharmacol. Res. 2019, 144, 1–7. [Google Scholar] [CrossRef]
- Christenson, S.A.; Brandsma, C.-A.; Campbell, J.D.; Knight, D.A.; Pechkovsk, D.V.; Hogg, J.C.; Timens, W.; Postma, D.S.; Lenburg, M.; Spirfa, A. miR-638 regulates gene expression networks associated with emphysematous lung destruction. Genome. Med. 2013, 5, 114. [Google Scholar] [CrossRef]
- Osei, E.T.; Florez-Sampedro, L.; Timens, W.; Postma, D.S.; Heijink, I.H.; Brandsma, C.A. Unravelling the complexity of COPD by microRNAs: It’s a small world after all. Eur. Respir. J. 2015, 46, 807–818. [Google Scholar] [CrossRef]
- Campbell, J.D.; McDonough, J.E.; Zeskind, J.E.; Hackett, T.L.; Pechkovsky, D.V.; Brandsma, C.-A.; Suzuki, M.; Gosselink, J.V.; Liu, G.; Alekseyev, Y.O.; et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome. Med. 2012, 4, 67. [Google Scholar] [CrossRef]
- Gower, A.C.; Steiling, K.; Brothers, J.F.; Lenburg., M.E.; Spira, A. Transcriptomic studies of the airway field of injury associated with smoking-related lung disease. Proc. Am. Thorac. Soc. 2011, 8, 173–179. [Google Scholar] [CrossRef]
- Lu, W.; You, R.; Yuan, X.; Yang, T.; Samuel, E.L.G.; Marcan, D.C.; Sikkema, W.K.A.; Tour, J.M.; Rodriguez, A.; Kheradmand, F.; et al. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat. Immunol. 2015, 16, 1185–1194. [Google Scholar] [CrossRef]
- Perez-Padilla, J.R.; Regalado-Pineda, J.; Vazquez-Garcia, J.C. Reproducibility of spirometry in Mexican workers and international reference values. Salud. Publica. Mex. 2001, 43, 113–121. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Marabita, F.; Candia, P.D.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief. Bioinform. 2015, 17, 204–212. [Google Scholar] [CrossRef]
- Faraldi, M.; Gomarasca, M.; Sansoni, V.; Perego, S.; Banfi, G.; Lombardi, G. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 2019, 9, 1584. [Google Scholar] [CrossRef]
- Pérez-Bautista, O.; Montaño, M.; Pérez-Padilla, R.; Zúñiga-Ramos, J.; Camacho-Priego, M.; Barrientos-Gutiérrez, T.; Buendía-Roldan, I.; Velasco-Torres, Y.; Carlos Ramos, C. Women with COPD by biomass show different serum profile of adipokines, incretins, and peptide hormones than smokers. Respir. Res. 2018, 19, 239. [Google Scholar] [CrossRef]
- Arias-Stella, J. The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation 2007, 115, 1132–1146. [Google Scholar]
- Stream, J.O.; Luks, A.M.; Grissom, C.K. Lung disease at high altitude. Exp. Rev. Respir. Med. 2009, 3, 635–650. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, G.; Zhao, T.C. Histone Deacetylase 4 (HDAC4): Mechanism of Regulations and Biological Functions. Epigenomics 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Wilson, D.O.; Weissfeld, J.L.; Balkan, A.; Schragin, J.G.; Fuhrman, C.R.; Fisher, S.N.; Wilson, J.; Leader, J.K.; Siegfried, J.M.; Shapir, S.D.; et al. Association of radiographic emphysema and airflow obstruction with lung cance. Am. J. Respir. Crit. Care. Med. 2008, 178, 738–744. [Google Scholar] [CrossRef]
- Gordon, J.W.; Pagiatakis, C.; Salma, J.; Du, M.; Andreucci, J.J.; Zhao, J.; Hou, G.; Perry, R.L.; Dan, Q.; Courtman, D.; et al. Protein kinase A-regulated assembly of a MEF2-HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 19027–19042. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Quintana-Carrillo, R.; Falfán-Valencia, R.; Vargas-Rojas, M.I. Chronic obstructive pulmonary disease induced by exposure to biomass smoke is associated with a Th2 cytokine production profile. Clin. Immunol. 2015, 161, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Golpe, R.; Martín-Robles, I.; Sanjuán-López, P.; Pérez-de-Llano, L.; González-Juanatey, C.; López-Campos, J.L.; Arellano-Orden, E. Differences in systemic inflammation between cigarette and biomass smoke-induced COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2639. [Google Scholar] [CrossRef]
- Díaz, A.A.; Pinto-Plata, V.; Hernández, C.; Peña, J.; Ramos, C.; Díaz, J.C.; Klaassend, C.; Patinoe, C.M.; Saldías, F.; Díaz, O. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir. Med. 2015, 109, 882–889. [Google Scholar] [CrossRef]
- Harvey, B.G.; Strulovici-Barel, Y.; Kaner, R.J.; Sanders, A.; Vincent, T.L.; Mezey, J.G.; Crystal, R.G. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur. Respir. J. 2015, 46, 1589–1597. [Google Scholar] [CrossRef]
- Balasubramanian, A.; MacIntyre, N.R.; Henderson, R.J.; Jensen, R.L.; Kinney, G.; Stringer, W.W.; Hersh, C.P.; Bowler, R.P.; Casaburi, R.; Han, M.K.; et al. Diffusing Capacity of Carbon Monoxide in Assessment of COPD. Chest 2019. [Google Scholar] [CrossRef]
- Cortez-Lugo, M.; Ramírez-Aguilar, M.; Pérez-Padilla, R.; Sansores-Martínez, R.; Ramírez-Venegas, A.; Barraza-Villarreal, A. Effect of personal exposure to PM2. 5 on respiratory health in a Mexican panel of patients with COPD. Int. J. Environ. Res. Public. Health. 2015, 12, 10635–10647. [Google Scholar] [CrossRef]


| Variable | COPD-TS | COPD-BS |
| Characteristics | ||
| Age (years) | 66.57 ± 6.37 | 73.27 ± 8.69* |
| Height (cm) | 155.31 ± 7.57 | 146.52 ± 5.69* |
| Weight (Kg) | 59.52 ± 12.13 | 56.25 ± 10.82 |
| BMI (Kg/m2) | 24.79 ± 5.78 | 26.33 ± 5.04 |
| Exposure Characteristics | ||
| Pack-years of smoking | 36.62 ± 23.1 | 0 |
| Hour-years of biomass smoke exposure | 0 | 366.88 ± 219.3 |
| Physiological characteristics | ||
| FEV1%P | 39.85 ± 5.36 | 39.92 ± 6.57 |
| FEV1/FVC ratio | 45.63 ± 11.41 | 45.23 ± 10.08 |
| DLCOsb %P | 50.96 ± 11.88 | 78.45 ± 20.47* |
| 6MWT (m) | 286.47 ± 174.23 | 318.91 ± 102.99 |
| GOLD grades | Case numbers (%) | |
| III | 16 (64) | 20 (80) |
| IV | 9 (36) | 5 (20) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Torres, Y.; Ruiz, V.; Montaño, M.; Pérez-Padilla, R.; Falfán-Valencia, R.; Pérez-Ramos, J.; Pérez-Bautista, O.; Ramos, C. Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass. Biomolecules 2019, 9, 837. https://doi.org/10.3390/biom9120837
Velasco-Torres Y, Ruiz V, Montaño M, Pérez-Padilla R, Falfán-Valencia R, Pérez-Ramos J, Pérez-Bautista O, Ramos C. Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass. Biomolecules. 2019; 9(12):837. https://doi.org/10.3390/biom9120837
Chicago/Turabian StyleVelasco-Torres, Yadira, Víctor Ruiz, Martha Montaño, Rogelio Pérez-Padilla, Ramcés Falfán-Valencia, Julia Pérez-Ramos, Oliver Pérez-Bautista, and Carlos Ramos. 2019. "Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass" Biomolecules 9, no. 12: 837. https://doi.org/10.3390/biom9120837
APA StyleVelasco-Torres, Y., Ruiz, V., Montaño, M., Pérez-Padilla, R., Falfán-Valencia, R., Pérez-Ramos, J., Pérez-Bautista, O., & Ramos, C. (2019). Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass. Biomolecules, 9(12), 837. https://doi.org/10.3390/biom9120837







