The Actual and Potential Aroma of Winemaking Grapes
Abstract
1. Introduction
2. The Actual Aroma of Grapes and Musts
- Free aroma, which refers to the aroma molecules found as such in the pulp and skin of the fruit, the grape in our case;
2.1. Key Aroma Compounds of Aromatic Grapes
2.2. Key Aroma Compounds of Raisins and of “Raisinized” Grapes
2.3. Aroma Compounds Responsible for Vegetal and Green Aroma and Flavors
2.4. Compounds Responsible for the Flavor of Neutral Grapes
- Fruity: ethyl isobutyrate, ethyl butyrate, ethyl 3-methylbutyrate, ethyl hexanoate, ethyl octanoate, and eventually others;
- Jammy, very sweet fruit: furaneol, homofuraneol, β-damascenone, γ-nonalactone, and massoia lactone;
- Sweet–floral: vanillin, ethyl vanillate, β-ionone, β-phenylethyl acetate, and phenylacetaldehyde;
- Floral–citric aroma compounds: linalool, geraniol, limonene, nonanal, and eventually others;
- Herbaceous: hexanal, (Z)-3-hexenal, (E)-2-hexenal, (Z)-3-hexenol, (E)-2-nonenal, (E,Z)-2,6-nonadienal;
- Peppery: rotundone;
- Unspecific: 3-methylbutanal, ethyl acetate, diacetyl.
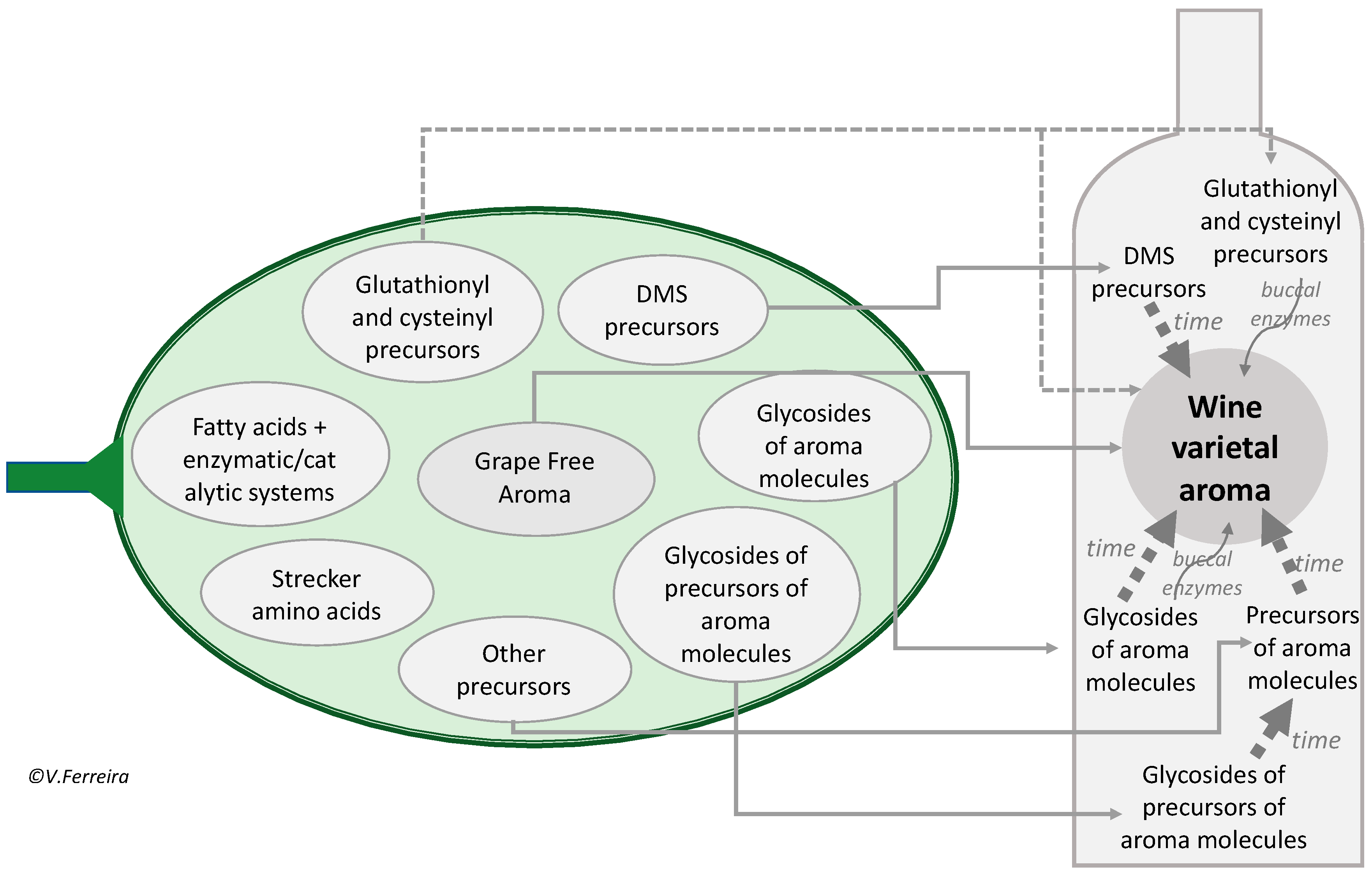
3. Grape Potential Aroma: Specific Aroma Precursors
3.1. Specific vs. Unspecific Precursors
3.2. Grape Aroma vs. Grape-Derived Wine Aroma
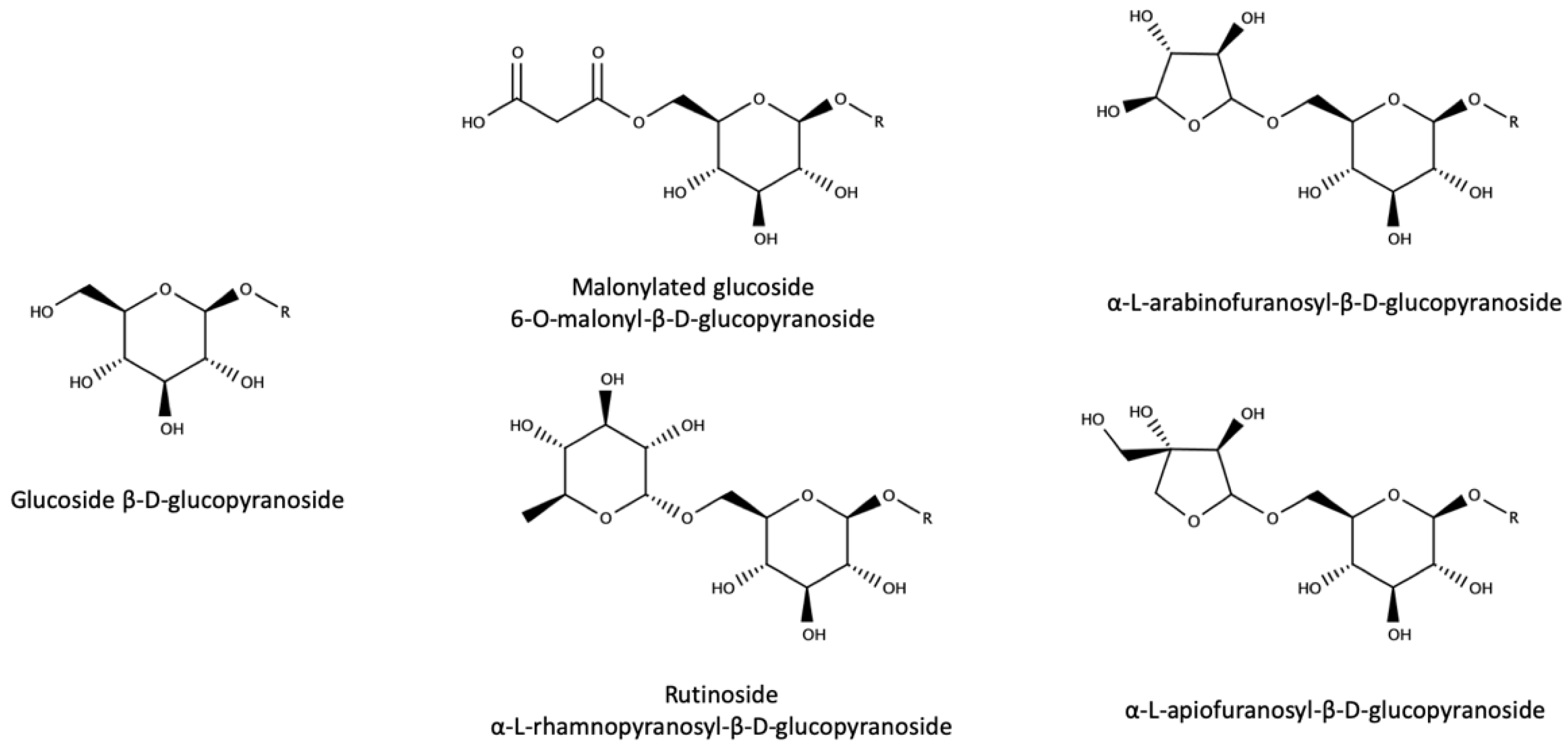
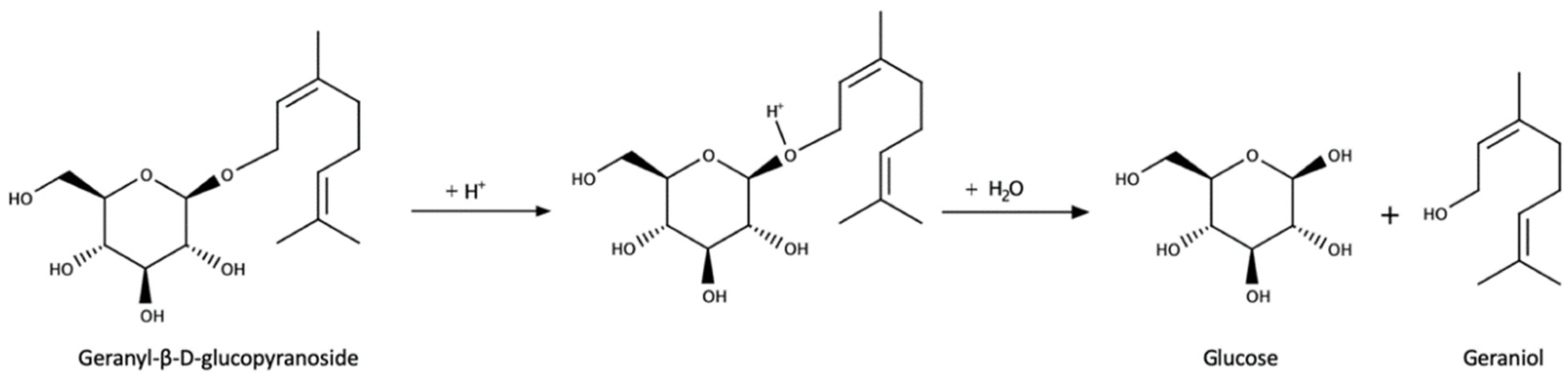
3.3. Glycoconjugates as Aroma Precursors
- Aliphatic alcohol derivatives;
- Terpenes;
- Norisoprenoids;
- Benzenoids, which can be further subdivided into:
- Benzyl and phenyl derivatives;
- Volatile phenols;
- Vanillins;
- Ethyl cinnamate.
- Miscellaneous compounds.
3.4. Other Precursors: Molecules Which by Chemical Rearrangement or Esterification Form the Aroma Molecule
3.5. S-Derivatives of Cysteine or Glutathione
3.6. S-Methylmethionine and Other DMS Precursors
3.7. The Action of Fungus and Other Exogenous Factors on Grape Actual and Potential Aroma
4. Final Conclusions
Funding
Conflicts of Interest
References
- Cordonnier, R.; Bayonove, C.L. Mise en evidence dans la baie de raisin, var. Muscat d’Alexandrie, de monoterpenes lies revelables par une ou plusieurs enzymes du fruit. Comptes Rendus de l’Académie des Sciences 1974, 278, 3387–3390. [Google Scholar]
- Williams, P.J.; Strauss, C.R.; Wilson, B. Hydroxylated Linalool Derivatives as Precursors of Volatile Monoterpenes of Muscat Grapes. J. Agric. Food Chem. 1980, 28, 766–771. [Google Scholar] [CrossRef]
- Cullere, L.; Lopez, R.; Ferreira, V. The Instrumental Analysis of Aroma-Active Compounds for Explaining the Flavor of Red Wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–307. [Google Scholar] [CrossRef]
- Ferreira, V.; De-la-Fuente, A.; Sáenz-Navajas, M.P. Wine aroma vectors and sensory attributes. In Managing Wine Quality, 2nd ed.; Reynolds, A., Ed.; Woodhead Publishing (Elsevier): Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Strauss, C.R.; Wilson, B.; Massy-Westropp, R.A. Use of C18 reversed-phase liquid chromatography for the isolation of monoterpene glycosides and nor-isoprenoid precursors from grape juice and wines. J. Chromatogr. A 1982, 235, 471–480. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Ibarz, M.J.; Ferreira, V.; Hernandez-Orte, P.; Loscos, N.; Cacho, J. Optimization and evaluation of a procedure for the gas chromatographic-mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J. Chromatogr. A 2006, 1116, 217–229. [Google Scholar] [CrossRef]
- Hampel, D.; Robinson, A.L.; Johnson, A.J.; Ebeler, S.E. Direct hydrolysis and analysis of glycosidically bound aroma compounds in grapes and wines: Comparison of hydrolysis conditions and sample preparation methods. Aust. J. Grape Wine Res. 2014, 20, 361–377. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. Changes in free and bound fractions of aromatic components in vine leaves during development of muscat grapes. Phytochemistry 1986, 25, 943–946. [Google Scholar] [CrossRef]
- Carro, N.; López, E.; Günata, Z.Y.; Baumes, R.L.; Bayonove, C.L. Free and glycosidically bound aroma compounds in grape must of four non-floral Vitis vinifera varieties. Analusis 1996, 24, 254–258. [Google Scholar]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Comparison of the Suitability of Different Hydrolytic Strategies to Predict Aroma Potential of Different Grape Varieties. J. Agric. Food Chem. 2009, 57, 2468–2480. [Google Scholar] [CrossRef]
- Francis, I.L.; Sefton, M.A.; Williams, P.J. Sensory Descriptive Analysis of the Aroma of Hydrolyzed Precursor Fractions from Semillon, Chardonnay and Sauvignon Blanc Grape Juices. J. Sci. Food Agric. 1992, 59, 511–520. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010, 120, 205–216. [Google Scholar] [CrossRef]
- Alegre, Y.; Arias-Pérez, I.; Hernandez-Orte, P.; Ferreira, V. Development of a new strategy for studying the aroma potential of winemaking grapes through the accelerated hydrolysis of phenolic and aromatic fractions (PAFs). Food Res. Int. 2019, in press. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F.G. Fatty acid hydroperoxide lyase: A plant cytochrome P450 enzyme involved in wound healing and pest resistance. ChemBioChem 2001, 2, 494–504. [Google Scholar] [CrossRef]
- Podolyan, A.; White, J.; Jordan, B.; Winefield, C. Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of Sauvignon Blanc. Funct. Plant Biol. 2010, 37, 767–784. [Google Scholar] [CrossRef]
- Starkenmann, C.; Le Calve, B.; Niclass, Y.; Cayeux, I.; Beccucci, S.; Troccaz, M. Olfactory Perception of Cysteine-S-Conjugates from Fruits and Vegetables. J. Agric. Food Chem. 2008, 56, 9575–9580. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Cueva, C.; Pozo-Bayon, M.A.; Moreno-Arribas, M.V. Ability of human oral microbiota to produce wine odorant aglycones from odourless grape glycosidic aroma precursors. Food Chem. 2015, 187, 112–119. [Google Scholar] [CrossRef]
- Parker, M.; Black, C.A.; Barker, A.; Pearson, W.; Hayasaka, Y.; Francis, I.L. The contribution of wine-derived monoterpene glycosides to retronasal odour during tasting. Food Chem. 2017, 232, 413–424. [Google Scholar] [CrossRef]
- Hatanaka, A. The Biogeneration of Green Odor by Green Leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Joslin, W.S.; Ough, C.S. Cause and fate of certain C6 compounds formed enzymatically in macerated grape leaves during harvest and wine fermentation. Am. J. Enol. Vitic. 1978, 29, 11–17. [Google Scholar]
- Wang, D.; Duan, C.Q.; Shi, Y.; Zhu, B.Q.; Javed, H.U.; Wang, J. Free and glycosidically bound volatile compounds in sun-dried raisins made from different fragrance intensities grape varieties using a validated HS-SPME with GC-MS method. Food Chem. 2017, 228, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Slegers, A.; Angers, P.; Ouellet, E.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Quebec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.L.; Xu, Y.; Jiang, W.G.; Li, J.M. Identification and Quantification of Impact Aroma Compounds in 4 Nonfloral Vids vinifera Varieties Grapes. J. Food Sci. 2010, 75, S81–S88. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Barker, A.; Black, C.A.; Hixson, J.; Williamson, P.; Francis, I.L. Don’t miss the marc: Phenolic-free glycosides from white grape marc increase flavour of wine. Aust. J. Grape Wine Res. 2019, 25, 212–223. [Google Scholar] [CrossRef]
- Ribereaugayon, P.; Boidron, J.N.; Terrier, A. Aroma of muscat grape varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhang, W.W.; Yu, W.J.; Zhao, L.P.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, L.; Wang, S.P. Study on the volatile composition of table grapes of three aroma types. LWT Food Sci. Technol. 2019, 115, 108450. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhang, W.W.; Duan, S.Y.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Bondada, B.; Ma, C.; Wang, S.P. In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars. Molecules 2018, 23, 1703. [Google Scholar] [CrossRef]
- Wu, Y.S.; Duan, S.Y.; Zhao, L.P.; Gao, Z.; Luo, M.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, S.P. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and Lychee (Litchi chinesis Sonn.) Fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsuda, H.; Utsumi, Y.; Hagiwara, T.; Kanisawa, T. Synthesis and odor of optically active rose oxide. Tetrahedron Lett. 2002, 43, 9077–9080. [Google Scholar] [CrossRef]
- Girard, B.; Fukumoto, L.; Mazza, G.; Delaquis, P.; Ewert, B. Volatile terpene constituents in maturing Gewurztraminer grapes from British Columbia. Am. J. Enol. Vitic. 2002, 53, 99–109. [Google Scholar]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Hellin, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef]
- Skinkis, P.A.; Bordelon, B.P.; Wood, K.V. Comparison of Monoterpene Constituents in Traminette, Gewurztraminer, and Riesling Winegrapes. Am. J. Enol. Vitic. 2008, 59, 440–445. [Google Scholar]
- Shure, K.B.; Acree, T.E. Changes in the Odor-Active Compounds in Vitis-Labruscana Cv Concord During Growth And Development. J. Agric. Food Chem. 1994, 42, 350–353. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sasaki, K.; Tanzawa, F.; Matsuyama, S.; Suzuki, S.; Takata, R.; Saito, H. Impact of harvest timing on 4-hydroxy-2,5-dimethyl-3(2H)-furanone concentration in ‘Muscat Bailey A’ grape berries. Vitis 2013, 52, 9–11. [Google Scholar]
- Sale, J.W.; Wilson, J.B. Distribution of volatile flavor in grapes and grape juices. J. Agric. Res. 1926, 33, 0301–0310. [Google Scholar] [CrossRef]
- Acree, T.E.; Lavin, E.H.; Nishida, R.; Watanabe, S. o-Amino Acetophenone the Foxy Smelling Component of Labruscana Grapes. In Flavour Science and Technology—6th Weurmann Symposium; Bessiere, Y., Thomas, A.F., Eds.; Wiley: Hoboken, NJ, USA, 1990; pp. 49–52. [Google Scholar]
- Massa, M.J.; Robacker, D.C.; Patt, J. Identification of grape juice aroma volatiles and attractiveness to the Mexican fruit fly (Diptera: Tephritidae). Fla. Entomol. 2008, 91, 266–276. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G.; Ullemeyer, H. 2-Aminoacetophenone—Causal Component of Untypical Aging Flavor (Naphthalene Note, Hybrid Note) Of Wine. Vitis 1993, 32, 61–62. [Google Scholar]
- Baek, H.H.; Cadwallader, K.R.; Marroquin, E.; Silva, J.L. Identification of predominant aroma compounds in muscadine grape juice. J. Food Sci. 1997, 62, 249–252. [Google Scholar] [CrossRef]
- Baek, H.H.; Cadwallader, K.R. Contribution of free and glycosidically bound volatile compounds to the aroma of muscadine grape juice. J. Food Sci. 1999, 64, 441–444. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.J.; Wu, B.H.; Fang, J.B.; Li, S.H. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and Sensory Studies on Tomato Paste Volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile Constituents of Apricot (Prunus-Armeniaca). J. Agric. Food Chem. 1990, 38, 471–477. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Importance Of 2-Aminoacetophenone to the Flavor of Masa Corn Flour Products. J. Agric. Food Chem. 1994, 42, 1–2. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E. The Volatiles of 2 New Strawberry Cultivars, Annelie and Alaska Pioneer, Obtained by Backcrossing of Cultivated Strawberries with Wild Strawberries, Fragaria-Vesca, Rugen and Fragaria-Virginiana. Z. Lebensmittel Unters. Forsch. 1982, 175, 113–116. [Google Scholar] [CrossRef]
- Iyer, M.M.; Sacks, G.L.; Padilla-Zakour, O.I. Assessment of the Validity of Maturity Metrics for Predicting the Volatile Composition of Concord Grape Juice. J. Food Sci. 2012, 77, C319–C325. [Google Scholar] [CrossRef]
- Depinho, P.G.; Bertrand, A. Analytical Determination of Furaneol (2,5-Dimethyl-4-Hydroxy-3(2h)-Furanone)—Application to Differentiation of White Wines from Hybrid and Various Vitis-Vinifera Cultivars. Am. J. Enol. Vitic. 1995, 46, 181–186. [Google Scholar]
- Rapp, A.; Engel, L. Determination and Detection of Furaneol (2,5-Dimethyl-4-Hydroxy-3-Furanon) in Wines from Vitis-Vinifera Varieties. Vitis 1995, 34, 71–72. [Google Scholar]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming-Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Moyano, L.; Zea, L. Changes in aroma profile of musts from grapes cv. Pedro Ximenez chamber-dried at controlled conditions destined to the production of sweet Sherry wine. LWT Food Sci. Technol. 2014, 59, 560–565. [Google Scholar] [CrossRef]
- Wang, D.; Cai, J.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS-SPME with GC-MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.U.; Wang, D.; Wu, G.F.; Kaleem, Q.M.; Duan, C.Q.; Shi, Y. Post-storage changes of volatile compounds in air- and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Res. Int. 2019, 119, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Cacho, J.; Ferreira, V. The chemical characterization of the aroma of dessert and sparkling white wines (Pedro Ximenez, Fino, Sauternes, and Cava) by gas chromatography-olfactometry and chemical quantitative analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Rocha, S.M.; Delgadillo, I.; Coimbra, M.A. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem. 2016, 192, 633–641. [Google Scholar] [CrossRef]
- Lukic, I.; Radeka, S.; Grozaj, N.; Staver, M.; Persuric, D. Changes in physico-chemical and volatile aroma compound composition of Gewurztraminer wine as a result of late and ice harvest. Food Chem. 2016, 196, 1048–1057. [Google Scholar] [CrossRef]
- Luo, J.Q.; Brotchie, J.; Pang, M.; Marriott, P.J.; Howell, K.; Zhang, P.Z. Free terpene evolution during the berry maturation of five Vitis vinifera L. cultivars. Food Chem. 2019, 299, 125101. [Google Scholar] [CrossRef]
- Šuklje, K.; Zhang, X.; Antalick, G.; Clark, A.C.; Deloire, A.; Schmidtke, L.M. Berry Shriveling Significantly Alters Shiraz (Vitis vinifera L.) Grape and Wine Chemical Composition. J. Agric. Food Chem. 2016, 64, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.C.; Šuklje, K.; Antalick, G.; Schmidtke, L.M.; Blackman, J.W. Late-Season Shiraz Berry Dehydration That Alters Composition and Sensory Traits of Wine. J. Agric. Food Chem. 2018, 66, 7750–7757. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma POtential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, M.J.; Riu, M.; Cotta, J.P.; Block, D.E.; Dokoozlian, N.K.; Ebeler, S.E. Vine microclimate and norisoprenoid concentration in cabernet sauvignon grapes and wines. Am. J. Enol. Vitic. 2007, 58, 291–301. [Google Scholar]
- Song, J.Q.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for beta-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and Aromatic Sensory Descriptors (Mainly Fresh and Dry Fruit Character) of Spanish Red Wines can be Predicted from their Aroma-Active Chemical Composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef]
- Juan, F.S.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma Chemical Composition of Red Wines from Different Price Categories and Its Relationship to Quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef]
- Black, C.; Francis, L.; Henschke, P.; Capone, D.; Anderson, S.; Day, M.; Holt, H.; Pearson, W.; Herderich, M.; Johnson, D. Aged Riesling and the development of TDN. Wine Vitic. J. 2012, 27, 20–26. [Google Scholar]
- Janusz, A.; Capone, D.L.; Puglisi, C.J.; Perkins, M.V.; Elsey, G.M.; Sefton, M.A. (E)-1-(2,3,6-trimethylphenyl)buta-1,3-diene: A potent grape-derived odorant in wine. J. Agric. Food Chem. 2003, 51, 7759–7763. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Capone, D.L.; Elsey, G.M.; Perkins, M.V.; Sefton, M.A. Quantitative analysis, occurrence, and stability of (E)-1-(2,3,6-Trimethylphenyl)buta-1,3-diene in wine. J. Agric. Food Chem. 2005, 53, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, E.; Dubourdieu, D.; Darriet, P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chem. 2007, 103, 536–545. [Google Scholar] [CrossRef]
- Tosi, E.; Fedrizzi, B.; Azzolini, M.; Finato, F.; Simonato, B.; Zapparoli, G. Effects of noble rot on must composition and aroma profile of Amarone wine produced by the traditional grape withering protocol. Food Chem. 2012, 130, 370–375. [Google Scholar] [CrossRef]
- Furdikova, K.; Machynakova, A.; Drtilova, T.; Klempova, T.; Durcanska, K.; Spanik, I. Comparison of volatiles in noble-rotten and healthy grape berries of Tokaj. LWT Food Sci. Technol. 2019, 105, 37–47. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Eric, F.; Darriet, P.; Dubourdieu, D. Identification of volatile compounds responsible for prune aroma in prematurely aged red wines. J. Agric. Food Chem. 2008, 56, 5285–5290. [Google Scholar] [CrossRef]
- Allamy, L.; Darriet, P.; Pons, A. Molecular interpretation of dried-fruit aromas in Merlot and Cabernet Sauvignon musts and young wines: Impact of over-ripening. Food Chem. 2018, 266, 245–253. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. I. Effects of harvest date. Food Res. Int. 2015, 76, 540–549. [Google Scholar] [CrossRef]
- Javed, H.U.; Wang, D.; Shi, Y.; Wu, G.F.; Xie, H.; Pan, Y.Q.; Duan, C.Q. Changes of free-form volatile compounds in pre-treated raisins with different packaging materials during storage. Food Res. Int. 2018, 107, 649–659. [Google Scholar] [CrossRef]
- Pons, A.; Allamy, L.; Lavigne, V.; Dubourdieu, D.; Darriet, P. Study of the contribution of massoia lactone to the aroma of Merlot and Cabernet Sauvignon musts and wines. Food Chem. 2017, 232, 229–236. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C. Changes in volatile compounds. In Sweet, Reinforced, and Fortified Wines; Mencarelli, F., Tonutti, P., Eds.; Wiley & Sons: Chichester, UK, 2013; pp. 91–103. [Google Scholar]
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of the aromatic profile in Garnacha Tintorera grapes during raisining and comparison with that of the naturally sweet wine obtained. Food Chem. 2013, 139, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C.; Matarese, F.; Scalabrelli, G.; Boss, P. Functional characterization of terpene synthases of ‘aromatic’ and ‘non-aromatic’ grapevine varieties. In Proceedings of the 10th International Conference on Grapevine Breeding and Genetics, Geneva, NY, USA, 1–5 August 2010; pp. 557–563. [Google Scholar]
- Ruiz, M.J.; Zea, L.; Moyano, L.; Medina, M. Aroma active compounds during the drying of grapes cv. Pedro Ximenez destined to the production of sweet Sherry wine. Eur. Food Res. Technol. 2010, 230, 429–435. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Smith, P.A.; Hranilovic, A.; Bindon, K.A.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on polysaccharide and tannin content and composition. Food Chem. 2018, 244, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bellincontro, A.; De Santis, D.; Botondi, R.; Villa, I.; Mencarelli, F. Different postharvest dehydration rates affect quality characteristics and volatile compounds of Malvasia, Trebbiano and Sangiovese grapes for wine production. J. Sci. Food Agric. 2004, 84, 1791–1800. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Bayonove, C.; Cordonnier, R.; Dubois, P. Study of an aromatic characteristic fraction of cabernet sauvignon grape variety, identification of 2-methoxy-3-isobutyl-pyrazine. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 1975, 281, 75–78. [Google Scholar]
- Lacey, M.J.; Allen, M.S.; Harris, R.L.N.; Brown, W.V. Methoxypyrazines in Sauvignon Blanc Grapes and Wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- De Boubee, D.R.; Van Leeuwen, C.; Dubourdieu, D. Organoleptic impact of 2-methoxy-3-isobutylpyrazine on red Bordeaux and Loire wines. Effect of environmental conditions on concentrations in grapes during ripening. J. Agric. Food Chem. 2000, 48, 4830–4834. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E. Methoxypyrazines in grapes and wines of Vitis vinifera cv. Carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Seasonal and Regional Variation of Green Aroma Compounds in Commercial Vineyards of Vitis vinifera L. Merlot in California. Am. J. Enol. Vitic. 2013, 64, 430–436. [Google Scholar] [CrossRef]
- Falcao, L.D.; de Revel, G.; Perello, M.C.; Moutsiou, A.; Zanus, M.C.; Bordignon-Luiz, M.T. A survey of seasonal temperatures and vineyard altitude influences on 2-methoxy-3-isobutylpyrazine, C-13-norisoprenoids, and the sensory profile of Brazilian Cabernet Sauvignon wines. J. Agric. Food Chem. 2007, 55, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Ryona, I.; Pan, B.S.; Intrigliolo, D.S.; Lakso, A.N.; Sacks, G.L. Effects of Cluster Light Exposure on 3-Isobutyl-2-methoxypyrazine Accumulation and Degradation Patterns in Red Wine Grapes (Vitis vinifera L. Cv. Cabernet Franc). J. Agric. Food Chem. 2008, 56, 10838–10846. [Google Scholar] [CrossRef] [PubMed]
- Gregan, S.M.; Jordan, B. Methoxypyrazine Accumulation and O-Methyltransferase Gene Expression in Sauvignon Blanc Grapes: The Role of Leaf Removal, Light Exposure, and Berry Development. J. Agric. Food Chem. 2016, 64, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Helwi, P.; Habran, A.; Guillaumie, S.; Thibon, C.; Hilbert, G.; Gomes, E.; Delrot, S.; Darriet, P.; van Leeuwen, C. Vine Nitrogen Status Does Not Have a Direct Impact on 2-Methoxy-3-isobutylpyrazine in Grape Berries and Wines. J. Agric. Food Chem. 2015, 63, 9789–9802. [Google Scholar] [CrossRef]
- Koegel, S.; Botezatu, A.; Hoffmann, C.; Pickering, G. Methoxypyrazine composition of Coccinellidae-tainted Riesling and Pinot noir wine from Germany. J. Sci. Food Agric. 2015, 95, 509–514. [Google Scholar] [CrossRef]
- Gracia-Moreno, E. Nuevos Métodos Analíticos para la Determinación Selectiva de Pirazinas, Ácidos y Otros Compuestos de Interés Aromático Presentes en Cantidades Traza. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2015. [Google Scholar]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of some volatile constituents of bell peppers. J. Agric. Food Chem. 1969, 17, 1322–1327. [Google Scholar] [CrossRef]
- Pickering, G.J.; Karthik, A.; Inglis, D.; Sears, M.; Ker, K. Detection thresholds for 2-isopropyl-3-methoxypyrazine in Concord and Niagara grape juice. J. Food Sci. 2008, 73, S262–S266. [Google Scholar] [CrossRef]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of Methoxypyrazines to Sauvignon Blanc Wine Aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine Analysis and Influence of Viticultural and Enological Procedures on their Levels in Grapes, Musts, and Wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Baumes, R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J. Agric. Food Chem. 2000, 48, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Faria, M.; Sa, F.; Barros, F.; Araujo, I.A. C-6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Martinez, M.C.; Santiago, J.L.; Simal-Gandara, J. Floral, spicy and herbaceous active odorants in Gran Negro grapes from shoulders and tips into the cluster, and comparison with Brancellao and Mouraton varieties. Food Chem. 2012, 135, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Li, X.L.; Yue, T.X.; Qin, M.Y.; Fang, Y.L.; Zhang, Z.W.; Xi, Z.M. Characteristic free aromatic components of nine clones of spine grape (Vitis davidii Foex) from Zhongfang County (China). Food Res. Int. 2013, 54, 1795–1800. [Google Scholar] [CrossRef]
- Feng, H.; Yuan, F.; Skinkis, P.A.; Qian, M.C. Influence of cluster zone leaf removal on Pinot noir grape chemical and volatile composition. Food Chem. 2015, 173, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Schreiner, R.P.; Qian, M.C. Soil Nitrogen, Phosphorus, and Potassium Alter β-Damascenone and Other Volatiles in Pinot noir Berries. Am. J. Enol. Vitic. 2018, 69, 157–166. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Hansen, M.; Cantwell, M.I.; Buttery, R.G.; Stern, D.J.; Ling, L.C. Broccoli Storage under Low-Oxygen Atmosphere: Identification of Higher Boiling Volatiles. J. Agric. Food Chem. 1992, 40, 850–852. [Google Scholar] [CrossRef]
- Teranishi, R.; Buttery, R.G.; Guadagni, D.G. Odor quality and chemical structure in fruit and vegetable flavors. Ann. N. Y. Acad. Sci. 1974, 237, 209–216. [Google Scholar] [CrossRef]
- Preston, L.D.; Block, D.E.; Heymann, H.; Soleas, G.; Noble, A.C.; Ebeler, S.E. Defining vegetal aromas in Cabernet Sauvignon using sensory and chemical evaluations. Am. J. Enol. Vitic. 2008, 59, 137–145. [Google Scholar]
- Escudero, A.; Campo, E.; Farina, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Jeffery, D.W.; Sefton, M.A. Vineyard and fermentation studies to elucidate the origin of 1,8-cineole in Australian red wine. J. Agric. Food Chem. 2012, 60, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Poitou, X.; Thibon, C.; Darriet, P. 1,8-Cineole in French Red Wines: Evidence for a Contribution Related to Its Various Origins. J. Agric. Food Chem. 2017, 65, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W.; Francis, I.L. Terroir or terpenoid transformation: The origin of 1,8-cineole (eucalyptol) in wine. In Proceedings of the 10th Wartburg Symposium on Flavor Chemistry and biology, Eisenach, Germany, 16–19 April 2013; pp. 130–136. [Google Scholar]
- Farina, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.L.; Bomben, J.L.; McFadden, W.H. Volatiles from Grapes. Vitis Vinifera (Linn.) Cultivar Grenache. J. Agric. Food Chem. 1967, 15, 378–380. [Google Scholar] [CrossRef]
- Gomez, E.; Martinez, A.; Laencina, J. Changes in volatile compounds during maturation of some grape varieties. J. Sci. Food Agric. 1995, 67, 229–233. [Google Scholar] [CrossRef]
- Ferrandino, A.; Carlomagno, A.; Baldassarre, S.; Schubert, A. Varietal and pre-fermentative volatiles during ripening of Vitis vinifera cv Nebbiolo berries from three growing areas. Food Chem. 2012, 135, 2340–2349. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Perestrelo, R.; Caldeira, M.; Camara, J.S. Solid phase microextraction as a reliable alternative to conventional extraction techniques to evaluate the pattern of hydrolytically released components in Vitis vinifera L. grapes. Talanta 2012, 95, 1–11. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef]
- Wood, C.; Siebert, T.E.; Parker, M.; Capone, D.L.; Elsey, G.M.; Pollnitz, A.P.; Eggers, M.; Meier, M.; Vossing, T.; Widder, S.; et al. From wine to pepper: Rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J. Agric. Food Chem. 2008, 56, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Barlow, S.; Krstic, M.; Herderich, M.; Fuentes, S.; Howell, K. Within-Vineyard, Within-Vine, and Within-Bunch Variability of the Rotundone Concentration in Berries of Vitis vinifera L. cv. Shiraz. J. Agric. Food Chem. 2015, 63, 4276–4283. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Descôtes, J.; Levassseur-Garcia, C.; Debord, C.; Denux, J.-P.; Dufourcq, T. A 2-year multisite study of viticultural and environmental factors affecting rotundone concentration in Duras red wine. OENO One 2019, 53, 457–470. [Google Scholar] [CrossRef]
- Huang, A.C.; Burrett, S.; Sefton, M.A.; Taylor, D.K. Production of the pepper aroma compound, (-)-rotundone, by aerial oxidation of alpha-guaiene. J. Agric. Food Chem. 2014, 62, 10809–10815. [Google Scholar] [CrossRef] [PubMed]
- Cullere, L.; Ontanon, I.; Escudero, A.; Ferreira, V. Straightforward strategy for quantifying rotundone in wine at ngL(-1) level using solid-phase extraction and gas chromatography-quadrupole mass spectrometry. Occurrence in different varieties of spicy wines. Food Chem. 2016, 206, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Descôtes, J.; Serrano, E.; Li Calzi, M.; Dagan, L.; Schneider, R. Can a certain concentration of rotundone be undesirable in Duras red wine? A study to estimate a consumer rejection threshold for the pepper aroma compound. Aust. J. Grape Wine Res. 2018, 24, 88–95. [Google Scholar] [CrossRef]
- Roberts, D.D.; Mordehai, A.P.; Acree, T.E. Detection and Partial Characterization of 8 Beta-Damascenone Precursors in Apples (Malus-Domestica Borkh, Cv Empire). J. Agric. Food Chem. 1994, 42, 345–349. [Google Scholar] [CrossRef]
- Picard, M.; de Revel, G.; Marchand, S. First identification of three p-menthane lactones and their potential precursor, menthofuran, in red wines. Food Chem. 2017, 217, 294–302. [Google Scholar] [CrossRef]
- Carlomagno, A.; Schubert, A.; Ferrandino, A. Screening and evolution of volatile compounds during ripening of ‘Nebbiolo’, ‘Dolcetto’ and ‘Barbera’ (Vitis vinifera L.) neutral grapes by SBSE-GC/MS. Eur. Food Res. Technol. 2016, 242, 1221–1233. [Google Scholar] [CrossRef]
- Garcia-Carpintero, E.G.; Sanchez-Palomo, E.; Gallego, M.A.G.; Gonzalez-Vinas, M.A. Free and bound volatile compounds as markers of aromatic typicalness of Moravia Dulce, Rojal and Tortosi red wines. Food Chem. 2012, 131, 90–98. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta 2008, 621, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Botelho, G.; Mendes-Faia, A.; Climaco, M.C. Characterisation of free and glycosidically bound odourant compounds of Aragonez clonal musts by GC-O. Anal. Chim. Acta 2010, 657, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.J.; Freitas, A.M.C.; Laureano, O.; Di Stefano, R. Glycosidic aroma compounds of some Portuguese grape cultivars. J. Sci. Food Agric. 2006, 86, 922–931. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv. Melon B. as precursors of odorants in Muscadet wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Lopez, R.; Ezpeleta, E.; Sanchez, I.; Cacho, J.; Ferreira, V. Analysis of the aroma intensities of volatile compounds released from mild acid hydrolysates of odourless precursors extracted from Tempranillo and Grenache grapes using gas chromatography-olfactometry. Food Chem. 2004, 88, 95–103. [Google Scholar] [CrossRef]
- Oliveira, I.; Ferreira, V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef]
- Wirth, J.; Guo, W.F.; Baumes, R.; Gunata, Z. Volatile compounds released by enzymatic hydrolysis of glycoconjugates of leaves and grape berries from Vitis vinifera Muscat of Alexandria and Shiraz cultivars. J. Agric. Food Chem. 2001, 49, 2917–2923. [Google Scholar] [CrossRef]
- Torchio, F.; Giacosa, S.; Vilanova, M.; Segade, S.R.; Gerbi, V.; Giordano, M.; Rolle, L. Use of response surface methodology for the assessment of changes in the volatile composition of Moscato bianco (Vitis vinifera L.) grape berries during ripening. Food Chem. 2016, 212, 576–584. [Google Scholar] [CrossRef]
- Crespo, J.; Rigou, P.; Romero, V.; Garcia, M.; Arroyo, T.; Cabellos, J.M. Effect of seasonal climate fluctuations on the evolution of glycoconjugates during the ripening period of grapevine cv. Muscat a petits grains blancs berries. J. Sci. Food Agric. 2018, 98, 1803–1812. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Study of the terpene profile at harvest and during berry development of Vitis vinifera L. aromatic varieties Aleatico, Brachetto, Malvasia di Candia aromatica and Moscato bianco. J. Sci. Food Agric. 2017, 97, 2898–2907. [Google Scholar] [CrossRef]
- Sefton, M.A.; Francis, I.L.; Williams, P.J. The Volatile Composition of Chardonnay Juices—A Study by Flavor Precursor Analysis. Am. J. Enol. Vitic. 1993, 44, 359–370. [Google Scholar]
- Picard, M.; Lytra, G.; Tempere, S.; Barbe, J.C.; de Revel, G.; Marchand, S. Identification of Piperitone as an Aroma Compound Contributing to the Positive Mint Nuances Perceived in Aged Red Bordeaux Wines. J. Agric. Food Chem. 2016, 64, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, S.; Asproudi, A.; Cabello, F.; Borsa, D. Aromatic characterization and enological potential of 21 minor varieties (Vitis vinifera L.). Eur. Food Res. Technol. 2011, 233, 473–481. [Google Scholar] [CrossRef]
- Garcia-Carpintero, E.G.; Sanchez-Palomo, E.; Gallego, M.A.G.; Gonzalez-Vinas, M.A. Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 2011, 44, 1549–1560. [Google Scholar] [CrossRef]
- Garcia-Carpintero, E.G.; Sanchez-Palomo, E.; Gonzalez-Vinas, M.A. Aroma characterization of red wines from cv. Bobal grape variety grown in La Mancha region. Food Res. Int. 2011, 44, 61–70. [Google Scholar] [CrossRef]
- Gracia-Moreno, E.; Lopez, R.; Ferreira, V. Determination of 2-, 3-, 4-methylpentanoic and cyclohexanecarboxylic acids in wine: Development of a selective method based on solid phase extraction and gas chromatography-negative chemical ionization mass spectrometry and its application to different wines and alcoholic beverages. J. Chromatogr. A 2015, 1381, 210–218. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef]
- Bowles, D.; Isayenkova, J.; Lim, E.-K.; Poppenberger, B. Glycosyltransferases: Managers of small molecules. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef]
- Jones, P.; Vogt, T. Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 2001, 213, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Hartl, K.; McGraphery, K.; Hoffmann, T.; Schwab, W. Attractive but Toxic: Emerging Roles of Glycosidically Bound Volatiles and Glycosyltransferases Involved in Their Formation. Mol. Plant 2018, 11, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Bonisch, F.; Frotscher, J.; Stanitzek, S.; Ruhl, E.; Wust, M.; Bitz, O.; Schwab, W. A UDP-Glucose: Monoterpenol Glucosyltransferase Adds to the Chemical Diversity of the Grapevine Metabolome. Plant Physiol. 2014, 165, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.K.; Zweigenbaum, J.; Ebeler, S.E. Profiling monoterpenol glycoconjugation in Vitis vinifera L. cv. Muscat of Alexandria using a novel putative compound database approach, high resolution mass spectrometry and collision induced dissociation fragmentation analysis. Anal. Chim. Acta 2015, 887, 138–147. [Google Scholar] [CrossRef]
- Godshaw, J.; Hjelmeland, A.K.; Zweigenbaum, J.; Ebeler, S.E. Changes in glycosylation patterns of monoterpenes during grape berry maturation in six cultivars of Vitis vinifera. Food Chem. 2019, 297. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The Aroma of Grapes—Localization and Evolution of Free and Bound Fractions of Some Grape Aroma Components Cv Muscat During 1st Development And Maturation. J. Sci. Food Agric. 1985, 36, 857–862. [Google Scholar] [CrossRef]
- Razungles, A.; Gunata, Z.; Pinatel, S.; Baumes, R.; Bayonove, C. Quantitative studies on terpenes, norisoprenoides and their precursors in several varieties of grapes. Sci. Aliments 1993, 13, 59–72. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Genisheva, Z.; Oliveira, J.M. Monoterpenic Characterization of White Cultivars from Vinhos Verdes Appellation of Origin (North Portugal). J. Inst. Brew. 2009, 115, 308–317. [Google Scholar] [CrossRef]
- Lamorte, S.A.; Gambuti, A.; Genovese, A.; Selicato, S.; Moio, L. Free and glycoconjugated volatiles of V. vinifera grape ‘Falanghina’. Vitis 2008, 47, 241–243. [Google Scholar]
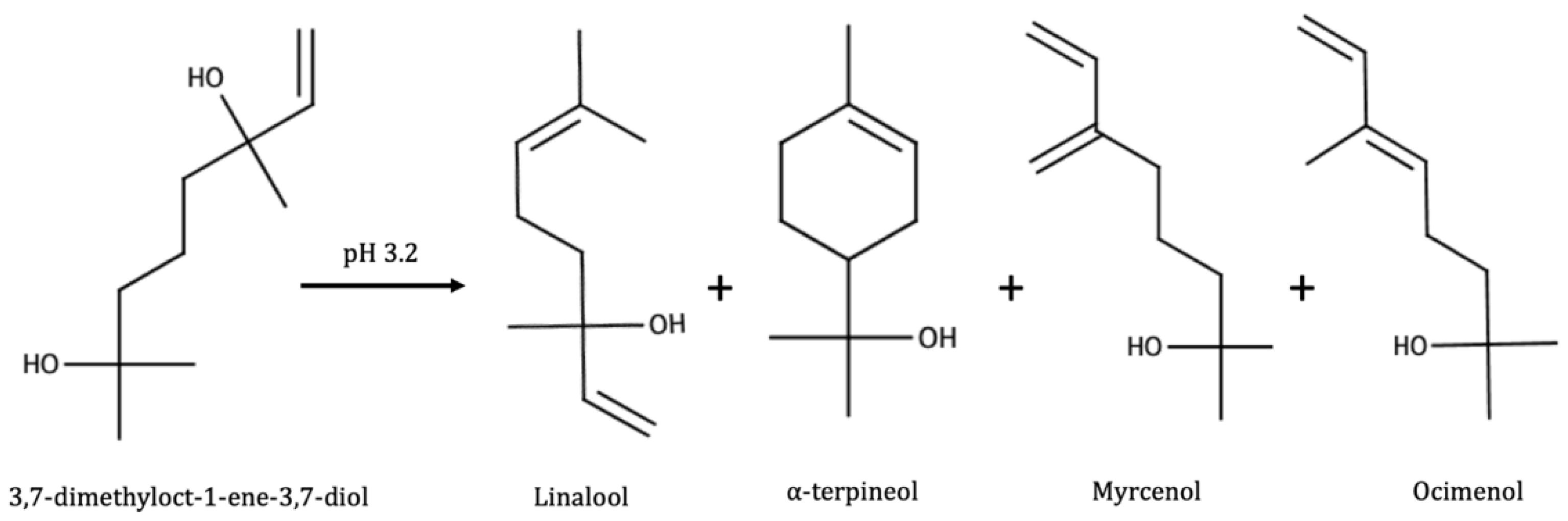
- Winterhalter, P. 1,1,6-Trimethyl-1,2-Dihydronaphthalene (Tdn) Formation in Wine. 1. Studies on the Hydrolysis of 2,6,10,10-Tetramethyl-1-Oxaspiro [4.5]Dec-6-Ene-2,8-Diol Rationalizing the Origin of Tdn and Related C-13 Norisoprenoids in Riesling Wine. J. Agric. Food Chem. 1991, 39, 1825–1829. [Google Scholar] [CrossRef]
- Salinas, M.R.; De La Hoz, K.S.; Zalacain, A.; Lara, J.F.; Garde-Cerdán, T. Analysis of red grape glycosidic aroma precursors by glycosyl glucose quantification. Talanta 2012, 89, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Qian, M.C. Aroma Potential in Early- and Late-Maturity Pinot noir Grapes Evaluated by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2016, 64, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N.; Lavigne, V. Synthesis of Volatile Phenols by Saccharomyces-Cerevisiae in Wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Saison, D.; Delvaux, F.; Delvaux, F.R. Decrease of 4-Vinylguaiacol during Beer Aging and Formation of Apocynol and Vanillin in Beer. J. Agric. Food Chem. 2008, 56, 11983–11988. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, S.J.; Lee, H.J.; Moon, J.H. Two novel glycosyl cinnamic and benzoic acids from Korean black raspberry (Rubus coreanus) wine. Food Sci. Biotechnol. 2014, 23, 1081–1085. [Google Scholar] [CrossRef][Green Version]
- Sasaki, K.; Takase, H.; Tanzawa, F.; Kobayashi, H.; Saito, H.; Matsuo, H.; Takata, R. Identification of Furaneol Glucopyranoside, the Precursor of Strawberry-like Aroma, Furaneol, in Muscat Bailey A. Am. J. Enol. Vitic. 2015, 66, 91–94. [Google Scholar] [CrossRef]
- Sasaki, K.; Takase, H.; Kobayashi, H.; Matsuo, H.; Takata, R. Molecular cloning and characterization of UDP-glucose: Furaneol glucosyltransferase gene from grapevine cultivar Muscat Bailey A (Vitis labrusca × V. vinifera). J. Exp. Bot. 2015, 66, 6167–6174. [Google Scholar] [CrossRef]
- Strauss, C.R.; Wilson, B.; Williams, P.J. Novel Monoterpene Diols and Diol Glycosides in Vitis-Vinifera Grapes. J. Agric. Food Chem. 1988, 36, 569–573. [Google Scholar] [CrossRef]
- Strauss, C.R.; Dimitriadis, E.; Wilson, B.; Williams, P.J. Studies on the Hydrolysis of 2 Megastigma-3,6,9-Triols Rationalizing the Origins of Some Volatile C-13 Norisoprenoids of Vitis-Vinifera Grapes. J. Agric. Food Chem. 1986, 34, 145–149. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Massywestropp, R.A.; Williams, P.J. Norisoprenoids in Vitis-Vinifera White Wine Grapes and the Identification of A Precursor of Damascenone in These Fruits. Aust. J. Chem. 1989, 42, 2071–2084. [Google Scholar] [CrossRef]
- Puglisi, C.J.; Elsey, G.M.; Prager, R.H.; Skouroumounis, G.K.; Sefton, M.A. Identification of a precursor to naturally occurring beta-damascenone. Tetrahedron Lett. 2001, 42, 6937–6939. [Google Scholar] [CrossRef]
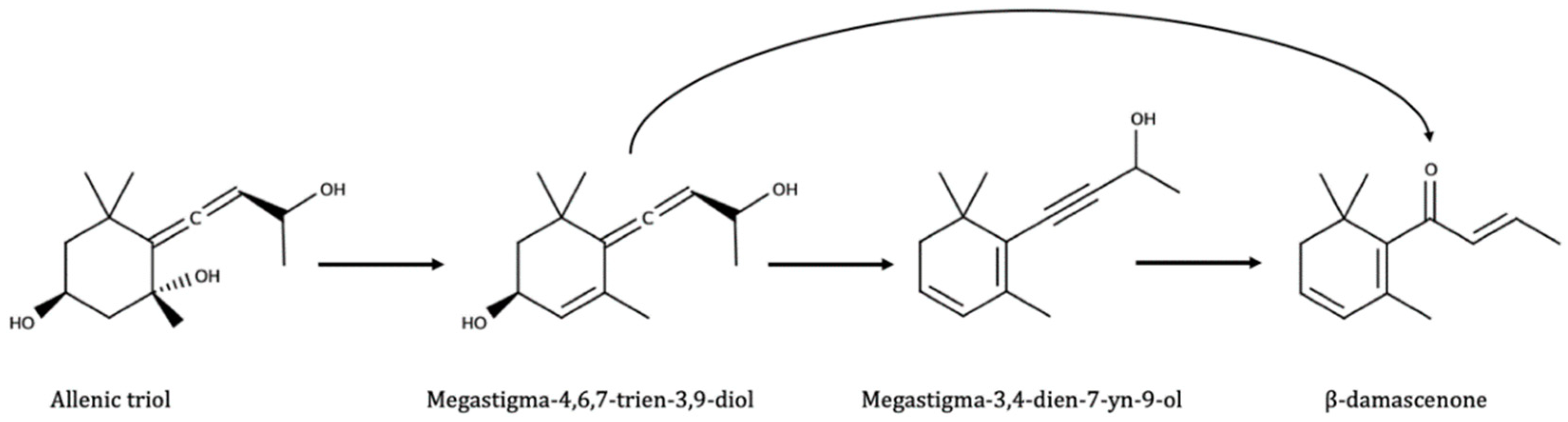
- Puglisi, C.J.; Daniel, M.A.; Capone, D.L.; Elsey, G.M.; Prager, R.H.; Sefton, M.A. Precursors to damascenone: Synthesis and hydrolysis of isomeric 3,9-dihydroxymegastigma-4,6,7-trienes. J. Agric. Food Chem. 2005, 53, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.A.; Puglisi, C.J.; Capone, D.L.; Elsey, G.M.; Sefton, M.A. Rationalizing the formation of damascenone: Synthesis and hydrolysis of damascenone precursors and their analogues, in both aglycone and glycoconjugate forms. J. Agric. Food Chem. 2008, 56, 9183–9189. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, N.D.R.; Capone, D.L.; Ugliano, M.; Taylor, D.K.; Skouroumounis, G.K.; Sefton, M.A.; Elsey, G.M. Formation of Damascenone under both Commercial and Model Fermentation Conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Wilkinson, K.L.; Elsey, G.A.; Raunkjaer, M.; Sefton, M.A. Identification of natural oak lactone precursors in extracts of American and french oak woods by liquid chromatography-tandem mass Spectrometry. J. Agric. Food Chem. 2007, 55, 9195–9201. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Prida, A.; Hayasaka, Y. Role of Glycoconjugates of 3-Methyl-4-hydroxyoctanoic Acid in the Evolution of Oak Lactone in Wine during Oak Maturation. J. Agric. Food Chem. 2013, 61, 4411–4416. [Google Scholar] [CrossRef]
- Gracia-Moreno, E.; Lopez, R.; Ferreira, V. Quantitative determination of five hydroxy acids, precursors of relevant wine aroma compounds in wine and other alcoholic beverages. Anal. Bioanal. Chem. 2015, 407, 7925–7934. [Google Scholar] [CrossRef]
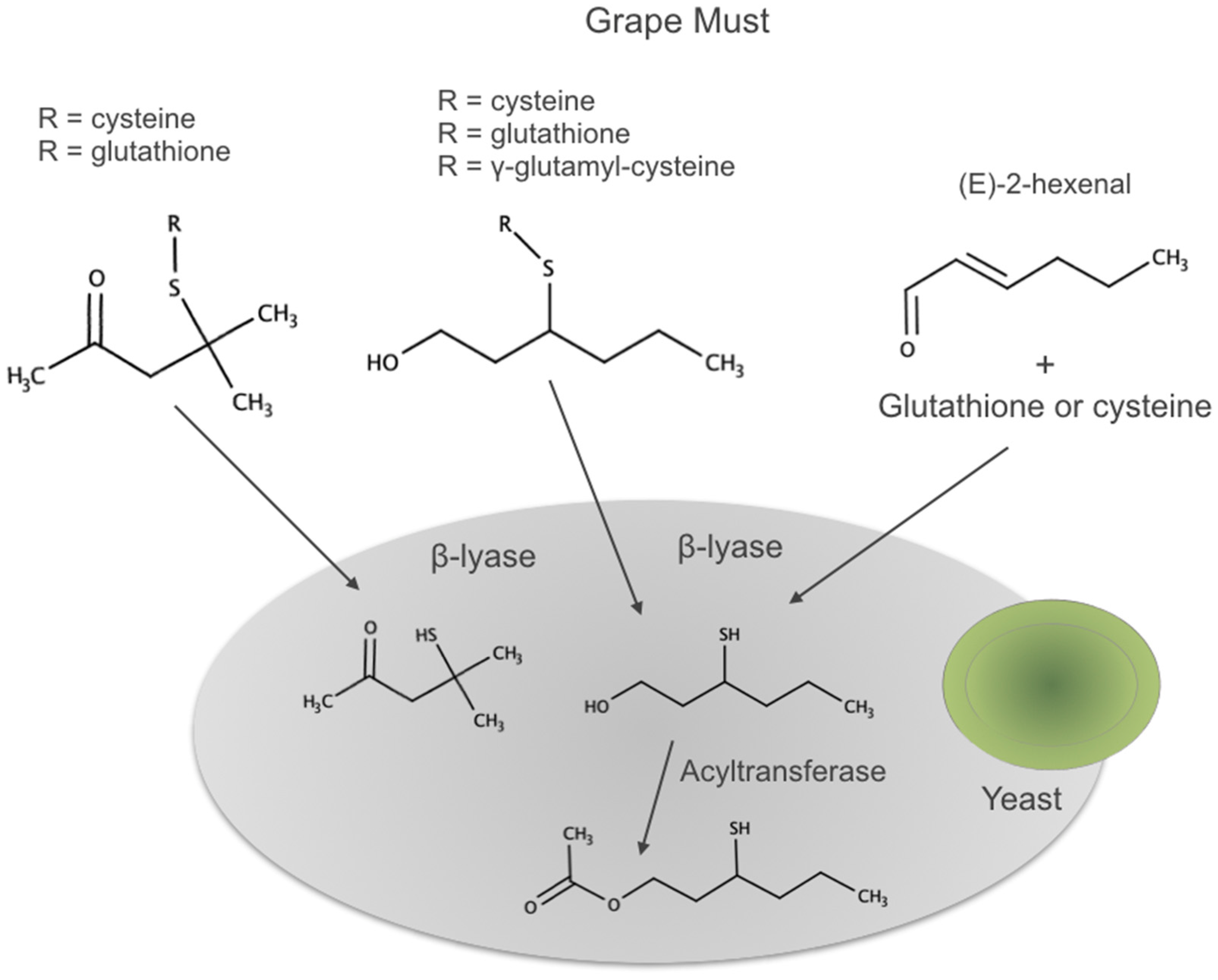
- Pena-Gallego, A.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. S-Cysteinylated and S-glutathionylated thiol precursors in grapes. A review. Food Chem. 2012, 131, 1–13. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Tominaga, T.; Murat, M.L.; Dubourdieu, D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv. Sauvignon Blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Zapata, J.; Cacho, J.; Ferreira, V. Analysis, Occurrence, and Potential Sensory Significance of Five Polyfunctional Mercaptans in White Wines. J. Agric. Food Chem. 2010, 58, 10184–10194. [Google Scholar] [CrossRef] [PubMed]
- Cerreti, M.; Esti, M.; Benucci, I.; Liburdi, K.; de Simone, C.; Ferranti, P. Evolution of S-cysteinylated and S-glutathionylated thiol precursors during grape ripening of Vitis vinifera L. cvs Grechetto, Malvasia del Lazio and Sauvignon Blanc. Aust. J. Grape Wine Res. 2015, 21, 411–416. [Google Scholar] [CrossRef]
- Thibon, C.; Boecker, C.; Shinkaruk, S.; Moine, V.; Darriet, P.; Dubourdieu, D. Identification of S-3-(hexanal)-glutathione and its bisulfite adduct in grape juice from Vitis vinifera L. cv. Sauvignon blanc as new potential precursors of 3SH. Food Chem. 2016, 199, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Peyrot des Gachons, C.; Dubourdieu, D. A new type of flavor precursors in Vitis vinifera L. cv. Sauvignon Blanc: S-cysteine conjugates. J. Agric. Food Chem. 1998, 46, 5215–5219. [Google Scholar] [CrossRef]
- Fedrizzi, B.; Pardon, K.H.; Sefton, M.A.; Elsey, G.M.; Jeffery, D.W. First Identification of 4-S-Glutathionyl-4-methylpentan-2-one, a Potential Precursor of 4-Mercapto-4-methylpentan-2-one, in Sauvignon Blanc Juice. J. Agric. Food Chem. 2009, 57, 991–995. [Google Scholar] [CrossRef]
- Subileau, M.; Schneider, R.; Salmon, J.-M.; Degryse, E. New insights on 3-mercaptohexanol (3MH) biogenesis in sauvignon Blanc wines: Cys-3MH and (E)-Hexen-2-al are not the major precursors. J. Agric. Food Chem. 2008, 56, 9230–9235. [Google Scholar] [CrossRef]
- Grant-Preece, P.A.; Pardon, K.H.; Capone, D.L.; Cordente, A.G.; Sefton, M.A.; Jeffery, D.W.; Elsey, G.M. Synthesis of Wine Thiol Conjugates and Labeled Analogues: Fermentation of the Glutathione Conjugate of 3-Mercaptohexan-1-ol Yields the Corresponding Cysteine Conjugate and Free Thiol. J. Agric. Food Chem. 2010, 58, 1383–1389. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Roland, A.; Rémond, E.; Delpech, S.; Schneider, R.; Cavelier, F. First identification and quantification of S-3-(hexan-1-ol)-γ-glutamyl-cysteine in grape must as a potential thiol precursor, using UPLC-MS/MS analysis and stable isotope dilution assay. Food Chem. 2017, 237, 877–886. [Google Scholar] [CrossRef]
- Concejero, B.; Pena-Gallego, A.; Fernandez-Zurbano, P.; Hernandez-Orte, P.; Ferreira, V. Direct accurate analysis of cysteinylated and glutathionylated precursors of 4-mercapto-4-methyl-2-pentanone and 3-mercaptohexan-1-ol in must by ultrahigh performance liquid chromatography coupled to mass spectrometry. Anal. Chim. Acta 2014, 812, 250–257. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Charrier, F.; Cavelier, F.; Rossignol, M.; Razungles, A. Distribution of varietal thiol precursors in the skin and the pulp of Melon B. and Sauvignon Blanc grapes. Food Chem. 2011, 125, 139–144. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsuyama, S.; Takase, H.; Sasaki, K.; Suzuki, S.; Takata, R.; Saito, H. Impact of Harvest Timing on the Concentration of 3-Mercaptohexan-1-ol Precursors in Vitis vinifera Berries. Am. J. Enol. Vitic. 2012, 63, 544–548. [Google Scholar] [CrossRef]
- Wang, L.; Harada, J.; Endo, Y.; Hisamoto, M.; Saito, F.; Okuda, T. Diurnal Changes in Amino Acid Concentrations in Riesling and Chardonnay Grape Juices and a Possible Role of Sunlight. Am. J. Enol. Vitic. 2014, 65, 435–442. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem. 2015, 183, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P.A. Influence of Grape-Harvesting Steps on Varietal Thiol Aromas in Sauvignon blanc Wines. J. Agric. Food Chem. 2011, 59, 10641–10650. [Google Scholar] [CrossRef]
- Maggu, M.; Winz, R.; Kilmartin, P.A.; Trought, M.C.T.; Nicolau, L. Effect of skin contact and pressure on the composition of Sauvignon Blanc must. J. Agric. Food Chem. 2007, 55, 10281–10288. [Google Scholar] [CrossRef]
- Capone, D.L.; Black, C.A.; Jeffery, D.W. Effects on 3-Mercaptohexan-1-ol Precursor Concentrations from Prolonged Storage of Sauvignon Blanc Grapes Prior to Crushing and Pressing. J. Agric. Food Chem. 2012, 60, 3515–3523. [Google Scholar] [CrossRef]
- Larcher, R.; Nicolini, G.; Tonidandel, L.; Villegas, T.R.; Malacarne, M.; Fedrizzi, B. Influence of oxygen availability during skin-contact maceration on the formation of precursors of 3-mercaptohexan-1-ol in Muller-Thurgau and Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2013, 19, 342–348. [Google Scholar] [CrossRef]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Application of a Modified Method for 3-Mercaptohexan-1-ol Determination To Investigate the Relationship between Free Thiol and Related Conjugates in Grape Juice and Wine. J. Agric. Food Chem. 2011, 59, 4649–4658. [Google Scholar] [CrossRef]
- Darriet, P.; Tominaga, T.; Demole, E.; Dubourdieu, D. Evidence of the Presence of a 4-Mercapto-4-Methylpentan-2-One Precursor in Vitis-Vinifera Sauvignon Blanc Grape Variety. C. R. Acad. Sci. III-Vie 1993, 316, 1332–1335. [Google Scholar]
- Segurel, M.A.; Razungles, A.J.; Riou, C.; Salles, M.; Baumes, R.L. Contribution of dimethyl sulfide to the aroma of Syrah and Grenache Noir wines and estimation of its potential in grapes of these varieties. J. Agric. Food Chem. 2004, 52, 7084–7093. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; Zhang, S.; Marchand, S.; de Revel, G.; Barbe, J.-C. Olfactory Impact of Dimethyl Sulfide on Red Wine Fruity Esters Aroma Expression in Model Solution. OENO One 2014, 48, 75–85. [Google Scholar] [CrossRef]
- Segurel, M.A.; Razungles, A.J.; Riou, C.; Trigueiro, M.G.L.; Baumes, R.L. Ability of possible DMS precursors to release DMS during wine aging and in the conditions of heat-alkaline treatment. J. Agric. Food Chem. 2005, 53, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Segurel, M.; Dagan, L.; Sommerer, N.; Marlin, T.; Baumes, R. Identification of S-methylmethionine in Petit Manseng grapes as dimethyl sulphide precursor in wine. Anal. Chim. Acta 2008, 621, 24–29. [Google Scholar] [CrossRef]
- Dupre, N.D.R.; Schneider, R.; Payan, J.C.; Salancon, E.; Razungles, A. Effects of Vine Water Status on Dimethyl Sulfur Potential, Ammonium, and Amino Acid Contents in Grenache Noir Grapes (Vitis vinifera). J. Agric. Food Chem. 2014, 62, 2760–2766. [Google Scholar] [CrossRef]
- Thibon, C.; Dubourdieu, D.; Darriet, P.; Tominaga, T. Impact of noble rot on the aroma precursor of 3-sulfanylhexanol content in Vitis vinifera L. cv Sauvignon blanc and Semillon grape juice. Food Chem. 2009, 114, 1359–1364. [Google Scholar] [CrossRef]
- Thibon, C.; Shinkaruk, S.; Jourdes, M.; Bennetau, B.; Dubourdieu, D.; Tominaga, T. Aromatic potential of botrytized white wine grapes: Identification and quantification of new cysteine-S-conjugate flavor precursors. Anal. Chim. Acta 2010, 660, 190–196. [Google Scholar] [CrossRef]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas Chromatography-Mass Spectrometry Method Optimized Using Response Surface Modeling for the Quantitation of Fungal Off-Flavors in Grapes and Wine. J. Agric. Food Chem. 2015, 63, 2877–2885. [Google Scholar] [CrossRef]
- Morales-Valle, H.; Silva, L.C.; Paterson, R.R.M.; Venancio, A.; Lima, N. Effects of the origins of Botrytis cinerea on earthy aromas from grape broth media further inoculated with Penicillium expansum. Food Microbiol. 2011, 28, 1048–1053. [Google Scholar] [CrossRef]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, J.H.; Gibberd, M.R. Smoke-derived taint in wine: Effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Dungey, K.A.; Baldock, G.A.; Kennison, K.R.; Wilkinson, K.L. Identification of a beta-d-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal. Chim. Acta 2010, 660, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke-derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef]
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography-tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Ristic, R.; van der Hulst, L.; Capone, D.L.; Wilkinson, K.L. Impact of Bottle Aging on Smoke-Tainted Wines from Different Grape Cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef]

| Compound | Structure | Grape | Odor Description | Threshold | Range of Occurrence in Grapes |
|---|---|---|---|---|---|
| Linalool | 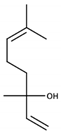 | Muscat | Hyacinth, Muscat wine | 6 μg/L [48] | 0.06–1.5 mg/L [28] |
| Geraniol | 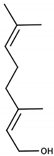 | Muscat | Citrus, rose | 40 μg/L [49] | 0.09–1.1 mg/L [28] |
| (Z)-Rose oxide | 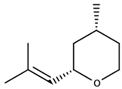 | Traminer | Rose, litchi | 0.5 (l form) or 50 μg/L (d form) [34] | 7–29 μg/L [35] |
| o-Aminoacetophenone | 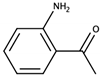 | Concord | Sweet, caramel | 0.2 μg/L [50] | 10–20 μg/L [46] |
| Methyl anthranilate | 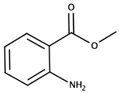 | Concord | Orangine, sweet | 3 μg/L [51] | 0.8 mg/kg [39] 0.5–6 mg/kg [52] |
| Compound | Structure | Odor Descriptor | Threshold in Wine | Range of Occurrence in Wine |
|---|---|---|---|---|
| β-Damascenone | 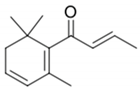 | Plum, cooked apple | 50 ng/L [32] | n.d. to 10.5 μg/L [71] |
| β-Ionone | 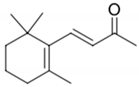 | Violet, woody | 90 ng/L [72] | n.d. to 1.2 μg/L [71] |
| TDN | 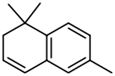 | Kerosene-like | 2 μg/L [73] | n.d. to 255 μg/L [74] |
| TPB | 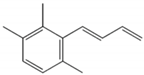 | Green, cut-grass | 40 ng/L [75] | n.d. to 233 ng/L [76] |
| Compound | Structure | Odor Descriptor | Odor Threshold | Range of Occurrence in Grape Juice |
|---|---|---|---|---|
| 3-Isobutyl-2-methoxypyrazine | 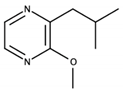 | Bell pepper, earthy | 2 ng/L (in water) [103]; 15 ng/L (in wine) [94] | n.d. to 79 ng/L [93] |
| 3-Isopropyl-2-methoxypyrazine | 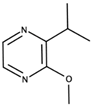 | Green pea, earthy | 0.74–1.11 (hybrid grape juice) [104]; 2 ng/L in wine [105] | n.d. to 6.8 ng/L [93] |
| 3-Secbutyl-2-methoxypyrazine | 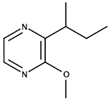 | Bell pepper | 1–2 ng/L (in water) [106] | n.d. to 1.3 ng/L [93] |
| Compound | Structure | Odor Descriptor | Threshold in Water | Ranges of Occurrence in Grape [23,25,57,61,110,111,112] |
|---|---|---|---|---|
| Hexanal | 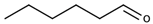 | Herbaceous | 5 μg/L [113] | 8–1300 μg/kg |
| (Z)-3-Hexenal | 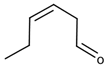 | Grass | 0.25 μg/L [48] | 4–20 μg/kg |
| (E)-2-Hexenal |  | Grass | 17 μg/L [113] | 13–3800 μg/kg |
| (E,E)-2,4-Hexadienal | 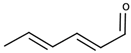 | Grass | 60 μg/L [114] | 50–120 μg/kg |
| (Z)-3-Hexenol | 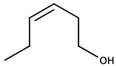 | Grass | 70 μg/L [48] | 4–79 μg/kg |
| (E)-2-Hexenol |  | Green | 400 μg/L [114] | |
| 1-Hexanol | 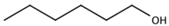 | Green | 2500 μg/L [113] | 45–214 μg/kg |
| E-2-Nonenal |  | Green, fatty | 0.17 μg/L [113] | |
| (E,Z)-2,6-Nonadienal | 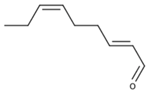 | Cucumber | 0.01 μg/L [115] | 113–482 μg/kg |
| Aroma Molecule | Enzymatic Hydrolysis | Harsh Acid Hydrolysis | Mild/Long Term Acid Hydrolysis |
|---|---|---|---|
| Norisoprenoids | |||
| β-Damascenone | Not found; yes in raisins [23,57] and frozen grapes [136]; not in wines [137]; 0.17–0.5 ppb in frozen grapes [12] | 26 ppb [138]; detected by GCO [139]; 4–28 ppb depending varieties, unclear pulp/skin distribution [140]; 4–20 ppb depending location [140]; levels correlated to total norisoprenoids by enzymatic [141]; 2–4.5 ppb depending varieties [12] | Detected by GCO [142]; maxima (3.3 ppb) after short aging, then steady decrease [14]; steady increase all the aging in fermented samples [143]; maxima 7.1–7.3 ppb after medium aging in unfermented controls [143]; formed soon and stable, maxima 17 ppb [15]; idem, with maxima 7 ppb [66] |
| β-Ionone | Not found; yes in frozen grapes [136]; not in wines [137]; <0.11 ppb in frozen grapes [12] | Generally yes; not found in [12] | Maxima (1.9 ppb) after short aging, stable with time [14]; formed soon, stable for a while, maxima 7.7 ppb [15] |
| TDN | Not found; yes in frozen grapes [136]; not in wines [137]; 1–6 ppb (5–30% of levels found in harsh acid hydrolysis) in frozen grapes [12] | 8 ppb [138]; detected by GCO [139]; 1–35 ppb depending on varieties, unclear pulp/skin distribution [140]; n.d. to 26 ppb depending on place [140]; 8–89 ppb depending on varieties [12] | Linear increase with time, max 140 ppb [143]; idem, max at 61 ppb [15]; idem [66] |
| TPB | Not found; 0.2–3 ppb (2–22% of levels found in harsh acid hydrolysis) in frozen grapes [12] | 3 ppb [138]; 2–23 ppb depending varieties [12] | Continuously formed, maxima 9 ppb [66] |
| Terpenes | |||
| Linalool | Generally present; not found in Portuguese reds [140]; not found in Melon B [141]; not found in Shiraz [144]; found at low levels (less than 7% geraniol 1% total terpenes) [144] | 3% levels found in enzymatic [138]; 10–50% of levels found in enzymatic [12] | Found only in mild acid hydrolysis [141]; maxima after fermentation, sharp decrease in aging [14]; in Grenache, maxima after short aging [143]; formed very soon, sharp decrease [15,66] |
| Geraniol | Always found; up to 10% of total terpenes in Shiraz, 14% in Muscat [144] | No [138]; 3–30% of levels found in enzymatic [12] | Maxima in fermentation, sharp decrease in aging [14,143]; formed very soon, sharp decrease [15,66] |
| (Z)-Rose oxide | 11–29 ppb in Muscat, depending on maturity [145]; unrelated to free form in raisins [23] | 0.04 ppb in Muscat, 0.01 ppb in Grenache; not found in Verdejo, Tempranillo, Chardonnay, Cabernet Sauvignon, or Merlot [12] | |
| Geranic acid | Up to 2–3 ppm [146,147]; also found in raisins [23]; <4 ppb [145]; up to 7.5% total terpenes in Shiraz, 18% in Muscat [144] | Not found [138]; 0.5–50% of levels found in enzymatic [12] | 1.5 ppb in Chardonnay juices [148] |
| Piperitone | Derived from limonene, unknown accumulation pattern [149]; limonene accumulates in the first periods of aging, then slight decrease [66] | ||
| Aroma Molecule | Enzymatic Hydrolysis | Harsh Acid Hydrolysis | Mild/Long Term Acid Hydrolysis |
|---|---|---|---|
| Volatile Phenols | |||
| Guaiacol | Not found [146]: only in Brachetto, not in Aleatico, Malvasia, or Moscato [147]; <2 ppb [125]; up to 60 ppb in Rojal wine [137]; 0–41 ppb [150]; 10–76 ppb depending on vintage [151]; 15–44 ppb depending on vintage [152]; 17 ppb in Shiraz [144]; 0.4–2.3 ppb depending on varieties [12] | Detected by GCO [139]; <0.61 ppb, unrelated to enzymatic levels [12] | Detected by GCO [142]; Steady increase with time, maxima 4.3 ppb [14]; idem, maxima 6.3 ppb [143]; idem, maxima 14 ppb [15] |
| Eugenol | 1–8.3 ppb [146,147]; not found [125]; up to 33 ppb in Rojal wine [137]; present in less than half varieties, up to 16 ppb [150]; 84–216 ppb depending on vintage [151]; 12–20 ppb in Bobal depending on vintage [152]; n.d. to 9.4 ppb depending on variety [140]; 2.7–18 ppb depending on location [140]; 10 ppb in Shiraz [144]; 0.4–7 ppb depending on variety [12] | Detected by GCO [139]; <0.36 ppb, unrelated to enzymatic levels [12] | Steady increase, maxima 1.25 ppb [15] |
| Isoeugenol | Up to 14 ppb in Rojal wine [137]; 7.6–26 ppb depending on vintage [151]; 5–25 ppb depending on vintage [152]; 0.4–4.8 ppb depending on varieties [12] | <0.58 ppb, unrelated to enzymatic levels [12] | Detected by GCO [142] |
| 2,6-Dimethoxyphenol | 3–60 ppb [147]; n.d. to 13 ppb depending on varieties [12] | n.d. to 5.5 ppb depending on varieties [12] | Detected by GCO [142]; steady increase with time, maxima 33 ppb [14]; idem, maxima 142 ppb [15] |
| 4-Vinylguaiacol | 65–357 ppb [147]; <24 ppb [150]; 56–378 ppb depending on vintage [151]; 56–64 ppb depending on vintage in Bobal [152]; 2–114 ppb depending on varieties [140]; 2–178 ppb depending on location [140]; 21 ppb in Shiraz [144]; 39–162 ppb on depending varieties [12] | 40% of enzymatic [138]; detected by GCO [139]; 10–38 ppb depending on varieties, unrelated to enzymatic [12] | A maxima (21 ppb) after short aging, then decrease and steady increase [14]; continuous increase, maxima 5.5 ppm [143]; formed soon and stable, maxima at 1.3 ppm [15] |
| 4-Vinylphenol | 28–266 ppb [150]; 5–222 ppb depending on varieties [140]; 19–310 ppb depending on location [140]; 6 ppb in Shiraz [144]; 121–1739 ppb depending on varieties [12] | 9–21 ppb depending on varieties, unrelated to enzymatic [12] | A maxima after short aging (45 ppb), then decrease and steady increase, maxima 80 ppb [14]; continuous increase, maxima 4.4 ppm [143]; formed very soon, later steady decrease, maxima at 102 ppb [15] |
| Vanillin Derivatives | |||
| Vanillin | 27–42 ppb [147]; 361 ppb in skin of Uva di Troia [125]; 31–61 ppb [137]; <37 ppb [150]; 48–68 ppb depending on vintage [151]; 60–160 ppb depending on vintage in Bobal [152]; 31 ppb in Shiraz [144]; 40 ppb in Muscat [144]; <4.1 ppb [12] | 50% enzymatic [138]; detected by GCO [139]; <1.5 ppb [12] | Detected by GCO [142]; linear increase with time, maxima 45 ppb [14]; idem, maxima 91 ppb [143]; idem, maxima 123 ppb [15] |
| Methyl vanillate | 4–7 ppb [147]; <7 ppb [125]; up to 205 ppb in Rojal wine [137]; <42 ppb [150]; 12–147 ppb depending on vintage [151]; 9–143 depending on vintage in Bobal [152]; 25 ppb in Shiraz [144]; 154 ppb in Muscat [144]; <18 ppb [12] | <3.4 ppb [12] | 6 ppb in Chardonnay juices [148] |
| Ethyl vanillate | Up to 45 ppb in Rojal wine [137]; n.d. to 10 ppb depending on vintage in Bobal [152]; <12 ppb [12] | <3.1 ppb | |
| Acetovanillone | Up to 205 and 260 ppb in Rojal and Tortosí wines [137]; 1–12 ppb depending on vintage [151]; 42 ppb in Muscat, none in Shiraz [144]; 8–34 ppb depending on variety [12] | Detected by GCO [139]; <2.5 ppb, unrelated to enzymatic [12] | Unclear pattern [15]; 5 ppb in Chardonnay juices [148] |
| Cinnamic Acid Derivatives | |||
| Ethyl cinnamate | 7 ppb only in pulp from Uva di Troia [125]; <0.8 ppb [12]; its precursor, cinnamic acid has been found up to 7 ppb in fractions from wine, levels depending on vintage [137,151,152] | 12 ppb [138]; <0.11 ppb [12] | Detected by GCO [142] [15]; steady increase with time in some varietals, maxima 3.3 ppb [14]; maxima 3.3 ppb after short aging [143] |
| Aroma Molecule | Enzymatic Hydrolysis | Harsh Acid Hydrolysis | Mild/Long Term Acid Hydrolysis |
|---|---|---|---|
| Ethyl cyclohexanoate | Its precursor, ethyl cyclohexanoic acid, found in unfermented mistellas [153] | ||
| Ethyl 4-methylpentanoate | Its precursor, ethyl 4-methylpentanoic acid, found in unfermented mistellas [153] | ||
| γ-Decalactone | No [125] | Identified [8] | Detected by GCO [15,142] |
| Massoia lactone | Detected by GCO [15] | ||
| Furaneol | Aglianico up to 2 ppm in pulp and 0.6 in skin, Uva di Troia 1,2 ppm in pulp, 90 ppb in skin [125]; 15–51 ppm in muscadine [46] | Detected by GCO [139] | Detected by GCO [15] |
| DMS | Only found in grape or grape mistellas not in precursor fractions [15] | ||
| Polyfunctional Mercaptans | |||
| 4-Methyl-4-mercaptopentan-2-one | Mostly released by yeast. | ||
| 3-Mercaptohexanol | Released by yeast. Detected by GCO in mild-acid hydrolyzates [15,142] | ||
| 3-Mercaptohexyl acetate | Formed by yeast from 3MH | ||
| Compound | Structure | Odor Descriptor | Threshold in Model Wine (ng/L) [188] | Range of Occurrence in Wine (ng/L) [189] |
|---|---|---|---|---|
| 4-Methyl-4-mercaptopentan-2-one | 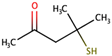 | Box tree | 0.8 | n.d. to 90 |
| 3-Mercaptohexanol | 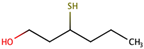 | Grapefruit | 60 | n.d. to 7300 |
| 3-Mercaptohexyl acetate | 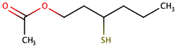 | Box tree, passion fruit | 4 | n.d. to 440 |
| Variety | CYS–MH | CYS–MMP | GLU–MH | GLU–MMP |
|---|---|---|---|---|
| Sauvignon Blanc | 174 ± 7 | 12.6 ± 1.4 | 1557 ± 86 | 7.7 ± 1.3 |
| Gewürztraminer | 89 ± 6 | 8.0 ± 1.5 | 1154 ± 56 | 6.6 ± 0.8 |
| Muscat | 157 ± 8 | n.d. | 1673 ± 71 | 8.3 ± 0.9 |
| Grenache | 172 ± 5 | 7.9 ± 1.2 | 1422 ± 63 | 9.4 ± 1.2 |
| Albariño | 158 ± 3 | 7.2 ± 0.7 | 1462 ± 80 | 8.4 ± 0.7 |
| Tempranillo | 205 ± 8 | 6.1 ± 1.8 | 1284 ± 76 | 10.3 ± 1.1 |
| Verdejo | 215 ± 9 | 7.3 ± 1.0 | 3397 ± 102 | n.d. |
| Chardonnay | 32 ± 4 | 0.4 ± 0.2 | 1405 ± 97 | n.d. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. https://doi.org/10.3390/biom9120818
Ferreira V, Lopez R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules. 2019; 9(12):818. https://doi.org/10.3390/biom9120818
Chicago/Turabian StyleFerreira, Vicente, and Ricardo Lopez. 2019. "The Actual and Potential Aroma of Winemaking Grapes" Biomolecules 9, no. 12: 818. https://doi.org/10.3390/biom9120818
APA StyleFerreira, V., & Lopez, R. (2019). The Actual and Potential Aroma of Winemaking Grapes. Biomolecules, 9(12), 818. https://doi.org/10.3390/biom9120818





