Artificial Neural Network (ANN) as a Tool to Reduce Human-Animal Interaction Improves Senegalese Sole Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
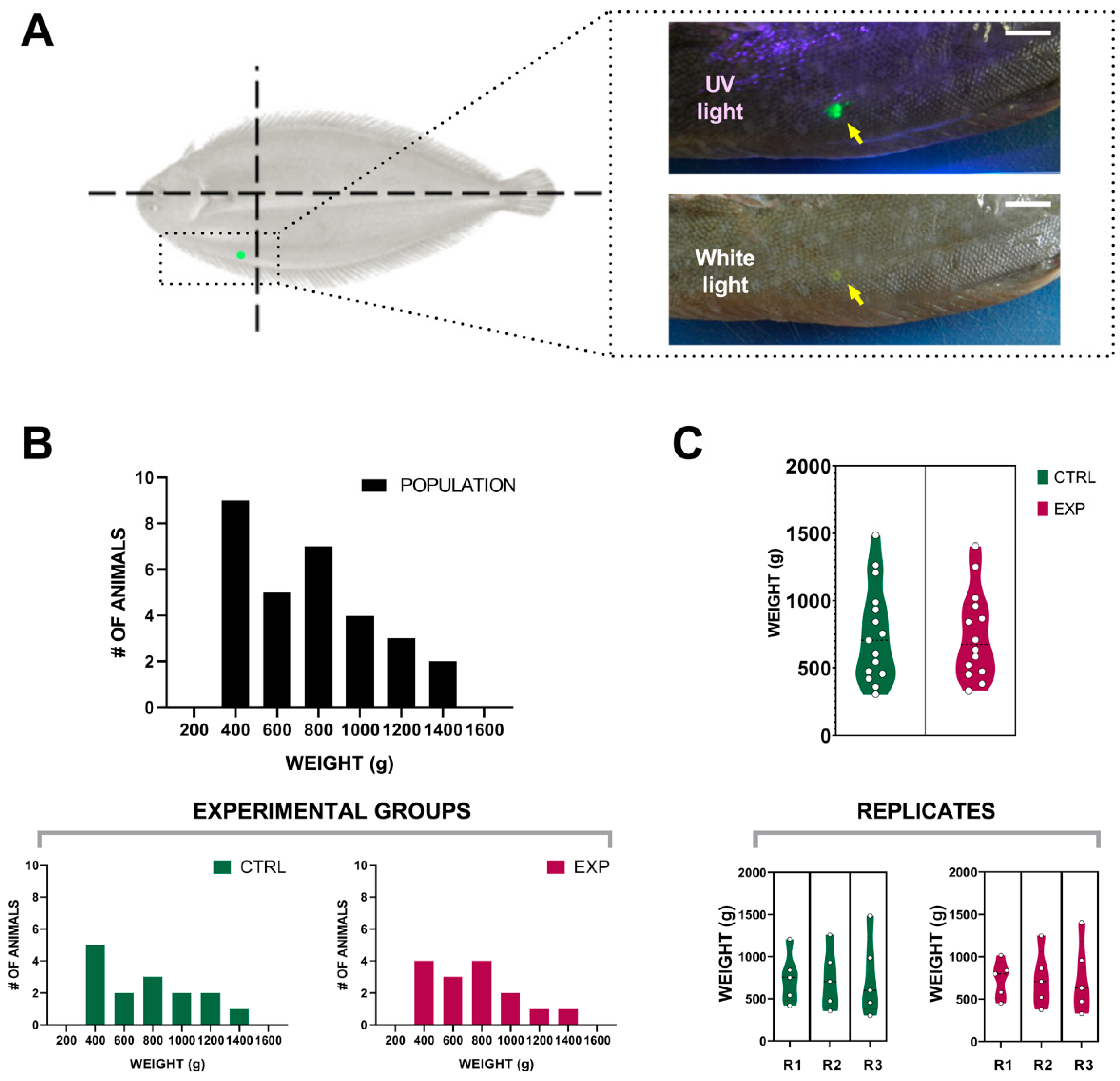
2.2. Group Formation and Animal Management
2.3. Manual and Visual Samplings
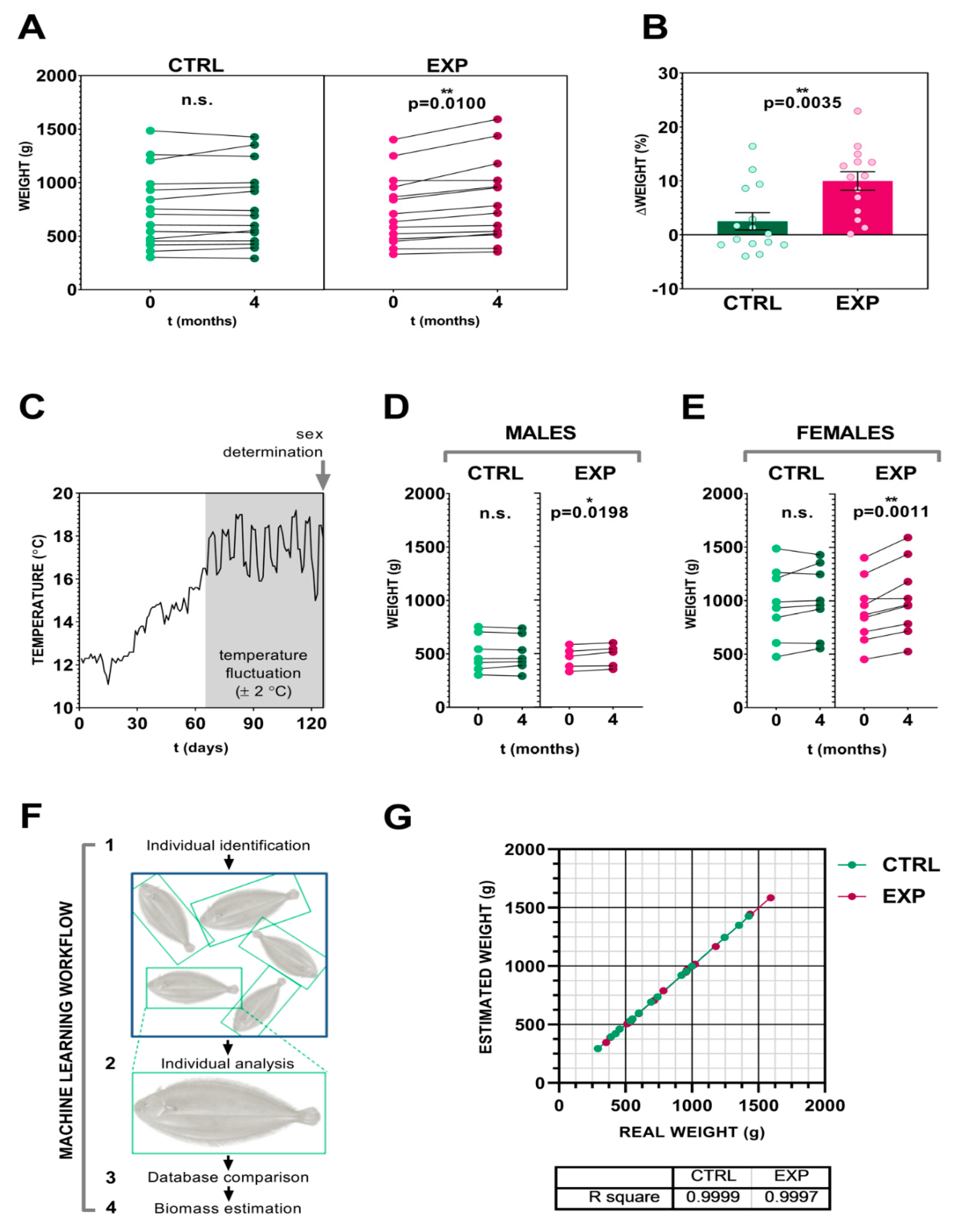
2.4. Biomass Estimation by Image Analysis
- (1)
- In the first phase, the user sends a top-view tank photo to our server. The server receives the image and resizes it to obtain, at least, the height or the width in 1024 pixels. The aspect of the image is not modified, and all of the colors of the original picture are retained.
- (2)
- In the second phase, we train the last layer of Inception_v4 to detect all fish in the tank. However, to train this CNN, we need to train it using sole fish images. To the best of our knowledge, there are no available datasets to detect sole fish. For this reason, we created a training dataset of 2000 sole fish images and 200 reference object images, contained in several fish tanks. This Inception_v4 model can identify the sole fish and the reference object, even if only a small part is visible, such as its head or tail. As a result, this CNN has 96% fish detection accuracy and 100% of reference object detection accuracy.
- (3)
- In the third phase, after all fish and the reference object in the picture have been identified and framed, our artificial vision system calculates the real size of all fish. This system uses the reference object framed in the picture to calculate the distance between the camera and the object. It calculates how many pixels the reference object framed contains, and makes a relation between its size in the real world and its pixels in the image. So, the system makes its own calibration and then calculates the size of every fish in the image. Thus, the application can determine the fish measurements, height and width, with a measurement error chance of 0.5% and 0.4%, respectively.
- (4)
- In the fourth phase, a novel CNN is created that is able to estimate the fish biomass using the height and the width of the fish. Supplementary Figure 1 shows the nine layer CNN developed for this purpose. This neural network has been trained with a dataset that contains the height, width, and biomass of 400 fish. Additionally, it has been used adam as an optimizer, together with a learning rate of 0.001 with a batch size of four.
2.5. Data Analysis
3. Results
3.1. Visible Implant Elastomer Tagging
3.2. Effect of the Intensity Human-Animal Interaction on Sole Biomass Gain
3.3. Image Analysis and Artificial Neural Networks as a Tool for Precise Fish Biomass Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- APROMAR. La Acuicultura en España; APROMAR: Cádiz, Spain, 2018. [Google Scholar]
- Sánchez, P.; Ambrosio, P.P.; Flos, R. Stocking density and sex influence individual growth of Senegalese sole (Solea senegalensis). Aquaculture 2010, 300, 93–101. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Saberioon, M.; Gholizadeh, A.; Cisar, P.; Pautsina, A.; Urban, J. Application of machine vision systems in aquaculture with emphasis on fish: State-of-the-art and key issues. Rev. Aquac. 2017, 9, 369–387. [Google Scholar] [CrossRef]
- Zion, B. The use of computer vision technologies in aquaculture—A review. Comput. Electron. Agric. 2012, 88, 125–132. [Google Scholar] [CrossRef]
- Torisawa, S.; Kadota, M.; Komeyama, K.; Suzuki, K.; Takagi, T. A digital stereo-video camera system for three-dimensional monitoring of free-swimming Pacific bluefin tuna, Thunnus orientalis, cultured in a net cage. Aquat. Living Resour. 2011, 24, 107–112. [Google Scholar] [CrossRef]
- Balaban, M.O.; Ünal Şengör, G.F.; Soriano, M.G.; Ruiz, E.G. Using Image Analysis to Predict the Weight of Alaskan Salmon of Different Species. J. Food Sci. 2010, 75, E157–E162. [Google Scholar] [CrossRef]
- Costa, C.; Scardi, M.; Vitalini, V.; Cataudella, S. A dual camera system for counting and sizing Northern Bluefin Tuna (Thunnus thynnus; Linnaeus, 1758) stock, during transfer to aquaculture cages, with a semi automatic Artificial Neural Network tool. Aquaculture 2009, 291, 161–167. [Google Scholar] [CrossRef]
- Almansa, C.; Reig, L.; Oca, J. The laser scanner is a reliable method to estimate the biomass of a Senegalese sole (Solea senegalensis) population in a tank. Aquac. Eng. 2015, 69, 78–83. [Google Scholar] [CrossRef]
- Sánchez, P.; Ambrosio, P.P.; Flos, R. Stocking density affects Senegalese sole (Solea senegalensis, Kaup) growth independently of size dispersion, evaluated using an individual photo-identification technique. Aquac. Res. 2013, 44, 231–241. [Google Scholar] [CrossRef]
- Anguis, V.; Cañavate, J.P. Spawning of captive Senegal sole (Solea senegalensis) under a naturally fluctuating temperature regime. Aquaculture 2005, 243, 133–145. [Google Scholar] [CrossRef]
- Soula, M.; Navarro, A.; Hildebrandt, S.; Zamorano, M.J.; Roo, J.; Hernández-Cruz, C.M.; Afonso, J.M. Evaluation of VIE (Visible Implant Elastomer) and PIT (Passive Integrated Transponder) physical tagging systems for the identification of red porgy fingerlings (Pagrus pagrus). Aquac. Int. 2012, 20, 571–583. [Google Scholar] [CrossRef]
- Astorga, N.; Afonso, J.M.; Zamorano, M.J.; Montero, D.; Oliva, V.; Fernandez, H.; Izquierdo, M.S. Evaluation of visible implant elastomer tags for tagging juvenile gilthead seabream (Sparus auratus L.); effects on growth, mortality, handling time and tag loss. Aquac. Res. 2005, 36, 733–738. [Google Scholar] [CrossRef]
- Navarro, A.; Oliva, V.; Zamorano, M.J.; Ginés, R.; Izquierdo, M.S.; Astorga, N.; Afonso, J.M. Evaluation of PIT system as a method to tag fingerlings of gilthead seabream (Sparus auratus L.): Effects on growth, mortality and tag loss. Aquaculture 2006, 257, 309–315. [Google Scholar] [CrossRef]
- Rasines, I.; Gómez, M.; Martín, I.; Rodríguez, C.; Mañanós, E.; Chereguini, O. Artificial fertilisation of cultured Senegalese sole (Solea senegalensis): Effects of the time of day of hormonal treatment on inducing ovulation. Aquaculture 2013, 392, 94–97. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Oyinlola, M.A. Vulnerability of flatfish and their fisheries to climate change. J. Sea Res. 2018, 140, 1–10. [Google Scholar] [CrossRef]
- Fernández, I.; Fernandes, J.M.O.; Roberto, V.P.; Kopp, M.; Oliveira, C.; Riesco, M.F.; Dias, J.; Cox, C.J.; Leonor Cancela, M.; Cabrita, E.; et al. Circulating small non-coding RNAs provide new insights into vitamin K nutrition and reproductive physiology in teleost fish. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 39–51. [Google Scholar] [CrossRef]
- Riesco, M.F.; Valcarce, D.G.; Martínez-Vázquez, J.M.; Martín, I.; Calderón-García, A.Á.; Gonzalez-Nunez, V.; Robles, V. Male reproductive dysfunction in Solea senegalensis: New insights into an unsolved question. Reprod. Fertil. Dev. 2019, 31, 1104. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Robles, V. Effect of captivity and cryopreservation on ROS production in Solea senegalensis spermatozoa. Reproduction 2016, 152, 439–446. [Google Scholar] [CrossRef]
- Carballo, C.; Pinto, P.I.S.; Mateus, A.P.; Berbel, C.; Guerreiro, C.C.; Martinez-Blanch, J.F.; Codoñer, F.M.; Mantecon, L.; Power, D.M.; Manchado, M. Yeast β-glucans and microalgal extracts modulate the immune response and gut microbiome in Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2019, 92, 31–39. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Mabrok, M.; Machado, M.; Azeredo, R.; Afonso, A.; Esteban, M.A.; Costas, B. Mucosal and systemic immune responses in Senegalese sole (Solea senegalensis Kaup) bath challenged with Tenacibaculum maritimum: A time-course study. Fish Shellfish Immunol. 2019, 87, 744–754. [Google Scholar] [CrossRef]
- Louro, B.; Marques, J.P.; Manchado, M.; Power, D.M.; Campinho, M.A. Sole head transcriptomics reveals a coordinated developmental program during metamorphosis. Genomics 2019. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Buchanan, B.; De Jong, G.; Dietterich, T.; Rosenbloom, P.; Waibel, A. Machine Learning. Annu. Rev. Comput. Sci. 1990, 4, 417–433. [Google Scholar] [CrossRef]
- Lynch, C. How do your data grow? Nature 2008, 455, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, K.; Xu, D.; Chen, L.; Guo, Q.; Sun, C.; Yang, X. Near infrared computer vision and neuro-fuzzy model-based feeding decision system for fish in aquaculture. Comput. Electron. Agric. 2018, 146, 114–124. [Google Scholar] [CrossRef]
- López-Cortés, X.A.; Nachtigall, F.M.; Olate, V.R.; Araya, M.; Oyanedel, S.; Diaz, V.; Jakob, E.; Ríos-Momberg, M.; Santos, L.S. Fast detection of pathogens in salmon farming industry. Aquaculture 2017, 470, 17–24. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Vázquez, J.M.; Valcarce, D.G.; Riesco, M.F.; Marco, V.S.; Matsuoka, M.; Robles, V. Artificial Neural Network (ANN) as a Tool to Reduce Human-Animal Interaction Improves Senegalese Sole Production. Biomolecules 2019, 9, 778. https://doi.org/10.3390/biom9120778
Martínez-Vázquez JM, Valcarce DG, Riesco MF, Marco VS, Matsuoka M, Robles V. Artificial Neural Network (ANN) as a Tool to Reduce Human-Animal Interaction Improves Senegalese Sole Production. Biomolecules. 2019; 9(12):778. https://doi.org/10.3390/biom9120778
Chicago/Turabian StyleMartínez-Vázquez, Juan M., David G. Valcarce, Marta F. Riesco, Vicent Sanz Marco, Morito Matsuoka, and Vanesa Robles. 2019. "Artificial Neural Network (ANN) as a Tool to Reduce Human-Animal Interaction Improves Senegalese Sole Production" Biomolecules 9, no. 12: 778. https://doi.org/10.3390/biom9120778
APA StyleMartínez-Vázquez, J. M., Valcarce, D. G., Riesco, M. F., Marco, V. S., Matsuoka, M., & Robles, V. (2019). Artificial Neural Network (ANN) as a Tool to Reduce Human-Animal Interaction Improves Senegalese Sole Production. Biomolecules, 9(12), 778. https://doi.org/10.3390/biom9120778




