Combined Use of Deep Eutectic Solvents, Macroporous Resins, and Preparative Liquid Chromatography for the Isolation and Purification of Flavonoids and 20-Hydroxyecdysone from Chenopodium quinoa Willd
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Instruments
2.2. Determination of Total Flavonoid (TF) and 20-Hydroxyecdysone Contents
2.3. Preparation and Selection of DESs
2.4. Response Surface Methodology (RSM)
2.5. Adsorption/Desorption Capacities of the Five Employed Resins
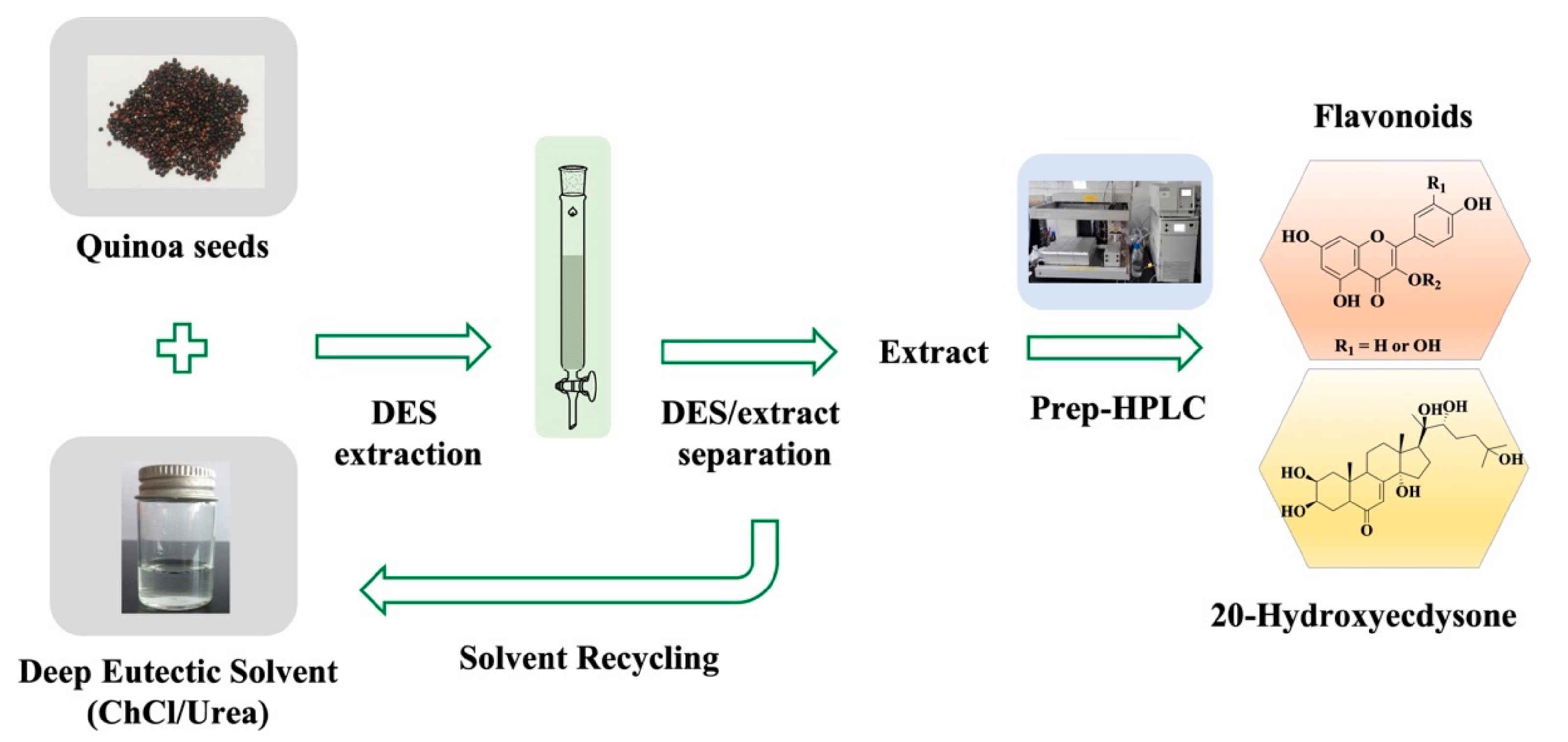
2.6. Small- and Preparative-Scale Sample Extraction
2.7. Analytical and Preparative HPLC
3. Results and Discussion
3.1. Preparation and Optimization of DES Systems
3.2. BBD Experimental Design
3.3. Macroporous Resin Selection
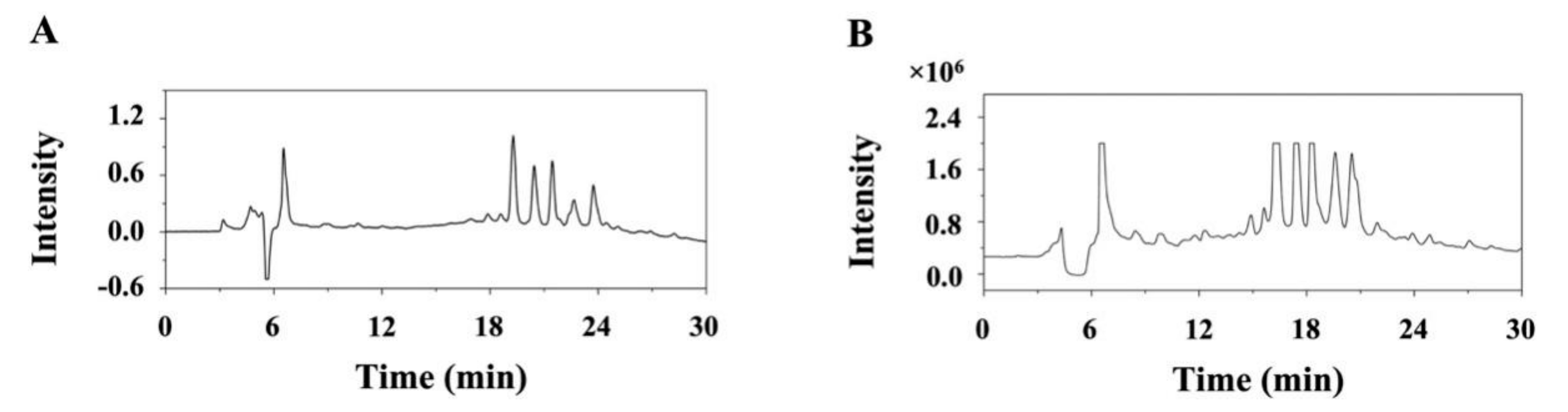
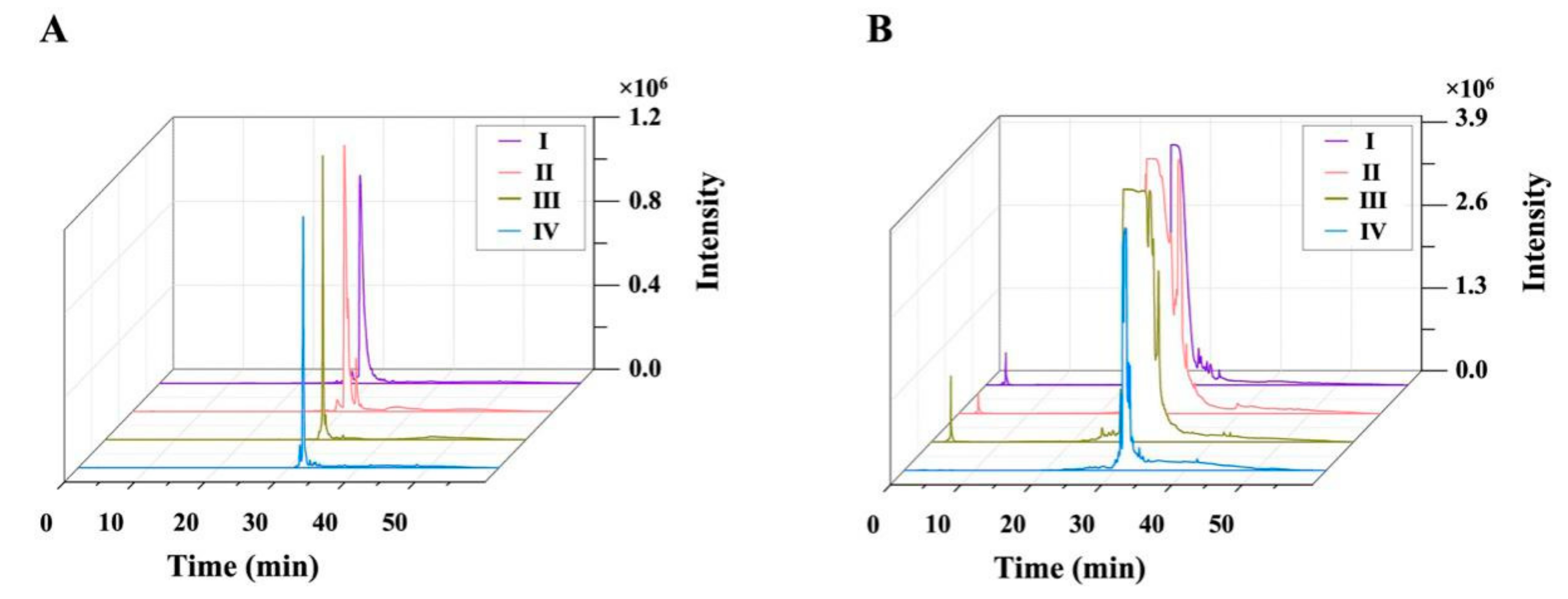
3.4. Preparative-Scale Isolation and Purification of Monomer Compounds
3.5. Identification of Isolated Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.W.; Zhang, Z.Q.; Sun, X.G.; Hu, Y.S.; Xing, H.B.; Dai, S. Ionic liquids and derived materials for lithium and sodium batteries. Chem. Soc. Rev. 2018, 47, 2020–2064. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: synthesis, properties and applications. Green Chem. 2013, 16, 1007–1033. [Google Scholar] [CrossRef]
- Carolin, R.; Burkhard, K. Low melting mixtures in organic synthesis – an alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Xu, J.; Huang, W.; Bai, R.; Queneau, Y.; Jérôme, F.; Gu, Y. Utilization of bio-based glycolaldehyde aqueous solution in organic synthesis: application to the synthesis of 2,3-dihydrofurans. Green Chem. 2019, 21, 2061–2069. [Google Scholar] [CrossRef]
- Wu, C.; Xiao, H.J.; Wang, S.W.; Tang, M.S.; Tang, Z.L.; Xia, W.; Li, W.F.; Cao, Z.; He, W.M. Natural Deep Eutectic Solvent-Catalyzed Selenocyanation of Activated Alkynes via an Intermolecular H-Bonding Activation Process. ACS Sustain. Chem. Eng. 2019, 7, 2169–2175. [Google Scholar] [CrossRef]
- Wu, C.; Hong, L.; Shu, H.; Zhou, Q.H.; Wang, Y.; Su, N.; Jiang, S.; Cao, Z.; He, W.M. Practical Approach for Clean Preparation of Z-β-Thiocyanate Alkenyl Esters. ACS Sustain. Chem. Eng. 2019, 7, 8798–8803. [Google Scholar] [CrossRef]
- Lu, L.H.; Wang, Z.; Xia, W.; Cheng, P.; Zhang, B.; Cao, Z.; He, W.M. Sustainable routes for quantitative green selenocyanation of activated alkynes. Chin. Chem. Lett. 2019, 30, 1237–1240. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.W.; Li, X.X.; Chen, L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: a review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for natural hydrophobic deep eutectic solvents based on natural components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd. (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Chen, Y.S.; Aluwi, N.A.; Saunders, S.R.; Ganjyal, G.M.; Medina-Meza, I.G. Metabolic fingerprinting unveils quinoa oil as a source of bioactive phytochemicals. Food Chem. 2019, 286, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Ruales, J.; Nair, B.M. Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa Willd.) seeds. Food Chem. 1993, 48, 137–143. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.Q.; Sheng, S.Q.; Sang, S.M.; Jhoo, J.W.; Bai, N.S.; Karwe, M.V.; Rosen, R.T.; Ho, C.T. Triterpene saponins from debittered quinoa. J. Agric. Food Chem. 2002, 50, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Cabanes, J.; Jimenez-Atienzar, M.; Ibanez-Tremolada, M.; Gomez-Pando, L.R.; Garcia-Carmona, F.; Gandia-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojo, L.E.; Delatorre-Herrera, J.; Poulev, A.; Calfio, C.; Raskin, I. Phytoecdysteroids and flavonoid glycosides among Chilean and commercial sources of Chenopodium quinoa: variation and correlation to physico-chemical characteristics. J. Sci. Food Agric. 2016, 96, 633–643. [Google Scholar] [CrossRef]
- Dini, I.; Carlo Tenore, G.; Dini, A. Phenolic constituents of Kancolla seeds. Food Chem. 2004, 84, 163–168. [Google Scholar] [CrossRef]
- Kumpun, S.; Maria, A.; Crouzet, S.; Evrard-Todeschi, N.; Girault, J.-P.; Lafont, R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011, 125, 1226–1234. [Google Scholar] [CrossRef]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Van den Driessche, J.J.; Plat, J.; Mensink, R.P. Effects of superfoods on risk factors of metabolic syndrome: a systematic review of human intervention trials. Food Funct. 2018, 9, 1944–1966. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Böhmdorfer, S.; Zengin, G.; Bacher, M.; Potthast, A.; Akramov, D.K.; Janibekov, A.; Rosenau, T. Phytochemical and biological activities of Silene viridiflora extractives. Development and validation of a HPTLC method for quantification of 20-hydroxyecdysone. Ind. Crop. Prod. 2019, 129, 542–548. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Coffee silverskin: a possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef]
- Wang, H.; Tan, J.; Shang, X.; Zheng, X.; Liu, X.; Wang, J.; Hou, X.; Du, Y. Porous organic cage incorporated monoliths for solid-phase extraction coupled with liquid chromatography-mass spectrometry for identification of ecdysteroids from Chenopodium quinoa Willd. J. Chromatogr. A 2019, 1583, 55–62. [Google Scholar] [CrossRef]
- Caussette, M.; Kershawb, J.L.; Sheltod, D.R. Survey of enzyme activities in desaponified quinoa Chenopodium quinoa Willd. Food Chem. 1997, 60, 587–592. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT-Food Sci. Technol. 2010, 43, 447–451. [Google Scholar] [CrossRef]
Availability of Isolated Substances: Not available. |
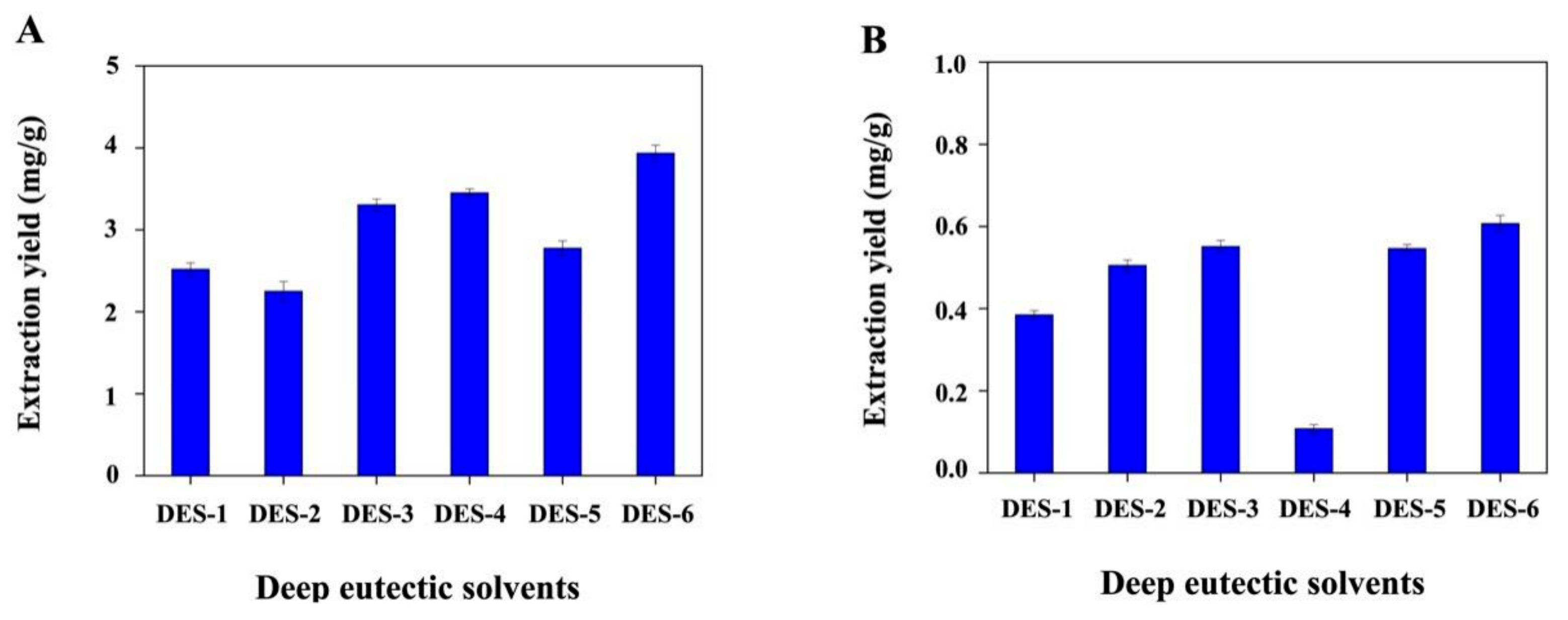


| DESs | Composite of DES | Molar Ratio | Viscosity a Pa·s | Conductivity μS/cm | Density g/cm3 |
|---|---|---|---|---|---|
| DES-1 | ChCl/aminoethyl alcohol | 1:6 | 0.037 | 3330 | 1.058 |
| DES-2 | ChCl/D-glucose | 1:1 | - c | 98 | 1.304 |
| DES-3 | ChCl/glycerol | 1:2 | 0.227 | 1750 | 1.171 |
| DES-4 | ChCl/oxalic acid b | 1:1 | 0.152 | 1043 | 1.230 |
| DES-5 | ChCl/lactic acid | 1:1 | 0.270 | 3670 | 1.133 |
| DES-6 | ChCl/urea | 1:2 | 0.658 | 1880 | 1.184 |
| No. | Experiment Design | Response | ||||||
|---|---|---|---|---|---|---|---|---|
| Coded Values | Variables | Extraction Yields (mg/g) | ||||||
| a | b | c | a (%) | b (°C) | c (mg/mL) | TF | 20-Hydroxyecdysone | |
| 1 | 1 | 0 | 1 | 50 | 50 | 125 | 2.90 | 0.29 |
| 2 | 0 | 1 | 1 | 30 | 60 | 125 | 3.20 | 0.25 |
| 3 | 0 | 0 | 0 | 30 | 50 | 100 | 4.49 | 0.51 |
| 4 | −1 | −1 | 0 | 10 | 40 | 100 | 2.08 | 0.33 |
| 5 | 0 | 0 | 0 | 30 | 50 | 100 | 4.29 | 0.53 |
| 6 | 1 | 1 | 0 | 50 | 60 | 100 | 3.07 | 0.34 |
| 7 | 0 | 0 | 0 | 30 | 50 | 100 | 4.13 | 0.50 |
| 8 | 1 | 0 | −1 | 50 | 50 | 75 | 3.20 | 0.34 |
| 9 | 0 | −1 | 1 | 30 | 40 | 125 | 2.72 | 0.38 |
| 10 | 0 | 1 | −1 | 30 | 60 | 75 | 3.27 | 0.42 |
| 11 | 1 | −1 | 0 | 50 | 40 | 100 | 2.78 | 0.47 |
| 12 | −1 | 0 | −1 | 10 | 50 | 75 | 2.53 | 0.30 |
| 13 | −1 | 1 | 0 | 10 | 60 | 100 | 2.25 | 0.34 |
| 14 | 0 | 0 | 0 | 30 | 50 | 100 | 4.28 | 0.51 |
| 15 | 0 | 0 | 0 | 30 | 50 | 100 | 3.70 | 0.47 |
| 16 | 0 | −1 | −1 | 30 | 40 | 75 | 3.40 | 0.32 |
| 17 | −1 | 0 | 1 | 10 | 50 | 125 | 2.03 | 0.29 |
| Optimal Variable Conditions | Optimum (mg/g) | |||
|---|---|---|---|---|
| a (%) | b (°C) | c (mg/mL) | ||
| TF | 32.4 | 50.7 | 97.2 | 4.23 |
| 20-Hydroxyecdysone | 32.6 | 47.8 | 99.7 | 0.51 |
| Numbers | Compound Names | R1 | R2 | |
|---|---|---|---|---|
| 1 | Quercetin-3-O-(2,6-di-α-L-rhamnopyranosyl)-β-D-galactopyranoside | 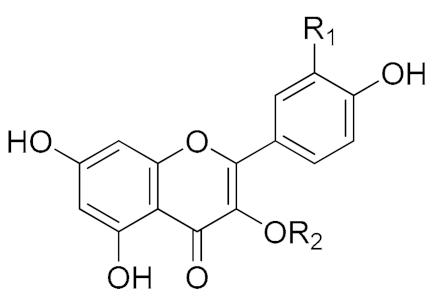 | OH | 3-O-(2,6-di-α-L-rhamnopyranosyl)-β-D-galactopyranoside |
| 2 | Quercetin-3-O-β-D-apiofuranosyl-(1‴→2")-O-[α-L-rhamnopyranosyl-(1″″→6″)]-β-D-galactopyranoside | OH | 3-O-β-D-apiofuranosyl-(1‴→2″)-O-[α-L-rhamnopyranosyl-(1″″→6″)]-β-D-galactopyranoside | |
| 3 | Kaempferol-3-O-(2,6-di-α-L- rhamnopyranosyl)-β-D-galactopyranoside | H | 3-O-(2,6-di-α-L-rhamnopyranosyl)-β-D-galactopyranoside | |
| 4 | 20-Hydroxyecdysone | 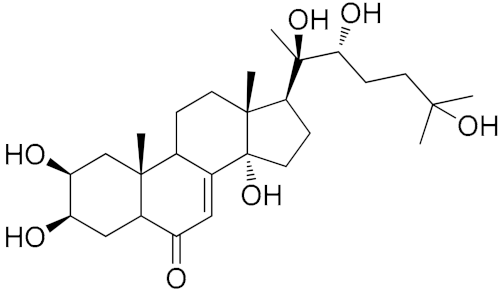 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Shang, X.; Zhang, P.; Wang, H.; Gu, Y.; Tan, J.-N. Combined Use of Deep Eutectic Solvents, Macroporous Resins, and Preparative Liquid Chromatography for the Isolation and Purification of Flavonoids and 20-Hydroxyecdysone from Chenopodium quinoa Willd. Biomolecules 2019, 9, 776. https://doi.org/10.3390/biom9120776
Zeng J, Shang X, Zhang P, Wang H, Gu Y, Tan J-N. Combined Use of Deep Eutectic Solvents, Macroporous Resins, and Preparative Liquid Chromatography for the Isolation and Purification of Flavonoids and 20-Hydroxyecdysone from Chenopodium quinoa Willd. Biomolecules. 2019; 9(12):776. https://doi.org/10.3390/biom9120776
Chicago/Turabian StyleZeng, Jia, Xianchao Shang, Peng Zhang, Hongwei Wang, Yanlong Gu, and Jia-Neng Tan. 2019. "Combined Use of Deep Eutectic Solvents, Macroporous Resins, and Preparative Liquid Chromatography for the Isolation and Purification of Flavonoids and 20-Hydroxyecdysone from Chenopodium quinoa Willd" Biomolecules 9, no. 12: 776. https://doi.org/10.3390/biom9120776
APA StyleZeng, J., Shang, X., Zhang, P., Wang, H., Gu, Y., & Tan, J.-N. (2019). Combined Use of Deep Eutectic Solvents, Macroporous Resins, and Preparative Liquid Chromatography for the Isolation and Purification of Flavonoids and 20-Hydroxyecdysone from Chenopodium quinoa Willd. Biomolecules, 9(12), 776. https://doi.org/10.3390/biom9120776





