Retinoic Acid and Germ Cell Development in the Ovary and Testis
Abstract
1. Introduction
2. Germ Cell Development in the Fetal Gonad
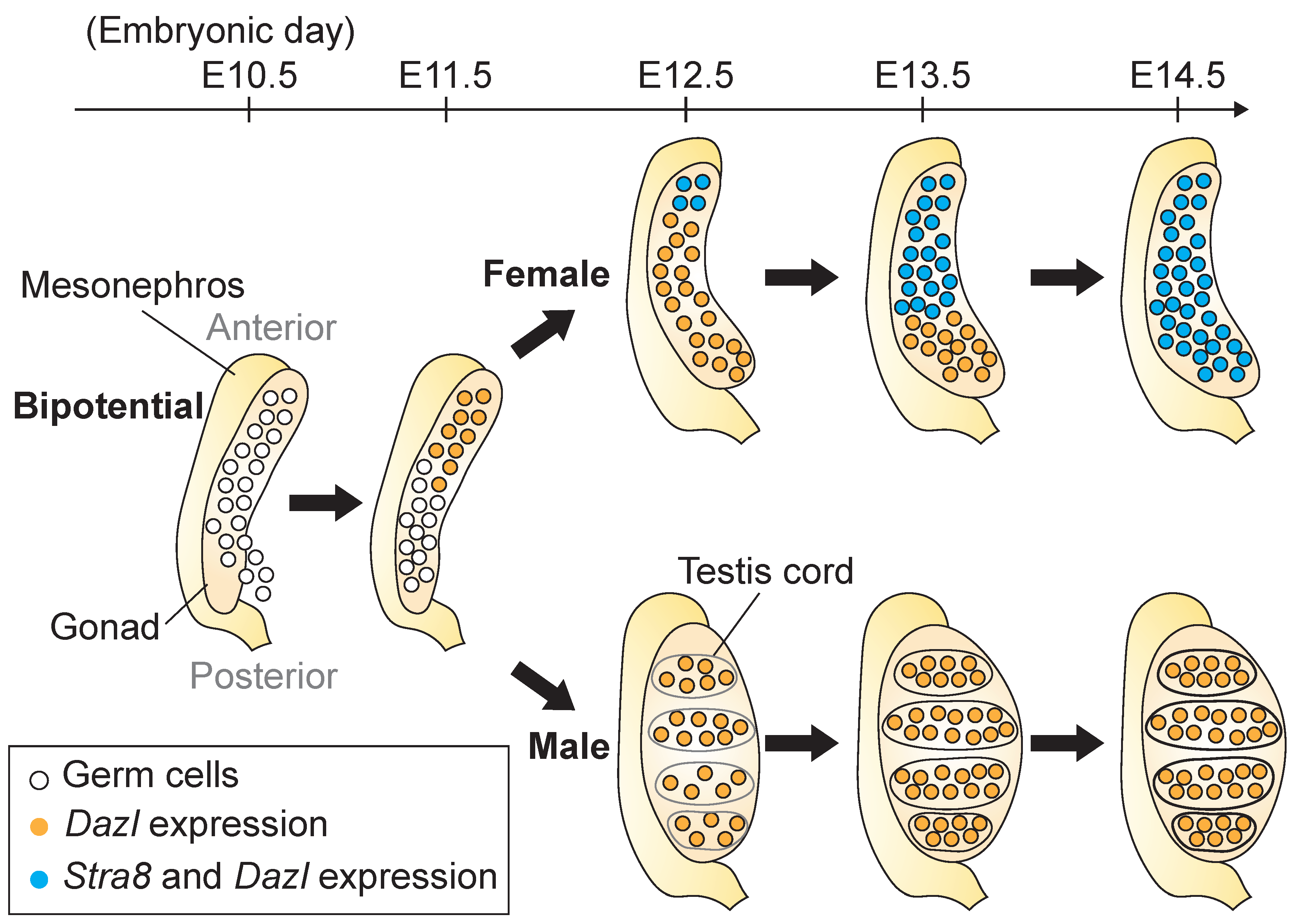
2.1. Formation of the Gonad and Migration of PGCs to the Gonad
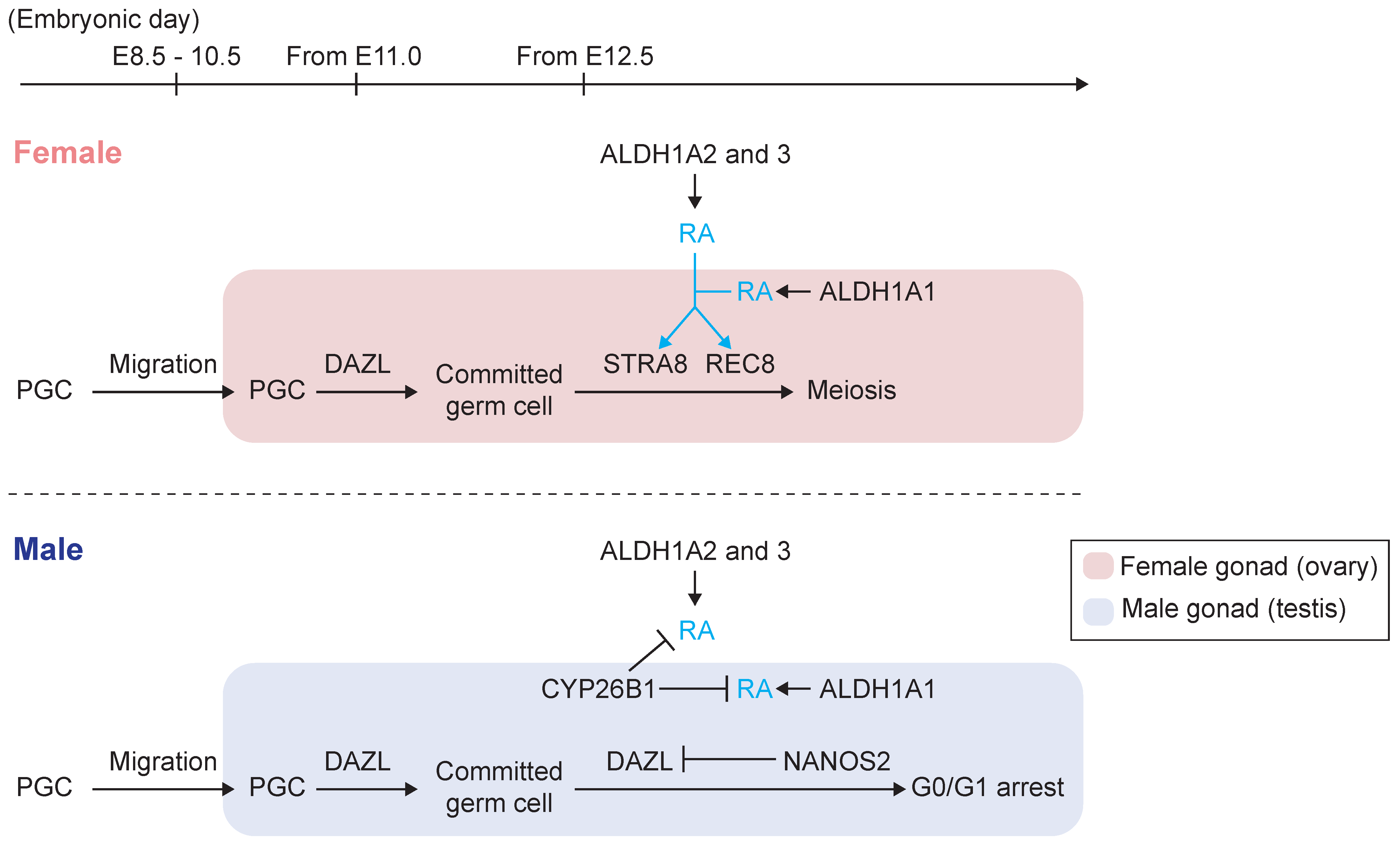
2.2. Initiation of Gametogenesis and Meiotic Entry
2.3. Stra8 and Its Inducer, RA, Regulate Meiotic Initiation in the Fetal Ovary
2.4. Source of RA in the Fetal Ovary
2.5. Prevention of Meiotic Initiation in the Fetal Testis
2.6. A Role for RA in the Ovary after Birth
3. Development of Male Germ Cells after Birth
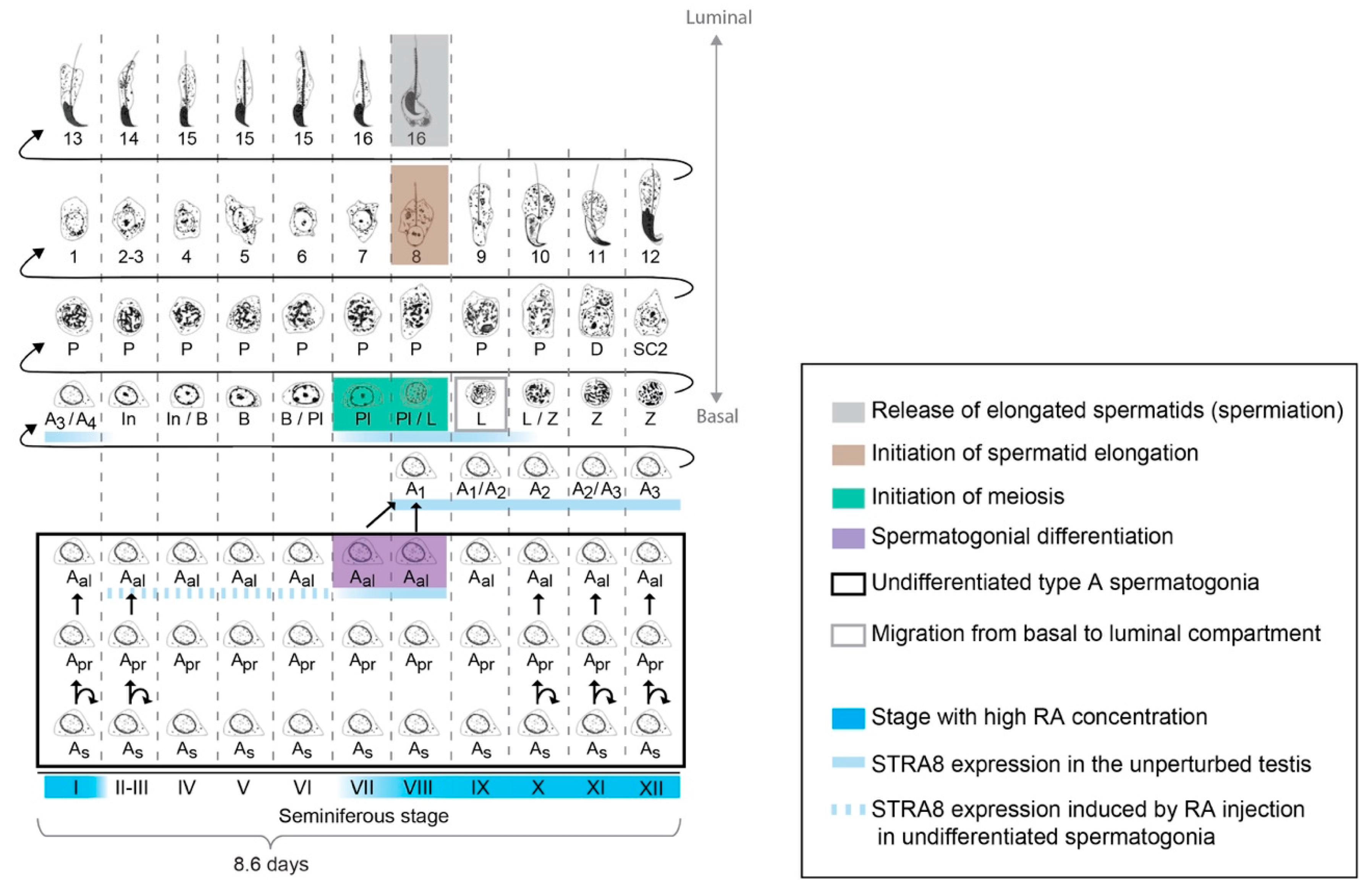
3.1. Organization of Spermatogenesis in the Postnatal and Adult Testis
3.2. Regulation of Spermatogenesis by Vitamin A and RA
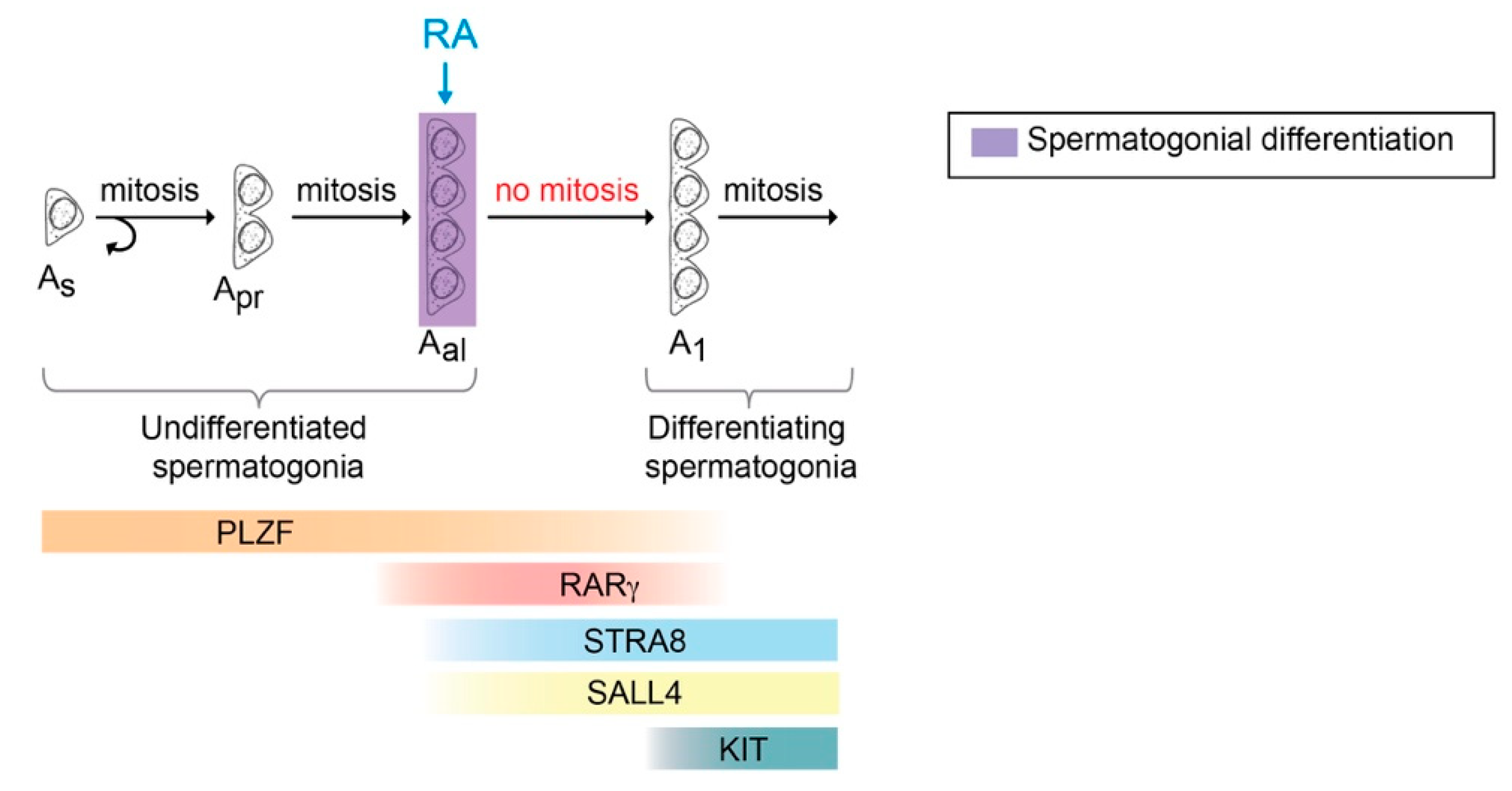
3.3. The Role of RA and Stra8 at Spermatogonial Differentiation and Meiotic Initiation
3.4. Role of RA at the Initiation of Spermatid Elongation and Spermiation
3.5. Source of RA in the Postnatal and Adult Testis
3.6. Periodicity of Spermatogenesis and RA Levels
3.7. Competence of Germ Cells for Spermatogonial Differentiation
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hacker, A.; Capel, B.; Goodfellow, P.; Lovell-Badge, R. Expression of Sry, the mouse sex determining gene. Development 1995, 121, 1603–1614. [Google Scholar] [PubMed]
- Schmahl, J.; Eicher, E.M.; Washburn, L.L.; Capel, B. Sry induces cell proliferation in the mouse gonad. Development 2000, 127, 65–73. [Google Scholar] [PubMed]
- Hu, Y.C.; Okumura, L.M.; Page, D.C. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013, 9, e1003629. [Google Scholar] [CrossRef]
- Hilscher, B.; Hilscher, W.; Bulthoff-Ohnolz, B.; Kramer, U.; Birke, A.; Pelzer, H.; Gauss, G. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974, 154, 443–470. [Google Scholar]
- McLaren, A. Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 1984, 38, 7–23. [Google Scholar]
- Byskov, A.G.; Saxen, L. Induction of meiosis in fetal mouse testis in vitro. Dev. Biol. 1976, 52, 193–200. [Google Scholar] [CrossRef]
- Koubova, J.; Menke, D.B.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2474–2479. [Google Scholar] [CrossRef]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef]
- Bowles, J.; Feng, C.W.; Miles, K.; Ineson, J.; Spiller, C.; Koopman, P. ALDH1A1 provides a source of meiosis-inducing retinoic acid in mouse fetal ovaries. Nat. Commun. 2016, 7, 10845. [Google Scholar] [CrossRef]
- Abu-Abed, S.; Dolle, P.; Metzger, D.; Beckett, B.; Chambon, P.; Petkovich, M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001, 15, 226–240. [Google Scholar] [CrossRef]
- Niederreither, K.; Abu-Abed, S.; Schuhbaur, B.; Petkovich, M.; Chambon, P.; Dolle, P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 2002, 31, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y.; Perey, B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am. J. Anat. 1957, 100, 241–267. [Google Scholar] [CrossRef]
- Huckins, C.; Clermont, Y. Evolution of gonocytes in the rat testis during late embryonic and early post-natal life. Arch. Anat. Histol. Embryol. 1968, 51, 341–354. [Google Scholar]
- Russell, L.D.; Ettlin, R.A.; Sinha Hikim, A.P.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis; Cache River Press: Clearwater, FL, USA, 1990. [Google Scholar]
- van Pelt, A.M.; de Rooij, D.G. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology 1991, 128, 697–704. [Google Scholar] [CrossRef]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef]
- Endo, T.; Freinkman, E.; de Rooij, D.G.; Page, D.C. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E10132–E10141. [Google Scholar] [CrossRef]
- McLaren, A. Primordial germ cells in the mouse. Dev. Biol. 2003, 262, 1–15. [Google Scholar] [CrossRef]
- Brambell, F.W.R. The development and morphology of the gonads of the mouse - Part I The morphogenesis of the indifferent gonad and of the ovary. Proc. R. Soc. B 1927, 101, 391–409. [Google Scholar] [CrossRef]
- Tam, P.P.; Snow, M.H. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 1981, 64, 133–147. [Google Scholar] [PubMed]
- Sapford, C.S. Changes in the cells of the Sex Cords and Seminiferous Tubules during the development of the Testis of the rat and mouse. Aust. J. Zool. 1962, 10, 178–192. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.C.; Fan, Y.; Carmell, M.A.; Dobrinski, I.; Watson, A.L.; Carlson, D.F.; Fahrenkrug, S.C.; et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Pesce, M.; Wang, X.; Wolgemuth, D.J.; Scholer, H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 1998, 71, 89–98. [Google Scholar] [CrossRef]
- Bullejos, M.; Koopman, P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol. Reprod. Dev. 2004, 68, 422–428. [Google Scholar] [CrossRef]
- Stevens, L.C. Development of resistance to teratocarcinogenesis by primordial germ cells in mice. J. Natl. Cancer Inst. 1966, 37, 859–867. [Google Scholar]
- Matsui, Y.; Tokitake, Y. Primordial germ cells contain subpopulations that have greater ability to develop into pluripotential stem cells. Dev. Growth Differ. 2009, 51, 657–667. [Google Scholar] [CrossRef]
- McLaren, A.; Southee, D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 1997, 187, 107–113. [Google Scholar] [CrossRef]
- Adams, I.R.; McLaren, A. Sexually dimorphic development of mouse primordial germ cells: Switching from oogenesis to spermatogenesis. Development 2002, 129, 1155–1164. [Google Scholar]
- Gill, M.E.; Hu, Y.C.; Lin, Y.; Page, D.C. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7443–7448. [Google Scholar] [CrossRef]
- Hu, Y.C.; Nicholls, P.K.; Soh, Y.Q.; Daniele, J.R.; Junker, J.P.; van Oudenaarden, A.; Page, D.C. Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 2015, 11, e1005019. [Google Scholar] [CrossRef] [PubMed]
- Seligman, J.; Page, D.C. The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem. Biophys. Res. Commun. 1998, 245, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Welling, M.; Bloch, D.B.; Munoz, J.; Mientjes, E.; Chen, X.; Tramp, C.; Wu, J.; Yabuuchi, A.; Chou, Y.F.; et al. DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Rep. 2014, 3, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gill, M.E.; Koubova, J.; Page, D.C. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 2008, 322, 1685–1687. [Google Scholar] [CrossRef]
- Baltus, A.E.; Menke, D.B.; Hu, Y.C.; Goodheart, M.L.; Carpenter, A.E.; de Rooij, D.G.; Page, D.C. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat. Genet. 2006, 38, 1430–1434. [Google Scholar] [CrossRef]
- Bannister, L.A.; Reinholdt, L.G.; Munroe, R.J.; Schimenti, J.C. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 2004, 40, 184–194. [Google Scholar] [CrossRef]
- Xu, H.; Beasley, M.D.; Warren, W.D.; van der Horst, G.T.; McKay, M.J. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 2005, 8, 949–961. [Google Scholar] [CrossRef]
- Koubova, J.; Hu, Y.C.; Bhattacharyya, T.; Soh, Y.Q.; Gill, M.E.; Goodheart, M.L.; Hogarth, C.A.; Griswold, M.D.; Page, D.C. Retinoic Acid activates two pathways required for meiosis in mice. PLoS Genet. 2014, 10, e1004541. [Google Scholar] [CrossRef]
- Menke, D.B.; Koubova, J.; Page, D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 2003, 262, 303–312. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Junker, J.P.; Gill, M.E.; Mueller, J.L.; van Oudenaarden, A.; Page, D.C. A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary. PLoS Genet. 2015, 11, e1005531. [Google Scholar] [CrossRef]
- Yao, H.H.; DiNapoli, L.; Capel, B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 2003, 130, 5895–5902. [Google Scholar] [CrossRef] [PubMed]
- Western, P.S.; van den Bergen, J.A.; Miles, D.C.; Sinclair, A.H. Male fetal germ cell differentiation involves complex repression of the regulatory network controlling pluripotency. FASEB J. 2010, 24, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, M.; Snow, M.H.; McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar] [PubMed]
- Kojima, M.L.; de Rooij, D.G.; Page, D.C. Amplification of a broad transcriptional program by a common factor triggers the meiotic cell cycle in mice. Elife 2019, 8, e43738. [Google Scholar] [CrossRef]
- Oulad-Abdelghani, M.; Bouillet, P.; Decimo, D.; Gansmuller, A.; Heyberger, S.; Dolle, P.; Bronner, S.; Lutz, Y.; Chambon, P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J. Cell Biol. 1996, 135, 469–477. [Google Scholar] [CrossRef]
- Zhou, Q.; Nie, R.; Li, Y.; Friel, P.; Mitchell, D.; Hess, R.A.; Small, C.; Griswold, M.D. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: An in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 2008, 79, 35–42. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.M.; Pouchnik, D.; Banasik, B.; McCarrey, J.R.; Small, C.; et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Li, H.; Clagett-Dame, M. Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol. Reprod. 2009, 81, 996–1001. [Google Scholar] [CrossRef]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P.; et al. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef]
- Ruhl, R.; Krzyzosiak, A.; Niewiadomska-Cimicka, A.; Rochel, N.; Szeles, L.; Vaz, B.; Wietrzych-Schindler, M.; Alvarez, S.; Szklenar, M.; Nagy, L.; et al. 9-cis-13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice. PLoS Genet. 2015, 11, e1005213. [Google Scholar] [CrossRef]
- Ruhl, R.; Krezel, W.; de Lera, A.R. 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: Vitamin A5. Nutr. Rev. 2018, 76, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.I.; Xia, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta 2012, 1821, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [CrossRef]
- Kastner, P.; Mark, M.; Chambon, P. Nonsteroid nuclear receptors: What are genetic studies telling us about their role in real life? Cell 1995, 83, 859–869. [Google Scholar] [CrossRef]
- Morita, Y.; Tilly, J.L. Segregation of retinoic acid effects on fetal ovarian germ cell mitosis versus apoptosis by requirement for new macromolecular synthesis. Endocrinology 1999, 140, 2696–2703. [Google Scholar] [CrossRef][Green Version]
- Dolle, P.; Ruberte, E.; Leroy, P.; Morriss-Kay, G.; Chambon, P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development 1990, 110, 1133–1151. [Google Scholar]
- Vernet, N.; Dennefeld, C.; Rochette-Egly, C.; Oulad-Abdelghani, M.; Chambon, P.; Ghyselinck, N.B.; Mark, M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006, 147, 96–110. [Google Scholar] [CrossRef]
- Boulogne, B.; Levacher, C.; Durand, P.; Habert, R. Retinoic acid receptors and retinoid X receptors in the rat testis during fetal and postnatal development: Immunolocalization and implication in the control of the number of gonocytes. Biol. Reprod. 1999, 61, 1548–1557. [Google Scholar] [CrossRef][Green Version]
- Childs, A.J.; Cowan, G.; Kinnell, H.L.; Anderson, R.A.; Saunders, P.T. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE 2011, 6, e20249. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Gely-Pernot, A.; Feret, B.; Dennefeld, C.; Benoit, G.; Davidson, I.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16582–16587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sirbu, I.O.; Mic, F.A.; Molotkova, N.; Molotkov, A.; Kumar, S.; Duester, G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 2009, 19, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chatzi, C.; Brade, T.; Cunningham, T.J.; Zhao, X.; Duester, G. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat. Commun. 2011, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Wen, J.; Guo, M.; Wang, J.; Li, G.; Wang, Z.; Wang, Y.; Teng, Z.; Cui, Y.; Xia, G. Retinoic acid derived from the fetal ovary initiates meiosis in mouse germ cells. J. Cell Physiol. 2013, 228, 627–639. [Google Scholar] [CrossRef]
- Bowles, J.; Feng, C.W.; Knight, D.; Smith, C.A.; Roeszler, K.N.; Bagheri-Fam, S.; Harley, V.R.; Sinclair, A.H.; Koopman, P. Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: Implications for retinoid balance in gonad development. Dev. Dyn. 2009, 238, 2073–2080. [Google Scholar] [CrossRef]
- Fan, X.; Molotkov, A.; Manabe, S.; Donmoyer, C.M.; Deltour, L.; Foglio, M.H.; Cuenca, A.E.; Blaner, W.S.; Lipton, S.A.; Duester, G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell Biol. 2003, 23, 4637–4648. [Google Scholar] [CrossRef]
- Bowles, J.; Koopman, P. Retinoic acid, meiosis and germ cell fate in mammals. Development 2007, 134, 3401–3411. [Google Scholar] [CrossRef]
- Spiller, C.; Koopman, P.; Bowles, J. Sex Determination in the Mammalian Germline. Annu. Rev. Genet. 2017, 51, 265–285. [Google Scholar] [CrossRef]
- Byskov, A.G. The anatomy and ultrastructure of the rete system in the fetal mouse ovary. Biol. Reprod. 1978, 19, 720–735. [Google Scholar] [CrossRef]
- Karl, J.; Capel, B. Three-dimensional structure of the developing mouse genital ridge. Philos. Trans. R Soc. Lond. B Biol. Sci. 1995, 350, 235–242. [Google Scholar] [PubMed]
- Miyauchi, H.; Ohta, H.; Nagaoka, S.; Nakaki, F.; Sasaki, K.; Hayashi, K.; Yabuta, Y.; Nakamura, T.; Yamamoto, T.; Saitou, M. Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J. 2017, 36, 3100–3119. [Google Scholar] [CrossRef] [PubMed]
- Abu-Abed, S.; MacLean, G.; Fraulob, V.; Chambon, P.; Petkovich, M.; Dolle, P. Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev. 2002, 110, 173–177. [Google Scholar] [CrossRef]
- Menke, D.B.; Page, D.C. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr. Patterns 2002, 2, 359–367. [Google Scholar] [CrossRef]
- MacLean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar] [PubMed]
- Suzuki, A.; Saga, Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008, 22, 430–435. [Google Scholar] [CrossRef]
- Suzuki, A.; Igarashi, K.; Aisaki, K.; Kanno, J.; Saga, Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 3594–3599. [Google Scholar] [CrossRef]
- Kato, Y.; Katsuki, T.; Kokubo, H.; Masuda, A.; Saga, Y. Dazl is a target RNA suppressed by mammalian NANOS2 in sexually differentiating male germ cells. Nat. Commun. 2016, 7, 11272. [Google Scholar] [CrossRef]
- Saga, Y. Function of Nanos2 in the male germ cell lineage in mice. Cell. Mol. Life Sci. 2010, 67, 3815–3822. [Google Scholar] [CrossRef]
- Suzuki, A.; Hirasaki, M.; Okuda, A. Does MAX open up a new avenue for meiotic research? Dev. Growth Differ. 2017, 59, 61–69. [Google Scholar] [CrossRef]
- Lehmann, R.; Nusslein-Volhard, C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development 1991, 112, 679–691. [Google Scholar] [PubMed]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved role of nanos proteins in germ cell development. Science 2003, 301, 1239–1241. [Google Scholar] [PubMed]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [PubMed]
- Handel, M.A.; Eppig, J.J. Sexual dimorphism in the regulation of mammalian meiosis. Curr. Top Dev. Biol. 1998, 37, 333–358. [Google Scholar] [PubMed]
- Bolcun-Filas, E.; Handel, M.A. Meiosis: The chromosomal foundation of reproduction. Biol. Reprod. 2018, 99, 112–126. [Google Scholar] [CrossRef]
- Dokshin, G.A.; Baltus, A.E.; Eppig, J.J.; Page, D.C. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat. Genet. 2013, 45, 877–883. [Google Scholar] [CrossRef]
- Su, Y.Q.; Sugiura, K.; Eppig, J.J. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 2009, 27, 32–42. [Google Scholar] [CrossRef]
- Duque, P.; Diez, C.; Royo, L.; Lorenzo, P.L.; Carneiro, G.; Hidalgo, C.O.; Facal, N.; Gomez, E. Enhancement of developmental capacity of meiotically inhibited bovine oocytes by retinoic acid. Hum. Reprod. 2002, 17, 2706–2714. [Google Scholar]
- Gomez, E.; Royo, L.J.; Duque, P.; Carneiro, G.; Hidalgo, C.; Goyache, F.; Lorenzo, P.L.; Alvarez, I.; Facal, N.; Diez, C. 9-cis-retinoic acid during in vitro maturation improves development of the bovine oocyte and increases midkine but not IGF-I expression in cumulus-granulosa cells. Mol. Reprod. Dev. 2003, 66, 247–255. [Google Scholar] [CrossRef]
- Hidalgo, C.O.; Diez, C.; Duque, P.; Facal, N.; Gomez, E. Pregnancies and improved early embryonic development with bovine oocytes matured in vitro with 9-cis-retinoic acid. Reproduction 2003, 125, 409–416. [Google Scholar]
- Deb, G.K.; Dey, S.R.; Bang, J.I.; Lee, J.G.; Kong, I.K. 9-cis Retinoic acid inhibits cumulus cell apoptosis during the maturation of bovine cumulus-oocyte-complexes. J. Anim. Sci. 2012, 90, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Read, C.C.; Dyce, P.W. All-trans retinoic acid exposure increases connexin 43 expression in cumulus cells and improves embryo development in bovine oocytes. Mol. Reprod. Dev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Wang, Z.; Bian, Y.; Zhang, F.; Yang, P.; Li, Y.; Zhang, Y.; Liu, Y.; Fang, F.; Cao, H.; et al. All-trans retinoic acid improves goat oocyte nuclear maturation and reduces apoptotic cumulus cells during in vitro maturation. Anim. Sci. J. 2014, 85, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Gil, M.A.; Cuello, C.; Caballero, I.; Roca, J.; Vazquez, J.M.; Gomez, E.; Martinez, E.A. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod. Fertil. Dev. 2008, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Suwa, H.; Kishi, H.; Imai, F.; Nakao, K.; Hirakawa, T.; Minegishi, T. Retinoic acid enhances progesterone production via the cAMP/PKA signaling pathway in immature rat granulosa cells. Biochem. Biophys. Rep. 2016, 8, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Tahaei, L.S.; Eimani, H.; Yazdi, P.E.; Ebrahimi, B.; Fathi, R. Effects of retinoic acid on maturation of immature mouse oocytes in the presence and absence of a granulosa cell co-culture system. J. Assist. Reprod. Genet. 2011, 28, 553–558. [Google Scholar] [CrossRef]
- Nasiri, E.; Mahmoudi, R.; Bahadori, M.H.; Amiri, I. The Effect of Retinoic Acid on in vitro Maturation and Fertilization Rate of Mouse Germinal Vesicle Stage Oocytes. Cell J. 2011, 13, 19–24. [Google Scholar]
- Ikeda, S.; Kitagawa, M.; Imai, H.; Yamada, M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J. Reprod. Dev. 2005, 51, 23–35. [Google Scholar] [CrossRef]
- Gomez, E.; Caamano, J.N.; Rodriguez, A.; De Frutos, C.; Facal, N.; Diez, C. Bovine early embryonic development and vitamin A. Reprod. Domest. Anim. 2006, 41, 63–71. [Google Scholar] [CrossRef]
- Mohan, M.; Thirumalapura, N.R.; Malayer, J. Bovine cumulus-granulosa cells contain biologically active retinoid receptors that can respond to retinoic acid. Reprod. Biol. Endocrinol. 2003, 1, 104. [Google Scholar] [CrossRef][Green Version]
- Kawai, T.; Yanaka, N.; Richards, J.S.; Shimada, M. De Novo-Synthesized Retinoic Acid in Ovarian Antral Follicles Enhances FSH-Mediated Ovarian Follicular Cell Differentiation and Female Fertility. Endocrinology 2016, 157, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, T.; Karino, S.; Tano, M.; Ibuki, Y.; Miyamoto, K. Regulation of midkine messenger ribonucleic acid levels in cultured rat granulosa cells. Biochem. Biophys. Res. Commun. 1996, 229, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Takesue, K.; Nishida, N.; Kato, Y.; Fujihara, N. Inhibitory effect of retinoic acid on the development of immature porcine granulosa cells to mature cells. J. Mol. Endocrinol. 2000, 25, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novi, A.M.; Saba, P. An electron microscopic study of the development of rat testis in the first 10 postnatal days. Z Zellforsch Mikrosk Anat 1968, 86, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am. J. Anat. 1956, 99, 391–413. [Google Scholar] [CrossRef]
- Oakberg, E.F. Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 1971, 169, 515–531. [Google Scholar] [CrossRef]
- de Rooij, D.G. Spermatogonial stem cell renewal in the mouse. I. Normal situation. Cell Tissue Kinet. 1973, 6, 281–287. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nabeshima, Y.; Yoshida, S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell 2007, 12, 195–206. [Google Scholar] [CrossRef]
- Chan, F.; Oatley, M.J.; Kaucher, A.V.; Yang, Q.E.; Bieberich, C.J.; Shashikant, C.S.; Oatley, J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014, 28, 1351–1362. [Google Scholar] [CrossRef]
- de Rooij, D.G. Stem cells in the testis. Int. J. Exp. Pathol. 1998, 79, 67–80. [Google Scholar] [CrossRef]
- Huckins, C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 1971, 169, 533–557. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar] [PubMed]
- de Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001, 121, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc. Natl. Acad. Sci. USA 2000, 97, 8346–8351. [Google Scholar] [CrossRef]
- Lok, D.; de Rooij, D.G. Spermatogonial multiplication in the Chinese hamster. III. Labelling indices of undifferentiated spermatogonia throughout the cycle of the seminiferous epithelium. Cell Tissue Kinet. 1983, 16, 31–40. [Google Scholar]
- Monesi, V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J. Cell Biol. 1962, 14, 1–18. [Google Scholar] [CrossRef]
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Griswold, M.D. The testicular iron shuttle: A “nurse” function of the Sertoli cells. J. Androl. 1994, 15, 381–385. [Google Scholar]
- Franca, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Oakberg, E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 1956, 99, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; de Rooij, D.G.; Page, D.C. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef] [PubMed]
- Muciaccia, B.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; de Rooij, D.G.; Vicini, E. Novel stage classification of human spermatogenesis based on acrosome development. Biol. Reprod. 2013, 89, 60. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.P.; Clermont, Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the “periodic acid-fuchsin sulfurous acid” technique. Am J Anat 1952, 90, 167–215. [Google Scholar] [CrossRef]
- Lok, D.; Weenk, D.; De Rooij, D.G. Morphology, proliferation, and differentiation of undifferentiated spermatogonia in the Chinese hamster and the ram. Anat. Rec. 1982, 203, 83–99. [Google Scholar] [CrossRef]
- Wolbach, S.B.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef]
- Mason, K.E. Differences in testis injury and repair after vitamin A-deficiency, vitamin E-deficiency, and inanition. Am. J. Anat. 1933, 52, 153–239. [Google Scholar] [CrossRef]
- Thompson, J.N.; Howell, J.M.; Pitt, G.A. Vitamin a and Reproduction in Rats. Proc R Soc Lond B Biol Sci 1964, 159, 510–535. [Google Scholar]
- Livera, G.; Rouiller-Fabre, V.; Pairault, C.; Levacher, C.; Habert, R. Regulation and perturbation of testicular functions by vitamin A. Reproduction 2002, 124, 173–180. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Griswold, M.D. Driving asynchronous spermatogenesis: Is retinoic acid the answer? Anim. Reprod. 2012, 9, 742–750. [Google Scholar]
- Mitranond, V.; Sobhon, P.; Tosukhowong, P.; Chindaduangrat, W. Cytological changes in the testes of vitamin-A-deficient rats. I. Quantitation of germinal cells in the seminiferous tubules. Acta Anat. 1979, 103, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Unni, E.; Rao, M.R.; Ganguly, J. Histological & ultrastructural studies on the effect of vitamin A depletion & subsequent repletion with vitamin A on germ cells & Sertoli cells in rat testis. Indian J. Exp. Biol. 1983, 21, 180–192. [Google Scholar] [PubMed]
- Morales, C.; Griswold, M.D. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 1987, 121, 432–434. [Google Scholar] [CrossRef] [PubMed]
- van Pelt, A.M.; De Rooij, D.G. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol. Reprod. 1990, 42, 677–682. [Google Scholar] [CrossRef]
- van Pelt, A.M.; de Rooij, D.G. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol. Reprod. 1990, 43, 363–367. [Google Scholar] [CrossRef]
- Huang, H.F.; Hembree, W.C. Spermatogenic response to vitamin A in vitamin A deficient rats. Biol. Reprod. 1979, 21, 891–904. [Google Scholar] [CrossRef]
- Amory, J.K.; Muller, C.H.; Shimshoni, J.A.; Isoherranen, N.; Paik, J.; Moreb, J.S.; Amory, D.W., Sr.; Evanoff, R.; Goldstein, A.S.; Griswold, M.D. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J. Androl. 2011, 32, 111–119. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Evanoff, R.; Snyder, E.; Kent, T.; Mitchell, D.; Small, C.; Amory, J.; Griswold, M.D. Suppression of Stra8 Expression in the Mouse Gonad by WIN 18,446. Biol. Reprod. 2011, 84, 957–965. [Google Scholar] [CrossRef]
- Brooks, N.L.; van der Horst, G. Short-term effects of N’N-bis(dichloroacetyl)-1,8-octamethylenediamine (WIN 18446) on the testes, selected sperm parameters and fertility of male CBA mice. Lab Anim. 2003, 37, 363–373. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Evanoff, R.; Mitchell, D.; Kent, T.; Small, C.; Amory, J.K.; Griswold, M.D. Turning a spermatogenic wave into a tsunami: Synchronizing murine spermatogenesis using WIN 18,446. Biol. Reprod. 2013, 88, 40. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Small, C.; Griswold, M.D. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol. Reprod. 2010, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Drumond, A.L.; Meistrich, M.L.; Chiarini-Garcia, H. Spermatogonial morphology and kinetics during testis development in mice: A high-resolution light microscopy approach. Reproduction 2011, 142, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Teletin, M.; Vernet, N.; Ghyselinck, N.B. Role of retinoic acid receptor (RAR) signaling in post-natal male germ cell differentiation. Biochim. et Biophys. Acta 2015, 1849, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015, 92, 37. [Google Scholar] [CrossRef]
- Pellegrini, M.; Filipponi, D.; Gori, M.; Barrios, F.; Lolicato, F.; Grimaldi, P.; Rossi, P.; Jannini, E.A.; Geremia, R.; Dolci, S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008, 7, 3878–3888. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Nishikawa, S.; Ogawa, M.; Hayashi, S.; Kunisada, T.; Fujimoto, T. Role of c-kit in mouse spermatogenesis: Identification of spermatogonia as a specific site of c-kit expression and function. Development 1991, 113, 689–699. [Google Scholar]
- Schrans-Stassen, B.H.; van de Kant, H.J.; de Rooij, D.G.; van Pelt, A.M. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- de Rooij, D.G.; Okabe, M.; Nishimune, Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol. Reprod. 1999, 61, 842–847. [Google Scholar] [CrossRef]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef]
- Buaas, F.W.; Kirsh, A.L.; Sharma, M.; McLean, D.J.; Morris, J.L.; Griswold, M.D.; de Rooij, D.G.; Braun, R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004, 36, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Filipponi, D.; Hobbs, R.M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Pandolfi, P.P.; Dolci, S. Repression of kit expression by Plzf in germ cells. Mol. Cell Biol. 2007, 27, 6770–6781. [Google Scholar] [CrossRef]
- Hobbs, R.M.; Fagoonee, S.; Papa, A.; Webster, K.; Altruda, F.; Nishinakamura, R.; Chai, L.; Pandolfi, P.P. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell 2012, 10, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Gely-Pernot, A.; Raverdeau, M.; Teletin, M.; Vernet, N.; Feret, B.; Klopfenstein, M.; Dennefeld, C.; Davidson, I.; Benoit, G.; Mark, M.; et al. Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor. PLoS Genet. 2015, 11, e1005501. [Google Scholar] [CrossRef] [PubMed]
- Teletin, M.; Vernet, N.; Ghyselinck, N.B.; Mark, M. Roles of Retinoic Acid in Germ Cell Differentiation. Curr. Top Dev. Biol. 2017, 125, 191–225. [Google Scholar]
- Busada, J.T.; Chappell, V.A.; Niedenberger, B.A.; Kaye, E.P.; Keiper, B.D.; Hogarth, C.A.; Geyer, C.B. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev. Biol. 2015, 397, 140–149. [Google Scholar] [CrossRef]
- Busada, J.T.; Geyer, C.B. The Role of Retinoic Acid (RA) in Spermatogonial Differentiation. Biol. Reprod. 2016, 94, 10. [Google Scholar] [CrossRef]
- Gely-Pernot, A.; Raverdeau, M.; Celebi, C.; Dennefeld, C.; Feret, B.; Klopfenstein, M.; Yoshida, S.; Ghyselinck, N.B.; Mark, M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology 2012, 153, 438–449. [Google Scholar] [CrossRef]
- Ikami, K.; Tokue, M.; Sugimoto, R.; Noda, C.; Kobayashi, S.; Hara, K.; Yoshida, S. Hierarchical differentiation competence in response to retinoic acid ensures stem cell maintenance during mouse spermatogenesis. Development 2015, 142, 1582–1592. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Harrison, C.A.; Rainczuk, K.E.; Wayne Vogl, A.; Stanton, P.G. Retinoic acid promotes Sertoli cell differentiation and antagonises activin-induced proliferation. Mol. Cell Endocrinol. 2013, 377, 33–43. [Google Scholar] [CrossRef]
- Huang, H.F.; Marshall, G.R. Failure of spermatid release under various vitamin A states - an indication of delayed spermiation. Biol. Reprod. 1983, 28, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Sung, W.; Wang, X.; Wolgemuth, D.J. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev. Dyn. 2004, 230, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.W.; Wang, X.Y.; Wolgemuth, D.J. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation 2005, 73, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wang, X.; Wolgemuth, D.J. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development 2009, 136, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wang, X.; Roberts, S.S.; Griffey, S.M.; Reczek, P.R.; Wolgemuth, D.J. Oral administration of a retinoic Acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 2011, 152, 2492–2502. [Google Scholar] [CrossRef]
- Hasegawa, K.; Saga, Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development 2012, 139, 4347–4355. [Google Scholar] [CrossRef]
- Chung, S.S.; Wang, X.; Wolgemuth, D.J. Prolonged Oral Administration of a Pan-Retinoic Acid Receptor Antagonist Inhibits Spermatogenesis in Mice With a Rapid Recovery and Changes in the Expression of Influx and Efflux Transporters. Endocrinology 2016, 157, 1601–1612. [Google Scholar] [CrossRef]
- Hosken, D.J.; Hodgson, D.J. Why do sperm carry RNA? Relatedness, conflict, and control. Trends Ecol. Evol. 2014, 29, 451–455. [Google Scholar] [CrossRef]
- Sugimoto, R.; Nabeshima, Y.I.; Yoshida, S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech. Dev. 2012, 128, 610–624. [Google Scholar] [CrossRef]
- Teletin, M.; Vernet, N.; Yu, J.; Klopfenstein, M.; Jones, J.W.; Feret, B.; Kane, M.A.; Ghyselinck, N.B.; Mark, M. Two functionally redundant sources of retinoic acid secure spermatogonia differentiation in the seminiferous epithelium. Development 2019, 146, 1–14. [Google Scholar] [CrossRef]
- Beedle, M.T.; Stevison, F.; Zhong, G.; Topping, T.; Hogarth, C.; Isoherranen, N.; Griswold, M.D. Sources of all-trans retinal oxidation independent of the aldehyde dehydrogenase 1A isozymes exist in the postnatal testis. Biol. Reprod. 2019, 100, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.; Sakai, M.; Kuroki, S.; Jo, J.I.; Hoshina, K.; Fujimori, Y.; Oka, K.; Amano, T.; Yamanaka, T.; Tachibana, M.; et al. FGF2 Has Distinct Molecular Functions from GDNF in the Mouse Germline Niche. Stem Cell Rep. 2018, 10, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.A.; Garcia, T.X.; Waheeb, R.; Jain, V.; Gandhi, P.; Meistrich, M.L.; Shetty, G.; Hofmann, M.C. Undifferentiated spermatogonia regulate Cyp26b1 expression through NOTCH signaling and drive germ cell differentiation. FASEB J. 2019, 33, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, T.; Mikedis, M.M.; Nicholls, P.K.; Page, D.C.; de Rooij, D.G. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules 2019, 9, 775. https://doi.org/10.3390/biom9120775
Endo T, Mikedis MM, Nicholls PK, Page DC, de Rooij DG. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules. 2019; 9(12):775. https://doi.org/10.3390/biom9120775
Chicago/Turabian StyleEndo, Tsutomu, Maria M. Mikedis, Peter K. Nicholls, David C. Page, and Dirk G. de Rooij. 2019. "Retinoic Acid and Germ Cell Development in the Ovary and Testis" Biomolecules 9, no. 12: 775. https://doi.org/10.3390/biom9120775
APA StyleEndo, T., Mikedis, M. M., Nicholls, P. K., Page, D. C., & de Rooij, D. G. (2019). Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules, 9(12), 775. https://doi.org/10.3390/biom9120775





