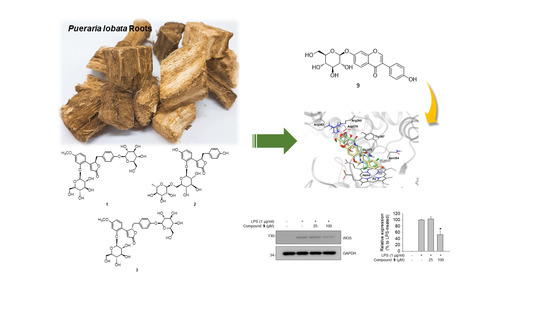
Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation
2.2.1. Lobatamunsolide A (1)
2.2.2. Lobatamunsolide B (2)
2.2.3. Lobatamunsolide C (3)
2.3. Acid Hydrolysis and Determination of the Absolute Configuration of Sugar Moieties
2.4. Computational Analysis
2.5. NO Production Assay
2.6. Cell Viability Assay
2.7. Preparation of Ligand and Receptor for Docking
2.8. Molecular Docking Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results and Discussion
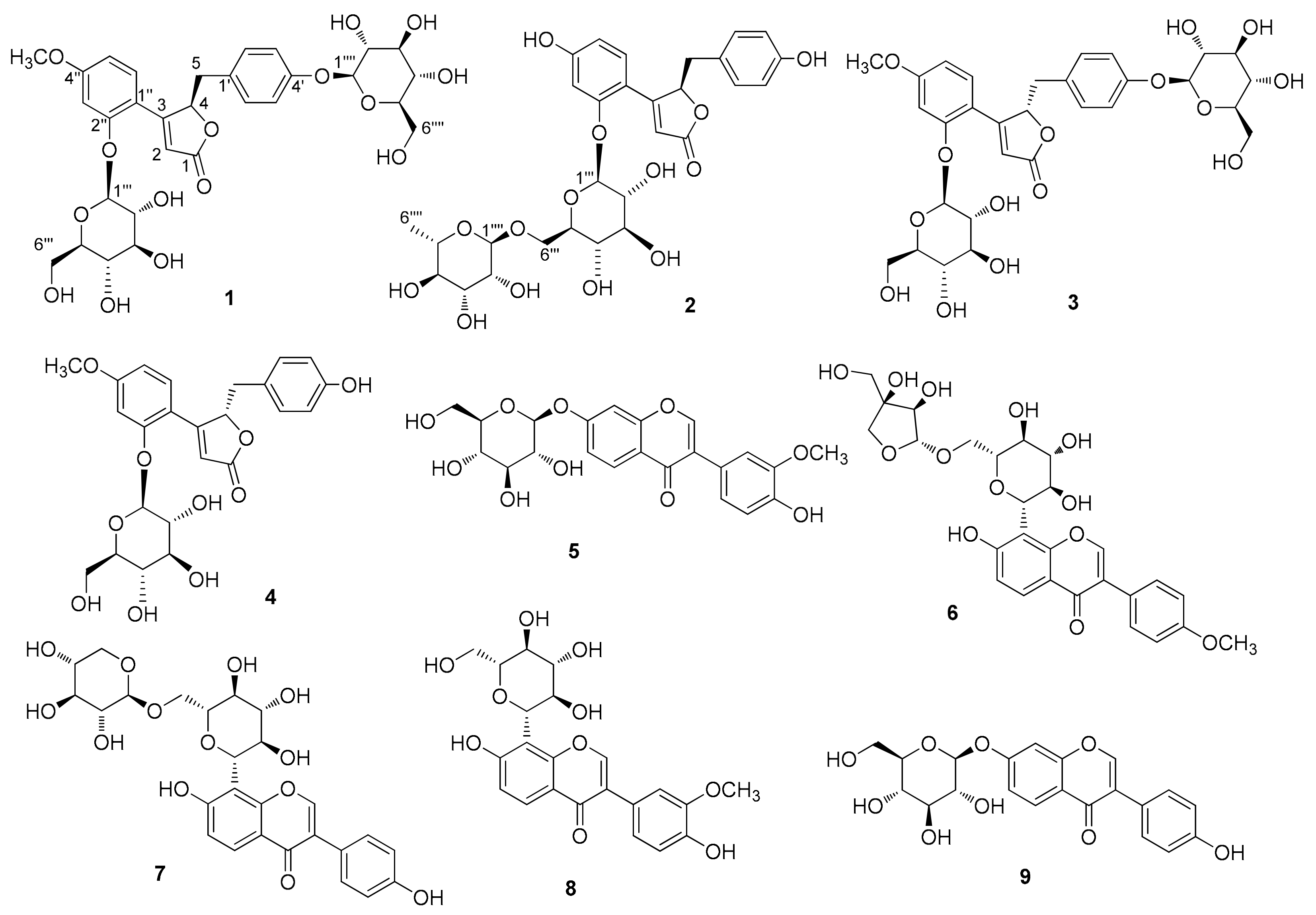
3.1. Isolation of Compounds
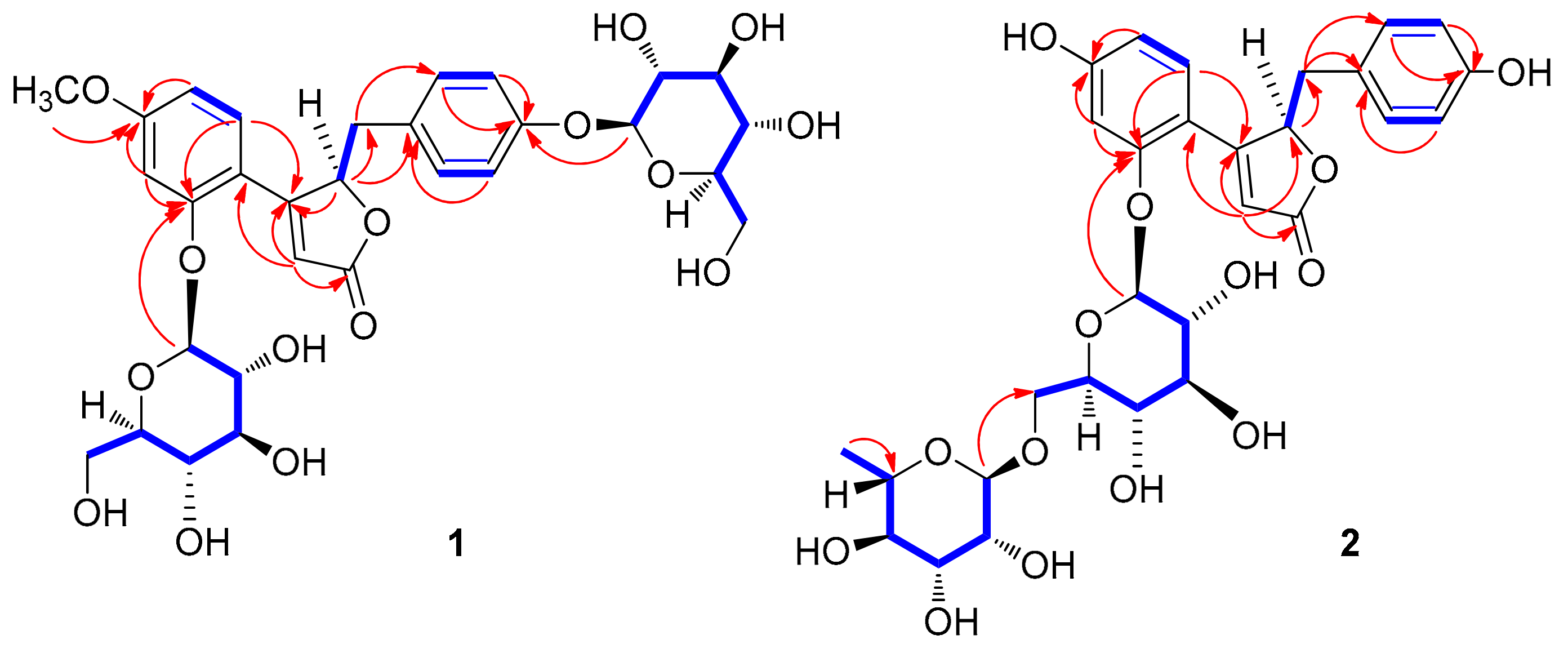
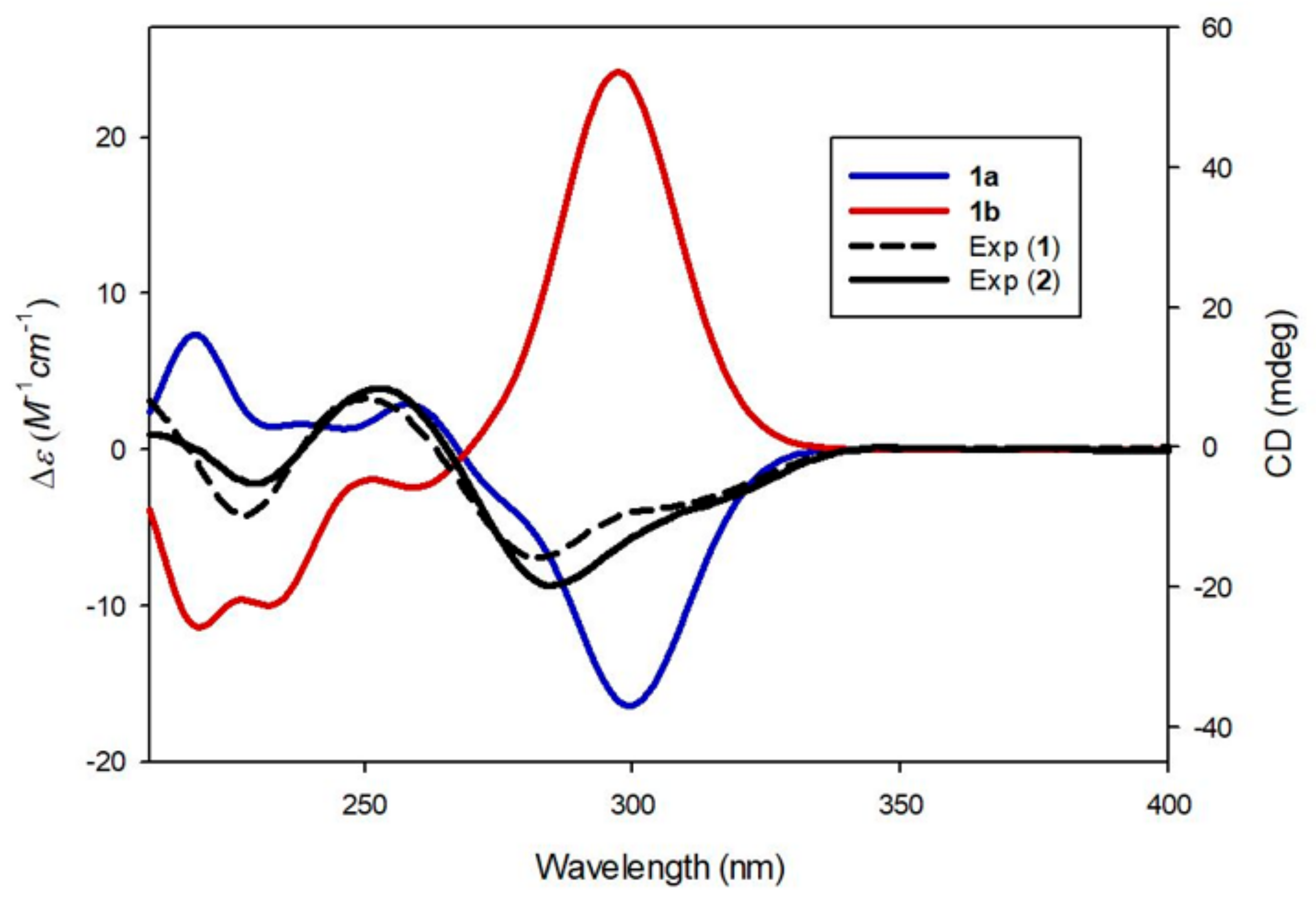
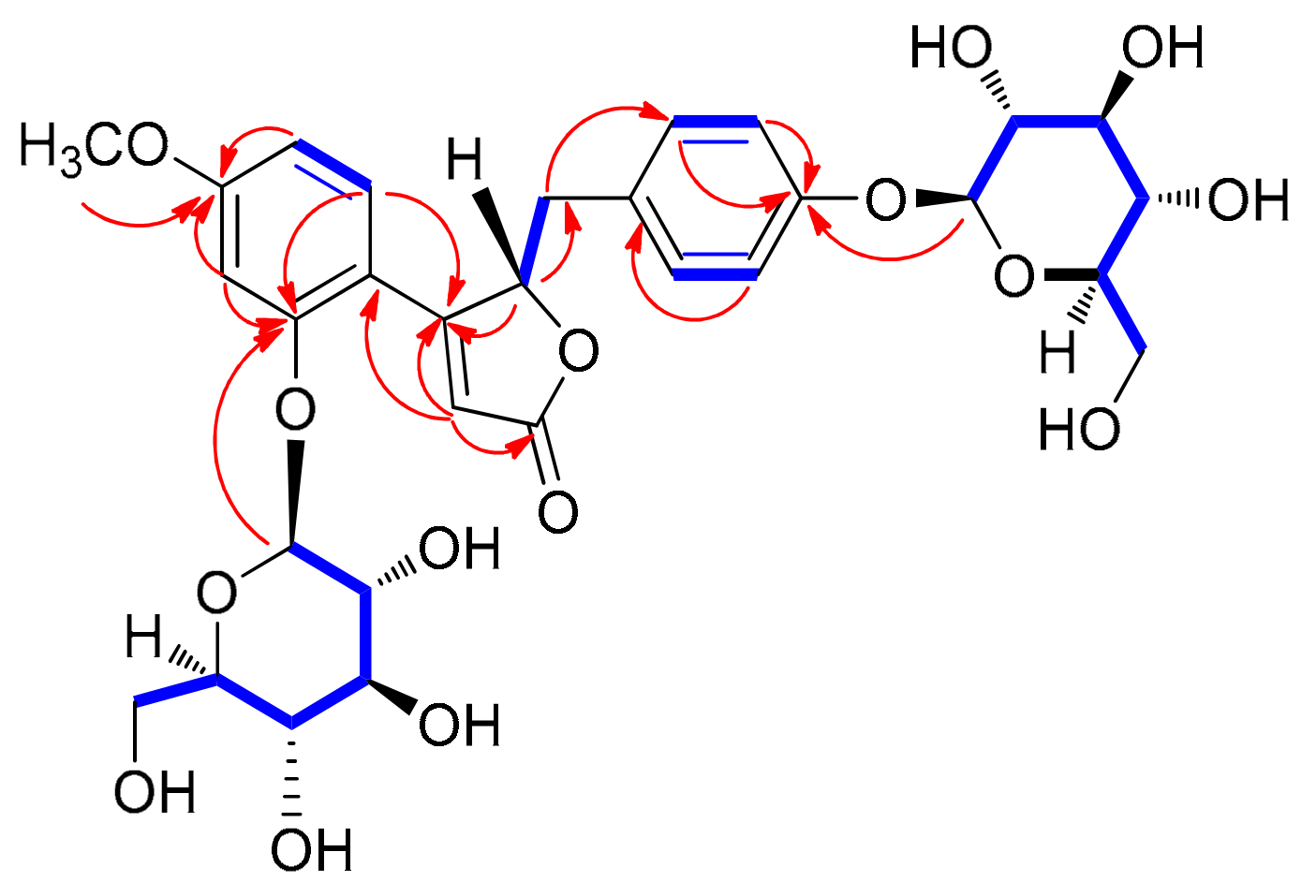
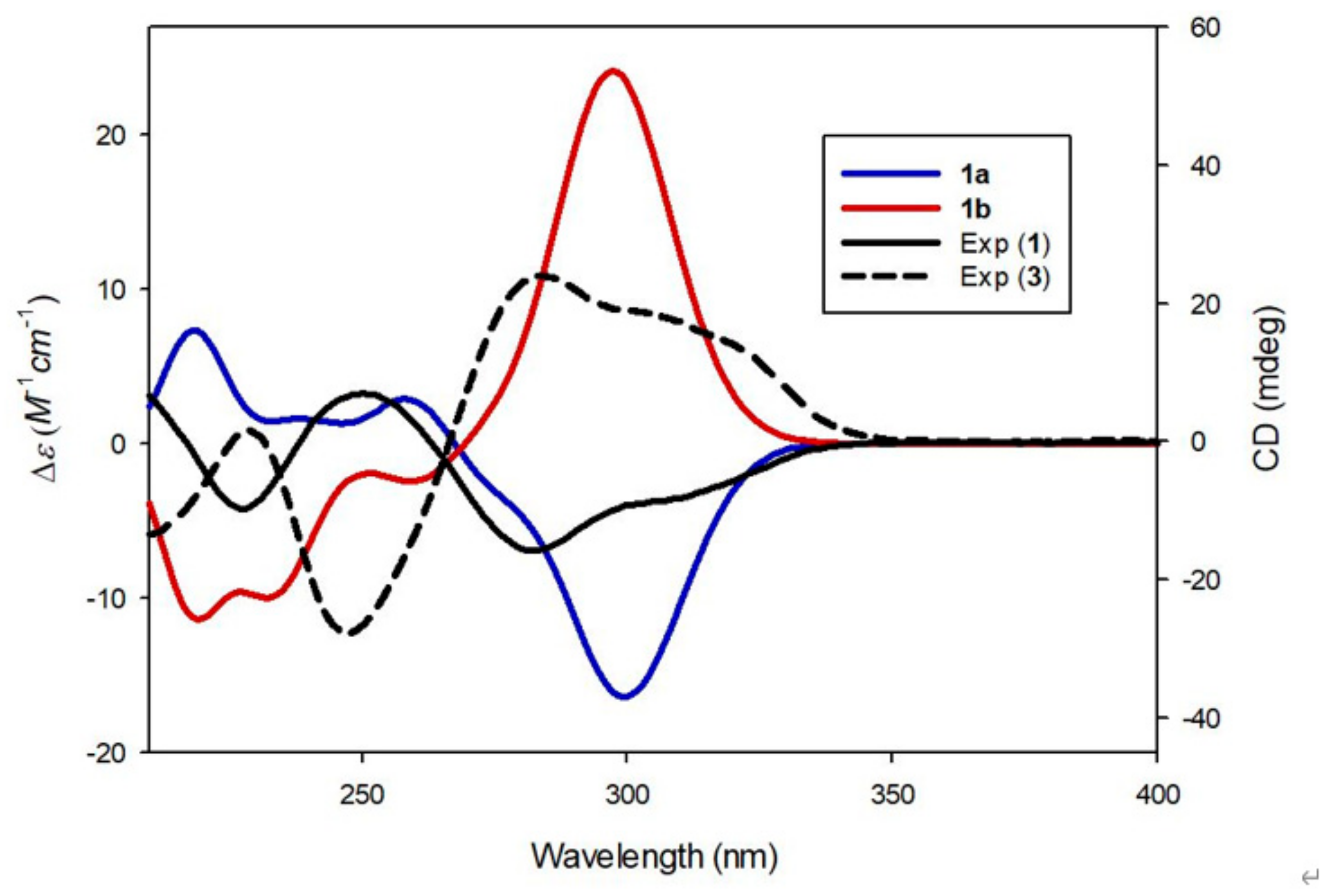
3.2. Structure Elucidation of Compounds
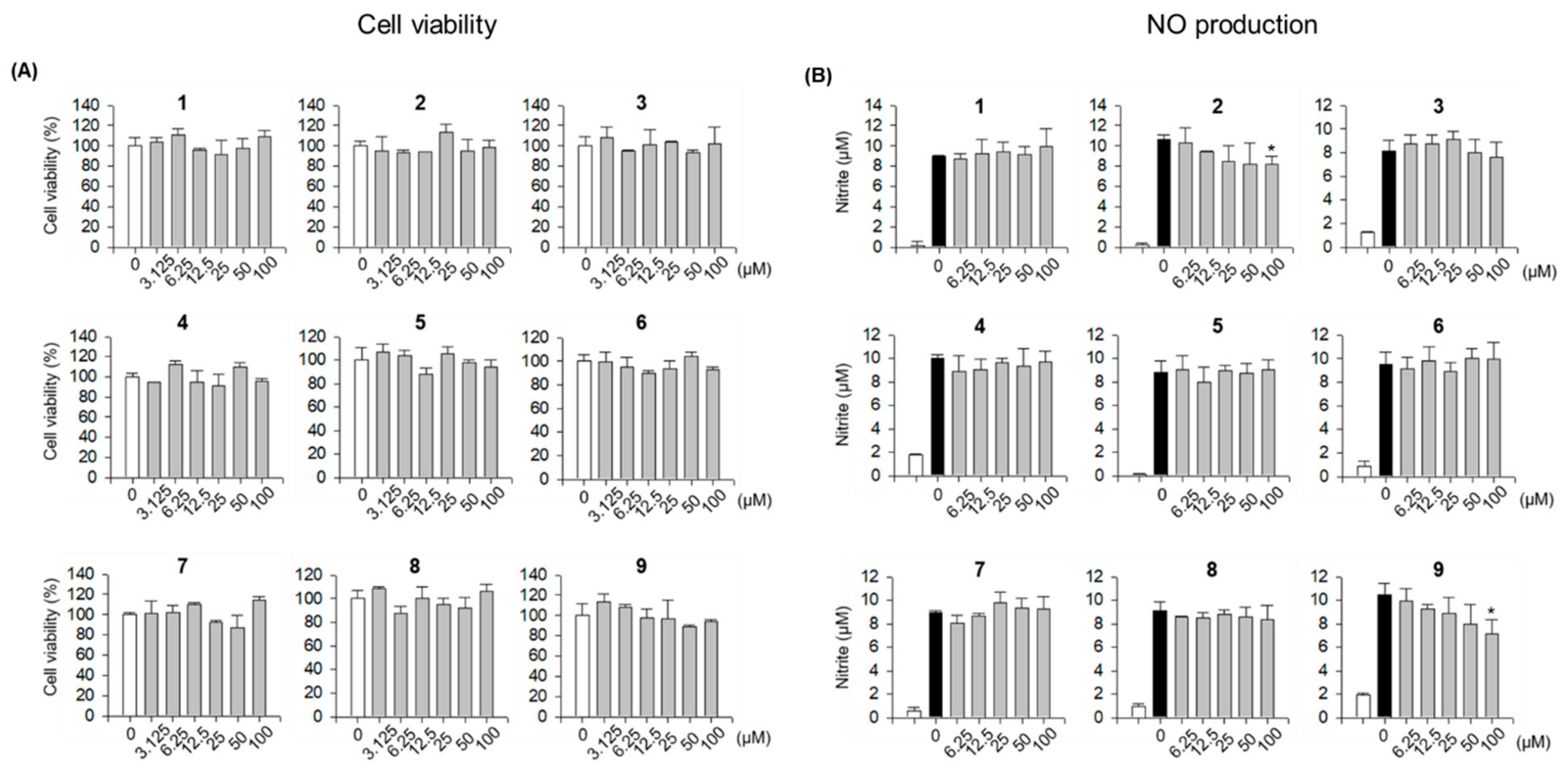
3.3. Inhibitory Effects of Compounds 1–6 on LPS-Induced NO Production in RAW 264.7 Cells
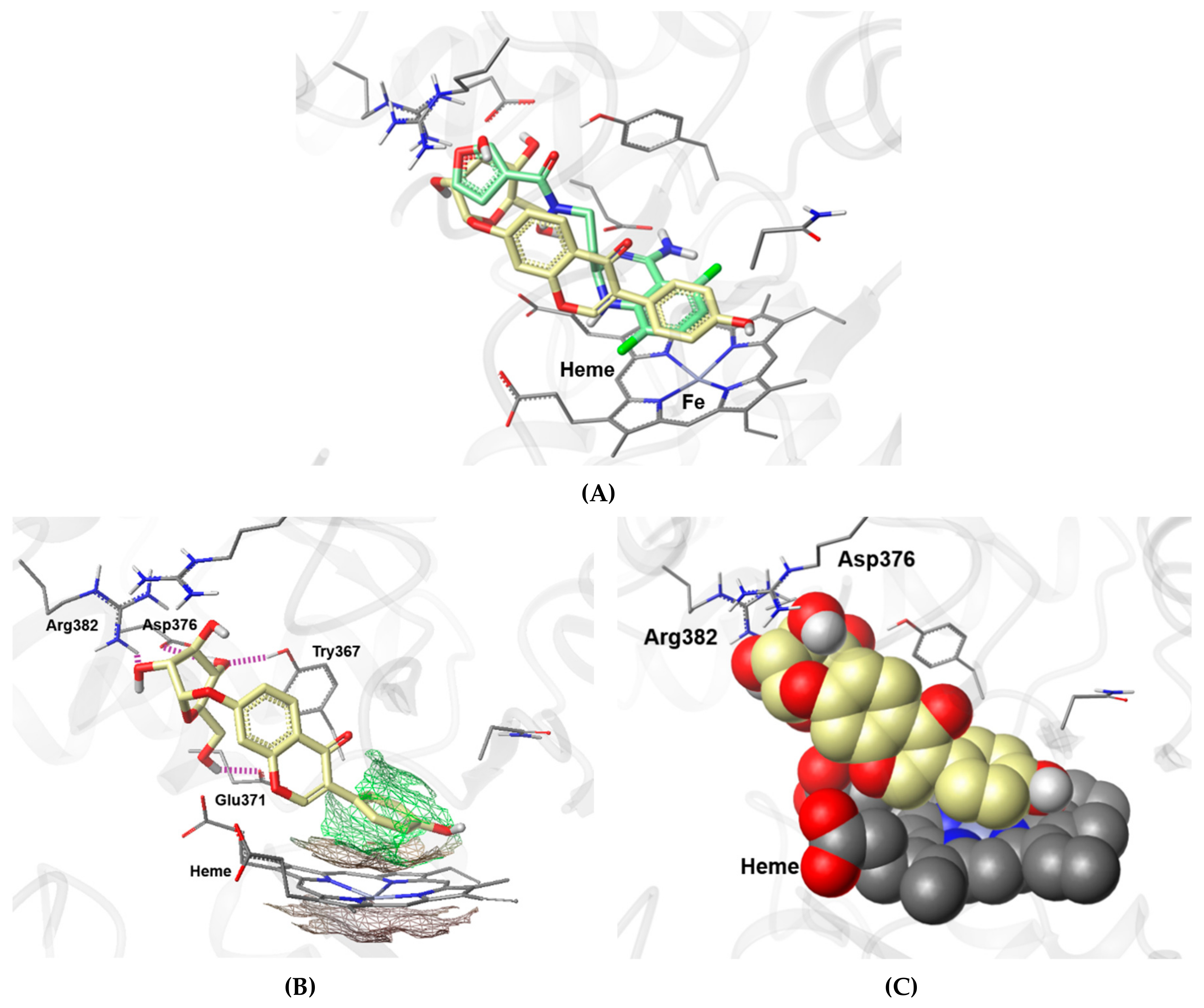
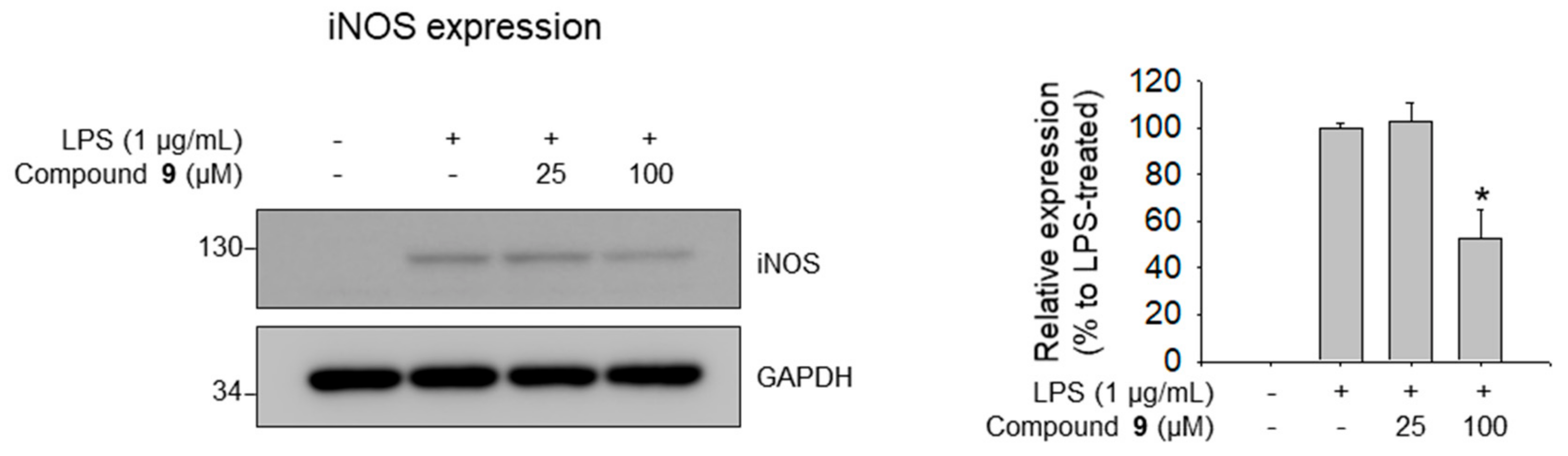
3.4. Molecular Docking Analysis of 9 and Its Inhibitory Effects on iNOS Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasain, J.K.; Jones, K.; Kirk, M.; Wilson, L.; Smith-Johnson, M.; Weaver, C.; Barnes, S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2018, 51, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.T. The history and distribution of five exotic weeds in North Carolina. Castanea 1976, 177–180. [Google Scholar]
- Tanaka, T.; Tang, H.; Yu, F.; Michihara, S.; Uzawa, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Kudzu (Pueraria lobata) vine ethanol extracts improve ovariectomy-induced bone loss in female mice. J. Agric. Food Chem. 2011, 59, 13230–13237. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.Y. The efficacy and tolerability of adjunctive alternative herbal medicine (Salvia miltiorrhiza and Pueraria lobata) on vascular function and structure in coronary patients. J. Altern. Complement. Med. 2009, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lam, T.N.; Zuo, Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2013, 53, 787–811. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kang, A.N.; Kang, S.; Park, Y.K.; Song, M. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Bebrevska, L.; Foubert, K.; Hermans, N.; Chatterjee, S.; Van Marck, E.; De Meyer, G.; Vlietinck, A.; Pieters, L.; Apers, S. In vivo antioxidative activity of a quantified Pueraria lobata root extract. J. Ethnopharmacol. 2010, 127, 112–117. [Google Scholar] [CrossRef]
- Reppert, A.; Yousef, G.G.; Rogers, R.B.; Lila, M.A. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. J. Agric. Food Chem. 2008, 56, 7860–7865. [Google Scholar] [CrossRef]
- Jin, S.E.; Son, Y.K.; Min, B.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 2012, 35, 823–837. [Google Scholar] [CrossRef]
- Keung, W.M.; Vallee, B.L. Kudzu root: An ancient Chinese source of modern antidipsotropic agents. Phytochemistry 1998, 47, 499–506. [Google Scholar] [CrossRef]
- Kayano, S.I.; Matsumura, Y.; Kitagawa, Y.; Kobayashi, M.; Nagayama, A.; Kawabata, N.; Kikuzaki, H.; Kitada, Y. Isoflavone C-glycosides isolated from the root of kudzu (Pueraria lobata) and their estrogenic activities. Food Chem. 2012, 134, 282–287. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Lee, D.; Lee, S.R.; Lee, J.W.; Choi, C.-I.; Jang, T.S.; Kang, K.S.; Kim, K.H. Chemical characterization of cytotoxic indole acetic acid derivative from Mulberry fruit (Morus alba L.) against human cervical cancer. Bioorg. Chem. 2018, 76, 28–36. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.-H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018, 41, 815–822. [Google Scholar] [PubMed]
- Yu, J.S.; Roh, H.-S.; Baek, K.-H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Song, J.H.; Song, J.-H.; Ko, H.-J.; Baek, J.Y.; Trinh, T.A.; Beemelmanns, C.; Yamabe, N.; Kim, K.H. Chemical identification of isoflavonoids from a termite-associated Streptomyces sp. RB1 and their neuroprotective effects in murine hippocampal HT22 cell line. Int. J. Mol. Sci. 2018, 19, 2640. [Google Scholar] [CrossRef]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar]
- Ahn, S.-Y.; Jo, M.S.; Lee, D.; Baek, S.-E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.-E.; et al. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef]
- Hirakura, K.; Morita, M.; Nakajima, K.; Sugama, K.; Takagi, K.; Niitsu, K.; Ikeya, Y.; Maruno, M.; Okada, M. Phenolic glucosides from the root of Pueraria lobata. Phytochemistry 1997, 46, 921–928. [Google Scholar] [CrossRef]
- Coxon, B. Two-dimensional J.-resolved proton nuclear magnetic resonance spectrometry of hydroxyl-coupled α- and β-d-glucose. Anal. Chem. 1983, 55, 2361–2366. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Muhit, M.A.; Umehara, K.; Mori-Yasumoto, K.; Noguchi, H. Furofuran lignan glucosides with estrogen-inhibitory properties from the Bangladeshi medicinal plant Terminalia citrina. J. Nat. Prod. 2016, 79, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; Kim, S.M.; Kang, U.; Chung, H.Y.; Seo, E.K. Peroxynitrite-scavenging glycosides from the stem bark of Catalpa ovata. J. Nat. Prod. 2017, 80, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Behar, I.; D’agostino, M.; Simone, F.D.; Schettino, O.; Pizza, C. Phytochemical investigation on Mercurialis annua. Biochem. Syst. Ecol. 1987, 15, 667–669. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chen, Y.P.; Chang, W.L.; Lin, H.C. Isoflavonoids and but-2-enolides from the roots of Pueraria lobata. Chin. Pharm. J. 2004, 56, 31–35. [Google Scholar]
- Wang, F.R.; Ge, X.Z.; Yang, X.W. Chemical constituents of Tongmai formula. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 61–69. [Google Scholar]
- Kinjo, J.E.; Furusawa, J.I.; Baba, J.; Takeshita, T.; Yamasaki, M.; Nohara, T. Studies on the constituents of Pueraria lobata. III. Isoflavonoids and related compounds in the roots and the voluble stems. Chem. Pharm. Bull. 1987, 35, 4846–4850. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Lee, J.C.; Kang, K.S.; Ryoo, R.; Park, H.J.; Kim, K.H. Bioactivity-guided Isolation of Anti-inflammatory Constituents of the Rare Mushroom Calvatia nipponica in LPS-stimulated RAW264.7 Macrophages. Chem. Biodivers. 2018, 15, e1800203. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Kim, K.H. Anti-inflammatory activity of the sclerotia of edible fungus, Poria cocos Wolf and their active lanostane triterpenoids. J. Funct. Foods 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar]
| Position | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | ||||
| 1 | 176.4 | s | 176.7 | s | 176.0 | s | |||
| 2 | 6.30 d (1.0) | 115.9 | d | 6.26 s | 114.9 | d | 6.17 s | 114.4 | d |
| 3 | 166.7 | s | 167.3 | s | 168.1 | s | |||
| 4 | 6.07 td (4.5, 1.0) | 84.6 | d | 6.04 td (5.0, 1.0) | 84.9 | d | 6.16 m | 85.5 | d |
| 5a | 2.92 dd (15.0, 5.0) | 38.7 | t | 2.86 dd (15.0, 5.0) | 38.9 | t | 2.85 dd (15.0. 6.0) | 38.8 | t |
| 5b | 3.24 dd (15.0, 4.5) | 3.20 dd (15.0, 4.0) | 3.24 dd (15.0, 3.5) | ||||||
| 1′ | 129.6 | s | 126.7 | s | 130.8 | s | |||
| 2′ | 6.85 d (8.5) | 131.9 | d | 6.78 d (8.5) | 131.9 | d | 7.00 d (8.5) | 131.7 | d |
| 3′ | 6.89 d (8.5) | 117.0 | d | 6.60 d (8.5) | 115.5 | d | 6.95 d (8.5) | 117.1 | d |
| 4′ | 158.0 | s | 157.0 | s | 157.9 | s | |||
| 5′ | 6.89 d (8.5) | 117.0 | d | 6.60 d (8.5) | 115.5 | d | 6.95 d (8.5) | 117.1 | d |
| 6′ | 6.85 d (8.5) | 131.9 | d | 6.78 d (8.5) | 131.9 | d | 7.00 d (8.5) | 131.7 | d |
| 1″ | 113.9 | s | 113.1 | s | 113.7 | s | |||
| 2″ | 158.5 | s | 158.5 | s | 158.1 | s | |||
| 3″ | 6.96 d (2.5) | 102.7 | d | 6.77 d (2.5) | 104.0 | d | 6.96 d (2.5) | 102.3 | d |
| 4″ | 164.8 | s | 163.0 | s | 165.0 | s | |||
| 5″ | 6.73 dd (8.5, 2.5) | 109.5 | d | 6.60 dd (8.5, 2.5) | 110.9 | d | 6.74 dd (8.5, 2.5) | 109.9 | d |
| 6″ | 7.34 d (8.5) | 131.8 | d | 7.26 d (8.5) | 132.1 | d | 7.45 d (8.5) | 132.2 | d |
| 1‴ | 5.12 d (7.5) | 101.6 | d | 5.03 d (7.5) | 101.6 | d | 5.07 d (7.5) | 101.8 | d |
| 2‴ | 3.48 m | 74.6 | d | 3.49 m | 74.5 | d | 3.48 m | 74.4 | d |
| 3‴ | 3.42 m | 77.9 | d | 3.46 m | 78.3 | d | 3.48 m | 78.0 | d |
| 4‴ | 3.36 m | 71.1 | d | 3.40 m | 71.0 | d | 3.36 m | 71.2 | d |
| 5‴ | 3.52 m | 78.4 | d | 3.59 m | 76.9 | d | 3.55 m | 78.4 | d |
| 6‴a | 3.92 dd (12.0, 2.0) | 62.4 | t | 4.03 dd (11.5, 2.0) | 67.5 | t | 3.95 dd (12.0, 2.0) | 62.4 | t |
| 6‴b | 3.68 dd (12.0, 6.0) | 3.65 dd (11.5, 6.0) | 3.71 dd (12.0, 6.0) | ||||||
| 1⁗ | 4.84 d (7.5) | 102.1 | d | 4.70 d (1.0) | 102.1 | d | 4.85 d (7.5) | 102.1 | d |
| 2⁗ | 3.42 m | 74.8 | d | 3.88 m | 71.8 | d | 3.44 m | 74.6 | d |
| 3⁗ | 3.44 m | 77.8 | d | 3.69 dd (9.5, 3.5) | 72.0 | d | 3.48 m | 78.0 | d |
| 4⁗ | 3.38 m | 71.1 | d | 3.33 m | 73.8 | d | 3.44 m | 71.1 | d |
| 5⁗ | 3.48 m | 78.5 | d | 3.63 dd (9.6, 6.5) | 69.6 | d | 3.39 m | 78.3 | d |
| 6⁗a | 3.86 dd (12.0, 2.0) | 62.3 | t | 1.18 d (6.0) | 17.6 | q | 3.90 dd (12.0, 2.0) | 62.2 | t |
| 6⁗b | 3.68 dd (12.0, 6.0) | 3.69 dd (12.0, 6.0) | |||||||
| 4″-OCH3 | 3.88 s | 56.0 | q | 3.88 s | 55.8 | q | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.S.; Yu, J.S.; Lee, J.C.; Lee, S.; Cho, Y.-C.; Park, H.-J.; Kim, K.H. Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages. Biomolecules 2019, 9, 755. https://doi.org/10.3390/biom9120755
Jo MS, Yu JS, Lee JC, Lee S, Cho Y-C, Park H-J, Kim KH. Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages. Biomolecules. 2019; 9(12):755. https://doi.org/10.3390/biom9120755
Chicago/Turabian StyleJo, Mun Seok, Jae Sik Yu, Joo Chan Lee, Seoyoung Lee, Young-Chang Cho, Hyun-Ju Park, and Ki Hyun Kim. 2019. "Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages" Biomolecules 9, no. 12: 755. https://doi.org/10.3390/biom9120755
APA StyleJo, M. S., Yu, J. S., Lee, J. C., Lee, S., Cho, Y.-C., Park, H.-J., & Kim, K. H. (2019). Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages. Biomolecules, 9(12), 755. https://doi.org/10.3390/biom9120755