Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr.
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Determination of Total Phenolic and Total Flavonoid Content
2.5. Antioxidant Activity
2.5.1. Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.5.2. Cellular Antioxidant Activity (CAA) Assay
2.6. Anti-Inflammatory Activity
2.6.1. Anti-Inflammatory Activity Assay for the Inhibition of iNOS
2.6.2. Reporter Gene Assay for the Inhibition of NF-κB
2.7. Antiprotozoal Assay
2.8. Antimicrobial Assay
2.9. Cytotoxicity Assays
2.10. Radioligand Displacement for Cannabinoid and Opioid Receptor Subtypes
2.11. Statistical Analysis
3. Results
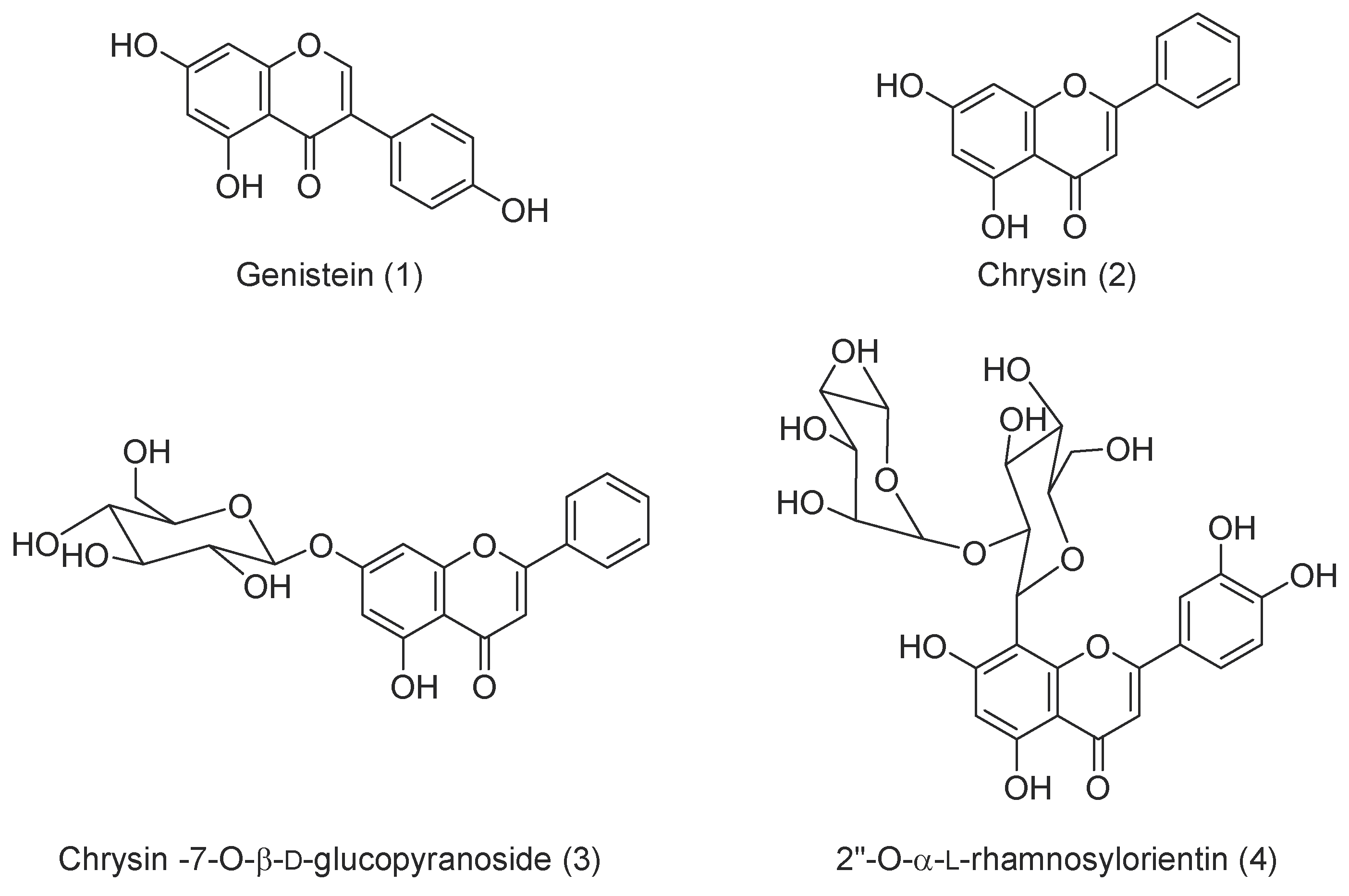
3.1. Chemistry
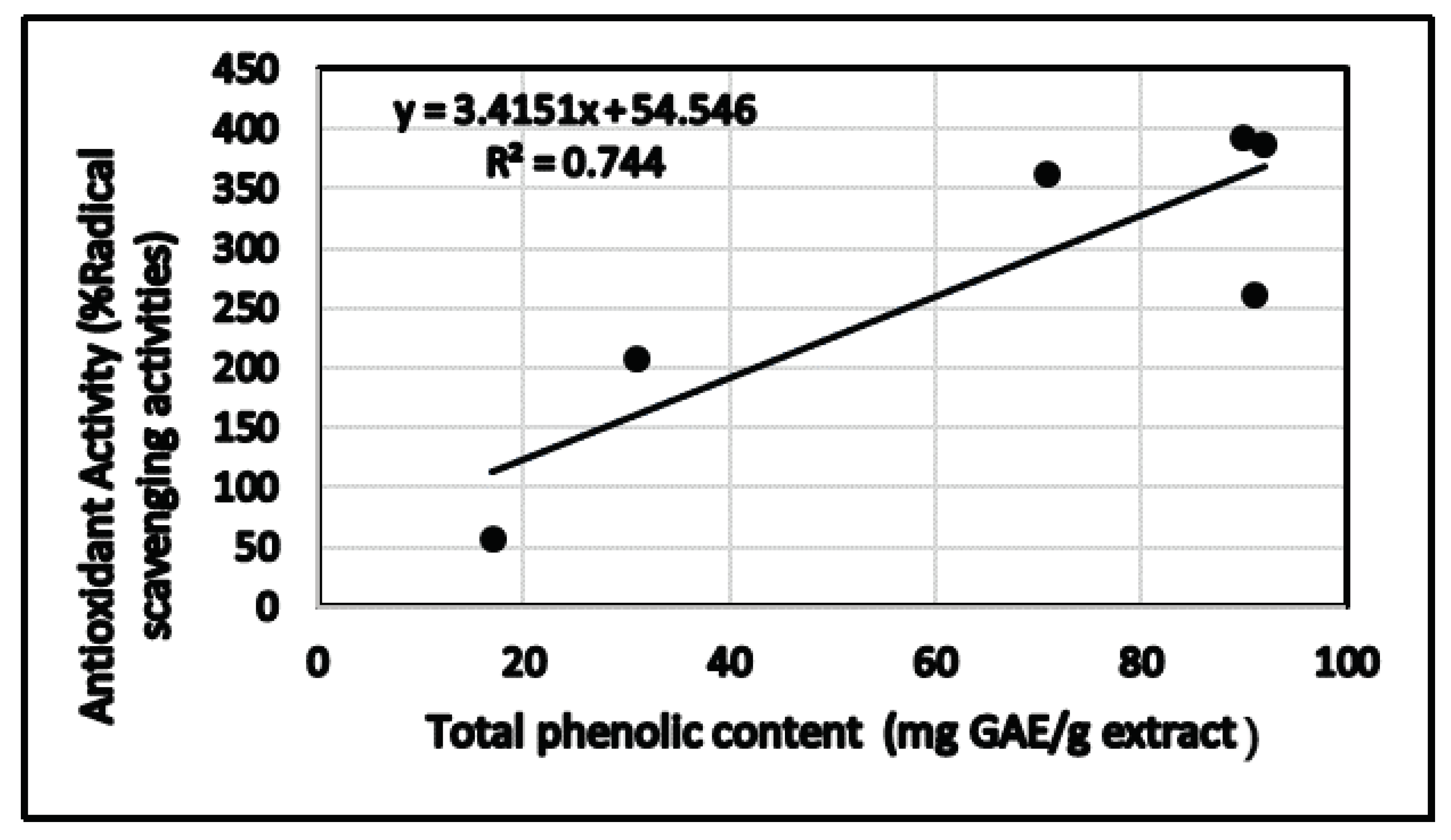
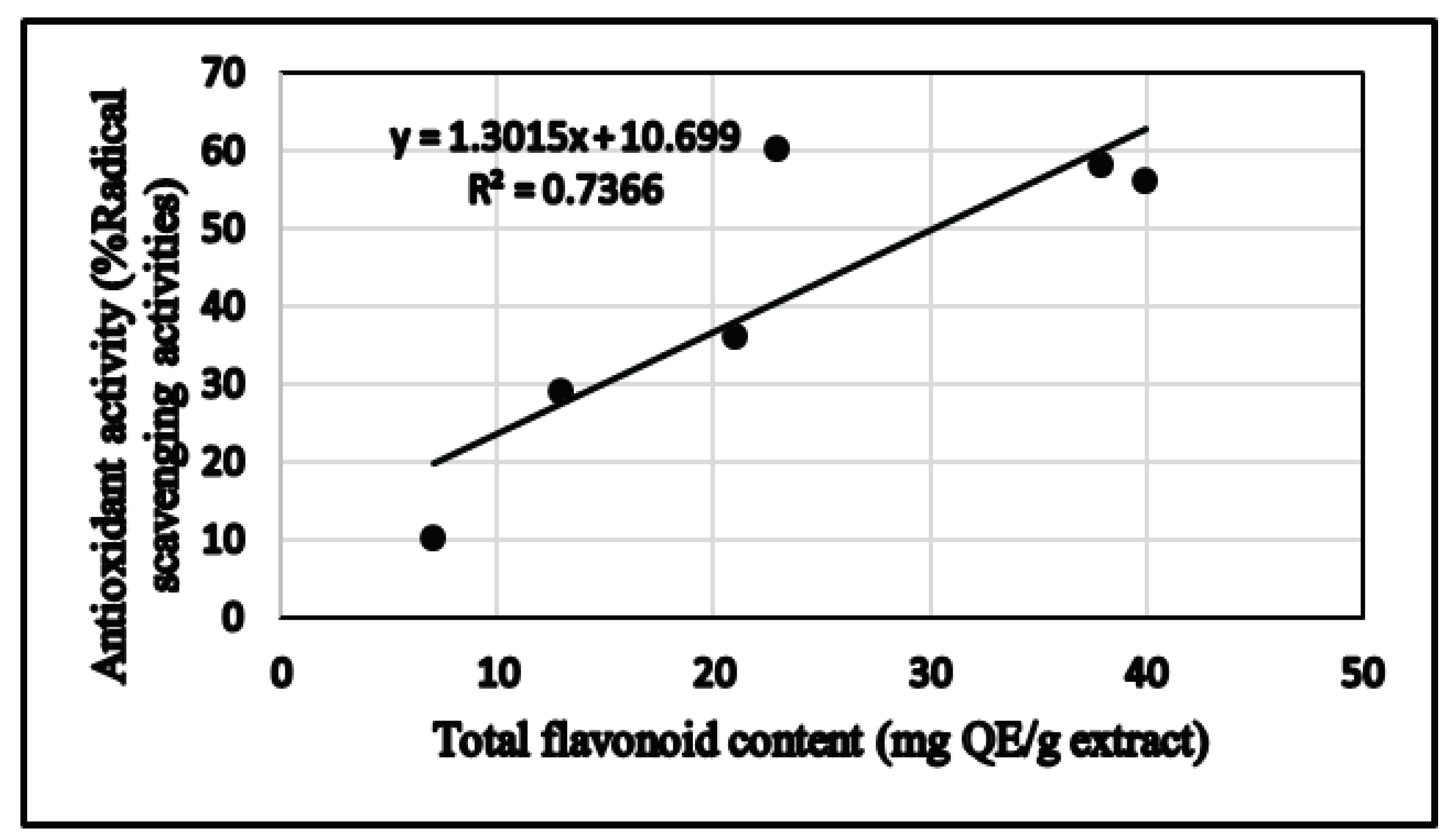
3.2. Determination of Total Phenolic and Total Flavonoid Contents
3.3. Determination of Antioxidant Activity
3.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
3.3.2. Cellular Antioxidant Activity (CAA) assay
3.4. Determination of Anti-Inflammatory Activity
3.5. Antiprotozoal Activity
3.6. Antimicrobial Activity
3.7. Anti-Malarial Activity
3.8. Cytotoxicity
3.9. Radioligand Displacement for Cannabinoid and Opioid Receptor Subtypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewdante Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Gutierrez, H.; Davies, A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011, 34, 316–325. [Google Scholar] [CrossRef]
- Lanzillotta, A.; Porrini, V.; Bellucci, A.; Benarese, M.; Branca, C.; Parrella, E.; Spano, P.F.; Pizzi, M. NF-κB in innate neuroprotection and age-related neurodegenerative diseases. Front. Neurol. 2015, 6, 98. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2018, 21, 101059. [Google Scholar] [CrossRef]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa, L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical structure alterations and social behavior impairment in p50-deficient mice. Cereb. Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Zhao, J.; Khan, S.I.; Wang, M.; Vasquez, Y.; Yang, M.H.; Avula, B.; Wang, Y.-H.; Avonto, C.; Smillie, T.J.; Khan, I.A. Octulosonic acid derivatives from Roman chamomile (Chamaemelum nobile) with activities against inflammation and metabolic disorder. J. Nat. Prod. 2014, 77, 509–515. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/leishmaniasis/en/ (accessed on 10 October 2019).
- Nardella, F.; Gallé, J.B.; Bourjot, M.; Weniger, B.; Vonthron-Sénécheau, C. Antileishmanial and Antitrypanosomal Activities of Flavonoids. In Natural Antimicrobial Agents; Mérillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; Volume 19. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Chemotherapy. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Gmünder, F.; Hamburger, M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Schrader, E. Equivalence of St John’s wort extract (Ze 117) and fluoxetine: A randomized, controlled study in mildmoderate depression. Int. Clin. Psychopharmacol. 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Song, J.-X.; Sze, S.C.-W.; Ng, T.-B.; Lee, C.K.-F.; Leung, G.P.; Shaw, P.-C.; Tong, Y.; Zhang, Y.-B. Anti-Parkinsonian drug discovery from herbal medicines: What have we got from neurotoxic models? J. Ethnopharmacol. 2012, 139, 698–711. [Google Scholar] [CrossRef]
- Lundstrom, K.; Pham, H.T.; Dinh, L.D. Interaction of plant extracts with central nervous system receptors. Medicines 2017, 4, 12. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Vaidya, B.; Sifat, A.E.; Karamyan, V.T.; Abbruscato, T.J. The neuroprotective role of the brain opioid system in stroke injury. Drug Discov. Today 2018, 23, 1385–1395. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Befort, K.; Nozaki, C.; Gaveriaux-Ruff, C.; Kieffer, B.L. The delta opioid receptor: An evolving target for the treatment of brain disorders. Trends Pharmacol. Sci. 2011, 32, 581–590. [Google Scholar] [CrossRef]
- Liska, M.G.; Crowley, M.G.; Lippert, T.; Corey, S.; Borlongan, C.V. Delta opioid receptor and peptide: A dynamic therapy for stroke and other neurological disorders. Handb. Exp. Pharmacol. 2018, 247, 277–299. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef]
- Mastinu, A.; Premoli, M.; Ferrari-Toninelli, G.; Tambaro, S.; Maccarinelli, G.; Memo, M.; Bonini, S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm. Mol. Biol. Clin. Investig. 2018, 36. [Google Scholar] [CrossRef]
- Iwaszkiewicz, K.; Schneider, J.; Hua, S. Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions. Front. Pharmacol. 2013, 4, 132. [Google Scholar] [CrossRef]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and pain: A clinical review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Cristofolini, G.; Troìa, A. A reassesment of the sections of the genus Cytisus Desf. (Cytiseae, Leguminosae). Taxon 2006, 55, 733–746. [Google Scholar] [CrossRef]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H.; Biology, P.B. Potential use of Cytisus scoparius extracts in topical applications for skin protection against oxidative damage. J. Photochem. Photobiol. B Biol. 2013, 125, 83–89. [Google Scholar]
- Sundararajan, R.; Haja, N.A.; Venkatesan, K.; Mukherjee, K.; Saha, B.P.; Bandyopadhyay, A.; Mukherjee, P.K. Cytisus scoparius link-A natural antioxidant. BMC Complementary Altern. Med. 2006, 6, 8. [Google Scholar] [CrossRef]
- Nirmal, J.; Babu, C.S.; Harisudhan, T.; Ramanathan, M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complementary Altern. Med. 2008, 8, 15. [Google Scholar] [CrossRef]
- Pereira, O.R.; Silva, A.M.; Domingues, M.R.; Cardoso, S.M. Identification of phenolic constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Larit, F.; Nael, M.A.; Benyahia, S.; Radwan, M.M.; Leon, F.; Jasicka-Misiak, I.; Poliwoda, A.; Wieczorek, D.; Benayache, F.; Benayache, S. Secondary metabolites from the aerial parts of Cytisus villosus Pourr. Phytochem. Lett. 2018, 24, 1–5. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. -Based Complementary Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Zaki, M.A.; Balachandran, P.; Khan, S.; Wang, M.; Mohammed, R.; Hetta, M.H.; Pasco, D.S.; Muhammad, I. Cytotoxicity and modulation of cancer-related signaling by (Z)-and (E)-3, 4, 3′, 5′-tetramethoxystilbene isolated from Eugenia rigida. J. Nat. Prod. 2013, 76, 679–684. [Google Scholar] [CrossRef]
- Ma, G.; Khan, S.I.; Benavides, G.; Schühly, W.; Fischer, N.H.; Khan, I.A.; Pasco, D.S. Inhibition of NF-κB-mediated transcription and induction of apoptosis by melampolides and repandolides. Cancer Chemother. Pharmacol. 2007, 60, 35–43. [Google Scholar] [CrossRef]
- Manda, S.; Khan, S.I.; Jain, S.K.; Mohammed, S.; Tekwani, B.L.; Khan, I.A.; Vishwakarma, R.A.; Bharate, S.B. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorganic Med. Chem. Lett. 2014, 24, 3247–3250. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 70. [Google Scholar] [CrossRef]
- Bharate, S.B.; Khan, S.I.; Yunus, N.A.; Chauthe, S.K.; Jacob, M.R.; Tekwani, B.L.; Khan, I.A.; Singh, I. P Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorganic Med. Chem. 2007, 15, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Jacob, M.R.; Khan, S.I.; Zhao, J.; Tekwani, B.L.; Midiwo, J.O.; Walker, L.A.; Muhammad, I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat. Prod. Commun. 2009, 4, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In Vitro Cell. Dev. Biol. 1990, 26, 1030–1034. [Google Scholar] [CrossRef]
- Tarawneh, A.; León, F.; Pettaway, S.; Elokely, K.M.; Klein, M.L.; Lambert, J.; Mansoor, A.; Cutler, S.J. Flavonoids from Perovskia atriplicifolia and their in vitro displacement of the respective radioligands for human opioid and cannabinoid receptors. J. Nat. Prod. 2015, 78, 1461–1465. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Haminiuk, C.W.I.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Granato, D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Verdrengh, M.; Jonsson, I.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef]
- Lu, H.; Shi, J.-X.; Zhang, D.-M.; Wang, H.-D.; Hang, C.-H.; Chen, H.-L.; Yin, H.-X. Inhibition of hemolysate-induced iNOS and COX-2 expression by genistein through suppression of NF-κB activation in primary astrocytes. J. Neurol. Sci. 2009, 278, 91–95. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Befort, K. Interactions of the opioid and cannabinoid systems in reward: Insights from knockout studies. Front. Pharmacol. 2015, 6, 6. [Google Scholar] [PubMed]
- Planas, M.E.; Rodriguez, L.; Sanchez, S.; Puig, M.M. Pharmacological evidence for the involvement of the endogenous opioid system in the response to local inflammation in the rat paw. Pain 1995, 60, 67–71. [Google Scholar] [CrossRef]
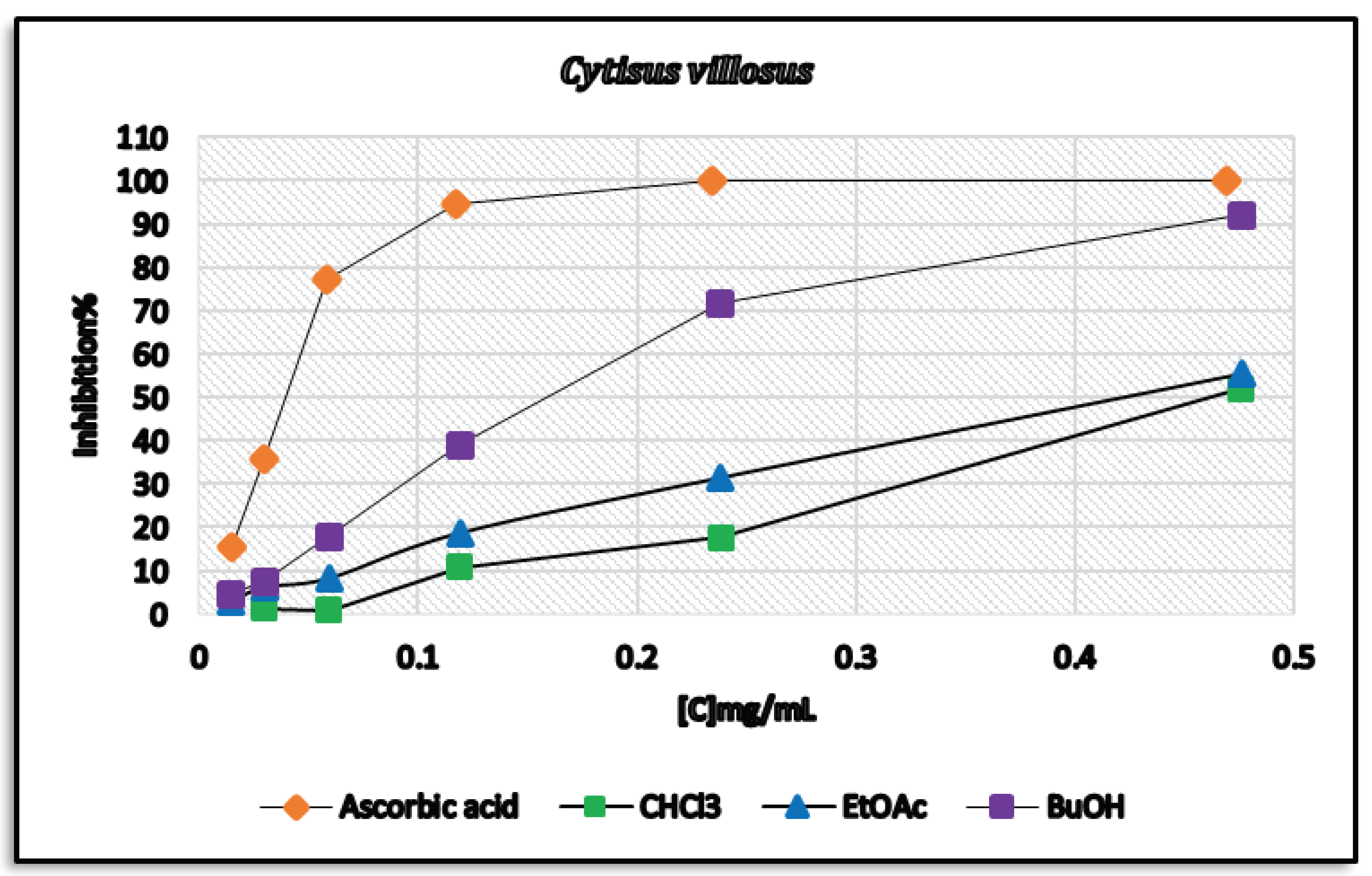
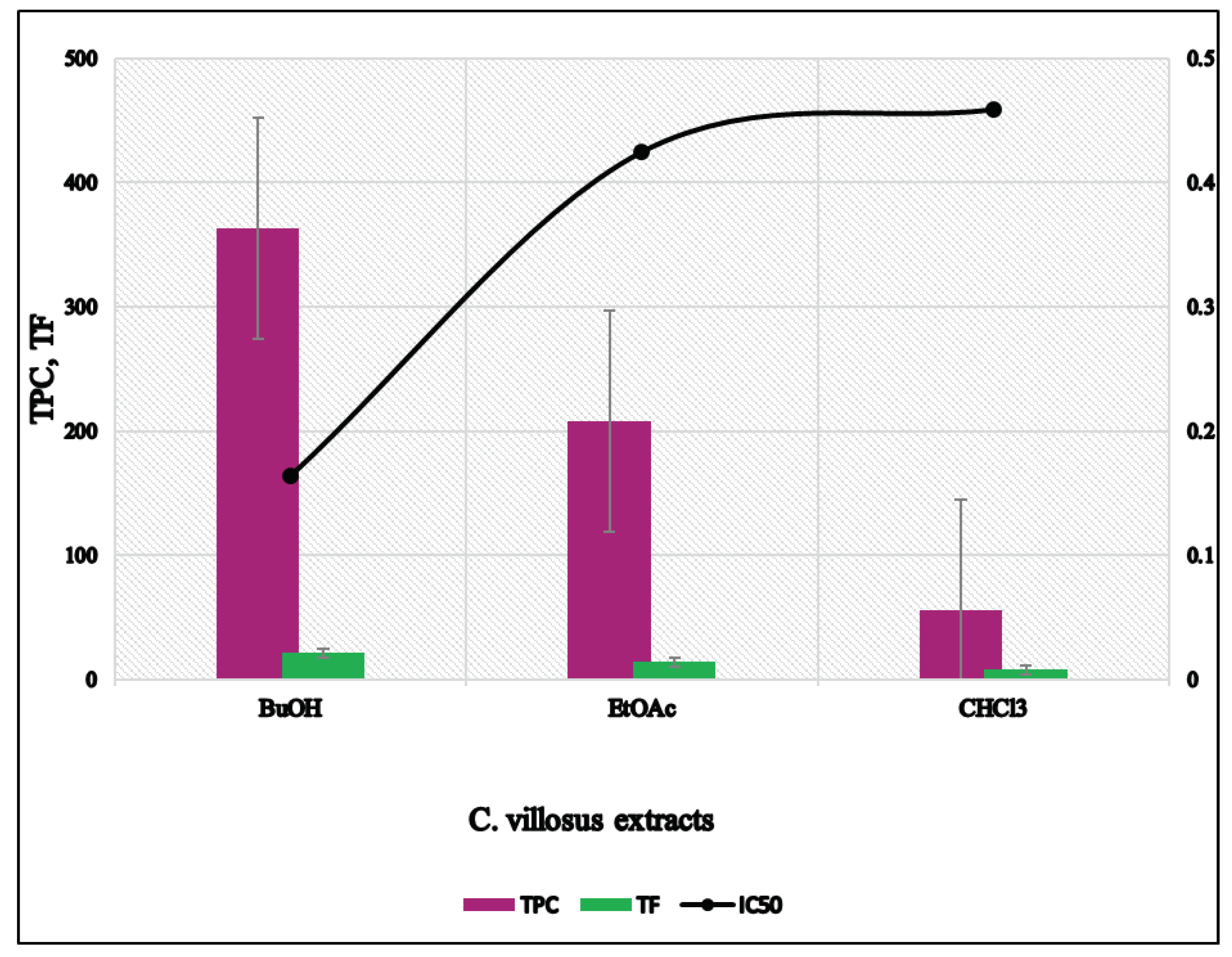
| Extract | Total Phenolic Content (mg GAE/g extract) | Total Flavonoid Content (mg QE/g extract) | DPPH Scavenging IC50 (mg/mL) | ARP = 1/IC50 | mg AAE/g Extract=ARP Extract/ARP Ascorbic Acid |
|---|---|---|---|---|---|
| CHCl3 | 56.0 ± 2.50 | 7.70 ± 0.547 | 0.459 ± 0.002 | 2.180 ± 0.01 | 0.093 ± 0.004 |
| EtOAc | 208.0 ± 8.49 | 13.95 ± 1.058 | 0.425 ± 0.003 | 2.355 ± 0.018 | 0.100 ± 0.001 |
| n-BuOH | 363.0 ± 8.32 | 21.16 ± 1.022 | 0.164 ± 0.004 | 6.113 ± 0.157 | 0.268 ± 0.007 |
| Ascorbic acid | 0.043 ± 0.006 | 23.761 ± 3.257 |
| % Decrease in Oxidative Stress | |||
|---|---|---|---|
| Tested Organism | Concentration (µg/mL) | ||
| 1000 | 500 | 250 | |
| xtracts | |||
| EtOAc | 47 | 37 | 29 |
| n-BuOH | 49 | 39 | 36 |
| Compounds | |||
| 1 | NA | NA | NA |
| 2 | NA | NA | NA |
| 3 | NA | NA | NA |
| 4 | 36 | 29 | 28 |
| Quercetin 25 µM | 77 | ||
| Tested Organism | Inhibition of iNOS IC50 (µg/mL) | Inhibition of NF-kB IC50 (µg/mL) | IC50 SP-1 |
|---|---|---|---|
| Extracts | |||
| EtOAc | 48 | NT | NT |
| n-BuOH | 90 | NT | NT |
| compounds | |||
| 1 | 9 | 28 | NA |
| 2 | >25 | 38 | NA |
| 3 | 20 | NA | NA |
| 4 | NA | NA | NA |
| Parthenolide | 0.2 | 1.63 | |
| Tested Organism | L. donovani Promastigote IC50 (μM) | L. donovani Promastigote IC90 (μM) | L. donovani Amastigote IC50 (μM) | L. donovani Amastigote IC90 (μM) | L. donovani Amastigote/THP1 IC50 (μM) | L. donovani Amastigote/THP1 IC90 (μM) | T. brucei IC50 (μM) | T. brucei IC90 (μM) | THP1 Cytotoxicity IC50 (μM) | THP1 Cytotoxicity C90 (μM) |
|---|---|---|---|---|---|---|---|---|---|---|
| Extracts | ||||||||||
| EtOH | >20 | >20 | >20 | >20 | >20 | >20 | 19.48 | >20 | >20 | >20 |
| BuOH | >20 | >20 | >20 | >20 | >20 | >20 | 7.99 | 12.61 | >20 | >20 |
| Compounds | ||||||||||
| 3 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| 4 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Amphotericin B | 0.136 | 0.215 | 0.211 | 0.374 | 0.188 | 0.421 | NT | NT | >2 | >2 |
| Pentamidine | 1.478 | 2.382 | 9.581 | >10 | 1.157 | 5.587 | 0.001 | 0.002 | >10 | >10 |
| DFMO | NT | NT | NT | NT | NT | NT | 3.634 | 8.804 | NT | NT |
| % Growth Inhibition 1,2/IC50 μg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-Fungal | Anti-Bacterial | ||||||||
| Extract/ Compound | C. albicans | C. glabrata | C. krusei | A. fumigatus | C. neoformans | S. aureus | MRSA | E. coli | P. aeruginosa |
| n-BuOH | 9 | 40 | 0 | 2 | 0 | 0 | 0 | 14 | 9 |
| EtOAc | 9 | 11 | 2 | 4 | 0 | 3 | 0 | 12 | 5 |
| 2 | >20 | NT | NT | NT | >20 | >20 | NT | >20 | >20 |
| 3 | >20 | NT | NT | NT | >20 | >20 | NT | >20 | >20 |
| 4 | >20 | NT | NT | >20 | >20 | >20 | >20 | >20 | >20 |
| AMB | 100 | NT | NT | 93 | 100 | NT | 1 | 0 | 0 |
| CIPRO | 0 | NT | NT | 8 | 0 | NT | 0 | 100 | 96 |
| Tested Organism | %Inhibition | ||
|---|---|---|---|
| Extract | P. falciparum (D6 Clone) | P. falciparum (W2 Clone) | Concentration ng/mL |
| BuOH | 0 | NT | 158667 |
| EtOAc | 0 | NT | 158667 |
| CQ | 100 | NT | 79.3 |
| IC50 | SI | IC50 | SI | IC50 | ||
|---|---|---|---|---|---|---|
| CQ | ˂26.0 | >9 | 116 | >2.1 | >238 | 238-26.4 |
| 2 | >4760 | 1 | >4760 | 1 | >4760 | 4760-528.9 |
| 3 | >4760 | 1 | >4760 | 1 | >4760 | 4760-528.9 |
| Cytotoxicity (IC50 µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Extract/Compound | SK-MEL | KB | BT-549 | SK-OV-3 | LLC-PK1 | Vero |
| EtOAc | NA | NA | NA | NA | NC | NC |
| BuOH | NA | NA | NA | NA | NC | NC |
| 3 | NA | NA | NA | NA | NC | NC |
| doxorubicin | 0.8 | 1.3 | 0.9 | 2 | 1.2 | NC |
| Cannabinoid Receptors (%) | Opioid Receptors (%) | ||||
|---|---|---|---|---|---|
| Extract/Compound | CB1 | CB2 | δ | κ | μ |
| EtOAc | 32.1 | 25.2 | 31.3 | 5.7 | 2.8 |
| n-BuOH | 33.7 | 26.1 | 24.8 | 10.3 | 5.7 |
| 2 | 7.7 | 0.8 | 8.7 | 12.8 | 12.2 |
| naloxone | 106.4 | 101.6 | 97.0 | ||
| CP 55,940 | 104.3 | 102.6 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larit, F.; León, F.; Benyahia, S.; Cutler, S.J. Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr. Biomolecules 2019, 9, 732. https://doi.org/10.3390/biom9110732
Larit F, León F, Benyahia S, Cutler SJ. Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr. Biomolecules. 2019; 9(11):732. https://doi.org/10.3390/biom9110732
Chicago/Turabian StyleLarit, Farida, Francisco León, Samira Benyahia, and Stephen J. Cutler. 2019. "Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr." Biomolecules 9, no. 11: 732. https://doi.org/10.3390/biom9110732
APA StyleLarit, F., León, F., Benyahia, S., & Cutler, S. J. (2019). Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr. Biomolecules, 9(11), 732. https://doi.org/10.3390/biom9110732






