Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Materials
2.2. Biodegradation of Commercial Xanthan
2.3. Extraction and Purification of LW-XG
2.4. Monosaccharide Composition Analysis
2.5. FT-IR and NMR Analysis
2.6. X-ray Diffraction (XRD) Analysis
2.7. Molecular Weight Analysis
2.8. Rheological Analysis
2.9. Antioxidant Activity Assay In Vitro
2.9.1. DPPH Radical Scavenging Activity Assay
2.9.2. Superoxide Anion Scavenging Activity Assay
2.9.3. Hydroxyl Radical Scavenging Activity Assay
2.9.4. Reducing Power Assay
2.10. Antioxidant Activity on H2O2-Induced Injury in Caco-2 Cells
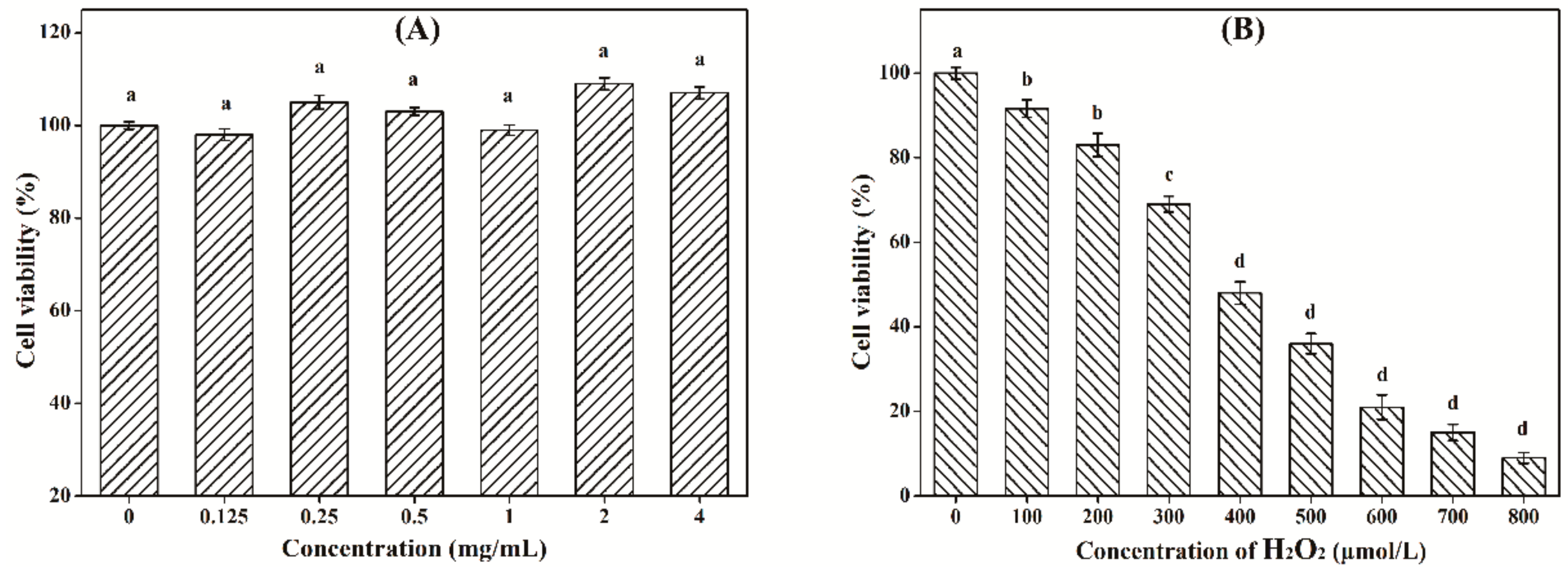
2.10.1. Cytotoxicity of LW-XG on Caco-2 Cells
2.10.2. Injured Cell Model Induced by H2O2
2.10.3. Effects of LW-XG on SOD, CAT, GSH-Px and MDA Activity
2.11. Statistical Analysis
3. Results and Discussion
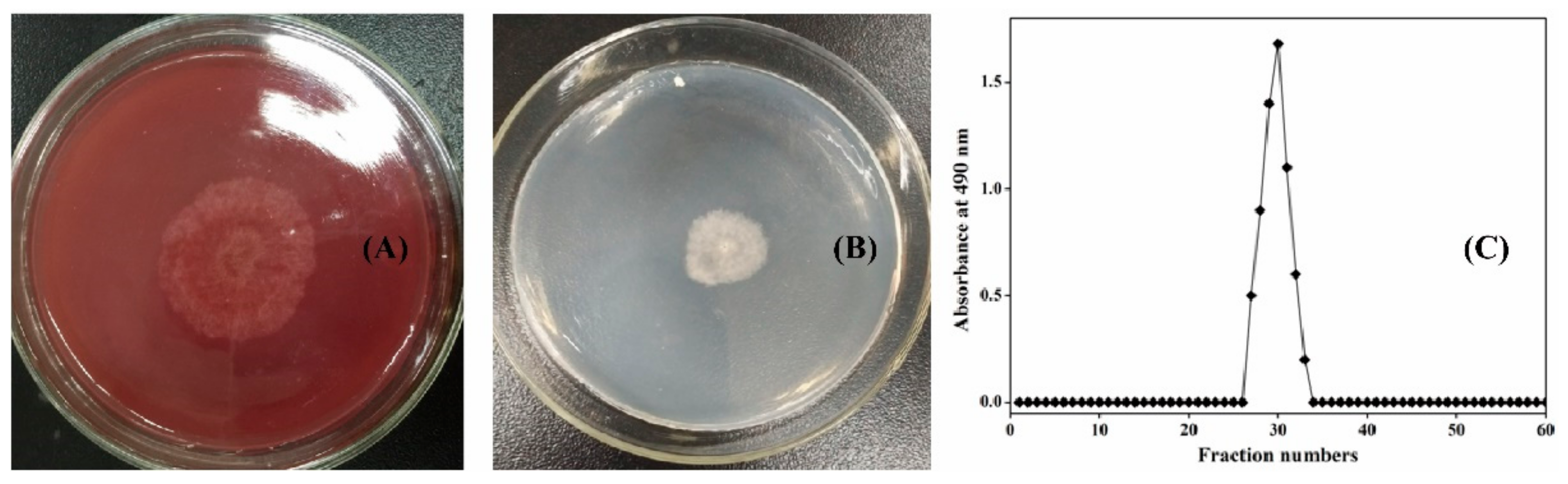
3.1. Production of LW-XG
3.2. Monosaccharide Composition
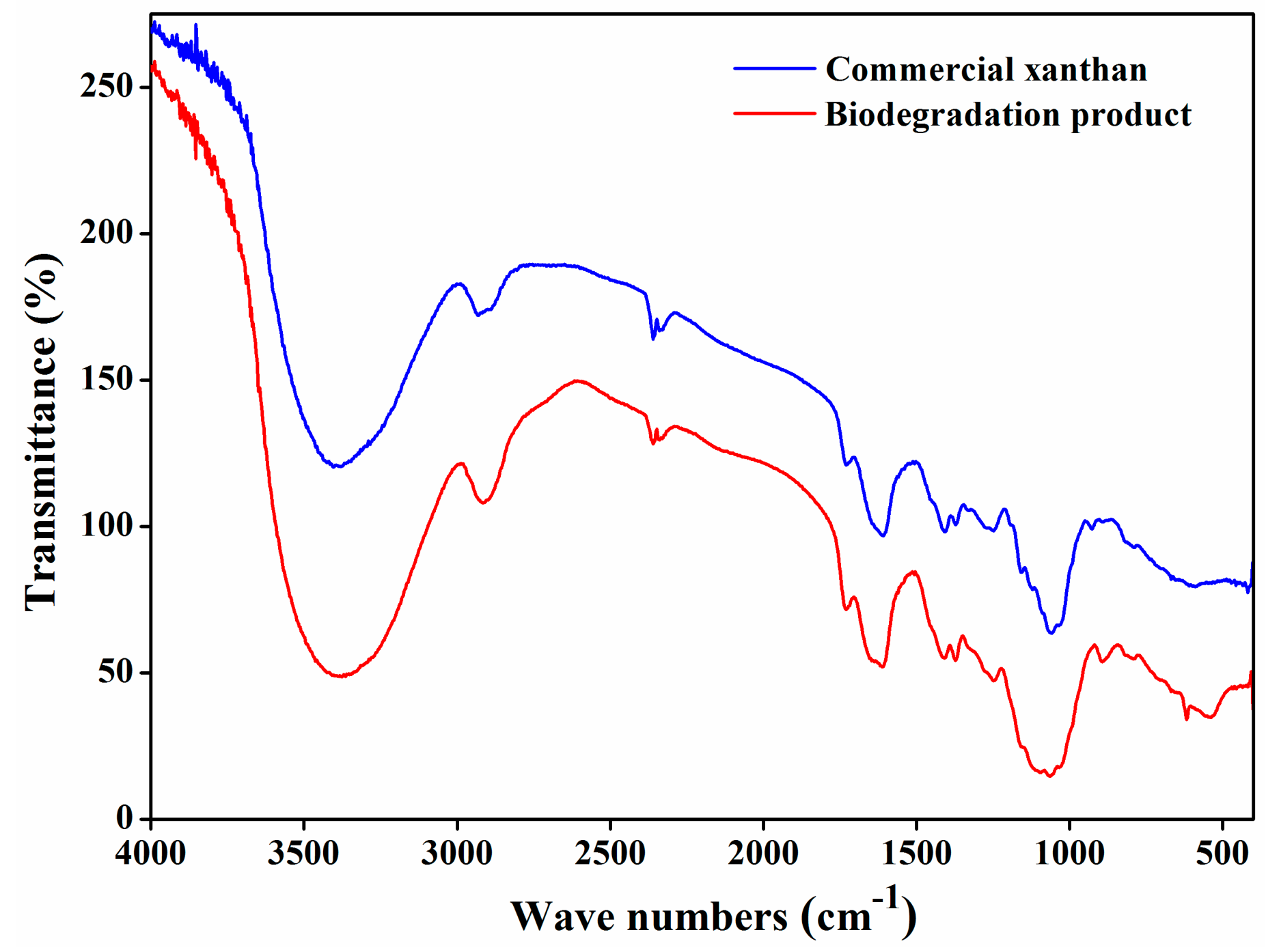
3.3. FT-IR
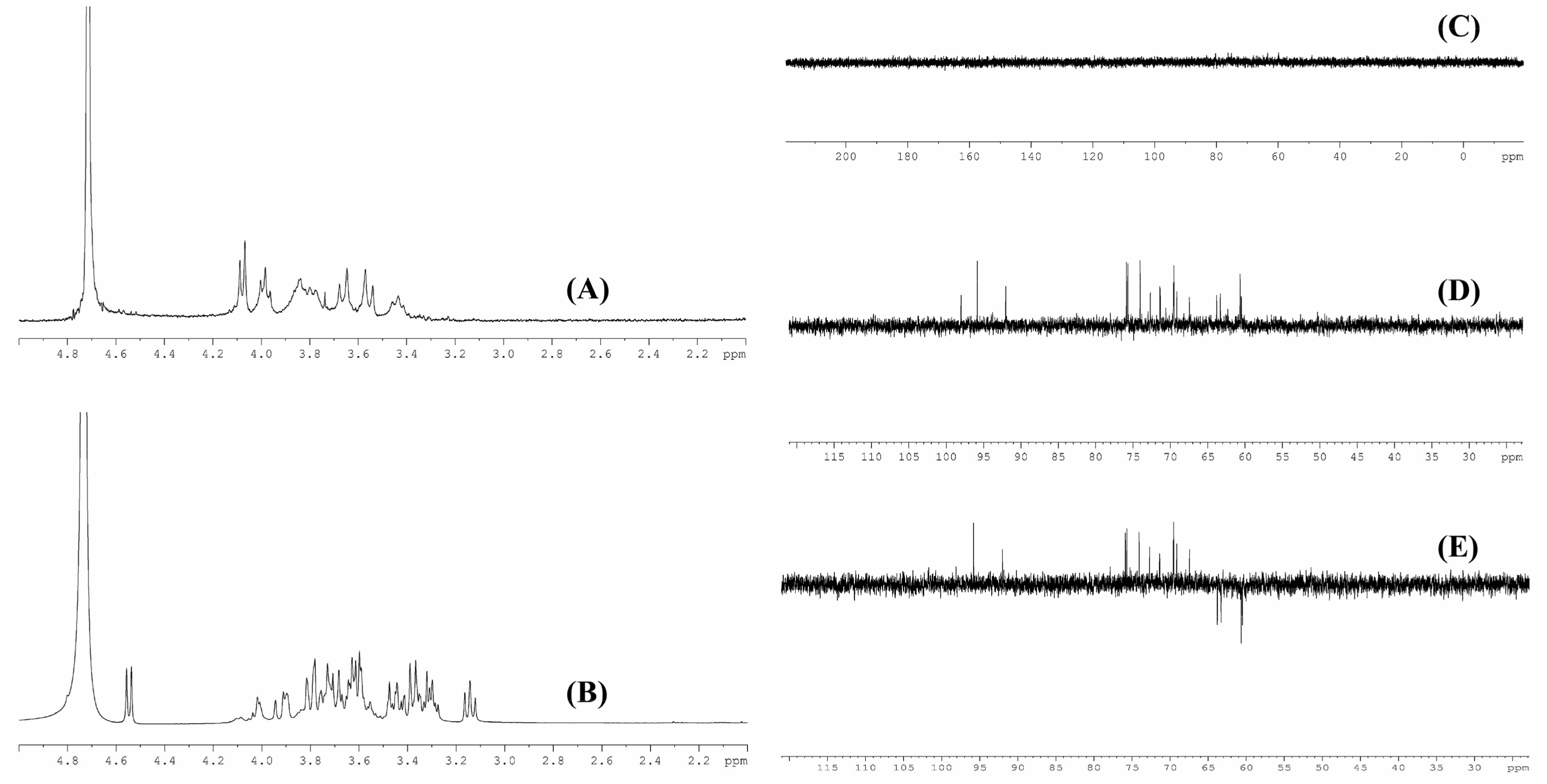
3.4. NMR Analysis
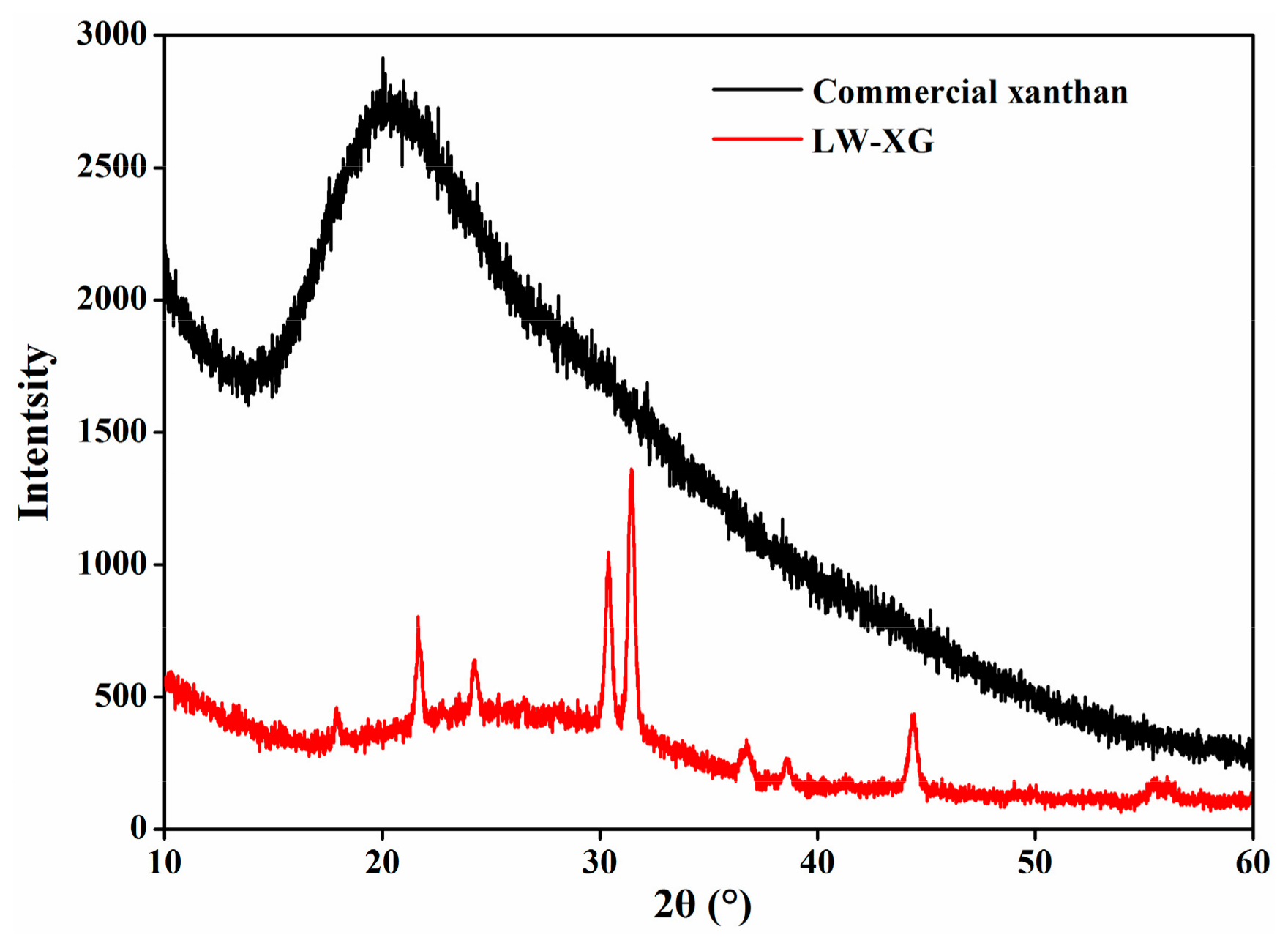
3.5. XRD Analysis
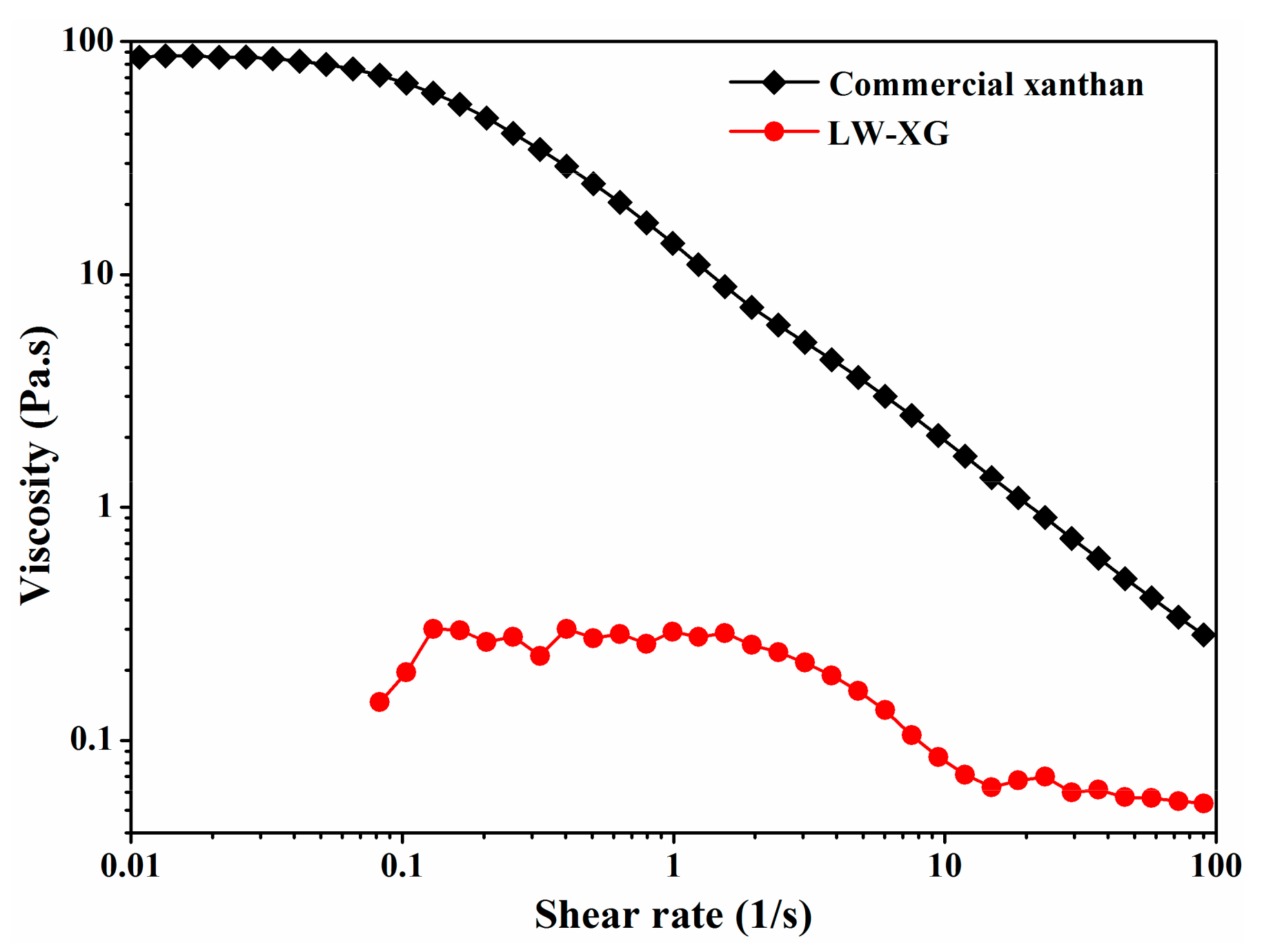
3.6. Molecular Weight and Rheological Analysis
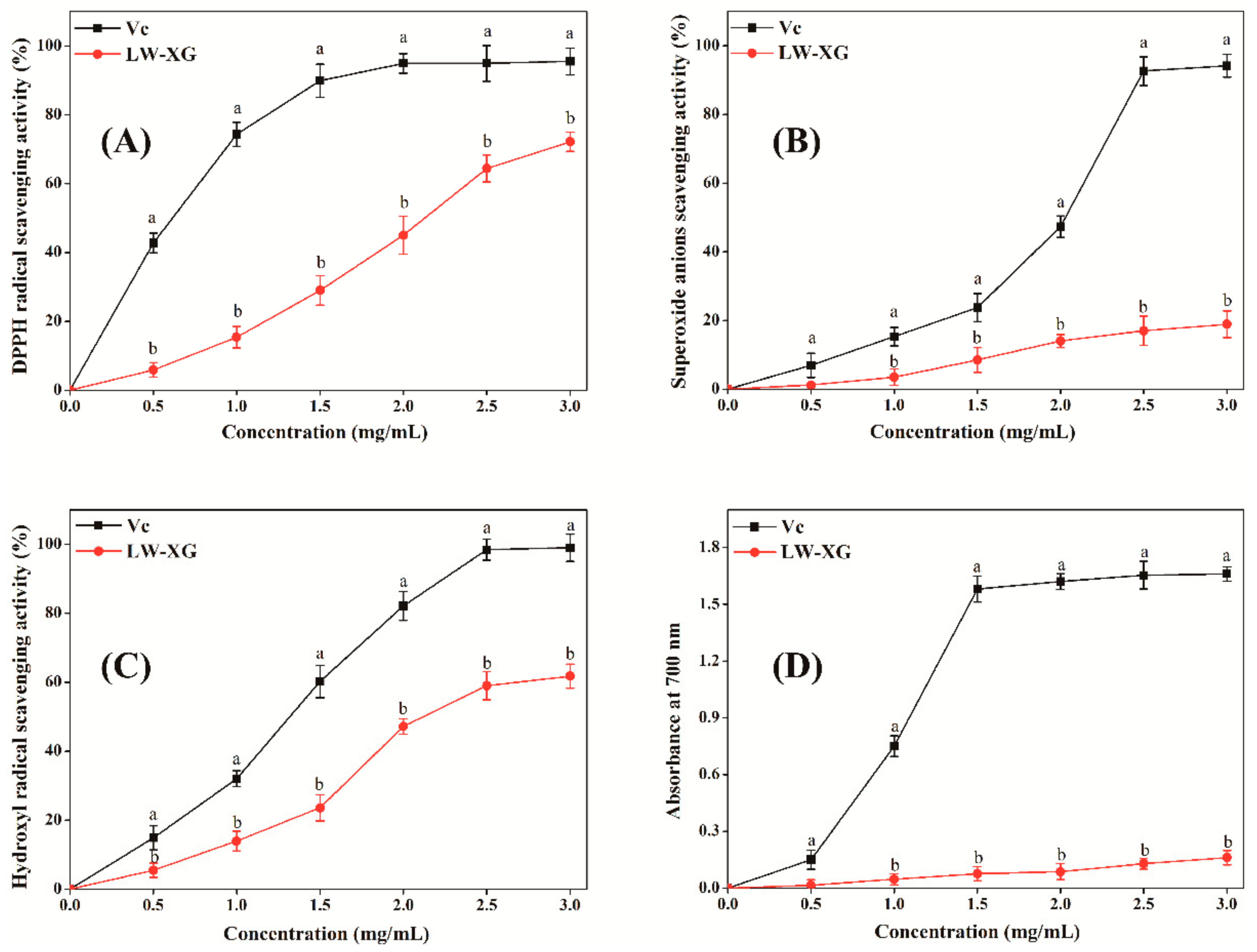
3.7. Antioxidant Activity Assay In Vitro
3.8. Effect of LW-XG on H2O2-Induced Injury in Caco-2 Cell Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Han, G.; Chen, Q.; Liu, F.; Cui, Z.; Shao, H.; Liu, F.; Ma, A.; Liao, J.; Guo, B.; Guo, Y.; et al. Low molecular weight xanthan gum for treating osteoarthritis. Carbohydr. Polym. 2017, 164, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Shao, H.; Ma, A.; Wu, J.; Zhang, W.; Liu, F.; Han, G.; et al. Low molecular weight xanthan gum suppresses oxidative stress-induced apoptosis in rabbit chondrocytes. Carbohydr. Polym. 2017, 169, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Chen, Q.; Dou, X.; Chen, L.; Wu, J.; Zhang, W.; Shao, H.; Ling, P.; Liu, F.; Wang, F. Lower range of molecular weight of xanthan gum inhibits cartilage matrix destruction via intrinsic bax-mitochondria cytochrome c-caspase pathway. Carbohydr. Polym. 2018, 198, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, J.; Zhang, F.; Dou, X.; Ma, A.; Zhang, X.; Shao, H.; Zhao, S.; Ling, P.; Liu, F.; et al. Lower range of molecular weight of xanthan gum inhibits apoptosis of chondrocytes through MAPK signaling pathways. Int. J. Biol. Macromol. 2019, 130, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Wu, J.H.; Xia, L.Z.; Chu, C.; Liu, D.; Gong, M. Preparation of xanthan-derived oligosaccharides and their hydroxyl radical scavenging activity. Carbohydr. Polym. 2013, 92, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Li, M.; Xie, J.; Jin, Q.; Xue, B.; Sun, T. Antioxidant activity of xanthan oligosaccharides prepared by different degradation methods. Carbohydr. Polym. 2013, 92, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Aminlari, M.; Moosavinasab, M. Preparation of and studies on the functional properties and bactericidal activity of the lysozyme-xanthan gum conjugate. LWT-Food Sci. Tech. 2014, 57, 594–602. [Google Scholar] [CrossRef]
- Saleh, H.M.; Annuar, M.S.M.; Simarani, K. Ultrasound degradation of xanthan polymer in aqueous solution: Its scission mechanism and the effect of NaCl incorporation. Ultrason. Sonochem. 2017, 39, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feke, D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solutions. Food Chem. 2015, 172, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Characterization of xanthan gum produced from glycerol by a mutant strain Xanthomonas campestris CCTCC M2015714. Carbohydr. Polym. 2017, 157, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, P.; Zeng, X.; Chen, X.; Xie, Y.; Zeng, Y.; Zhang, Y.; Xie, T. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Liu, S.; Zhang, Y.; Wu, Z.; Xu, W.; Sun, Q.; Yang, L.; Zhang, H. Efficient biosynthesis of anticancer polysaccharide by a mutant Chaetomium globosum ALE20 via non-sterilized fermentation. Int. J. Biol. Macromol. 2019, 136, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, P.; Tao, N.; Zhang, H.; Li, R.; Zhan, X.; Wang, F.; Shen, Y. Anticancer activity of polysaccharides produced from glycerol and crude glycerol by an endophytic fungus Chaetomium globosum CGMCC 6882 on human lung cancer A549 cells. Biomolecules 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Shen, M.; Xie, J.; Liu, D.; Du, M.; Lin, L.; Gao, H.; Hamaker, B.R.; Xie, M. Physicochemical characterization, antioxidant activity of polysaccharides from Mesona chinensis Benth and their protective effect on injured NCTC-1469 cells induced by H2O2. Carbohydr. Polym. 2017, 175, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lin, L.; Xie, J.; Wang, Z.; Wang, H.; Dong, Y.; Shen, M.; Xie, M. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. The antioxidant activity of derivatized cushaw polysaccharides. Int. J. Biol. Macromol. 2019, 128, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Chovanová, K.; Šimonovičová, A.; Ferianc, P. Investigation of microbial community isolated from indoor artworks and air environment: Identification, biodegradative abilities, and DNA typing. Can. J. Microb. 2009, 55, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Umikalsom, M.S.; Ariff, A.B.; Karim, M.I.A. Saccharification of pretreated oil palm empty fruit bunch fiber using cellulase of Chaetomium globosum. J. Agric. Food Chem. 1998, 46, 3359–3364. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Activation of glycerol metabolism in Xanthomonas campestris by adaptive evolution to produce a high-transparency and low-viscosity xanthan gum from glycerol. Bioresour. Technol. 2016, 211, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Fornari, R.C.G.; Mazutti, M.A.; de Oliveira, D.; Padilha, F.F.; Cichoski, A.J.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Production and characterization of xantham gum by Xanthomonas campestris using cheese whey as sole carbon source. J. Food Eng. 2009, 90, 119–123. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Zeng, Y.; Li, X.; Jiang, X.; Wang, Y.; Xie, T.; Zhang, Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr. Polym. 2016, 151, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G. The antioxidant activities of carboxymethylated cushaw polysaccharide. Int. J. Biol. Macromol. 2019, 121, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Alencar, P.O.C.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocolloid. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, R.; Cui, J.; Wang, J.; Fan, W.; Zhang, H.; Zhan, X. Antibacterial activity of a polysaccharide produced from Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Elella, M.H.A.; Mohamed, R.R.; ElHafeez, E.A.; Sabaa, M.W. Synthesis of novel biodegradable antibacterial grafted xanthan gum. Carbohydr. Polym. 2017, 173, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cong, S.; Zhao, J.; Dong, Y.; Li, T.; Zhu, B.; Song, S.; Wen, C. The combination between cations and sulfated polysaccharide from abalone gonad (Haliotis discus hannai Ino). Carbohydr. Polym. 2018, 188, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, W.; Zhang, H.; Ding, C.; Huang, Y.; Liao, J.; Zhang, Z.; Yuan, S.; Chen, Y.; Yuan, M. Antioxidant and immunomodulatory activities of polysaccharides from the rhizome of Dryopteris crassirhizoma Nakai. Int. J. Biol. Macromol. 2019, 130, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Pang, X.; Wang, P.G.; Chen, M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.Y.; Howarth, G.S.; Bindon, K.A.; Kennedy, J.A.; Bastian, S.E.P. Low molecular weight procyanidins from grape seeds enhance the impact of 5-fluorouracil chemotherapy on Caco-2 human colon cancer cells. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Liu, J.; Liang, Z. Probiotic Bacillus subtilis CW14 reduces disruption of the epithelial barrier and toxicity of ochratoxin A to Caco-2 cells. Food Chem. Toxicol. 2019, 126, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Poormontaseri, M.; Hosseinzadeh, S.; Shekarforoush, S.S.; Kalantari, T. The effects of probiotic Bacillus subtilis on the cytotoxicity of Clostridium perfringens type a in Caco-2 cell culture. BMC Microbiol. 2017, 17, 150. [Google Scholar] [CrossRef] [PubMed]

| Parameters (U/mg protein) | Normal Control Group | Model Control Group | LW-XG Concentration (mg/mL) | ||
|---|---|---|---|---|---|
| 0.75 | 1.50 | 3.00 | |||
| SOD | 52.1 ± 1.95 a | 21.8 ± 2.06 b | 29.4 ± 1.79 c | 39.8 ± 3.15 c | 51.4 ± 2.63 c |
| CAT | 43.5 ± 2.17 a | 22.9 ± 2.89 b | 27.2 ± 2.75 c | 35.9 ± 1.97 c | 40.9 ± 2.14 c |
| GSH-Px | 41.1 ± 3.13 a | 20.1 ± 2.57 b | 25.0 ± 2.91 c | 33. 7 ± 2.10 c | 38.8 ± 2.83 c |
| MDA | 0.5 ± 0.08 a | 1.6 ± 0.15 b | 1.3 ± 0.10 c | 0.8 ± 0.07 c | 0.6 ± 0.03 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Wang, K.; Yu, M.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum. Biomolecules 2019, 9, 730. https://doi.org/10.3390/biom9110730
Hu X, Wang K, Yu M, He P, Qiao H, Zhang H, Wang Z. Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum. Biomolecules. 2019; 9(11):730. https://doi.org/10.3390/biom9110730
Chicago/Turabian StyleHu, Xiaolong, Kangli Wang, Miao Yu, Peixin He, Hanzhen Qiao, Huiru Zhang, and Zichao Wang. 2019. "Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum" Biomolecules 9, no. 11: 730. https://doi.org/10.3390/biom9110730
APA StyleHu, X., Wang, K., Yu, M., He, P., Qiao, H., Zhang, H., & Wang, Z. (2019). Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum. Biomolecules, 9(11), 730. https://doi.org/10.3390/biom9110730





