The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix dioica Roxb.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, Extraction, and Fractionation
2.2. Animals and Their Housing
2.3. Chemicals
2.4. Phytochemical Screening
2.4.1. Primary Phytochemical Examination
2.4.2. Total Phenolic Content
2.4.3. Total Flavonoid Content
2.4.4. HPLC-DAD Analysis
2.5. In Vivo Experiments
Antidiarrheal Activity in Rats
2.6. Ex Vivo Experiments Using Isolated Tissues
2.6.1. Rabbit Jejunum Preparation
2.6.2. Rabbit Tracheal Preparation
2.6.3. Rabbit Aortic Ring Preparation
2.6.4. Isolated Paired Atria
2.6.5. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.1.1. Preliminary Phytochemical Study
3.1.2. Total Flavonoid Content
3.1.3. Total Phenolic Content
3.1.4. HPLC-DAD Analysis
3.2. In Vivo Experiments
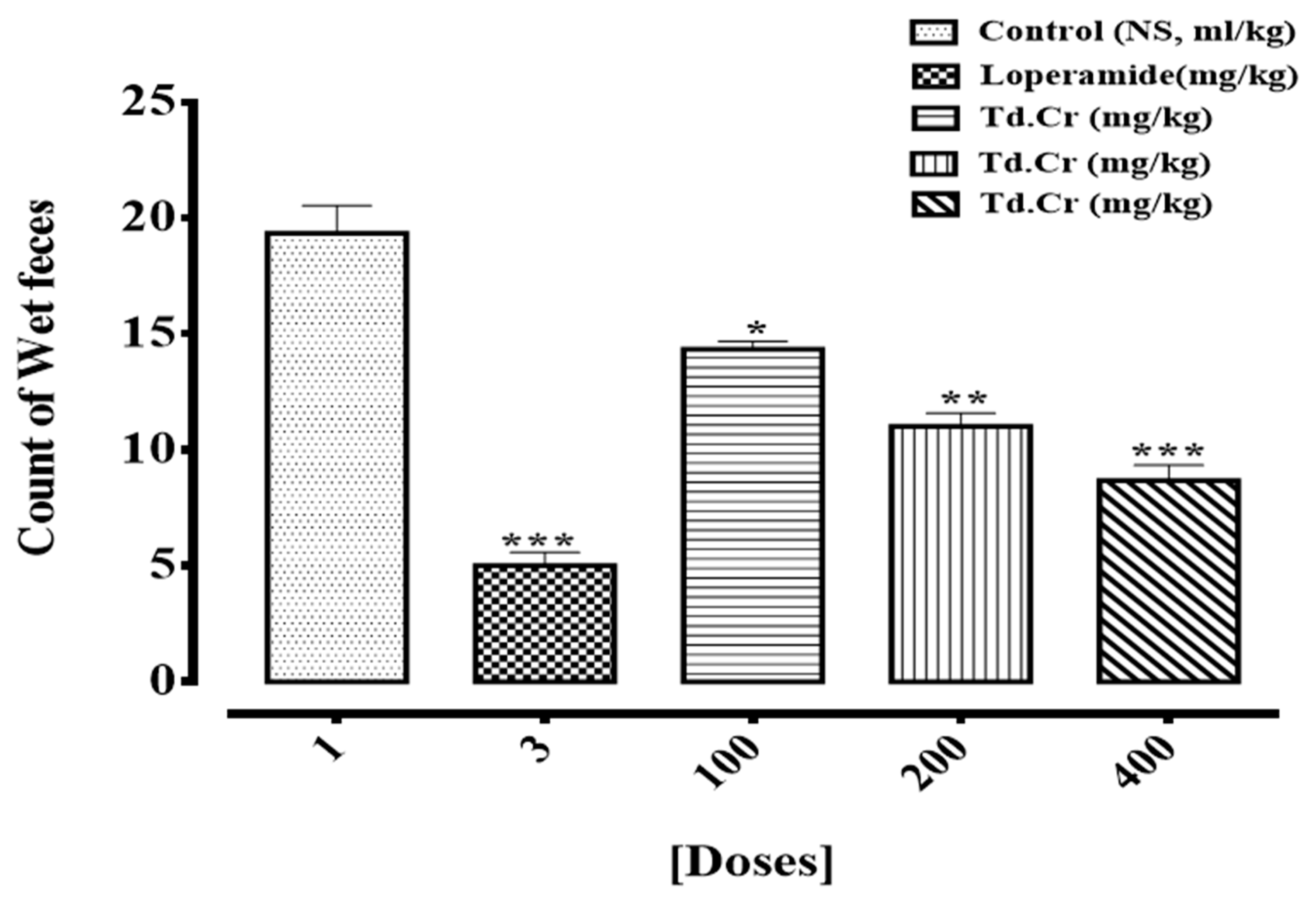
Antidiarrheal Activity in Rats
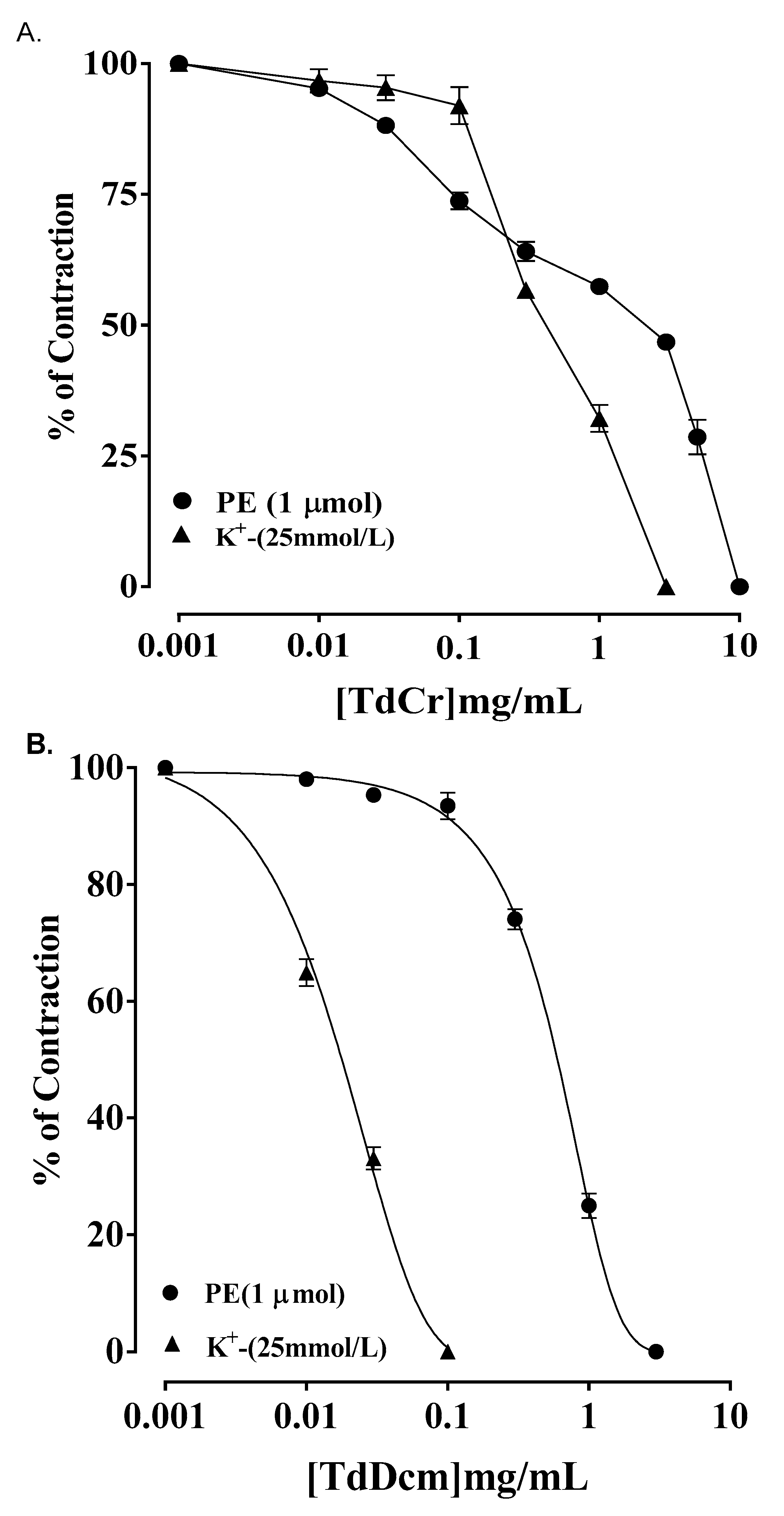
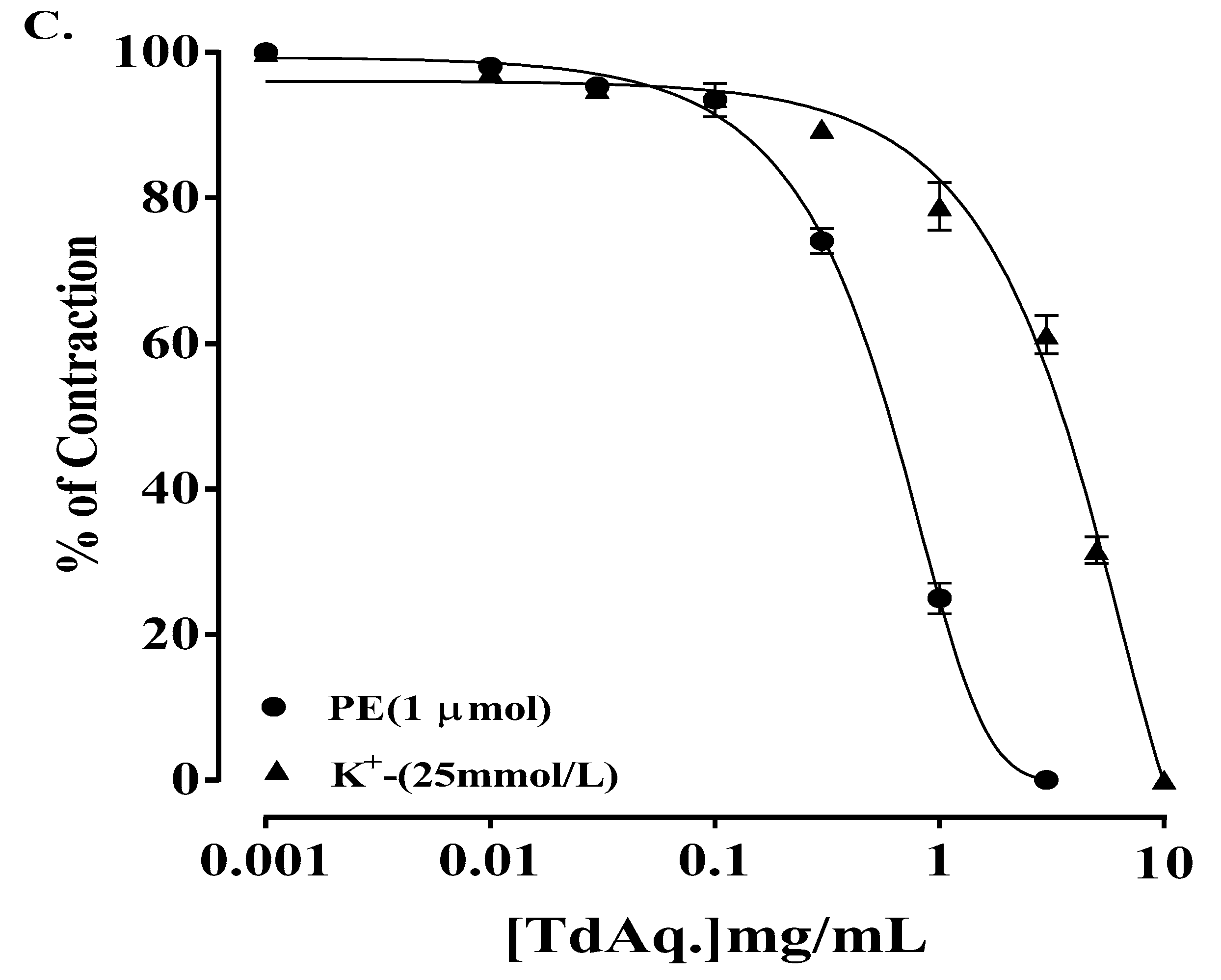
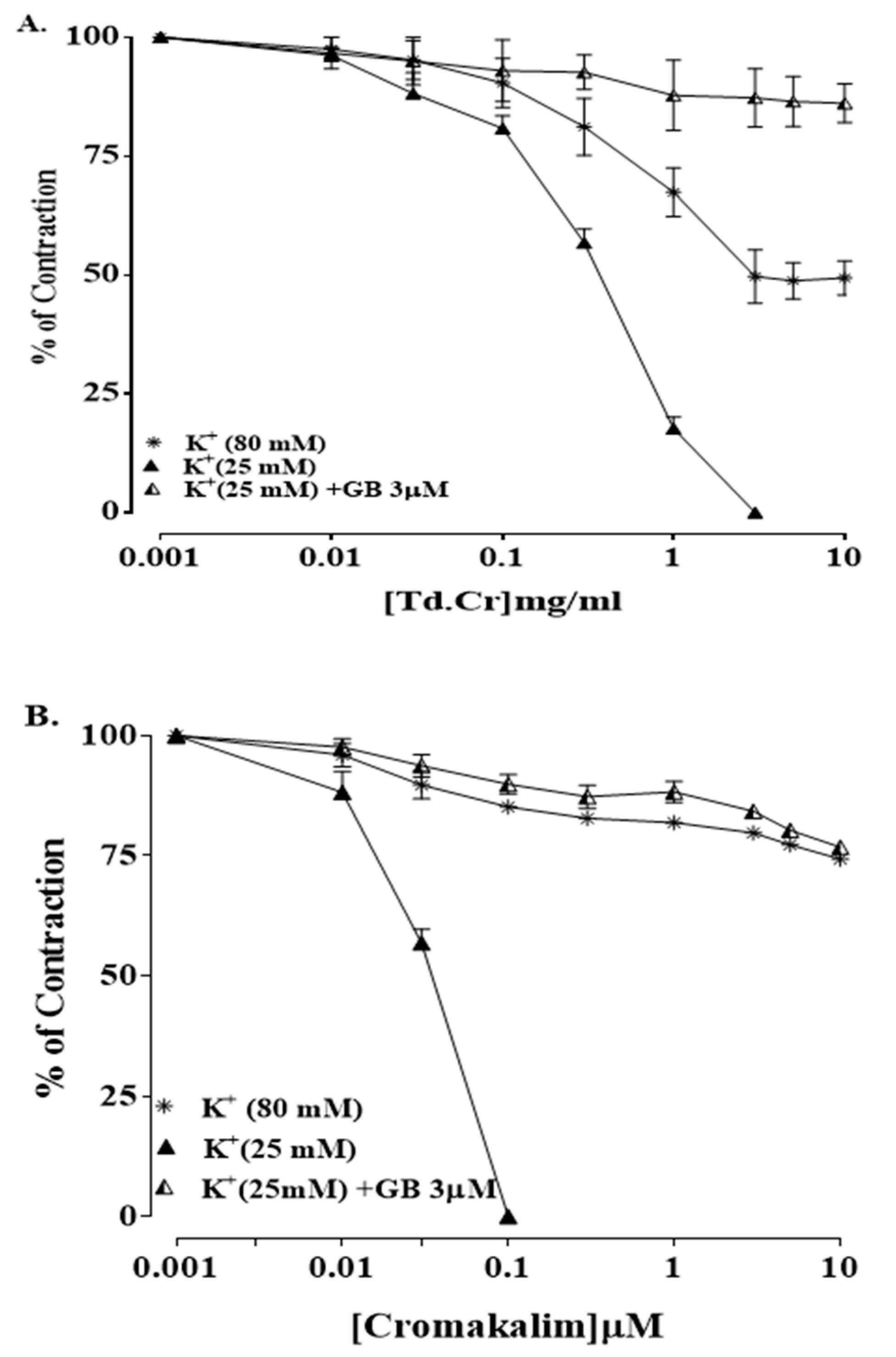
3.3. Ex Vivo Experiments
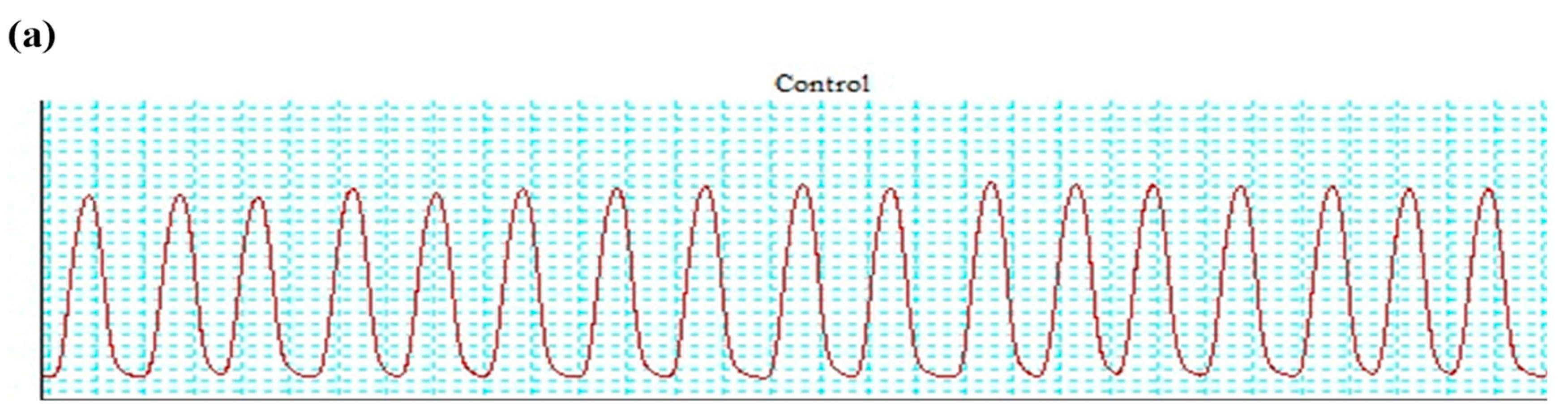
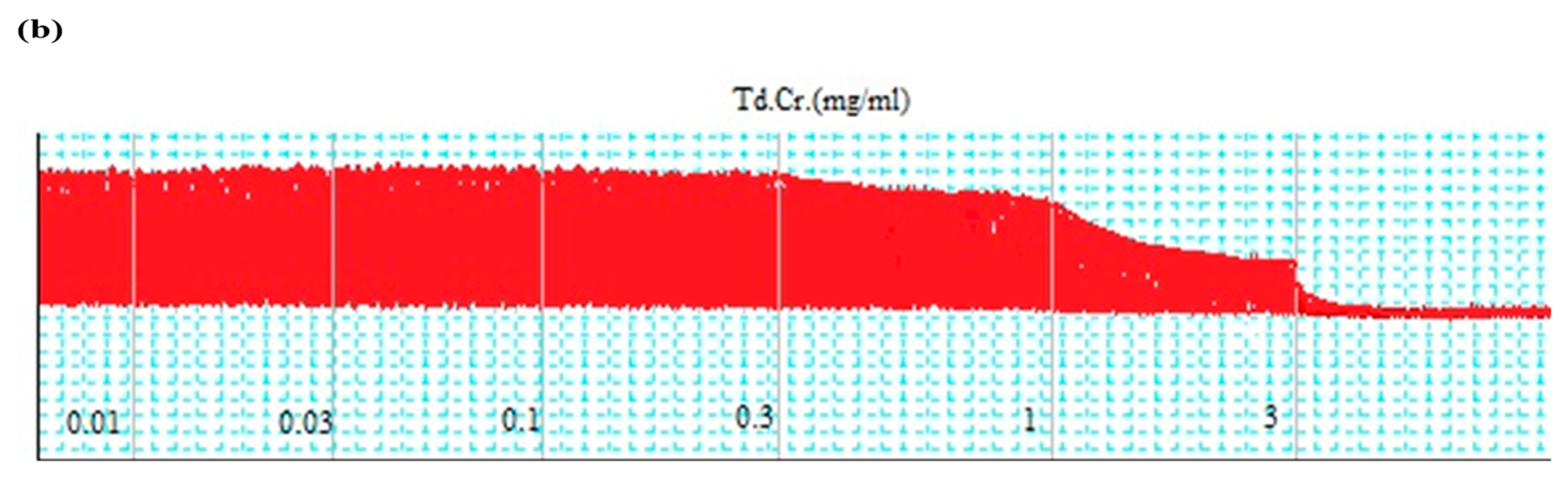
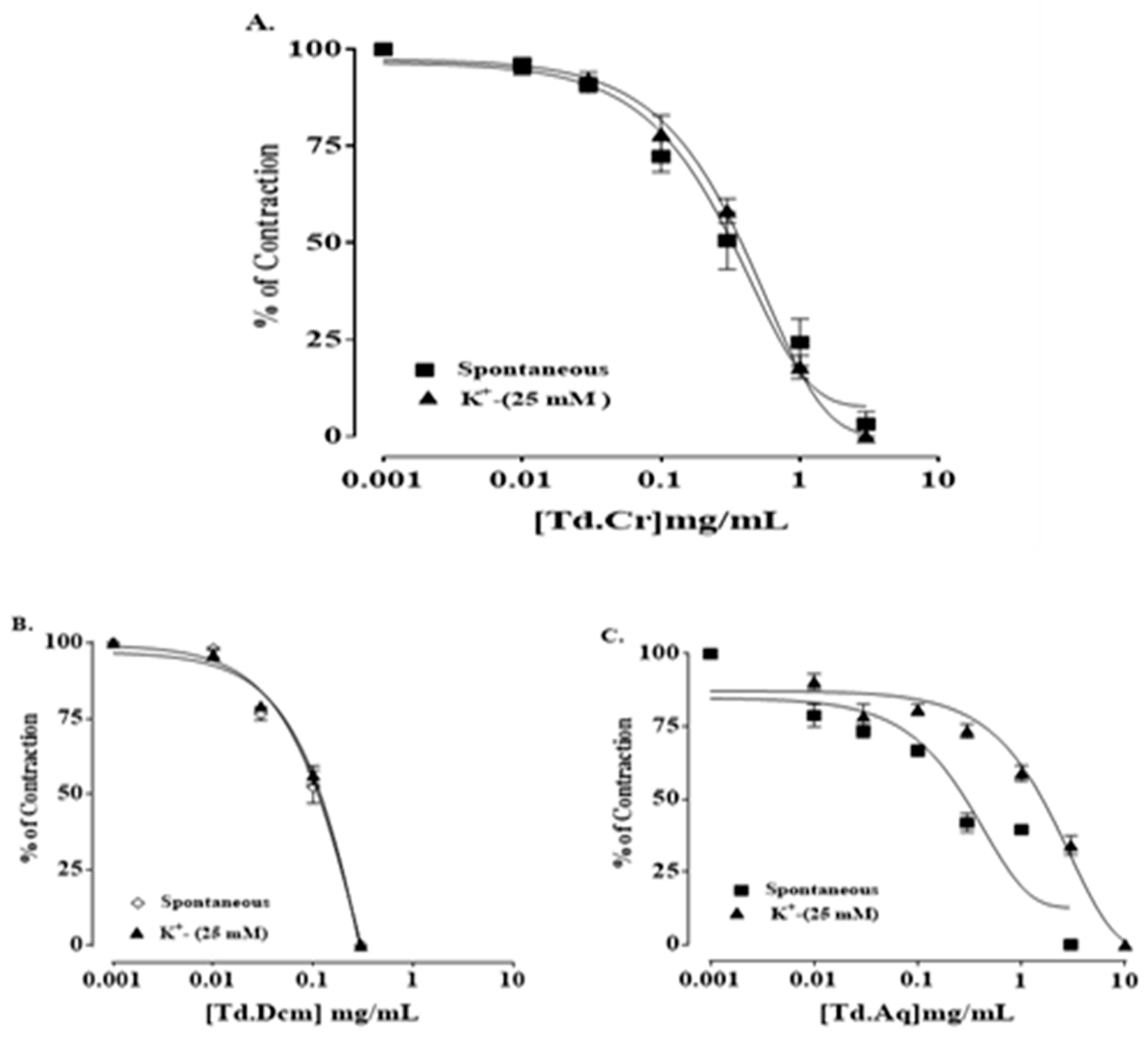
3.3.1. Rabbit Jejunum Preparation
3.3.2. Isolated Tracheal Strip Preparation
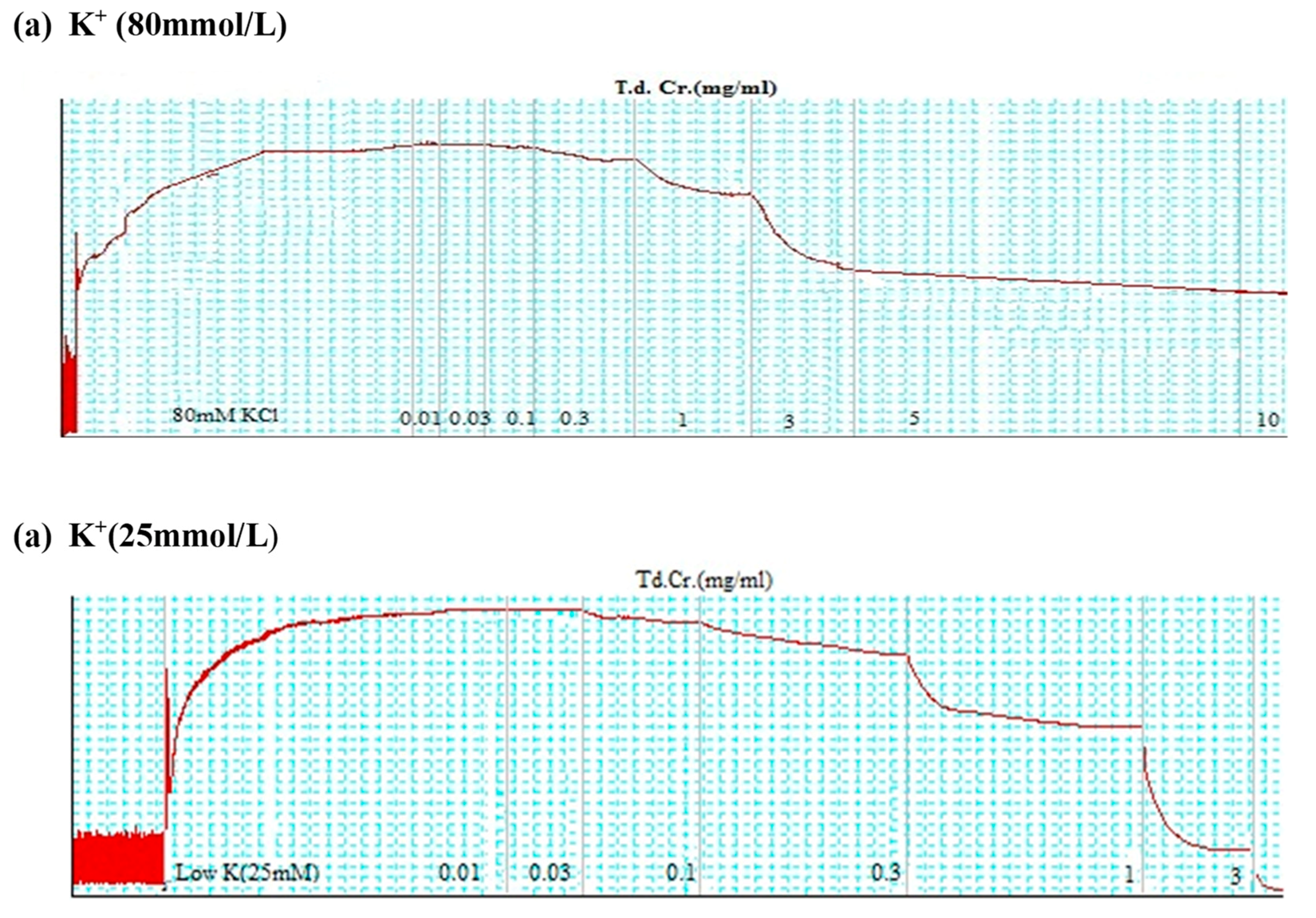
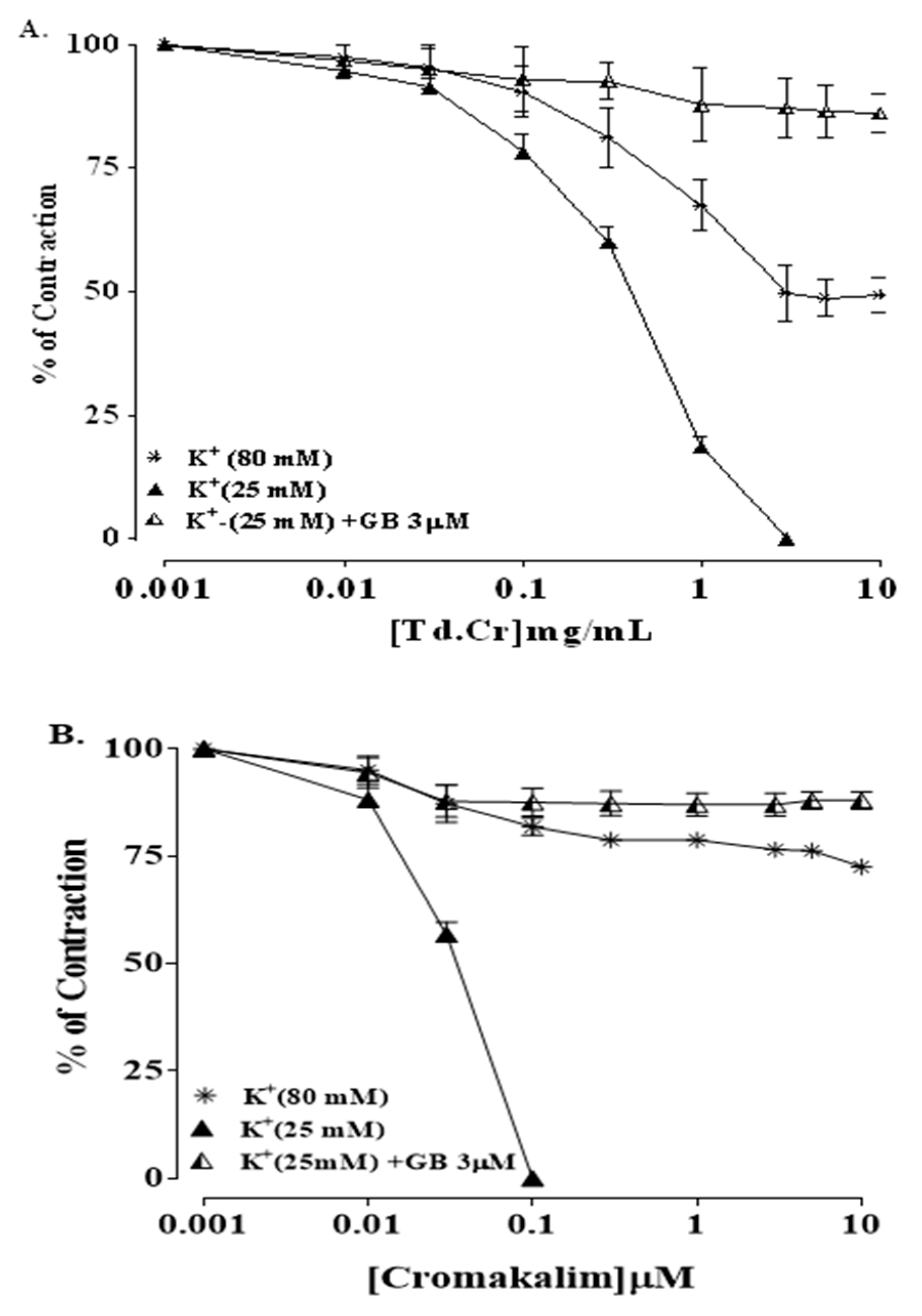
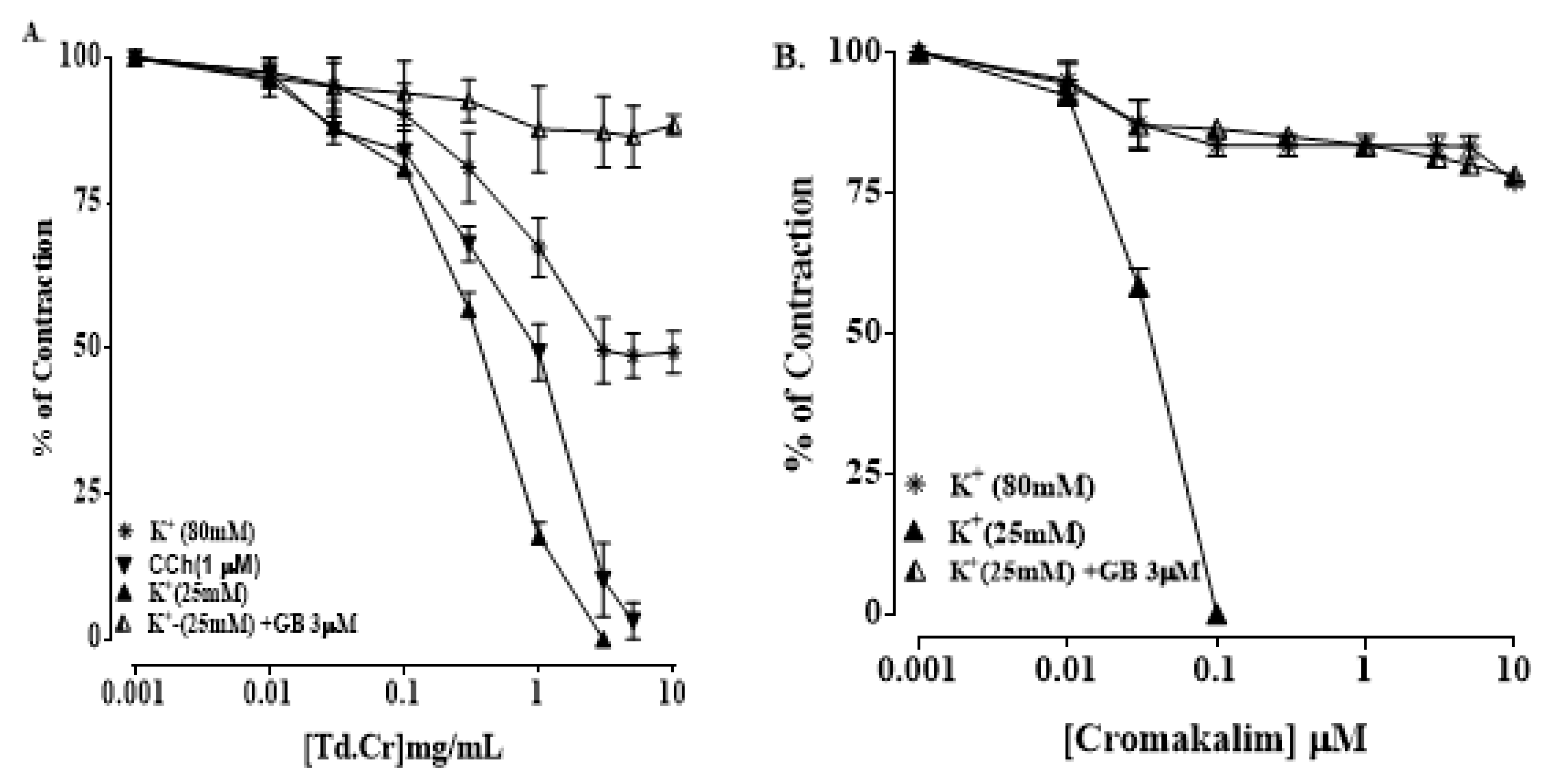
3.3.3. Isolated Preparations of Aortic Ring
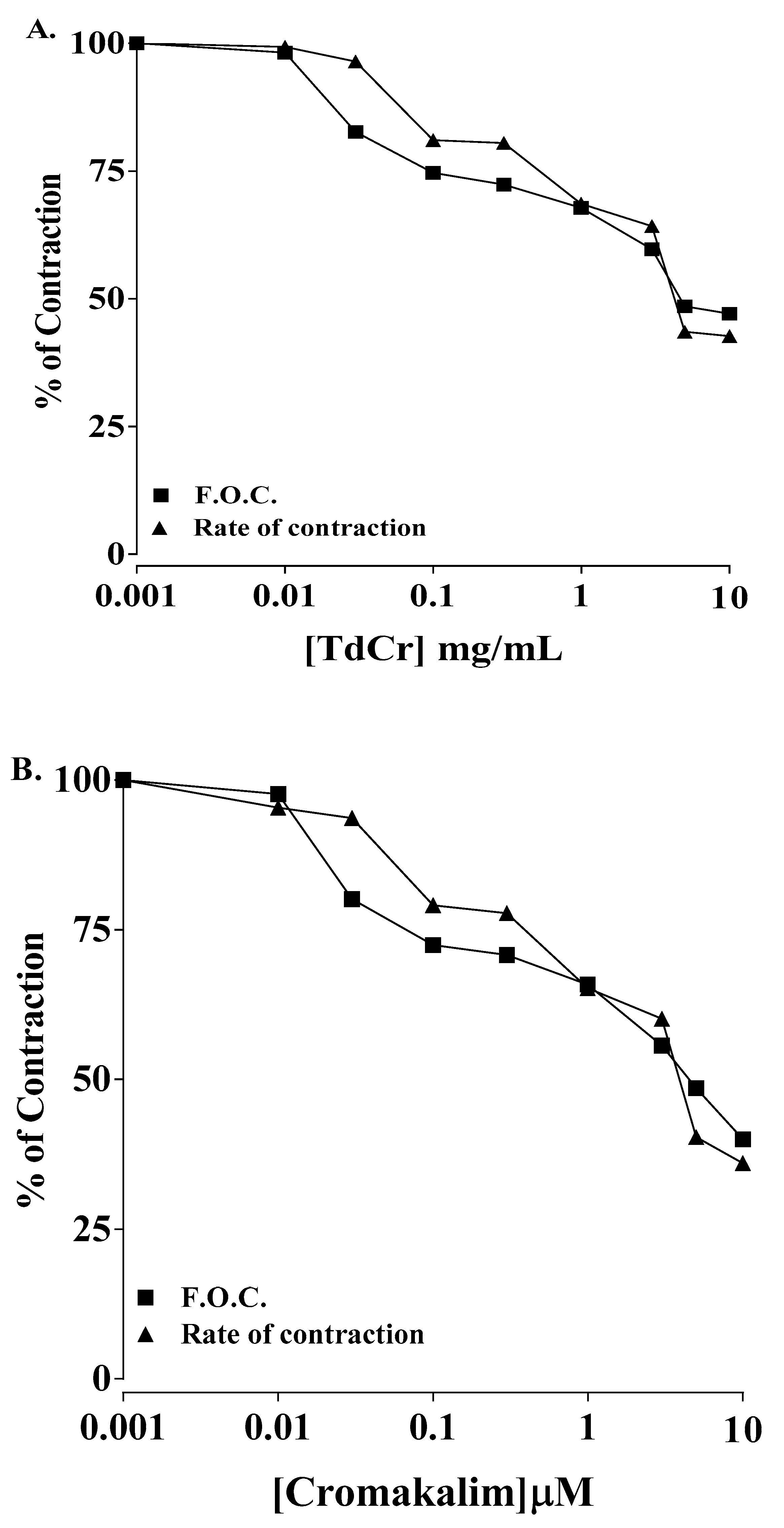
3.3.4. Effects on Paired Atrial Preparation
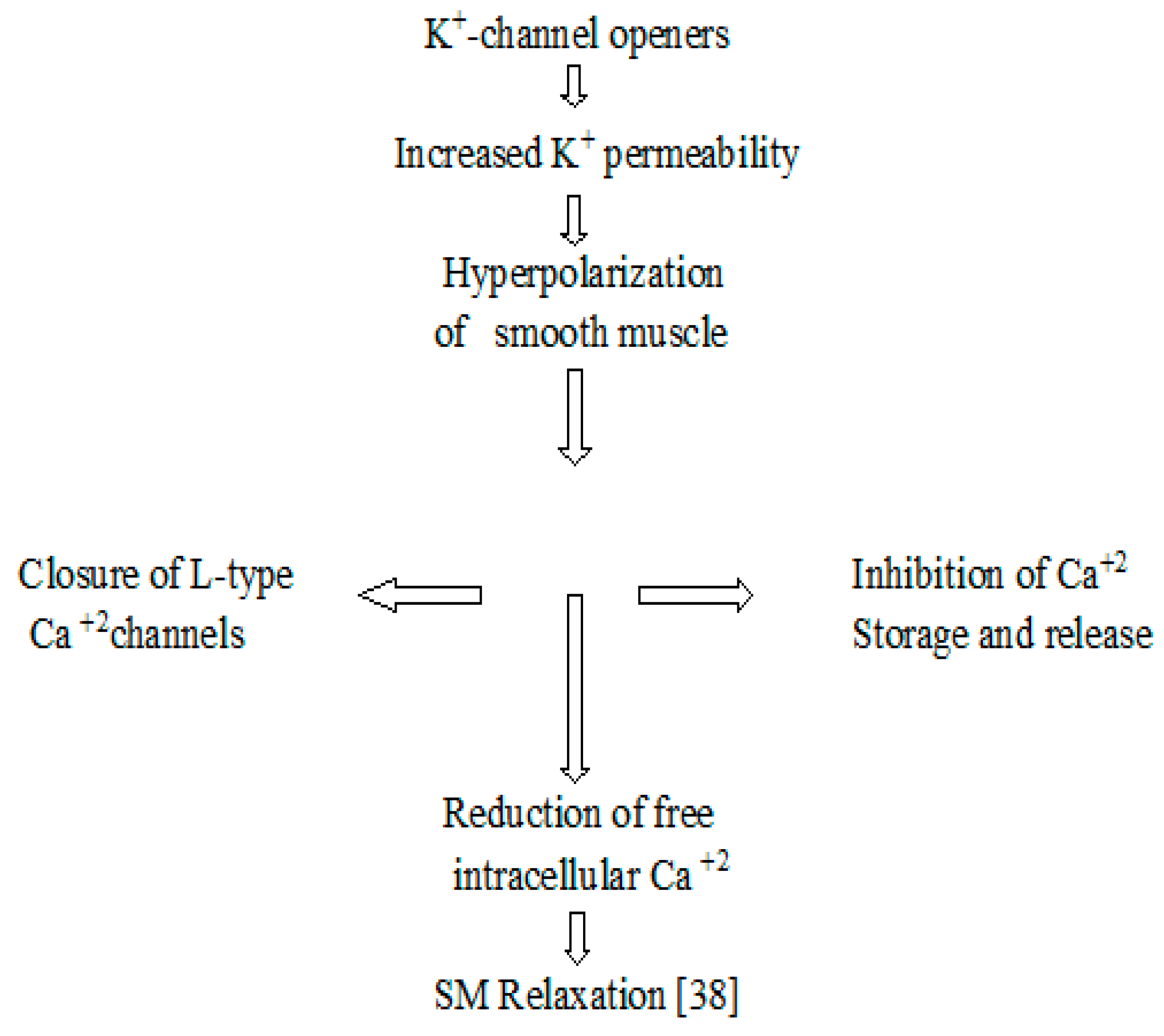
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine; |
| CCh | Carbamylcholine; |
| CCB | Calcium channel blockade; |
| CI | Confidence interval; |
| EC50 | Effective concentration that gives half the maximum response; |
| FOC | Force of contraction; |
| GB | Glibenclamide; |
| KATP | ATP-dependent potassium channel; |
| K+(80 mmol/L) | High K; |
| K+(25 mmol/L) | Low K’; |
| TdCr | Methanolic crude extract of Tamarix dioica; |
| TdDcm | Dichloromethane fraction of Tamarix dioica; |
| TdAq | Aqueous fraction of Tamarix dioica; |
| MLC | Myosin light chain; |
| NS | Normal saline; |
| PE | Phenylephrine; |
| SEM | Standard error mean; |
| TPC | Total phenolic content; |
| TFC | Total flavonoid content. |
References
- Gilani, A.H.; Rahman, A.-U. Trends in ethnopharmacology. J. Ethnopharmacol. 2005, 100, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Samejo, M.Q.; Sumbul, A.; Shah, S.; Memon, S.B.; Chundrigar, S. Phytochemical Screening of Tamarix dioica Roxb. Ex Roch. J. Pharm. Res. 2013, 181–183. [Google Scholar] [CrossRef]
- Mahmood, A.; Shaheen, H.; Qureshi, R.A.; Sangi, Y.; Gilani, S.A. Ethnomedicinal survey of plants from district Bhimber Azad Jammu and Kashmir. Pak. J. Med. Plants Res. 2011, 5, 2348–2360. [Google Scholar]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hirko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.F.; Muhammad, J.S.; Shahryar, S.; Usmanghani, K.; Gilani, A.-H.; Jafri, W.; Sugiyama, T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012, 141, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Said, M. Potential of herbal medicines in modern medical therapy. Anc. Sci. Life 1984, 4, 36–47. [Google Scholar] [PubMed]
- Shah, M.; Hussain, F. Ethnomedicinal plant wealth of Mastuj valley, Hindukush range, District Chitral, Pakistan. J. Med. Plants Res. 2012, 6, 4328–4337. [Google Scholar] [CrossRef][Green Version]
- Chaudhary, S.; Kumar, R. Ethnomedicinal plants of the district bijnor (u.p.) india. J. Indian Bot. Soc. 2015, 94, 97–103. [Google Scholar]
- Waaris, H.M.; Ahmad, S.; Anjum, S.; Alam, K. Ethnobotanical Studies of Dicotyledonous Plants of Lal Suhanra National Park, Bahawalpur. Intl. J. Sci. Res. 2014, 3, 2452–2460. [Google Scholar]
- Anis, M.; Sharma, M.; Iqbal, M. Herbal Ethnomedicine Of The Gwalior Forest Division In Madhya Pradesh, India. Pharm. Boil. 2000, 38, 241–253. [Google Scholar] [CrossRef]
- Khan, S.; Khan, G.M.; Mehsud, S.; Rahman, A.; Gomal, F.K. Antifungal activity of Tamarix dioica-an in vitro study. Glob. J. Math. 2004, 2, 40–42. [Google Scholar]
- Nidavani, R.B.; Mahalakhshmi, A.M.; Shalawadi, M. Potent ulcer protective action of anti inflammatory herbs: A short review. W. J. Pharm. Res. 2014, 3, 2057–2066. [Google Scholar]
- Najmi, A.K.; Pillai, K.K.; Pal, S.N.; Aqil, M. Free radical scavenging and hepatoprotective activity of jigrine against galactosamine induced hepatopathy in rats. J. Ethnopharmacol. 2004, 97, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Pillai, K.K.; Najmi, A.K.; Ahmad, S.J.; Pal, S.N.; Balani, D.K. Evaluation of hepatoprotective potential of jigrine post-treatment against thioacetamide induced hepatic damage. J. Ethnopharmacol. 2002, 79, 35–41. [Google Scholar] [CrossRef]
- Khan, S.F.; Mahmood, T. In vitro antimicrobial and cytotoxic activity of Tamarix dioica Roxb. Leaves. Turk. J. Biol. 2013, 37, 329–335. [Google Scholar] [CrossRef]
- Ashour, O.M.; Abdel-Naim, A.B.; Abdallah, H.M.; Nagy, A.A.; Mohamadin, A.M.; Abdel-Sattar, E.A. Evaluation of the Potential Cardioprotective Activity of Some Saudi Plants against Doxorubicin Toxicity. Zeitschrift für Naturforschung C 2012, 67, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Bisht, K.S.; Sharma, S.K.; Jain, R.; Taneja, P.; Singh, S.; Simonsen, O.; Boll, P.M. Highly oxygenated bioactive flavones from Tamarix. Phytochemistry 1994, 36, 507–511. [Google Scholar] [CrossRef]
- Malhotra, S. Antioxidant, antiinflammatory and antiinvasive activities of biopolyphenolics. Arkivoc 2008, 2008, 119–139. [Google Scholar]
- Bashir, S.; Janbaz, K.H.; Qaiser, J.; Gilani, A.H. Studies on spasmogenic and spasmolytic activities of Calendula officinalis flowers. Phytothr. Res. 2006, 20, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996; pp. 1–7. [Google Scholar]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A.J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef]
- Haq, I.U.; Bibi, G.; Kanwal, S.; Ahmad, M.S.; Mirza, B. Antioxidant/anticancer activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharma. Res. 2012, 11, 241–249. [Google Scholar]
- Jafri, L.; Saleem, S.; Haq, I.U.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab. J. Chem. 2014, 10, S3699–S3706. [Google Scholar] [CrossRef]
- Aleem, A.; Mehmood, M.H.; Jawed, F.; Gilani, A.-H.; Ja, K.H.; Bashir, S. Pharmacological Studies on Antidiarrheal, Gut Modulatory, Bronchodilatory and Vasodilatory Activities of Myrica nagi. Int. J. Pharmacol. 2015, 11, 888–898. [Google Scholar]
- Janbaz, K.H.; Arif, J.; Saqib, F.; Imran, I.; Ashraf, M.; Zia-Ul-Haq, M.; Jaafar, H.Z.; De Feo, V. In-vitro and in-vivo validation of ethnopharmacological uses of methanol extract of Isodon rugosus Wall. ex Benth. (Lamiaceae). BMC Complement. Altern. Med. 2014, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Akram, S.; Saqib, F.; Khalid, M. Antispasmodic activity of Symplocos paniculata is mediated through opening of ATP-dependent K+ channels. Bangladesh J. Pharmacol. 2016, 11, 495–500. [Google Scholar] [CrossRef]
- Camerino, D.C.; Tricarico, D.; Desaphy, J.F. Ion channel pharmacology. Neurotherapeutics 2007, 4, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Janbaz, K.H.; Jabeen, Q. Hypotensive and diuretic activities of aqueous-ethanol extract of Asphodelus tenuifolius. Bangladesh J. Pharmacol. 2016, 11, 830. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Choi, J.-H. Effect of Methyl Jasmonate on Phenolics, Isothiocyanate, and Metabolic Enzymes in Radish Sprout (Raphanus sativusL.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Parthenium hysterophorus: A Probable Source of Anticancer, Antioxidant and Anti-HIV Agents. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar]
- Agrawal, A.D. Pharmacological activities of flavonoids. a review. Int. J. Pharma. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar]
- Montaño, J.M.; Morón, B.C. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agri. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Gaginella, T.S.; Phillips, S.F.; Phillips, D.S.F. Ricinoleic acid: Current view of an ancient oil. Dig. Dis. Sci. 1975, 20, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Croci, T.; Landi, M.; Emonds-Alt, X.; Le Fur, G.; Maffrand, J.-P.; Manara, L. Role of tachykinins in castor oil diarrhoea in rats. Br. J. Pharmacol. 1997, 121, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Janbaz, K.H. Ethnopharmacological evaluation of Cenchrus ciliaris for multiple gastrointestinal disorders. Bangladesh J. Pharmacol. 2017, 12, 15. [Google Scholar] [CrossRef]
- Meite, S.; N’Guessan, J.; Bahi, C.; Yapi, H.; Djaman, A.; Guina, F. Antidiarrheal Activity of the Ethyl Acetate Extract of Morinda morindoides in Rats. Trop. J. Pharm. Res. 2009, 8, 201–207. [Google Scholar] [CrossRef]
- Karaki, H.; Wiess, G. Mini-review: Calcium release in smooth muscles. Life Sci. 1983, 42, 111–112. [Google Scholar] [CrossRef]
- Khan, T.; Ali, S.; Qayyum, R.; Hussain, I.; Wahid, F.; Shah, A.J. Intestinal and vascular smooth muscle relaxant effect of Viscum album explains its medicinal use in hyperactive gut disorders and hypertension. BMC Complement. Altern. Med. 2016, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Khan, A.-U.; Ghayur, M.N.; Ali, S.F.; Herzig, J.W. Antispasmodic Effects of Rooibos Tea (Aspalathus linearis) is Mediated Predominantly through K+-Channel Activation. Basic Clin. Pharmacol. Toxicol. 2006, 99, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Weston, A.H. Pharmacology of the potassium channel openers. Cardiovasc. Drugs Ther. 1995, 9, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Franck, H.; Puschmann, A.; Schusdziarra, V.; Allescher, H.-D. Functional evidence for a glibenclamide-sensitive K+ channel in rat ileal smooth muscle. Eur. J. Pharmacol. 1994, 271, 379–386. [Google Scholar] [CrossRef]
- Shah, A.J.; Gowani, S.A.; Zuberi, A.J.; Ghayur, M.N.; Gilani, A.H. Antidiarrhoeal and spasmolytic activities of the methanolic crude extract ofAlstonia scholarisL. are mediated through calcium channel blockade. Phytother. Res. 2010, 24, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.-U.; Gilani, A.H. Antidiarrhoeal and bronchodilatory potential ofValeriana wallichii. Nat. Prod. Res. 2012, 26, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Teshigawara, T.; Taira, N. Cytoplasmic calcium and the relaxation of canine coronary arterial smooth muscle produced by cromakalim, pinacidil and nicorandil. Br. J. Pharmacol. 1990, 101, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Lederer, W.J.; Nichols, C.G. Modulation of ATP-sensitive K+-channel activity and contractile behavior in mammalian ventricles by the potassium channel openers: Cromokalim and RP49356. J. Pharmacol. Exp. Ther. 1990, 255, 429–435. [Google Scholar] [PubMed]
- Grossett, A.; Hicks, P.E. Evidence for blood vessel selectivity of BRL34915. British J. Pharmacol 1986, 89. [Google Scholar]
- Osterrieder, W. Modification of K+ conductance of heart cell membrane by BRL 34915. Naunyn-Schmiedebergs Arch Pharmacol. 1988, 337, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Porter, L.M.; Liu, G.; Dhar-Chowdhury, P.; Srivastava, S.; Pountney, D.J.; Yoshida, H.; Artman, M.; Fishman, G.I.; Yu, C.; et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am. J. Physiol. Circ. Physiol. 2006, 291, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Jacquet, H.; Prost, A.; D’Hahan, N.; Vivaudou, M. The molecular basis of the specificity of action of KATP channel openers. EMBO J. 2000, 19, 6644–6651. [Google Scholar] [CrossRef] [PubMed]
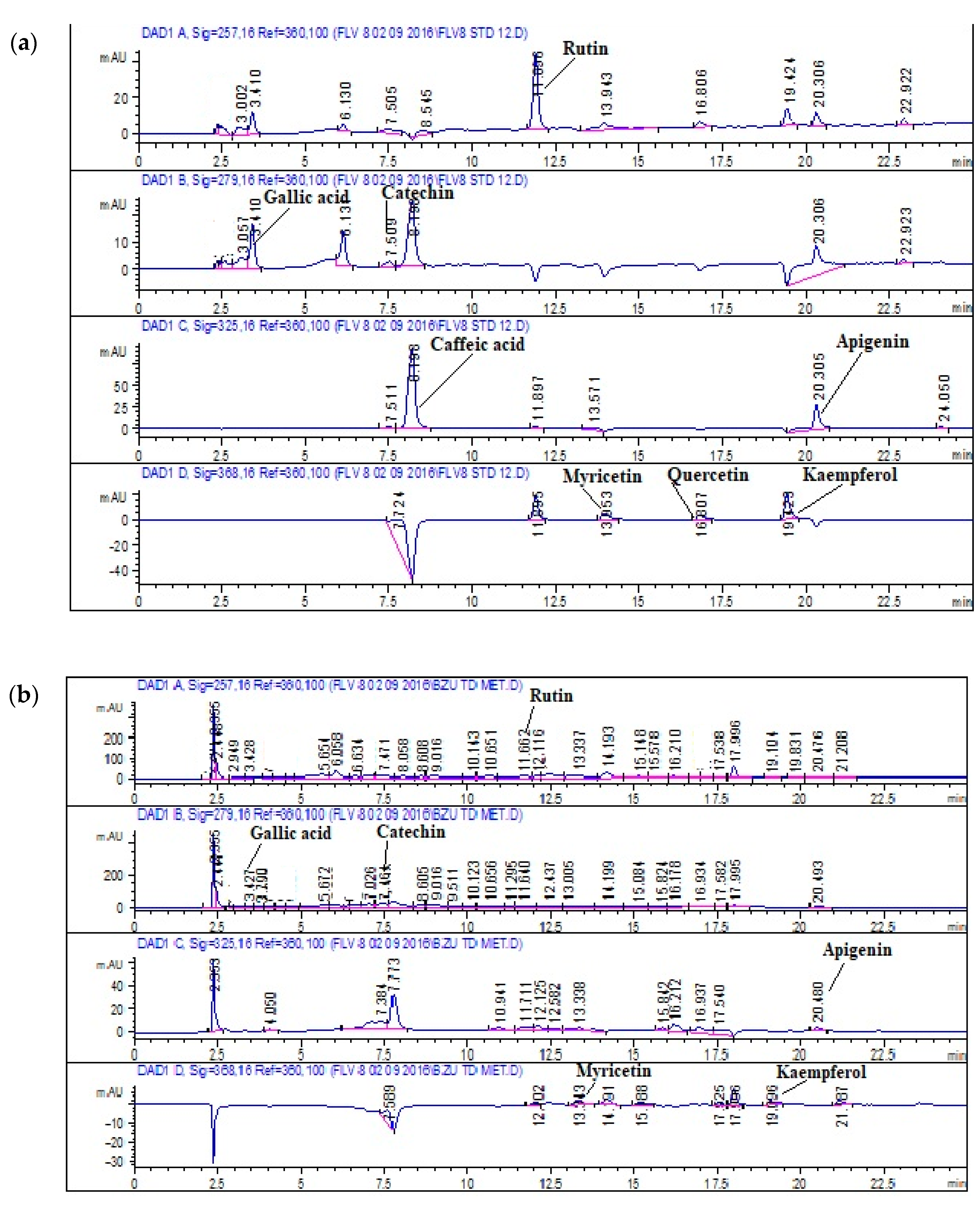

| Compound | Signal Wavelength | Retention Time as Per TdCr (min) |
|---|---|---|
| Rutin | 257 | 4.35 |
| Gallic acid | 257 | 15.91 |
| Catechin | 279 | 9.7 |
| Caffeic acid | 325 | 49.15 |
| Apigenin | 325 | 23.53 |
| Myricetin | 368 | 18.72 |
| Quercetin | 368 | 23.59 |
| Kaempferol | 368 | 21.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, S.M.; Aleem, A.; Saqib, F.; Ormenisan, A.N.; Elena Neculau, A.; Anastasiu, C.V. The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix dioica Roxb. Biomolecules 2019, 9, 722. https://doi.org/10.3390/biom9110722
Imtiaz SM, Aleem A, Saqib F, Ormenisan AN, Elena Neculau A, Anastasiu CV. The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix dioica Roxb. Biomolecules. 2019; 9(11):722. https://doi.org/10.3390/biom9110722
Chicago/Turabian StyleImtiaz, Syeda Madiha, Ambreen Aleem, Fatima Saqib, Alexe Nicolae Ormenisan, Andrea Elena Neculau, and Costin Vlad Anastasiu. 2019. "The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix dioica Roxb." Biomolecules 9, no. 11: 722. https://doi.org/10.3390/biom9110722
APA StyleImtiaz, S. M., Aleem, A., Saqib, F., Ormenisan, A. N., Elena Neculau, A., & Anastasiu, C. V. (2019). The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix dioica Roxb. Biomolecules, 9(11), 722. https://doi.org/10.3390/biom9110722





