On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective
Abstract
1. Introduction
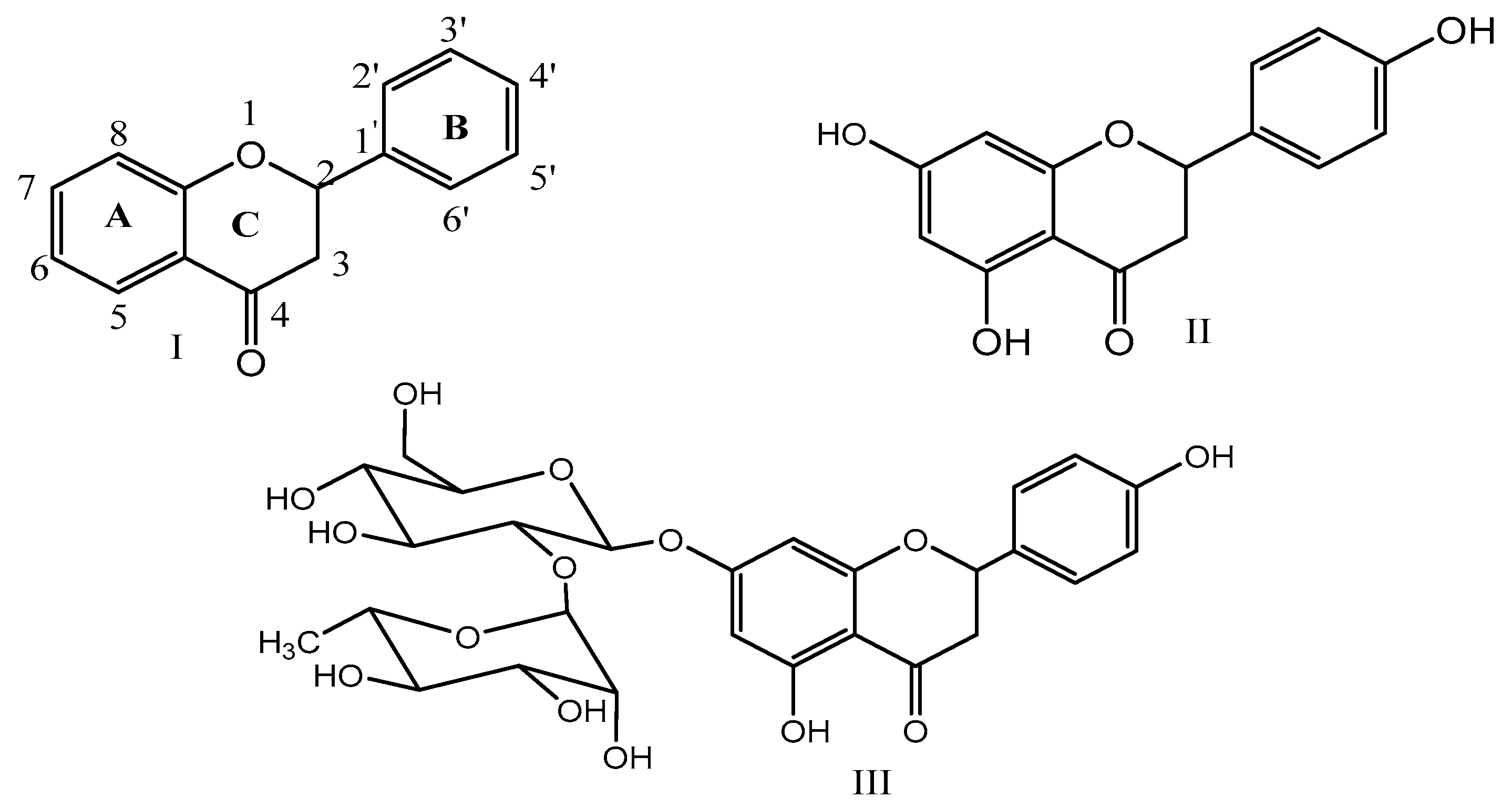
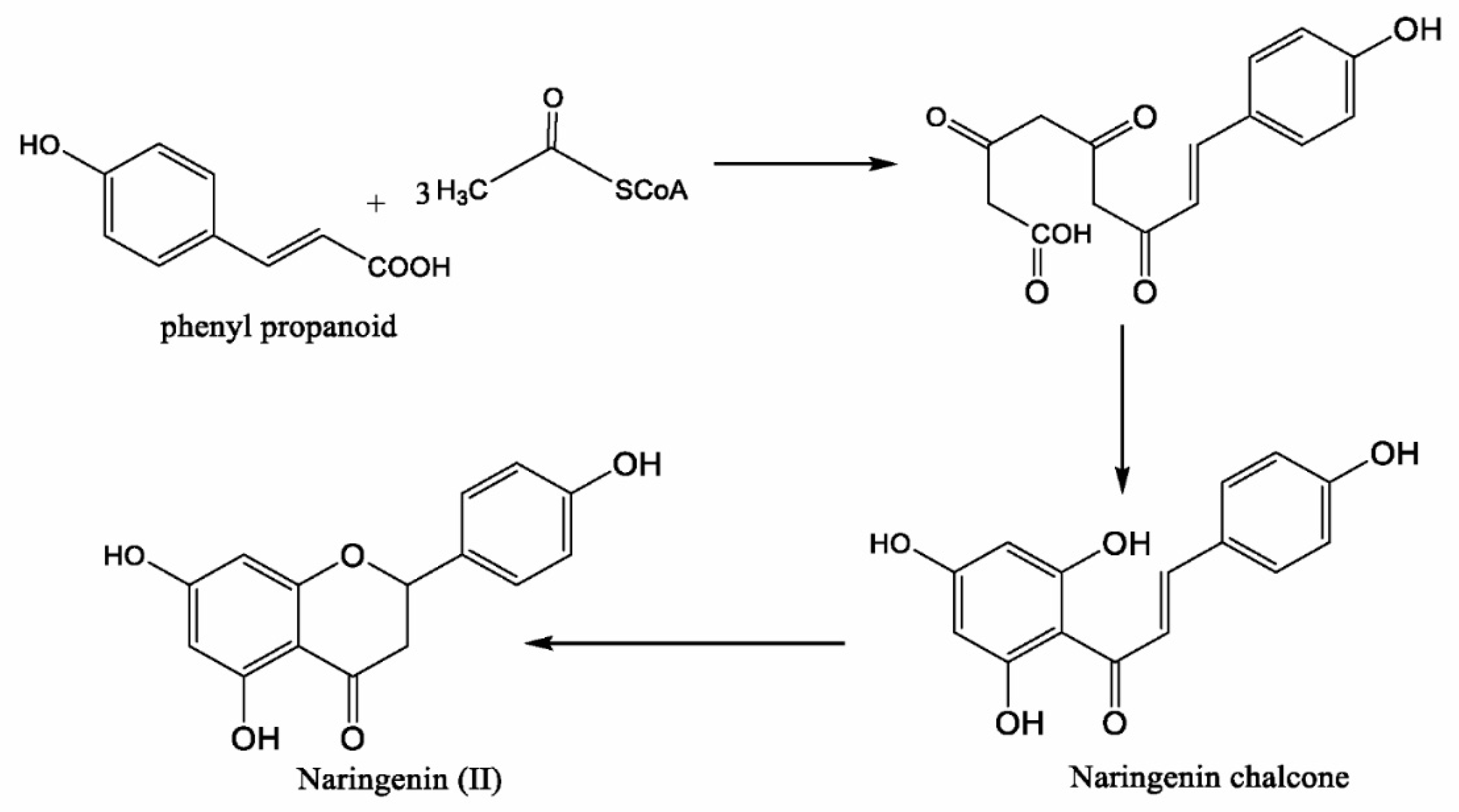
2. Chemistry of Naringenin and Its Sources
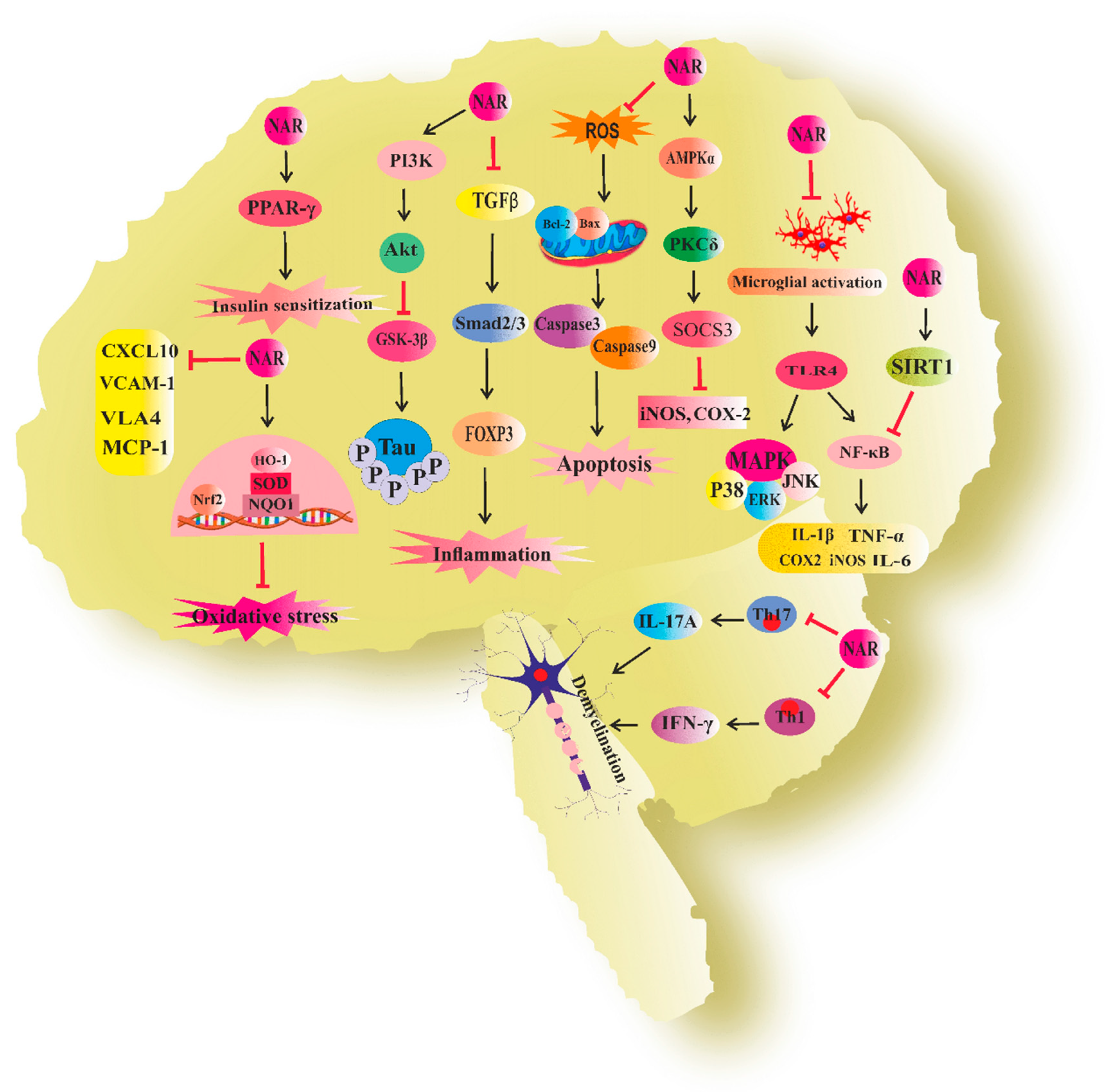
3. Naringenin in Neurodegenerative Diseases
3.1. Naringenin and Alzheimer’s Disease
3.2. Naringenin and Parkinson’s Disease
3.3. Naringenin and Neuroinflammation
3.4. Naringenin and Multiple Sclerosis
3.5. Naringenin and Cognitive Deficit
3.6. Naringenin and Neurotoxicity
3.7. Naringenin and Other Neurodegenerative Diseases
4. Nanostructured Formulations of Naringenin for Management of Neurodegenerative Diseases.
5. Conclusions
Abbreviation
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase |
| SOCS-3 | suppressor of cytokine signaling 3 |
| AMPKα | (AMP)-activated protein kinase α |
| PKC | protein kinase C |
| JNK | c-Jun N terminal kinase |
| ERK | extracellular-signal-regulated kinase |
| MAPK | mitogen-activated protein kinase |
| TNF- α | tumor necrosis factor α |
| INF-γ | interferon γ |
| IL-1β | interleukin 1β |
| MCP-1 | monocyte chemoattractant protein-1 |
| STAT-1 | signal transducer and activator of transcription-1 |
| PI3K/Akt | phosphatidylinositol-3 kinase/Akt |
| ER | estrogen receptor |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| BACE | β-Site amyloid precursor protein (APP) cleaving enzyme |
| APP | amyloid precursor protein |
| GSK-3β | glycogen synthase kinase 3β |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| AchE | acetylcholinesterase |
| RIP2 | receptor interacting protein-2 |
| NF-κB | nuclear factor-κB |
| MMP-9 | matrix metallopeptidase 9 |
| SOD | superoxide dismutase |
| GSH | glutathione |
| Nrf-2/ARE | nuclear factor E2-related factor 2/ antioxidant response element |
| HO-1 | hemoxygenase-1 |
| NQO-1 | NAD(P)H quinone dehydrogenase1 |
| TBARS | thiobarbituric acid-reactive substances |
| BDNF | brain-derived neurotrophic factor |
| TrkB | tropomyosin-related kinase B |
| GRP78 | glucose-regulated protein |
| Foxp3 | forkhead box P3 |
| DOPAC | dihydroxyphenylacetic acid |
| HVA | homovanillic acid |
| TH | tyrosine hydroxylase |
| CHIP | C terminus Hsp70 interacting protein |
| LPO | lipid peroxidation |
| GDNF | glial cell line-derived neurotrophic factor |
| HIF1a | hypoxia inducible factor1a |
| VEGF | vascular endothelial growth factor |
| 5-HT | 5-hydroxytryptamine |
| GFAP | glial fibrillary acidic protein |
| 6-OHDA | 6-hydroxydopamine |
| ICV-STZ | intracerebroventricular-streptozotocine |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| LPS | lipopolysaccharide |
| MeHg | methyl mercury |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenylpyridinium |
| pMCAO | permanent middle cerebral artery occlusion |
| tMCAO | transient middle cerebral artery occlusion |
| EAE | experimental autoimmune encephalomyelitis |
Author Contributions
Funding
Conflicts of Interest
References
- Hardy, J.; Gwinn-Hardy, K. Genetic classification of primary neurodegenerative disease. Science 1998, 282, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Thrall, J.H. Prevalence and costs of chronic disease in a health care system structured for treatment of acute illness. Radiology 2005, 235, 9–12. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kashyap, M.P.; Tripathi, V.K.; Singh, S.; Garg, G.; Rizvi, S.I. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol. Neurobiol. 2017, 54, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Giráldez-Pérez, R.M.; Antolín-Vallespín, M.; Muñoz, M.D.; Sánchez-Capelo, A. Models of α-synuclein aggregation in Parkinson’s disease. Acta Neuropathol. Commun. 2014, 2, 176. [Google Scholar] [CrossRef]
- Nah, J.; Yuan, J.; Jung, Y.-K. Autophagy in neurodegenerative diseases: From mechanism to therapeutic approach. Mol. Cells 2015, 38, 381–389. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Effects of cannabidiol interactions with Wnt/beta-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J. Neuroinflammation 2015, 12, 203. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell. Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A.; Nabavi, S.M. Naringenin and its nano-formulations for fatty liver: Cellular modes of action and clinical perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Naringenin and the liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 633–651. [Google Scholar]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl 4-induced acute liver failure. Pharm. Res. 2009, 26, 893–902. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Croft, K.D. The Chemistry and Biological Effects of Flavonoids and Phenolic Acidsa. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef]
- Verma, A.K.; Pratap, R. Chemistry of biologically important flavones. Tetrahedron 2012, 68, 8523–8538. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B 2007, 848, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.A.; Remsberg, C.M.; Miranda, N.D.; Vega-Villa, K.R.; Andrews, P.K.; Davies, N.M. Pharmacokinetics of selected chiral flavonoids: Hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 2008, 29, 63–82. [Google Scholar] [CrossRef]
- Krause, M.; Galensa, R. Analysis of enantiomeric flavanones in plant extracts by high-performance liquid chromatography on a cellulose triacetate based chiral stationary phase. Chromatographia 1991, 32, 69–72. [Google Scholar] [CrossRef]
- Wistuba, D.; Trapp, O.; Gel-Moreto, N.; Galensa, R.; Schurig, V. Stereoisomeric Separation of Flavanones and Flavanone-7- O -glycosides by Capillary Electrophoresis and Determination of Interconversion Barriers. Anal. Chem. 2006, 78, 3424–3433. [Google Scholar] [CrossRef]
- Krause, M.; Galensa, R. High-performance liquid chromatography of diastereomeric flavanone glycosides in Citrus on a β-cyclodextrin-bonded stationary phase (Cyclobond I). J. Chromatogr. A 1991, 588, 41–45. [Google Scholar] [CrossRef]
- Gaggeri, R.; Rossi, D.; Collina, S.; Mannucci, B.; Baierl, M.; Juza, M. Quick development of an analytical enantioselective high performance liquid chromatography separation and preparative scale-up for the flavonoid Naringenin. J. Chromatogr. A 2011, 1218, 5414–5422. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar] [CrossRef]
- Ho, P.C.; Saville, D.J.; Coville, P.F.; Wanwimolruk, S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm. Acta Helv. 2000, 74, 379–385. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12. [Google Scholar] [CrossRef] [PubMed]
- Gel-Moreto, N.; Streich, R.; Galensa, R. Chiral separation of diastereomeric flavanone-7-O-glycosides in citrus by capillary electrophoresis. Electrophoresis 2003, 24, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.; Strasburg, G.M.; Booren, A.; Ian Gray, J. Antioxidant Polyphenols from Tart Cherries (Prunus cerasus). J. Agric. Food Chem. 1999, 47, 840–844. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.; Gil, M.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the Polyphenol Profile of Tomato Juices Processed by Pulsed Electric Fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Exarchou, V.; Godejohann, M.; van Beek, T.A.; Gerothanassis, I.P.; Vervoort, J. LC-UV-solid-phase extraction-NMR-MS combined with a cryogenic flow probe and its application to the identification of compounds present in Greek oregano. Anal. Chem. 2003, 75, 6288–6294. [Google Scholar] [CrossRef]
- Olsen, H.T.; Stafford, G.I.; Van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, D.-S.; Yang, W.-K.; Nho, K.J.; Seo, H.S.; Kim, Y.S.; Kim, H.K. Inhibitory effects of Drynaria fortunei extract on house dust mite antigen-induced atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2012, 144, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Johnston, A.; Phillips, D.A. Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Mol Plant. Microbe Interact. 1992, 5, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wang, W.; Qian, Y.; Jin, H.J. Anti-Aβ antibodies induced by Aβ-HBc virus-like particles prevent Aβ aggregation and protect PC12 cells against toxicity of Aβ1–40. J. Neurosci. Methods 2013, 218, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, W.A.; McMillan, P.J.; Kulstad, J.J.; Leverenz, J.B.; Craft, S.; Haynatzki, G.R. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006, 199, 265–273. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimer’s Dis. JAD 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Tong, M.; Daiello, L.A.; Ott, B.R. Early-Stage Alzheimer’s Disease Is Associated with Simultaneous Systemic and Central Nervous System Dysregulation of Insulin-Linked Metabolic Pathways. J. Alzheimers Dis. 2019, 68, 657–668. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A Mechanistic Review on its Biological Activities and Health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Yang, W.; Ma, J.; Liu, Z.; Lu, Y.; Hu, B.; Yu, H. Effect of naringenin on brain insulin signaling and cognitive functions in ICV-STZ induced dementia model of rats. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014, 35, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2019, 152906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, Z.; Zhang, Z.; Liu, G.; Wang, Y.; Ren, Y.; Wu, X.; Geng, F. Protective Role Of Naringenin Against Aβ25-35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell. Mol. Neurobiol. 2018, 38, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kuboyama, T.; Tohda, C. A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer’s Disease and Its Target Molecule. Front. Pharmacol. 2017, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Imasaki, T.; Aoki, M.; Sakai, N.; Nitta, E.; Shirouzu, M.; Nitta, R. Structural Insights into the Altering Function of CRMP2 by Phosphorylation. Cell Struct. Funct. 2018, 43, 15–23. [Google Scholar] [CrossRef]
- Wilson, S.M.; Ki Yeon, S.; Yang, X.F.; Park, K.D.; Khanna, R. Differential regulation of collapsin response mediator protein 2 (CRMP2) phosphorylation by GSK3ss and CDK5 following traumatic brain injury. Front. Cell. Neurosci. 2014, 8, 135. [Google Scholar] [CrossRef]
- Lawal, M.F.; Olotu, F.A.; Agoni, C.; Soliman, M.E. Exploring the C-Terminal Tail Dynamics: Structural and Molecular Perspectives into the Therapeutic Activities of Novel CRMP-2 Inhibitors, Naringenin and Naringenin-7-O-glucuronide, in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2018, 15, e1800437. [Google Scholar] [CrossRef]
- Lawal, M.; Olotu, F.A.; Soliman, M.E.S. Across the blood-brain barrier: Neurotherapeutic screening and characterization of naringenin as a novel CRMP-2 inhibitor in the treatment of Alzheimer’s disease using bioinformatics and computational tools. Comput. Biol. Med. 2018, 98, 168–177. [Google Scholar] [CrossRef]
- Zambrano, P.; Suwalsky, M.; Jemiola-Rzeminska, M.; Strzalka, K.; Sepúlveda, B.; Gallardo, M.J.; Aguilare, L.F. The acetylcholinesterase (AChE) inhibitor and anti-Alzheimer drug donepezil interacts with human erythrocytes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1078–1085. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, M.J.; Lee, J.M.; Choi, S.J.; Cho, H.Y.; Hong, B.; Kim, H.K.; Kim, E.; Shin, D.H. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement. Geriatr. Cogn. Disord. 2004, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ciro, A.; Park, J.; Burkhard, G.; Yan, N.; Geula, C. Biochemical differentiation of cholinesterases from normal and Alzheimer’s disease cortex. Curr. Alzheimer Res. 2012, 9, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Jedrejek, D.; Senol, F.S.; Salmas, R.E.; Durdagi, S.; Kowalska, I.; Pecio, L.; Oleszek, W. Molecular modeling and in vitro approaches towards cholinesterase inhibitory effect of some natural xanthohumol, naringenin, and acyl phloroglucinol derivatives. Phytomedicine: Int. J. Phytother. Phytopharm. 2018, 42, 25–33. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef]
- Zella, M.A.S.; Metzdorf, J.; Ostendorf, F.; Maass, F.; Muhlack, S.; Gold, R.; Haghikia, A.; Tönges, L. Novel immunotherapeutic approaches to target alpha-synuclein and related neuroinflammation in Parkinson’s disease. Cells 2019, 8, 105. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Meeran, M.F.N.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease. Molecules 2019, 24, 2182. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Hu, J.; Bi, J.; Zhou, T.; Guo, X.; Han, C.; Huang, J.; Wang, T.; Xiong, N. Multiple pathways for natural product treatment of Parkinson’s disease: A mini review. Phytomedicine. 2019, 60, 152954. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Pascua Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcon-Perez, J.M.; Sanchez, D. Extracellular vesicles secreted by astroglial cells transport Apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front. Cell. Neurosci. 2018, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Q.; Zhang, B.; He, X.-M.; Li, D.-D.; Shi, J.-S.; Zhang, F. Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol. Res. 2019, 139, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Toska, K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol 1998, 16, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Angeline, M.S.; Sarkar, A.; Anand, K.; Ambasta, R.K.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef]
- Wegrzynowicz, M.; Bar-On, D.; Anichtchik, O.; Iovino, M.; Xia, J.; Ryazanov, S.; Leonov, A.; Giese, A.; Dalley, J.W.; Griesinger, C. Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 2019, 1–21. [Google Scholar] [CrossRef]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin decreases α-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef]
- Mani, S.; Sekar, S.; Chidambaram, S.B.; Sevanan, M. Naringenin protects against 1-methyl-4-phenylpyridinium-induced neuroinflammation and resulting reactive oxygen species production in SH-SY5Y cell line: An in Vitro model of parkinson’s disease. Pharmacogn. Mag. 2018, 14, 458–464. [Google Scholar]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019, 129, 534–539. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Tejera, D.; Heneka, M.T. Microglia in Neurodegenerative Disorders. Methods Mol. Biol. 2019, 2034, 57–67. [Google Scholar] [PubMed]
- Qin, H.; Roberts, K.L.; Niyongere, S.A.; Cong, Y.; Elson, C.O.; Benveniste, E.N. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J. Immunol. 2007, 179, 5966–5976. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Mc Guire, C.; Hagemeyer, N.; Martens, A.; Schroeder, A.; Wieghofer, P.; Daems, C.; Staszewski, O.; Vande Walle, L.; Jordao, M.J.C.; et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 2018, 9, 2036. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Lin, C.; Lin, H.Y.; Liu, Y.S.; Wu, C.Y.; Tsai, C.F.; Chang, P.C.; Yeh, W.L.; Lu, D.Y. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016, 53, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, G.Y.; Choi, Y.H. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases. Int. J. Mol. Med. 2012, 30, 204–210. [Google Scholar]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Socias, B.; Ouidja, M.O.; Sepulveda-Diaz, J.E.; Acuna, L.; Silva, R.L.; Michel, P.P.; Del-Bel, E.; Cunha, T.M.; Raisman-Vozari, R. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox. Res. 2016, 29, 447–459. [Google Scholar] [CrossRef]
- Kim, G.; Ouzounova, M.; Quraishi, A.A.; Davis, A.; Tawakkol, N.; Clouthier, S.G.; Malik, F.; Paulson, A.K.; D’Angelo, R.C.; Korkaya, S.J.O. SOCS3-mediated regulation of inflammatory cytokines in PTEN and p53 inactivated triple negative breast cancer model. Oncogene 2015, 34, 671–680. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Yang, S. Lidocaine Potentiates SOCS3 to Attenuate Inflammation in Microglia and Suppress Neuropathic Pain. Cell. Mol. Neurobiol. 2019, 39, 1081–1092. [Google Scholar] [CrossRef]
- Bair, A.M.; Thippegowda, P.B.; Freichel, M.; Cheng, N.; Richard, D.Y.; Vogel, S.M.; Yu, Y.; Flockerzi, V.; Malik, A.B.; Tiruppathi, C.J. Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-κB activation in endothelial cells through AMP-activated protein kinase and protein kinase Cδ. J. Biol. Chem. 2009, 284, 563–574. [Google Scholar] [CrossRef]
- Gautam, S.; Ishrat, N.; Yadav, P.; Singh, R.; Narender, T.; Srivastava, A.K. 4-Hydroxyisoleucine attenuates the inflammation-mediated insulin resistance by the activation of AMPK and suppression of SOCS-3 coimmunoprecipitation with both the IR-β subunit as well as IRS-1. Mol. Cell. Biochem. 2016, 414, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet. Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Lee, M.J.; Jang, M.; Choi, J.; Chang, B.S.; Kim, D.Y.; Kim, S.H.; Kwak, Y.S.; Oh, S.; Lee, J.H.; Chang, B.J.; et al. Korean Red Ginseng and Ginsenoside-Rb1/-Rg1 Alleviate Experimental Autoimmune Encephalomyelitis by Suppressing Th1 and Th17 Cells and Upregulating Regulatory T Cells. Mol Neurobiol 2016, 53, 1977–2002. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Zoheir, K.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Bakheet, S.A.; Attia, S.M.; Abd-Allah, A.R. Amelioration of autoimmune arthritis by naringin through modulation of T regulatory cells and Th1/Th2 cytokines. Cell. Immunol. 2014, 287, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, A.L.; Ortiz, G.G.; Pacheco-Moises, F.P.; Mireles-Ramirez, M.A.; Bitzer-Quintero, O.K.; Delgado-Lara, D.L.C.; Ramirez-Jirano, L.J.; Velazquez-Brizuela, I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch. Med Res. 2018, 49, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Y.; Niu, X.; Tang, H.; Meydani, S.N.; Wu, D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2018, 54, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gong, W.; Chen, J.; Xie, H.-W.; Wang, M.; Yin, X.-P.; Wu, W. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting Th1 and Th17 cell differentiation and proliferation. Int. Immunopharmacol. 2018, 62, 227–236. [Google Scholar] [CrossRef]
- De Araújo Farias, V.; Carrillo-Gálvez, A.B.; Martín, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Wu, C.; Wu, D. Naringenin Modifies the Development of Lineage-Specific Effector CD4(+) T Cells. Front. Immunol. 2018, 9, 2267. [Google Scholar] [CrossRef]
- Zhan, Z.; Song, L.; Zhang, W.; Gu, H.; Cheng, H.; Zhang, Y.; Yang, Y.; Ji, G.; Feng, H.; Cheng, T. Absence of cyclin-dependent kinase inhibitor p27 or p18 increases efficiency of iPSC generation without induction of iPSC genomic instability. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Lee, Y.; Lahens, N.F.; Zhang, S.; Bedont, J.; Field, J.M.; Sehgal, A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019, 17, e3000228. [Google Scholar] [CrossRef]
- Niu, X.; Wu, C.; Li, M.; Zhao, Q.; Meydani, S.N.; Wang, J.; Wu, D.J.T. Naringenin is an inhibitor of T cell effector functions. J. Nutr. Biochem. 2018, 58, 71–79. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marracci, G.H.; Bourdette, D.N. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006, 175, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Struyf, S.; Van Damme, J.; Fiten, P.; Ugarte-Berzal, E.; Opdenakker, G. Chemokine isoforms and processing in inflammation and immunity. J. Autoimmun. 2017, 85, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Quinones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef]
- Aguirre, A.; Dupree, J.L.; Mangin, J.M.; Gallo, V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 2007, 10, 990–1002. [Google Scholar] [CrossRef]
- Joshi, M.; Singh, S.; Patel, S.; Shah, D.; Krishnakumar, A. Identification of small molecule activators for ErbB 4 receptor to enhance oligodendrocyte regeneration by in silico approach. Comput. Toxicol. 2018, 8, 13–20. [Google Scholar] [CrossRef]
- Khajevand-Khazaei, M.-R.; Ziaee, P.; Motevalizadeh, S.-A.; Rohani, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M.J.E. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018, 826, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lannert, H.; Hoyer, S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci. 1998, 112, 1199. [Google Scholar] [CrossRef]
- Pathan, A.R.; Viswanad, B.; Sonkusare, S.K.; Ramarao, P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006, 79, 2209–2216. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yin, Y.; Tan, Y.; Hong, K.; Zhou, H. The Flavanone, Naringenin, Modifies Antioxidant and Steroidogenic Enzyme Activity in a Rat Model of Letrozole-Induced Polycystic Ovary Syndrome. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2019, 25, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rahigude, A.; Bhutada, P.; Kaulaskar, S.; Aswar, M.; Otari, K.J.N. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience 2012, 226, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Baluchnejadmojarad, T.; Roghani, M.J.P. Effect of naringenin on intracerebroventricular streptozotocin-induced cognitive deficits in rat: A behavioral analysis. Pharmacology 2006, 78, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Sadhu, A.; Singh, P.K.; Agrawal, A.; Ilango, K.; Purohit, S.; Dubey, G.P.J.B. Revalidation of the neuroprotective effects of a United States patented polyherbal formulation on scopolamine induced learning and memory impairment in rats. Biomed. Pharmacother. 2018, 97, 1046–1052. [Google Scholar] [CrossRef]
- Zaki, H.F.; Abd-El-Fattah, M.A.; Attia, A.S. Naringenin protects against scopolamine-induced dementia in rats. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 15–25. [Google Scholar] [CrossRef]
- Khan, M.B.; Khan, M.M.; Khan, A.; Ahmed, M.E.; Ishrat, T.; Tabassum, R.; Vaibhav, K.; Ahmad, A.; Islam, F. Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem. Int. 2012, 61, 1081–1093. [Google Scholar] [CrossRef]
- Liaquat, L.; Batool, Z.; Sadir, S.; Rafiq, S.; Shahzad, S.; Perveen, T.; Haider, S. Naringenin-induced enhanced antioxidant defence system meliorates cholinergic neurotransmission and consolidates memory in male rats. Life Sci. 2018, 194, 213–223. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, M.; Chen, W.; Liu, C.; Wang, F.; Han, X.; Zuo, Z.; Peng, S. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS ONE 2014, 9, e93639. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.-Z.; Ying, J.; Zhang, J.; Wang, X.-F.; Hu, Y.-H.; Liang, Y.-P.; Liu, Q.; Xu, G.-H. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int. J. Mol. Med. 2016, 38, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S.J.B. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ahshin-Majd, S.; Zamani, S.; Kiamari, T.; Kiasalari, Z.; Baluchnejadmojarad, T.; Roghani, M.J.P. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 2016, 86, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D.J. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.-Y.; Mao, Y.-W.; Gräff, J.; Guan, J.-S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.-H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M.; Rocha, J.B. Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Yumvihoze, E.; Chan, H.M. Superoxide produced in the matrix of mitochondria enhances methylmercury toxicity in human neuroblastoma cells. Toxicol. Appl. Pharmacol. 2015, 289, 371–380. [Google Scholar] [CrossRef]
- Sumathi, T.; Christinal, J. Neuroprotective effect of Portulaca oleraceae ethanolic extract ameliorates methylmercury induced cognitive dysfunction and oxidative stress in cerebellum and cortex of rat brain. Biol. Trace Elem. Res. 2016, 172, 155–165. [Google Scholar] [CrossRef]
- Manu, K.A.; Christina, H.; Das, S.; Mumbrekar, K.D.; Rao, B.S. Neuroprotective Role of Naringenin against Methylmercury Induced Cognitive Impairment and Mitochondrial Damage in a Mouse Model. Environ. Toxicol. Pharmacol. 2019, 103224. [Google Scholar] [CrossRef]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res. Bull. 2018, 143, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dusek, P.; Roos, P.M.; Litwin, T.; Schneider, S.A.; Flaten, T.P.; Aaseth, J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015, 31, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jain, V.; Kushwah, N.; Dheer, A.; Mishra, K.P.; Prasad, D.; Singh, S.B. Role of DNA Methylation in Hypobaric Hypoxia-Induced Neurodegeneration and Spatial Memory Impairment. Ann. Neurosci. 2018, 25, 191–200. [Google Scholar] [CrossRef]
- Cheraghi, G.; Hajiabedi, E.; Niaghi, B.; Nazari, F.; Naserzadeh, P.; Hosseini, M.J. High doses of sodium tungstate can promote mitochondrial dysfunction and oxidative stress in isolated mitochondria. J. Biochem. Mol. Toxicol. 2019, 33, e22266. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. Biofactors 2019. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Angeline, M.S.; Anand, K.; Ambasta, R.K.; Kumar, P. Naringenin and quercetin reverse the effect of hypobaric hypoxia and elicit neuroprotective response in the murine model. Brain Res. 2012, 1481, 59–70. [Google Scholar] [CrossRef]
- Muthaiah, V.P.K.; Venkitasamy, L.; Michael, F.M.; Chandrasekar, K.; Venkatachalam, S.J. Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. J. Pharmacol. Pharmacother. 2013, 4, 192–197. [Google Scholar]
- Chtourou, Y.; Fetoui, H.; Gdoura, R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol. Trace Elem. Res. 2014, 158, 376–383. [Google Scholar] [CrossRef]
- Chtourou, Y.; Slima, A.B.; Gdoura, R.; Fetoui, H. Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat hippocampus. Neurochem. Res. 2015, 40, 1563–1575. [Google Scholar] [CrossRef]
- Sachdeva, S.; Pant, S.C.; Kushwaha, P.; Bhargava, R.; Flora, S.J.J.F.; Toxicology, C. Sodium tungstate induced neurological alterations in rat brain regions and their response to antioxidants. Food Chem. Toxicol. 2015, 82, 64–71. [Google Scholar] [CrossRef]
- Xue, L.; Murray, J.H.; Tolkovsky, A.M.J. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J. Biol. Chem. 2000, 275, 8817–8824. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur. J. Pain 2019, 23, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Ma, C.-M.; Han, Y.-Z.; Li, Y.; Liu, C.; Duan, Z.-H.; Wang, H.-L.; Liu, D.-Q.; Liu, R.-H. Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch. Biol. Sci. 2015, 67, 639–646. [Google Scholar] [CrossRef]
- Kipp, M.; Clarner, T.; Gingele, S.; Pott, F.; Amor, S.; Van Der Valk, P.; Beyer, C. Brain lipid binding protein (FABP7) as modulator of astrocyte function. Physiol. Res. 2011, 60, S49. [Google Scholar]
- Hegazy, H.G.; Ali, E.H.; Sabry, H.A.J.T.; Zoology, A. The neuroprotective action of naringenin on oseltamivir (Tamiflu) treated male rats. J. Basic. Appl. Zool. 2016, 77, 83–90. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Yousefi, M.; Dolati, S.; Kasmaei, H.D.; Charsouei, S.; Nouri, M.; Shakouri, S.K. Stem cell-based cell therapy for neuroprotection in stroke: A review. J. Cell. Biochem. 2019, 120, 8849–8862. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, C.; Wei, C.; Yao, Y.; Ma, X.; Gong, Z.; Liu, S.; Zang, D.; Chen, J.; Shi, F.-D. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl. Stroke Res. 2018, 9, 174–184. [Google Scholar] [CrossRef]
- Nakano, T.; Nishigami, C.; Irie, K.; Shigemori, Y.; Sano, K.; Yamashita, Y.; Myose, T.; Tominaga, K.; Matsuo, K.; Nakamura, Y.J.J.; et al. Goreisan prevents brain edema after cerebral ischemic stroke by inhibiting aquaporin 4 upregulation in mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 758–763. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Shih, P.-H.; Yen, G.-C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Chen, L.; Zhang, J.; Zhang, L.; Zhao, X.; Zhao, T.; Zhao, Y. Protective effect of naringenin in experimental ischemic stroke: Down-regulated NOD2, RIP2, NF-kappaB, MMP-9 and up-regulated claudin-5 expression. Neurochem. Res. 2014, 39, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, Y.G.; McDonald, C.; Kanneganti, T.D.; Hasegawa, M.; Body-Malapel, M.; Inohara, N.; Nunez, G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 2007, 178, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, V.; Gunawan, P.Y.; Wiradarma, H.D.; Hartoyo, V.J.O.A.M. Glial Fibrillary Acidic Protein Serum Level as a Predictor of Clinical Outcome in Ischemic Stroke. J. Med. Sci. 2019, 7, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Khan, M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.; Safhi, M. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef]
- Saleh, T.M.; Saleh, M.C.; Connell, B.J.; Song, Y.H. A co-drug conjugate of naringenin and lipoic acid mediates neuroprotection in a rat model of oxidative stress. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1008–1016. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, D.; Xia, W.; Gu, C.; Lahm, T.; Xu, X.; Qiu, Q.; Zhang, Z. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) contributes to the neuroprotective effects of histone deacetylase inhibitors in retinal ischemia-reperfusion injury. Neuroscience 2019, 418, 25–36. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Rose, J.; Brian, C.; Woods, J.; Pappa, A.; Panayiotidis, M.I.; Powers, R.; Franco, R. Mitochondrial dysfunction in glial cells: Implications for neuronal homeostasis and survival. Toxicology 2017, 391, 109–115. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, C.; Ju, Z.; Zheng, S.; Wen, Z.; Xu, S.; Chen, Y.; Ma, Z. The mitochondrial membrane protein FgLetm1 regulates mitochondrial integrity, production of endogenous reactive oxygen species and mycotoxin biosynthesis in Fusarium graminearum. Mol. Plant Pathol. 2018, 19, 1595–1611. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Song, Q.; Fang, Z.; Chen, Z.; Zhang, Y.; Zhang, L.; Zhang, L.; Niu, N.; Ma, S. Mitochondrial dysfunction causes oxidative stress and Tapetal apoptosis in chemical hybridization reagent-induced male sterility in wheat. Front. Plant Sci. 2018, 8, 2217. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Huang, J.; Huang, L.; Luo, N.; Liang, X.; Liang, M.; Xie, W. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clin. Exp. Pharmacol. Physiol. 2017, 44, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, S.; Augustine, J.; Cunning, R.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M.J.I. Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7. Int. J. Mol. Sci. 2019, 20, 2101. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Alhomida, A.S.; LaNoue, K.F. Gabapentin Attenuates Oxidative Stress and Apoptosis in the Diabetic Rat Retina. Neurotox. Res. 2019, 36, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants 2018, 7, 133. [Google Scholar] [CrossRef]
- Cui, X.; Fu, Z.; Wang, M.; Nan, X.; Zhang, B. Pitavastatin treatment induces neuroprotection through the BDNF-TrkB signalling pathway in cultured cerebral neurons after oxygen-glucose deprivation. Neurol. Res. 2018, 40, 391–397. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Q.; Chen, Y.; Liu, F.; Zheng, Y.; Tang, J.; Zhang, J.; Zhang, J.H. Neuroprotective role of an N-acetyl serotonin derivative via activation of tropomyosin-related kinase receptor B after subarachnoid hemorrhage in a rat model. Neurobiol. Dis. 2015, 78, 126–133. [Google Scholar] [CrossRef][Green Version]
- Reijonen, S.; Putkonen, N.; Norremolle, A.; Lindholm, D.; Korhonen, L. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp. Cell Res. 2008, 314, 950–960. [Google Scholar] [CrossRef]
- Jiang, Y.; Chadwick, S.R.; Lajoie, P. Endoplasmic reticulum stress: The cause and solution to Huntington’s disease? Brain Res. 2016, 1648, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lv, H.; Liao, M.; Xu, X.; Huang, S.; Tan, H.; Peng, T.; Zhang, Y.; Li, H. GRP78 counteracts cell death and protein aggregation caused by mutant huntingtin proteins. Neurosci. Lett. 2012, 516, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Kitamura, A.; Nagata, K. Analyzing the aggregation of polyglutamine-expansion proteins and its modulation by molecular chaperones. Methods (San DiegoCalif.) 2011, 53, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, N.; Yamamoto, Y.; Noda, C.; Hatayama, T. Naringenin inhibits the aggregation of expanded polyglutamine tract-containing protein through the induction of endoplasmic reticulum chaperone GRP78. Biol. Pharm. Bull. 2012, 35, 1836–1840. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639. [Google Scholar] [CrossRef]
- Bruijn, L.I.; Miller, T.M.; Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004, 27, 723–749. [Google Scholar] [CrossRef]
- Kim, S.H.; Shi, Y.; Hanson, K.A.; Williams, L.M.; Sakasai, R.; Bowler, M.J.; Tibbetts, R.S. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. J. Biol. Chem. 2009, 284, 8083–8092. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.-X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59. [Google Scholar] [CrossRef]
- Xia, P.; Gao, X.; Duan, L.; Zhang, W.; Sun, Y.-F. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed. Pharmacother. 2018, 107, 1480–1487. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, L.; Liu, Y.; Peng, T. Oxysophoridine rescues spinal cord injury via anti-inflammatory, anti-oxidative stress and anti-apoptosis effects. Mol. Med. Rep. 2018, 17, 2523–2528. [Google Scholar] [CrossRef]
- Shi, L.-B.; Tang, P.-F.; Zhang, W.; Zhao, Y.-P.; Zhang, L.-C.; Zhang, H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene 2016, 592, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Roy, S. The physical approximation of APP and BACE-1: A key event in alzheimer’s disease pathogenesis. Dev. Neurobiol. 2018, 78, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Drenscko, M.; Loverde, S.M. Molecular dynamics simulations of the interaction of phospholipid bilayers with polycaprolactone. Mol. Simul. 2019, 45, 859–867. [Google Scholar] [CrossRef]
- Ahmad, A.; Fauzia, E.; Kumar, M.; Mishra, R.K.; Kumar, A.; Khan, M.A.; Raza, S.S.; Khan, R. Gelatin-Coated Polycaprolactone Nanoparticle-Mediated Naringenin Delivery Rescue Human Mesenchymal Stem Cells from Oxygen Glucose Deprivation-Induced Inflammatory Stress. ACS Biomater. Sci. Eng. 2018, 5, 683–695. [Google Scholar] [CrossRef]
| Type of Diseases | Method | Model | Neuropharmacological Mechanisms and Outcome | References |
|---|---|---|---|---|
| LPS Induced neuroinflammation | In vitro: BV2 microgelia cells | ↓iNOS ↓COX-2 ↑SOCS3 ↑ AMPKα ↑ PKCδ | [85] | |
| Neuroinflammation | Induced by LPS | In vitro: BV2 microgelia cells | ↓JNK ↓ERK ↓p38 ↓MAPK ↓TNF-α ↓IL-1β ↑ Arg-1↑IL-10 | [12] |
| Induced by LPS | In vitro: BV2 microgelia cells | ↓TNF-α ↓IL-6 ↓IL-1β ↓MCP-1 ↓NfκB ↓MAPK ↓Akt ↓iNOS ↓ COX-2 | [86] | |
| Induced by LPS/ IFN-γ | In vitro: microglia | ↓p38 MAPK ↓ERK1/2 ↓STAT1 ↓iNOS ↓ TNF-α | [87] | |
| Aβ25-35-induced AD | In vitro: PC12 cells | ↓apoptosis, ↓caspase3, ↑ PI3K/AKT, ↑ER | [54] | |
| AD | Aβ25-35-induced AD | In vivo: Wistar rats | ↓MDA ↓apoptosis, ↑ER, ↑spatial memory and cognition | [55] |
| ICV-STZ- induced AD Induced by Aβ1-42 and Aβ25-35 | In vivo: Sprague–Dawley rats In vitro: cultured cortical neurons In vivo: 5XFAD Mice | ↓Tau hyper-phosphorylation, ↑ PI3K/AKT ↓GSK3-β ↑ PPAR- γ ↑insulin signaling ↑spatial learning and memory ↓amyloid plaques, ↓Tau hyper-phosphorylation | [52] [56] | |
| Amnesia | Induced by scopolamine | In vivo: ICR mice | ↓AchE activity ↑spontaneous alteration behavior | [62] |
| pMCAO- induced cerebral ischemic | In vivo: Sprague–Dawley rats | ↓infarct size, ↓brain water content, ↓ NOD2, RIP2, NF-κB, MMP-9, ↑ claudin-5 | [152] | |
| Induced by hypoxia | In vitro: neurons isolated from the brain of Sprague–Dawley rats | ↓ROS, MDA, ↑SOD, GSH↓caspase-3, Bax, ↑ Bcl-2, ↑AMP, ADP, ATP, ANT↑ Nrf2, HO-1, NQO1 | [160] | |
| Ischemic stroke | MCAO/R-induced ischemic stroke | In vivo: Sprague–Dawley rats | ↓brain water content, ↓TUNEL-positive cells | [163] |
| Induced by MCAO/R | In vivo: Wistar rats | ↓infarct size, neurological deficits, brain water, ↑ motor, and somatosensory function ↑SOD, GSH, MPO, TBARS, ↓COX-2, iNOS, ↓IL-1β, TNF-α ↓ NF-κB | [155] | |
| Diabetic retinopathy | STZ-induced diabetic retinopathy | In vivo: Wistar albino rats | ↓ TBARS, ↑GSH, ↓caspase-3, Bax, ↑Bcl-2 ↑ BDNF, TrkB, synaptophysin, | [169] |
| Polyglutamine diseases | - | In vitro: mouse C3H10T1/2 cells, COS-7 cells, and HeLa-tetQ97 Cells | ↑GRP78 | [175] |
| (MOG)35-55-induced EAE | In vivo: C57BL/6 mice | ↓ Th1, Th9, Th17, ↑ Treg, ↓T-bet, PU.1, and RORγt, | [97] | |
| EAE | Induced by anti-CD3/CD28 | In vivo: C57BL/6 mice | ↓IFNγ, ↓STAT1, STAT3, STAT4, ↓IL-6, ↑gp-130, ↓Foxp3 | [101] |
| Induced by anti-CD3/CD28 and (MOG)35-55 | In vitro: mouse T cells | ↓T cells proliferation, ↓ IFN-γ, IL-17A ↓ TNF-α, IL-6, block T cells at G0/G1 phase ↑ P27, ↓ retinoblastoma protein phosphorylation, ↓IL-2, CD25 ↓ STAT5 | [104] | |
| Induced by 6-OHDA | In vitro: Human neuroblastoma SH-SY5Y cells. In vivo: C57BL/6 mice | ↑Nrf2/ARE ↑HO-1, ↓ROS ↑GSH ↓ JNK and p38 | [71] | |
| PD | MPTP-induced PD | In vivo: C57BL/6J mice | ↓α-synuclein ↑dopamine transporter ↑DOPAC ↑HVA ↑TH ↓TNFα & IL1β ↑SOD | [78] |
| Rotenone-induced PD | In vivo: Wistar rats | ↓ubiquitin and caspase3 improvement of motor skills ↑parkin ↑CHIP ↑PARK 7 protein ↑TH | [76] | |
| MPTP-induced PD | In vivo: C57BL/6J mice | ↑GRx & CAT ↓LPO& iNOS ↓ nuclear pigmentation and cytoplasmic vacuolation | [80] | |
| - | In vitro: primary rat midbrain neuron-glia co-cultures | ↑ BDNF, GDNF↑ Nrf2 ↑Dopaminergic neurons survival | [73] | |
| 6-OHDA-induced PD | In vivo: Sprague-Dawley rats | ↑DOPAC, ↑HVA, ↑Dopamine↑TH | [74] | |
| Induced by MPP+ | In vitro: Human neuroblastoma SH-SY5Y cells | ↓ ROS ↓NF-κB ↓TNF-α ↓Bax ↑Bcl-2 | [79] | |
| Induced by sodium tungstate | In vivo: Wistar rat | ↑GSH ↓ROS ↓ TBARS ↑Dopamine | [141] | |
| Induced by glutamate | In vitro: primary culture of mouse hippocampal neurons | ↑ Erk1/2 & Akt phosphorylation ↓calpain-1 & caspase-3 | [144] | |
| Neurotoxicity | Induced by hypobaric hypoxia | In vivo: Swiss albino mice | ↓HIF1a ↓VEGF ↓caspase-3 ↓ ubiquitin ↑CHIP ↑ parkin | [137] |
| iron-induced neurotoxicity | In vivo: Wistar rat | ↑ SOD, CAT ↓ROS ↑ AChE ↓MDA ↑Na+/K+ ATPase | [139] | |
| Induced by oseltamivir | In vivo: Wistar rat | ↑FABP7 ↑Ca ATPase, ↑TAC ↓TOC ↓TNO ↓ cytochrome P450 | [146] | |
| Induced by iron | In vivo: Wistar rat | ↓ROS ↑GSH, CAT, SOD ↑AchE ↑ectonucleotidase enzymes ↑mitochondrial complex I–V enzymes ↑ mitochondrial membrane potential | [140] | |
| Induced by carbaryl | In vitro: mouse neuroblastoma cells | ↓ROS ↓Bax, caspase-3 ↑Bcl-2 ↑mitochondrial membrane potential | [138] | |
| Induced by ICV-STZ | In vivo: Wistar rats | ↑ Learning and memory performance | [116] | |
| Induced by ICV-STZ | In vivo: Wistar rats | ↑learning & memory ↓TBARS, MDA, 4-HNE, H2O2, protein carbonyl, ↑GSH, SOD, CAT ↑Na+/K+ ATPase activity | [119] | |
| Induced by scopolamine | In vivo: albino Wistar rats | ↓AChE ↑GSH ↓TBARS ↓TNFα ↓5HT, NE ↑spontaneous alternation performance & conditioned avoidance response | [118] | |
| Induced by isoflurane | In vivo: Sprague–Dawley rats | ↓Bad, caspase-3, Bax ↑ Bcl-2, Bcl-xL ↓TUNEL ↑ PI3K/Akt ↓PTEN ↓NF-κB, TNF-α, IL -6, IL-1β, Improvement of cognitive dysfunction | [123] | |
| Induced by LPS | In vivo: albino Wistar rats | ↓TLR4, NF-κB, TNF-α, COX2 and iNOS ↑Nrf2, SOD, CAT, and GSH ↓MDA and AChE ↓GFAP ↑ spatial recognition memory, discrimination ratio & retention and recall capability | [110] | |
| Cognitive deficit | Age-induced cognitive deficit | In vivo: Sprague–Dawley rats | ↑ SIRT1 ↓ NF-κB ↑serotonin, noradrenaline, dopamine, TH | [126] |
| Induced by MeHg | In vivo: Swiss Albino mice | ↑ mitochondrial complex I- IV activities, ↓lesions /10kb ↑GSH, GST ↓MDA & protein carbonyl ↑spatial and recognition memory | [131] | |
| - | In vivo: young adult male Albino Wistar rats | ↓AChE, ↑ 5HT | [120] | |
| Induced by type 2 diabetes mellitus | In vivo: Young Sprague–Dawley rats | ↓AChE, ↓hyperglycemia ↑memory performance | [115] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. https://doi.org/10.3390/biom9110690
Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, Chen J-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules. 2019; 9(11):690. https://doi.org/10.3390/biom9110690
Chicago/Turabian StyleNouri, Zeinab, Sajad Fakhri, Fardous F. El-Senduny, Nima Sanadgol, Ghada E. Abd-ElGhani, Mohammad Hosein Farzaei, and Jen-Tsung Chen. 2019. "On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective" Biomolecules 9, no. 11: 690. https://doi.org/10.3390/biom9110690
APA StyleNouri, Z., Fakhri, S., El-Senduny, F. F., Sanadgol, N., Abd-ElGhani, G. E., Farzaei, M. H., & Chen, J.-T. (2019). On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules, 9(11), 690. https://doi.org/10.3390/biom9110690








