Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cell Vitality Assays
2.3. Gene Expression Relative Quantification
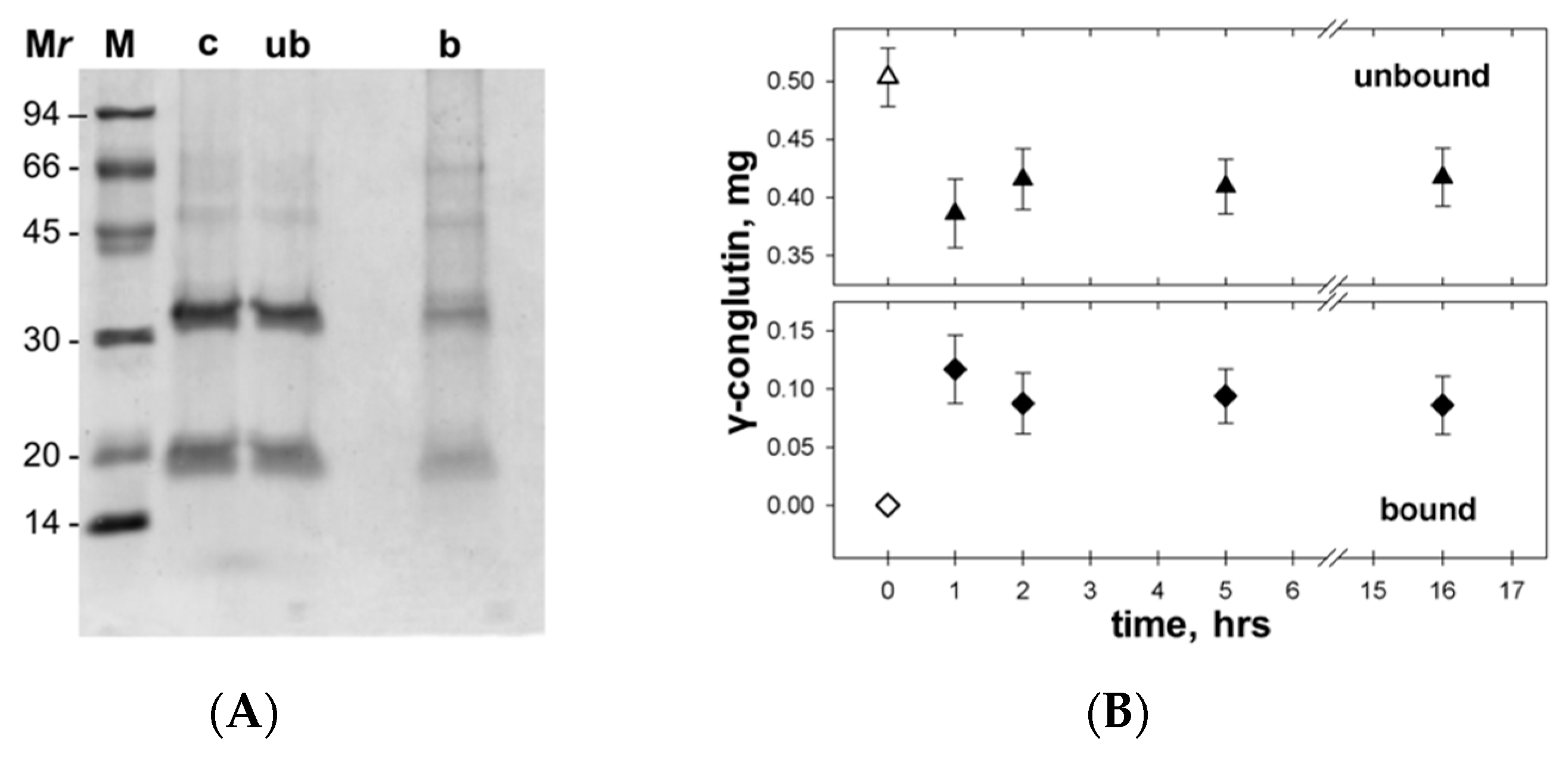
2.4. Preparation of γC Samples and Interaction with Nanoparticles
2.5. SDS-PAGE
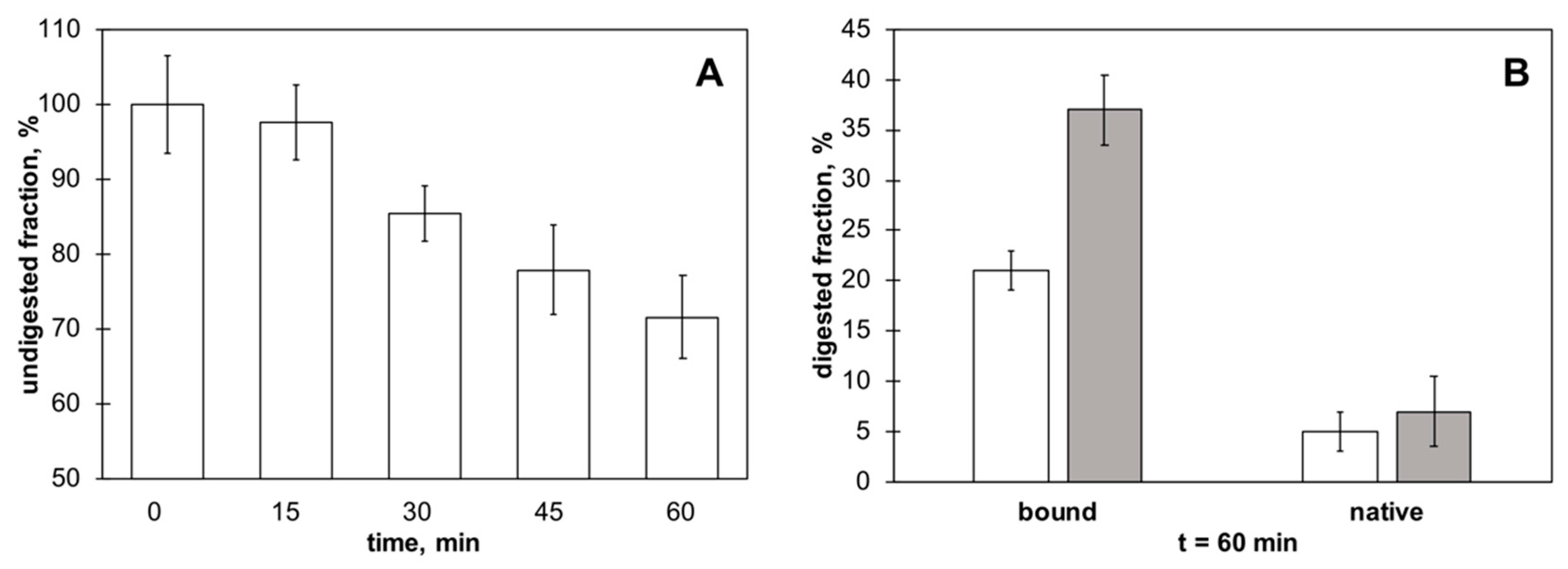
2.6. Proteolytic Digestions
2.7. Protein Densitometric Quantification
2.8. In-Silico Predictions
2.9. Statistical Analysis
3. Results and Discussion
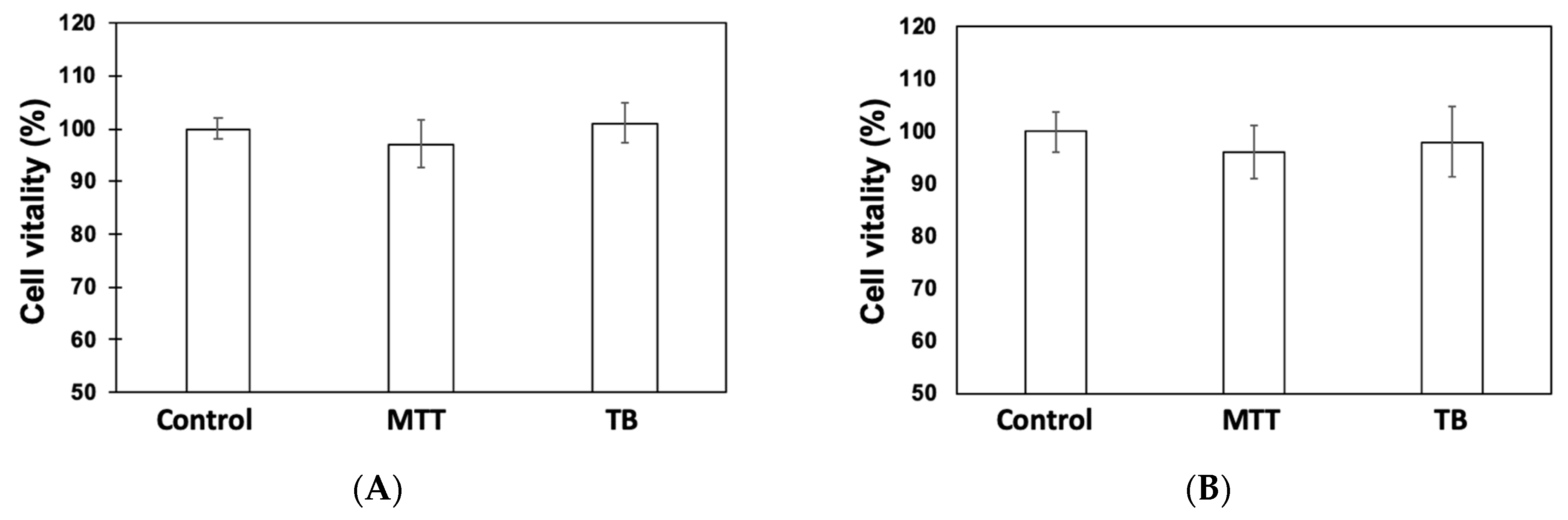
3.1. Cytotoxicity Assessments
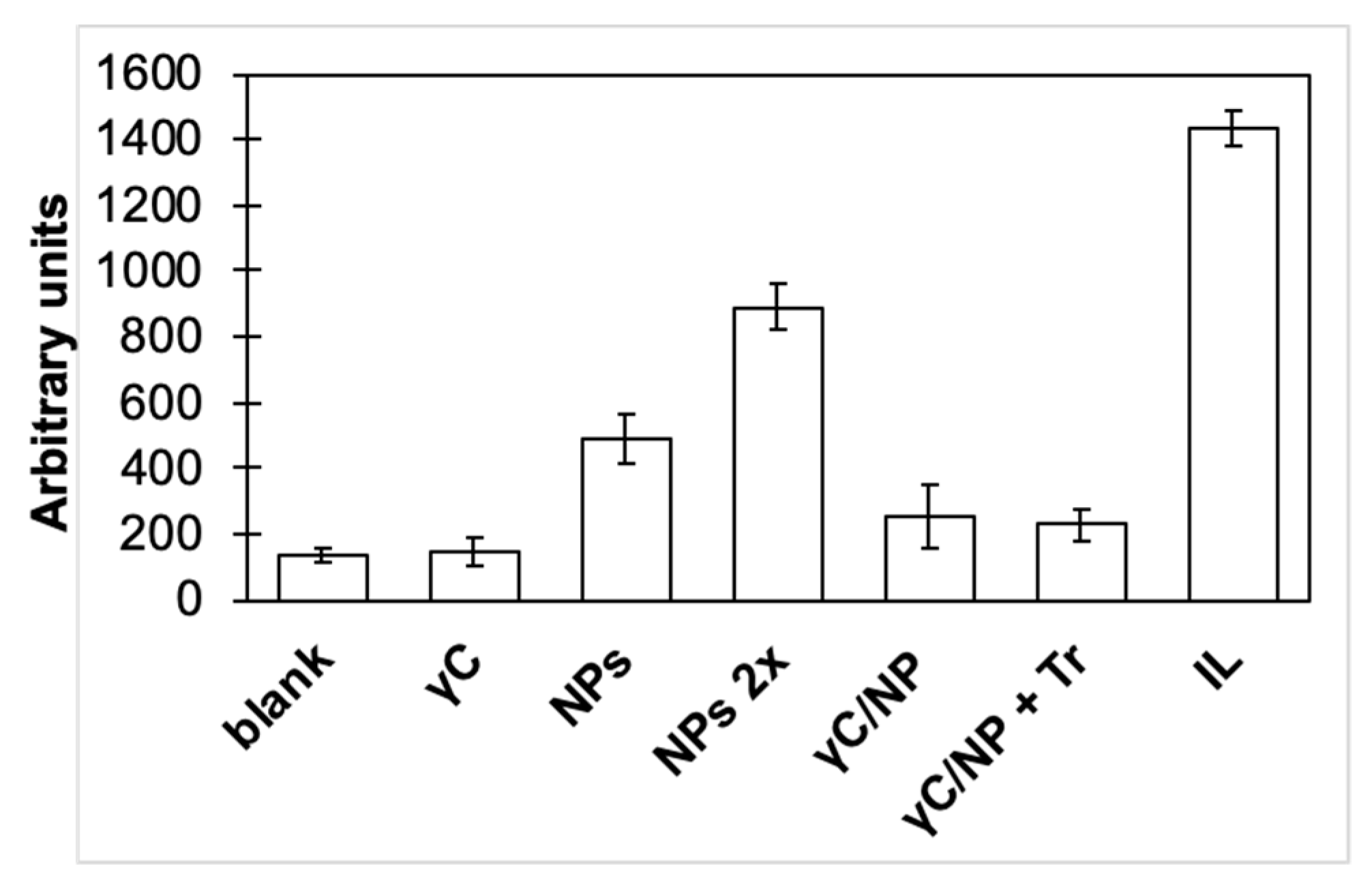
3.2. γC effects on Inflammatory Pathways
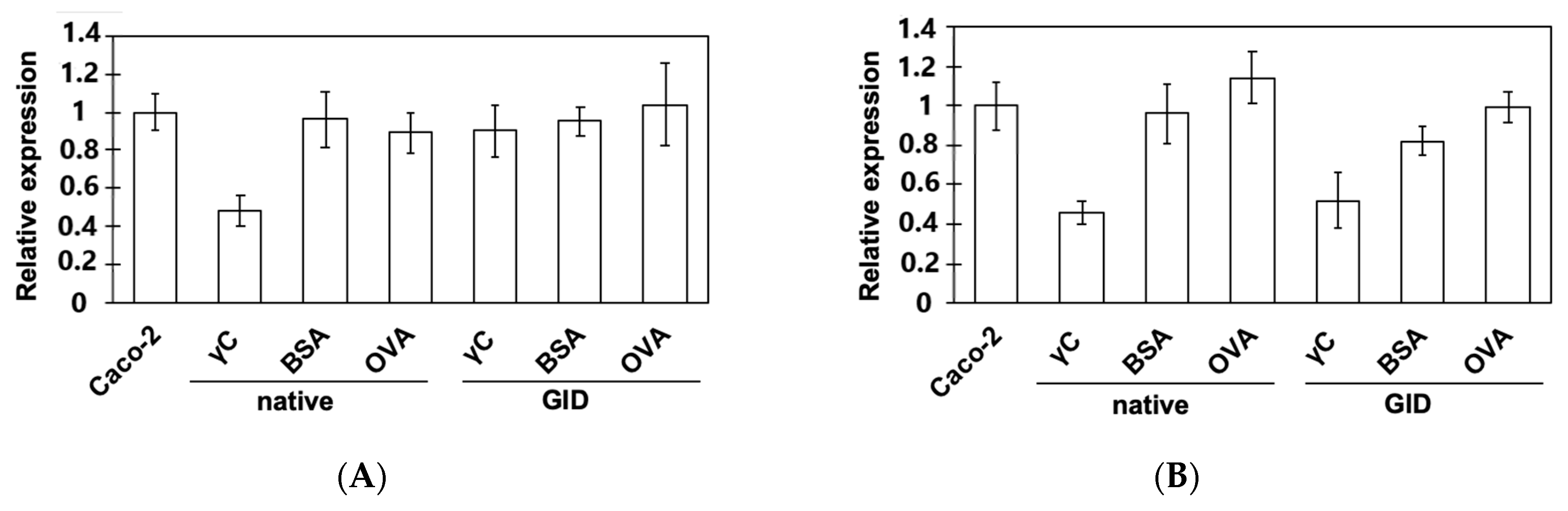
3.3. Effects on the Expression of Intestinal Peptide Transporters
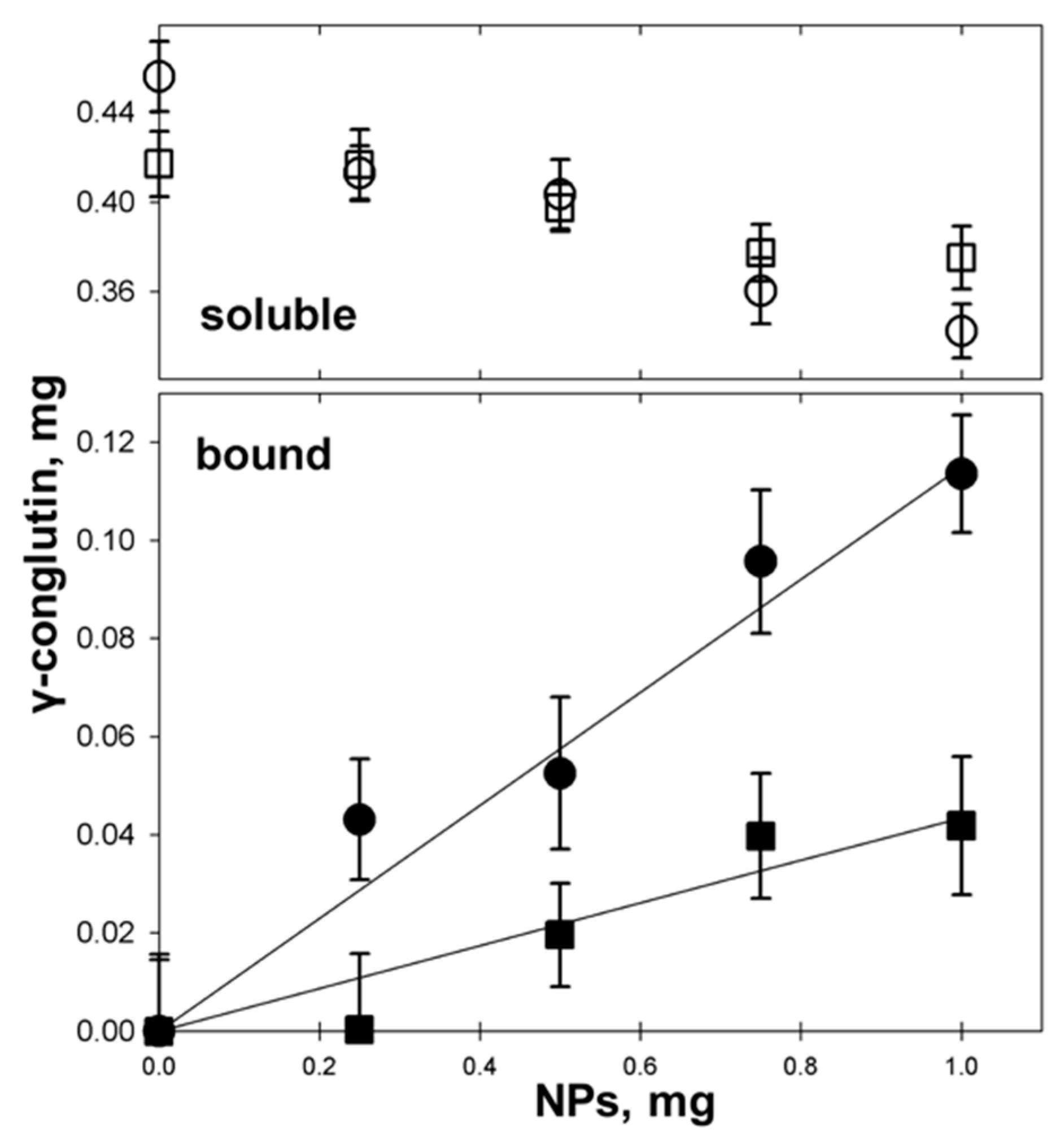
3.4. γC molecular Features and Biological Effects Following Adsorbtion to Polystyrene NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| γC | γ-Conglutin |
| MTT | 3-[4–dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| NFkB | Nuclear Factor kappa B |
| TB | Tryphan Blue |
| IL1β | Interleukin 1β |
References
- Capraro, J.; Spotti, P.; Magni, C.; Scarafoni, A.; Duranti, M. Spectroscopic studies on the pH-dependent structural dynamics of γ-conglutin, the blood glucose-lowering protein of lupin seeds. Int. J. Biol. Macromol. 2010, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Scarafoni, A.; Di Cataldo, A.; Vassilevskaia, T.D.; Bekman, E.P.; Rodrigues-Pousada, C.; Ceciliani, F.; Duranti, M. Cloning, sequencing and expression in the seeds and radicles of two Lupinus albus conglutin gamma genes. Biochim. Biophys. Acta 2001, 1519, 147–151. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin gamma, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Parolari, A.; Magni, C.; Duranti, M. Lupin seed γ-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br. J. Nutr. 2012, 107, 67–73. [Google Scholar] [CrossRef]
- Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Administration of Lupinus albus gamma conglutin (Cγ) to n5 STZ rats augmented Ins-1 gene expression and pancreatic insulin content. Plant Foods Hum. Nutr. 2014, 69, 241–247. [Google Scholar] [CrossRef]
- González-Santiago, A.E.; Vargas-Guerrero, B.; García-López, P.M.; Martínez-Ayala, A.L.; Domínguez-Rosales, J.A.; Gurrola-Díaz, C.M. Lupinus albus conglutin gamma modifies the gene expressions of enzymes involved in glucose hepatic production in vivo. Plant Foods Hum. Nutr. 2017, 72, 134–140. [Google Scholar] [CrossRef]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human health. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef]
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, A.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-mimetic action of conglutin-γ, a lupin seed protein, in mouse myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205. [Google Scholar] [CrossRef]
- Capraro, J.; Clemente, A.; Rubio, L.A.; Magni, C.; Scarafoni, A.; Duranti, M. Assessment of the lupin seed glucose-lowering protein intestinal absorption by using in vitro and ex vivo models. Food Chem. 2011, 125, 1279–1283. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Faoro, F.; Maffi, D.; Scarafoni, A.; Tedeschi, G.; Maffioli, E.; Parolari, A.; Manzoni, C.; Lovati, M.R.; et al. Internalisation and multiple phosphorylation of γ-conglutin, the lupin seed glycaemia-lowering protein, in HepG2 cells. Biochem. Biophys. Res. Commun. 2013, 437, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Scirè, A.; Baldassarre, M.; Tanfani, M.; Capraro, J.; Duranti, M.; Scarafoni, A. Interaction of γ-conglutin from Lupinus albus with model phospholipid membranes: Investigations on structure, thermal stability and oligomerization status. Biochim. Biophys. Acta 2018, 1866, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Brehme, M.; Voisine, C. Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis. Models Mech. 2016, 9, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; Miriani, M.; Ferranti, P.; Bonomi, F.; Iametti, S.; Barbiroli, A. Structural changes in emulsion-bound bovine beta-lactoglobulin affect its proteolysis and immunoreactivity. Biochim. Biophys. Acta 2016, 1864, 805–813. [Google Scholar] [CrossRef]
- Kristo, E.; Corredig, M. Functional Properties of Food Proteins. In Applied Food Protein Chemistry; Ustunol, Z., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2015; pp. 47–73. [Google Scholar] [CrossRef]
- Stefani, M. Protein folding and misfolding on surfaces. Int. J. Mol. Sci. 2008, 9, 2515–2542. [Google Scholar] [CrossRef]
- Miriani, M.; Eberini, I.; Iametti, S.; Ferranti, P.; Sensi, C.; Bonomi, F. Unfolding of beta-lactoglobulin on the surface of polystyrene nanoparticles: Experimental and computational approaches. Proteins 2014, 82, 1272–1282. [Google Scholar] [CrossRef]
- Miriani, M.; Iametti, S.; Kurtz, D.M.; Bonomi, F. Rubredoxin refolding on nanostructured hydrophobic surfaces: Evidence for a new type of biomimetic chaperones. Proteins 2014, 82, 3154–3162. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Bekdemir, A.; Liao, S.; Stellacci, F. On the effect of ligand shell heterogeneity on nanoparticle/protein binding thermodynamics. Colloids Surf. B Biointerfaces 2019, 174, 367–373. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Walker, D.; Thwaites, D.T.; Simmons, N.L.; Gilbert, H.J.; Hirst, B.H. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. 1998, 507, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Dantzig, A.H.; Hoskins, J.A.; Tabas, L.B.; Bright, S.; Shepard, R.L.; Jenkins, I.L.; Duckworth, D.C.; Sportsman, J.R.; Mackensen, D.; Rosteck, P.R., Jr.; et al. Association of intestinal peptide transport with a protein related to the cadherin superfamily. Science 1994, 264, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Kissel, T. Do cell culture conditions influence the carrier-mediated transport of peptides in Caco-2 cell monolayers? Eur. J. Pharm. Sci. 2003, 19, 433–442. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Duranti, M.; Sessa, F.; Scarafoni, A.; Bellini, T.; Dallocchio, F. Thermal stabilities of lupin seed conglutin γ protomers and tetramers. J. Agric. Food Chem. 2000, 48, 1118–1123. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Open Source Drug Discovery Consortium; Raghava, G.P. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Berlin, Germany, 2015; pp. 103–111. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.J. Food proteins as a source of bioactive peptides with diverse functions. Br. J. Nutr. 2012, 108, S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Fitzgerald, R.J. Strategies for the discovery and identification of food protein-derived biologically active peptides. Trends Food Sci. Technol. 2017, 69, 289–305. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Diamond, J.M. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu. Rev. Physiol. 1989, 51, 125–141. [Google Scholar] [CrossRef]
- Leibach, F.H.; Ganapathy, V. Peptide transporters in the intestine and the kidney. Annu. Rev. Nutr. 1996, 16, 99–119. [Google Scholar] [CrossRef]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Thamotharan, M.; Bawani, S.Z.; Zhou, X.; Adibi, S.A. Hormonal regulation of oligopeptide transporter Pept-1 in a human intestinal cell line. Am. J. Physiol. Cell Physiol. 1999, 276, C821–C826. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Lee, N.P.; Poon, R.T.; Shek, F.H.; Ng, I.O.; Luk, J.M. Role of cadherin-17 in oncogenesis and potential therapeutic implications in hepatocellular carcinoma. Biochim. Biophys. Acta 2010, 1806, 138–145. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Scarafoni, A.; Duranti, M. Susceptibility of lupin gamma-conglutin, the plasma glucose-lowering protein of lupin seeds, to proteolytic enzymes. J. Agric. Food Chem. 2009, 57, 8612–8616. [Google Scholar] [CrossRef]
- Duranti, M.; Gius, C.; Sessa, F.; Vecchio, G. The saccharide chain of lupin seed conglutin γ is not responsible for the protection of the native protein from degradation by trypsin, but facilitates the refolding of the acid-treated protein to the resistant conformation. Eur. J. Biochem. 1995, 230, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Biological reactivity of zinc oxide nanoparticles with mammalian test systems: An overview. Nanomedicine 2015, 10, 2075–2092. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbiroli, A.; Capraro, J.; Marulo, S.; Gamba, M.; Scarafoni, A. Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix. Biomolecules 2019, 9, 606. https://doi.org/10.3390/biom9100606
Barbiroli A, Capraro J, Marulo S, Gamba M, Scarafoni A. Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix. Biomolecules. 2019; 9(10):606. https://doi.org/10.3390/biom9100606
Chicago/Turabian StyleBarbiroli, Alberto, Jessica Capraro, Serena Marulo, Marta Gamba, and Alessio Scarafoni. 2019. "Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix" Biomolecules 9, no. 10: 606. https://doi.org/10.3390/biom9100606
APA StyleBarbiroli, A., Capraro, J., Marulo, S., Gamba, M., & Scarafoni, A. (2019). Effects on the Caco-2 Cells of a Hypoglycemic Protein from Lupin Seeds in a Solution and Adsorbed on Polystyrene Nanoparticles to Mimic a Complex Food Matrix. Biomolecules, 9(10), 606. https://doi.org/10.3390/biom9100606






