Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation of Iso-Mukaadial Acetate from W. salutaris
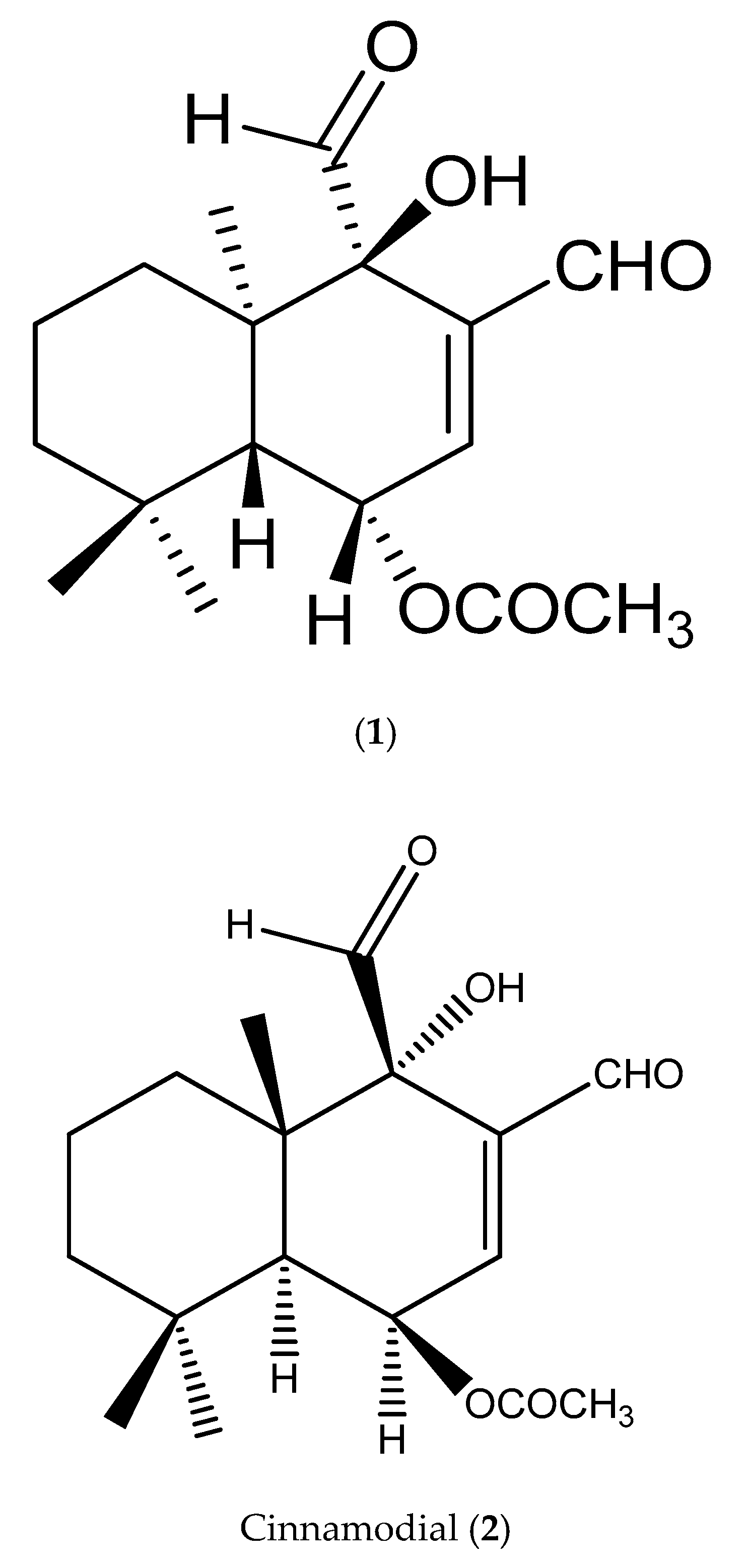
2.2. Structural Confirmation
2.3. Cell Culture and Treatment Conditions
2.4. Preparation of Extracts for Cell Culture
2.5. Cytotoxicity Evaluation
2.6. Glucose Utilisation Screening
2.7. Westen Blot Analyses
2.8. α-Amylase Inhibition Assay
2.9. α-Glucosidase Inhibition Assay
2.10. Antioxidant Activity
2.11. Statistical Analysis
3. Results
3.1. Structural Elucidation
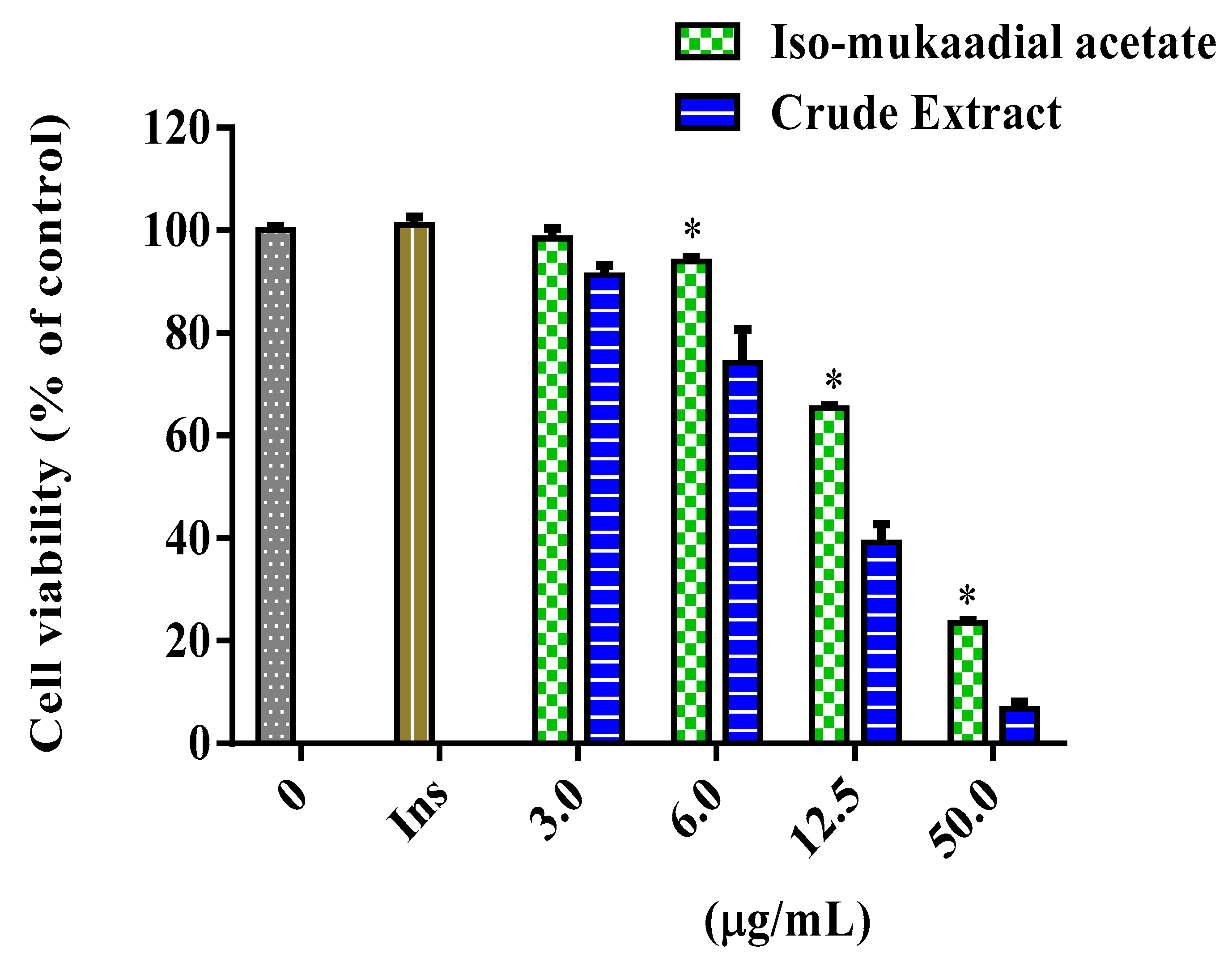
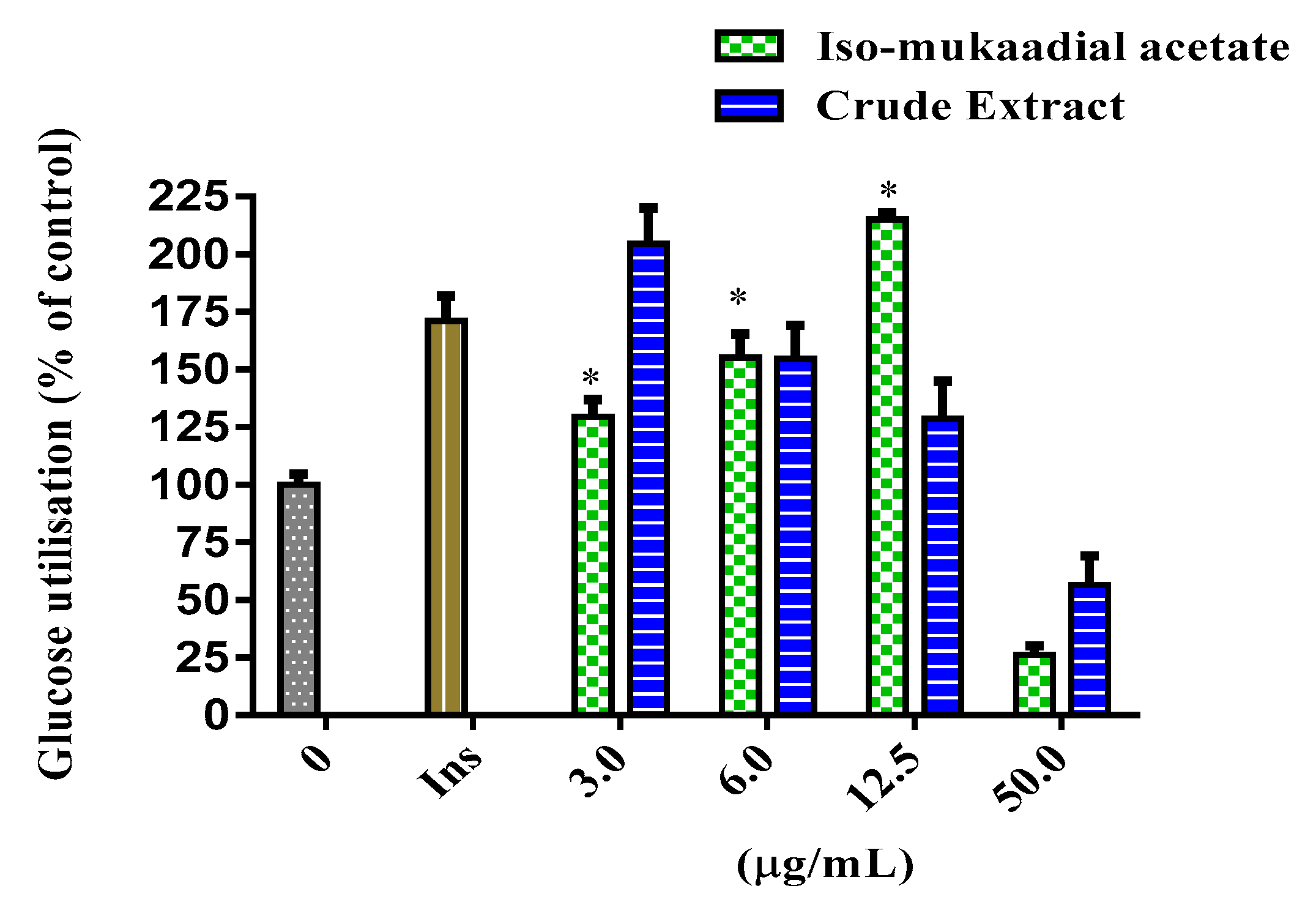
3.2. Effect of Dichloromethane Crude Extract and Iso-Mukaadial Acetate on Cytotoxic Evaluation and Glucose Utilisation
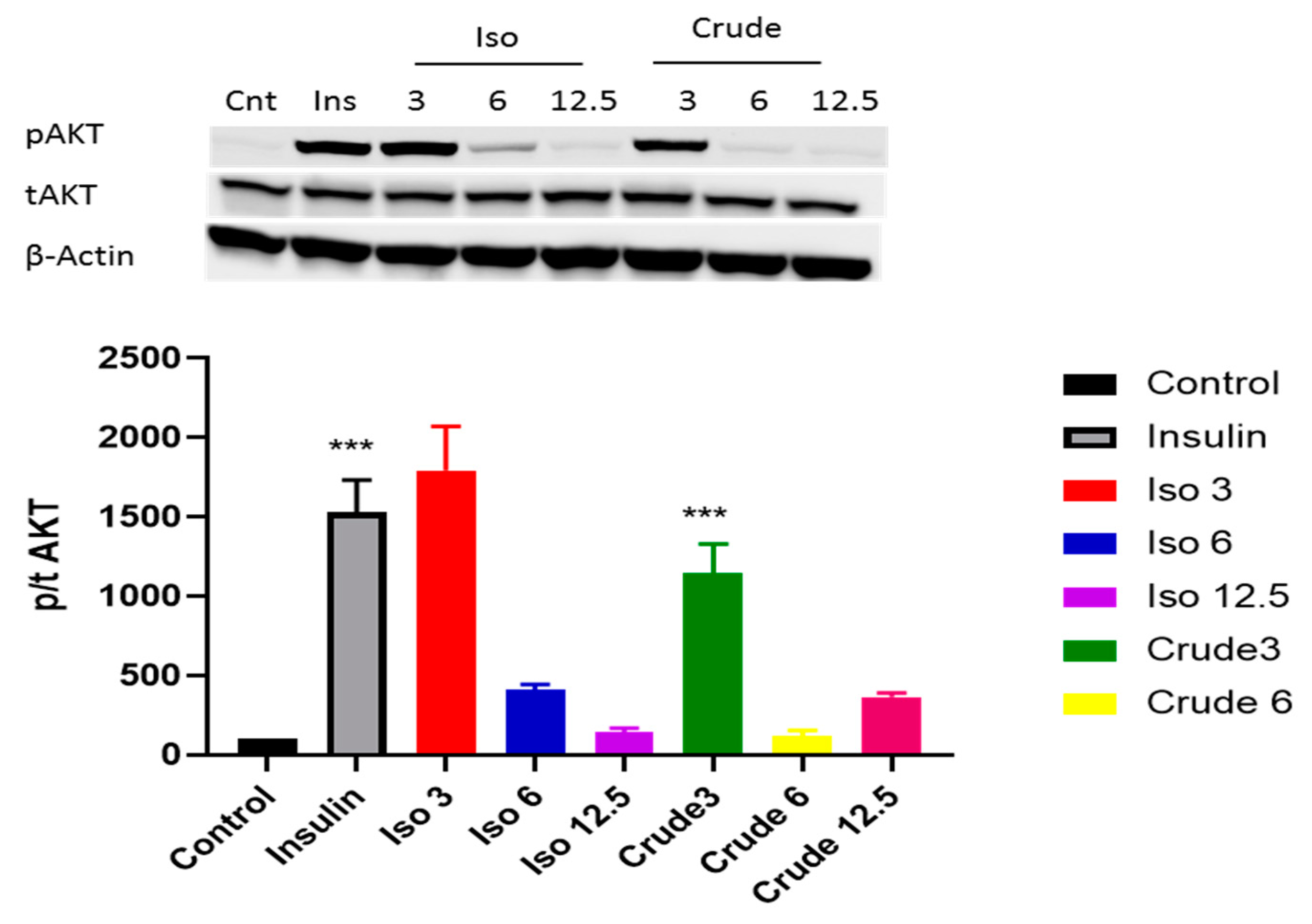
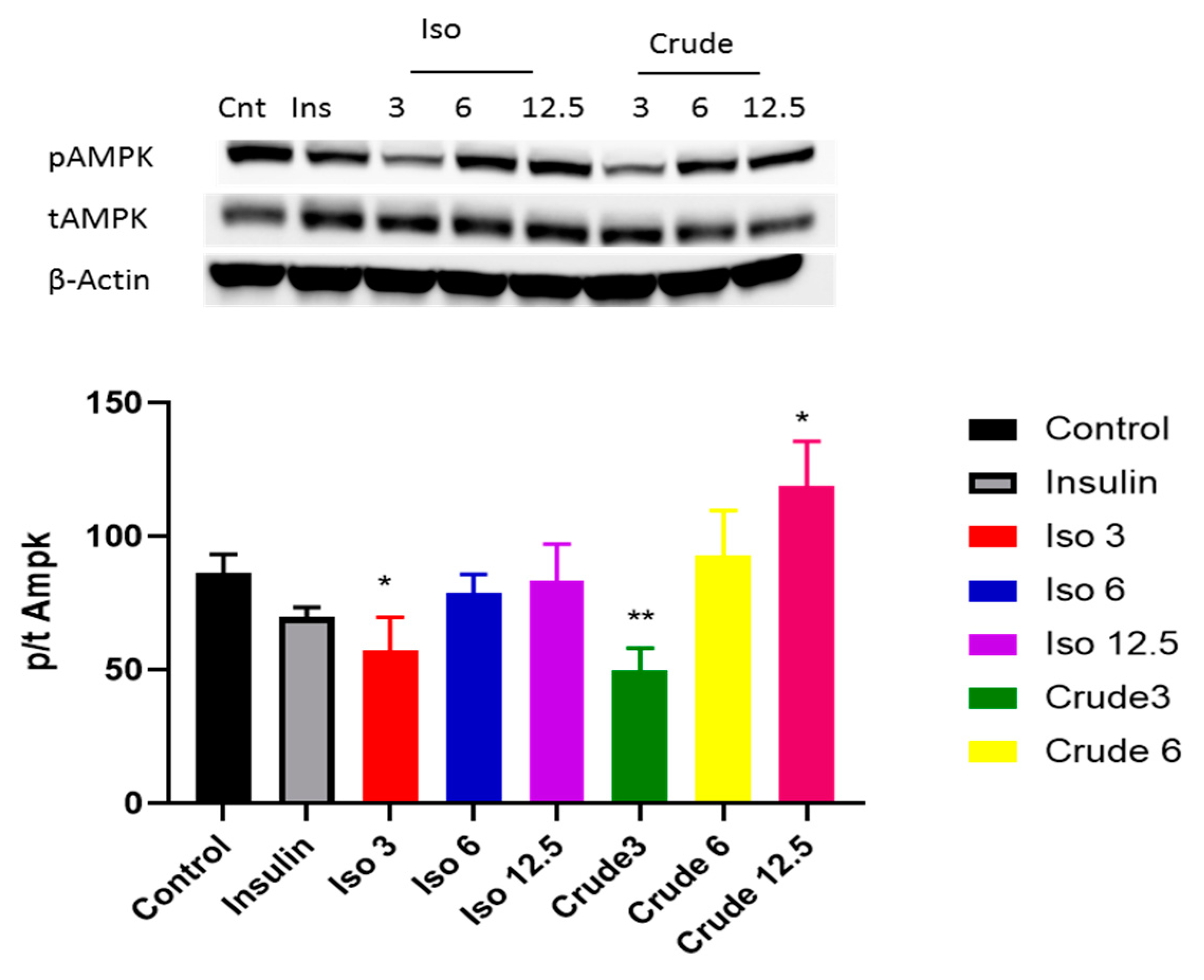
3.3. DCM Crude Extract and Iso-Mukaadial Acetate Activate the AMPK and AKT Pathway
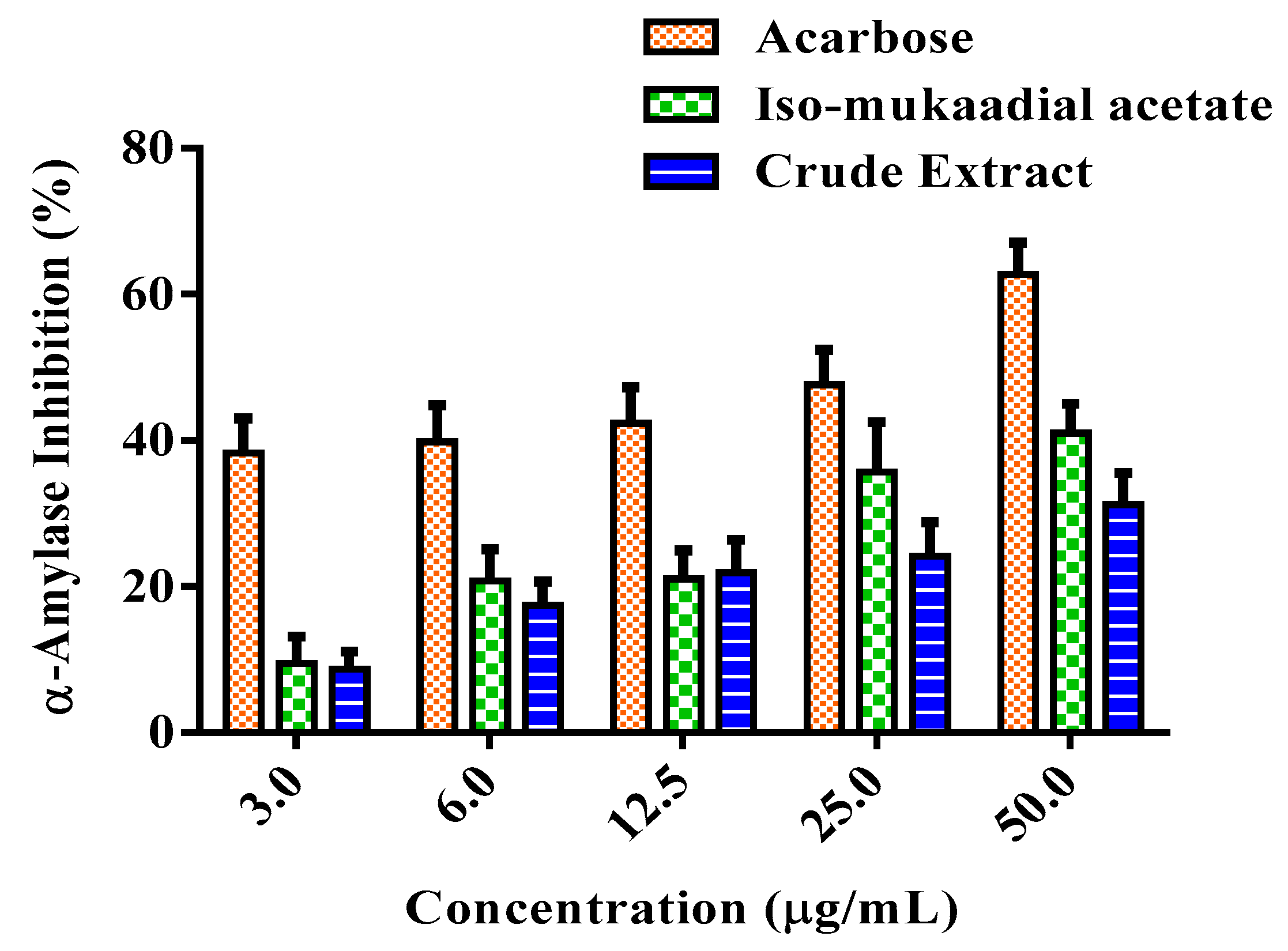
3.4. DCM Crude Extract and Iso-Mukaadial Acetate Inhibit α-Amylase
3.5. DCM Crude Extract and Iso-Mukaadial Acetate Inhibit α-Glucosidase
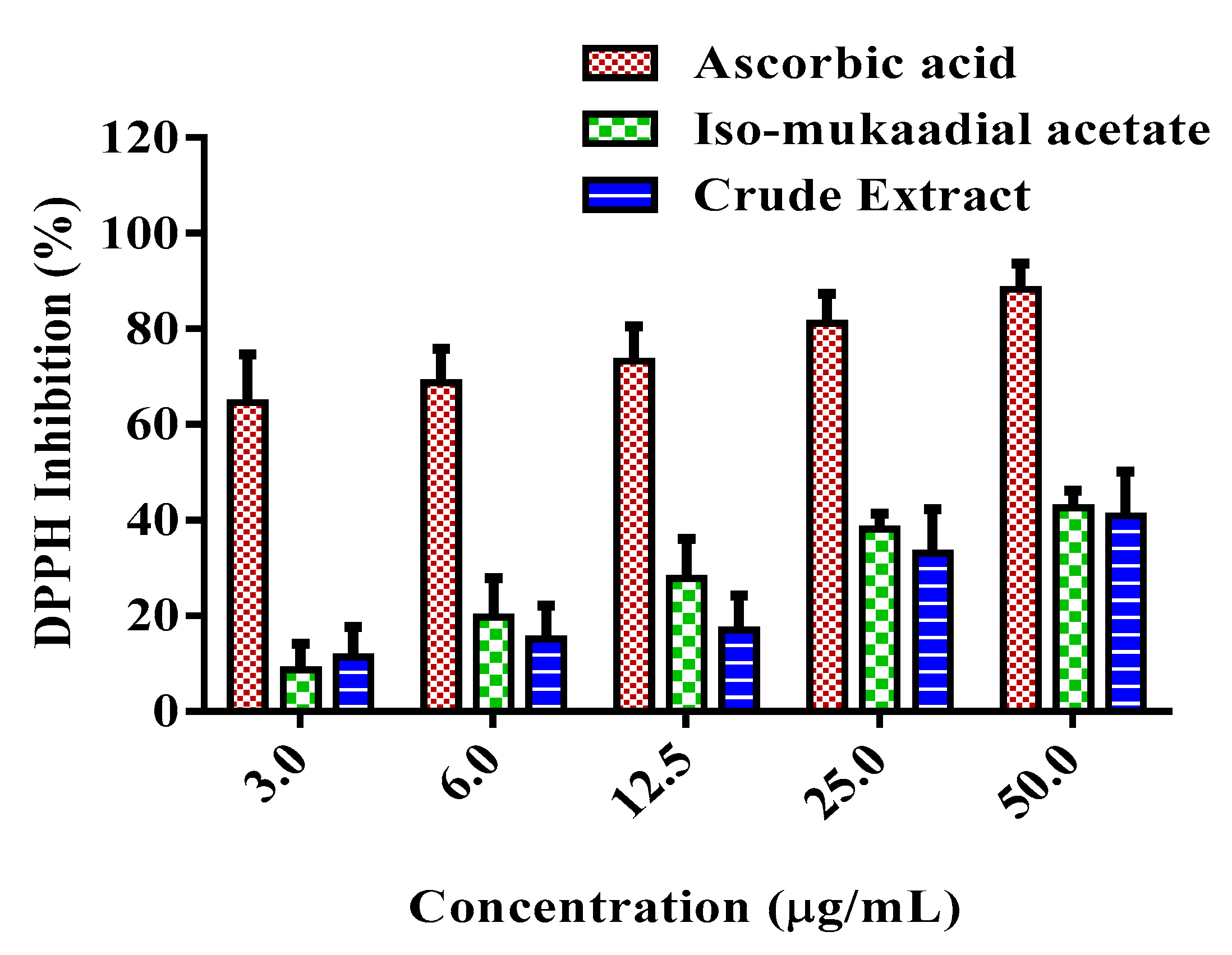
3.6. DCM Crude Extract and Iso-Mukaadial Acetate Inhibit DPPH Radical
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elberry, A.A.; Harraz, F.M.; Gharei, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel-Sattar, E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015, 3, 37–44. [Google Scholar] [CrossRef]
- Kumaresan, P.T.; Saravanan, S.; Subish, R. In vitro antidiabetic activity of Morinda Tinctoria fruits extracts. Asian J. Pharm. Clin. Res. 2014, 7, 90–92. [Google Scholar]
- Chan, M. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; pp. 1–88. [Google Scholar]
- Alvim, R.O.; Cheuhen, M.R.; Machado, S.R.; Sousa, A.G.P.; Santos, P.C. General aspects of muscle glucose uptake. An. Acad. Bras. Ciências 2015, 87, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Mootoosamy, A.; Mahomoodally, M.F. Ethnomedicinal application of native remedies used against diabetes and related complications in Mauritius. J. Ethnopharmacol. 2014, 151, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hyunb, T.K.; Kim, M.J. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011, 124, 1647–1651. [Google Scholar] [CrossRef]
- Musabayane, C. The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc. J. Afr. 2012, 23, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kumar, R.; Laloo, D.; Hemalatha, S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac. J. Trop. Dis. 2012, 2, 239–250. [Google Scholar] [CrossRef]
- Leem, K.H.; Kim, M.G.; Hahm, Y.T.; Kim, H.K. Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway. Nutrients 2016, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.J.; Soumyanat, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Sangeetha, R.; Vedasree, N. In Vitro α-Amylase Inhibitory Activity of the Leaves of Thespesia populnea. ISRN Pharmacol. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Malviya, N.; Jain, S.; Malviya, S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. Drug Res. 2010, 67, 113–118. [Google Scholar]
- Wadkar, K.A.; Magdum, C.S.; Patil, S.S.; Naikwade, N.S. Anti-diabetic potential and indian medicinal plants. J. Herb. Med. Toxicol. 2008, 2, 45–50. [Google Scholar]
- Trinh, B.T.D.; Staerk, D.; Jäger, A.K. Screeningforpotential α-glucosidase and α-amylaseinhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. J. Ethnopharmacol. 2016, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.M.; De Groot, A. The occurrence and biological activity of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Nyaba, Z.N.; Murambiwa, P.; Opoku, A.R.; Mukaratirwa, S.; Shode, F.O.; Simelane, M.B. Isolation, characterization, and biological evaluation of a potent anti-malarial drimane sesquiterpene from Warburgia salutaris stem bark. Malar. J. 2018, 17, 296. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Warburgia salutaris (Bertol. f.) Chiov.: A multi-use ethnomedicinal plant species. J. Med. Plants Res. 2013, 7, 53–60. [Google Scholar] [CrossRef]
- Botha, J.; Witkowski, E.T.F.; Shackleton, C.M. The impact of commercial harvesting on Warburgia salutaris (‘pepper-bark tree’) in Mpumalanga, South Africa. Biodivers. Conserv. 2004, 13, 1675–1698. [Google Scholar] [CrossRef]
- Frum, Y.; Viljoen, A.; Drewes, S.; Houghton, P. In vitro 5-lipoxygenase and anti-oxidant activities of Warburgia salutaris and drimane sesquiterpenoids. S. Afr. J. Bot. 2005, 71, 447–449. [Google Scholar] [CrossRef]
- Rabe, T.; Van Staden, J.V. Isolation of an antibacterial sesquiterpenoid from Warburgia salutaris. J. Ethnopharmacol. 2000, 73, 171–174. [Google Scholar] [CrossRef]
- Maroyi, A. The genus Warburgia: A review of its traditional uses and pharmacology. Pharm. Biol. 2014, 52, 378–391. [Google Scholar] [CrossRef]
- Brahmachari, G. Discovery and Development of Antidiabetic Agents from Natural Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 198–201. [Google Scholar]
- Van de Venter, M.; Roux, S.; Bungu, L.C.; Louw, J.; Crouch, N.R.; Grace, O.M.; Maharaj, V.; Pillay, P.; Sewnarian, P.; Bhagwandin, N.; et al. Antidiabetic screening and scoring of 11 plants traditionally. J. Ethnopharmacol. 2008, 119, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, S.E.; Joubert, E.; Johnson, R.; Louw, J.; Opoku, A.R.; Muller, C.J. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015, 59, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Sathiavelu, A.; Sangeetha, S.; Archit, R.; Mythili, S. In Vitro anti-diabetic activity of aqueous extract of the medicinal plants Nigella sativa, Eugenia jambolana, Andrographis paniculata and Gymnema sylvestre. Int. J. Drug Dev. Res. 2013, 5, 323–328. [Google Scholar]
- Bajpai, V.K.; Park, Y.H.; Na, M.; Kang, S.C. α-Glucosidase and tyrosinase inhibitory effectsof an abietane type diterpenoid taxoquinonefrom Metasequoia glyptostroboides. BMC Complement. Altern. Med. 2015, 15, 1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LET Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacology active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.; Neelapu, N.R.R.; Challa, S. Role of Antidiabetic compounds on glucose metabolism—A special focus on medicinal plant: Salacia sps. Med. Chem. 2014, 4, 373–381. [Google Scholar]
- Das, M.S.; Devi, G. In vitro Cytotoxicity and Glucose Uptake Activity of Fruits of Terminalia bellirica in Vero, L-6 and 3T3 cell lines. J. Appl. Pharm. Sci. 2015, 5, 92–95. [Google Scholar] [CrossRef][Green Version]
- Gupta, R.; Pareek, A.; Suthar, M.; Rathore, G.; Basniwal, P.; Jain, D. Study of glucose uptake activity of Helicteres isora Linn. Fruits in L-6 cell lines. Int. J. Diab. Dev. Ctries. 2010, 29, 170–173. [Google Scholar]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids Isolated from Ziziphus jujuba Enhance Glucose Uptake Activity in Skeletal Muscle Cells. J. Nutr. Sci. Vitam. 2017, 63, 193–199. [Google Scholar] [CrossRef]
- Kong, D.; Song, G.; Wang, C.; Ma, H.; Ren, L.; Nie, Q.; Zhang, X.; Gan, K. Overexpression of mitofusin 2 improves translocation of glucose transporter 4 in skeletal muscle of high-fat diet-fed rats through AMP-activated protein kinase signalling. Mol. Med. Rep. 2013, 8, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Adam, Z.; Khamis, S.; Ismail, A.; Hamid, M. Ficus deltoidea enhance glucose uptake activity in cultured muscle cells. J. Nucl. Relat. Technol. 2015, 12, 54–65. [Google Scholar]
- Hardie, D.G. Regulation of AMP-activated protein kinase. Acta Pharm. Sin. B 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taukoorah, U.; Mahomoodally, M.F. Crude Aloe vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement In Vitro. Adv. Pharmacol. Sci. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Picot, C.M.N.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory Potential of Five Traditionally Used Native Antidiabetic Medicinal Plants on α-Amylase, α-Glucosidase, Glucose Entrapment, and Amylolysis Kinetics In Vitro. Adv. Pharmacol. Sci. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Kitahara, K.; Suganuma, T.; Hashimoto, F.; Tadera, K. Antioxidant and α-Amylase Inhibitory Compounds from Aerial Parts of Varthemia iphionoides Boiss. Biosci. Biotechnol. Biochem. 2006, 70, 2178–2184. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msomi, N.Z.; Shode, F.O.; Pooe, O.J.; Mazibuko-Mbeje, S.; Simelane, M.B.C. Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line. Biomolecules 2019, 9, 520. https://doi.org/10.3390/biom9100520
Msomi NZ, Shode FO, Pooe OJ, Mazibuko-Mbeje S, Simelane MBC. Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line. Biomolecules. 2019; 9(10):520. https://doi.org/10.3390/biom9100520
Chicago/Turabian StyleMsomi, Nontokozo Z., Francis O. Shode, Ofentse J. Pooe, Sithandiwe Mazibuko-Mbeje, and Mthokozisi B. C. Simelane. 2019. "Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line" Biomolecules 9, no. 10: 520. https://doi.org/10.3390/biom9100520
APA StyleMsomi, N. Z., Shode, F. O., Pooe, O. J., Mazibuko-Mbeje, S., & Simelane, M. B. C. (2019). Iso-Mukaadial Acetate from Warburgia salutaris Enhances Glucose Uptake in the L6 Rat Myoblast Cell Line. Biomolecules, 9(10), 520. https://doi.org/10.3390/biom9100520




