Versatility of Cyclophilins in Plant Growth and Survival: A Case Study in Arabidopsis
Abstract
1. Introduction
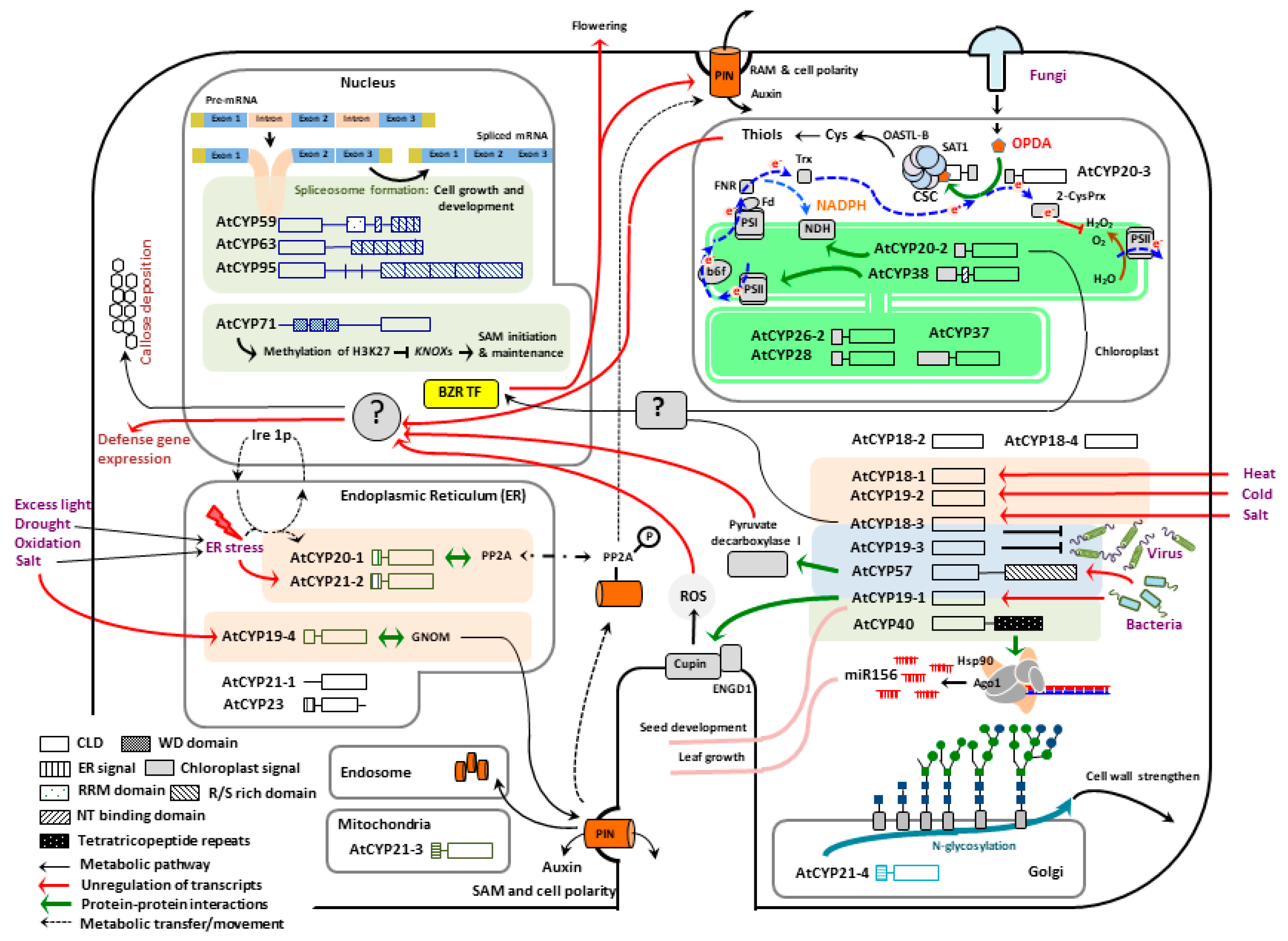
2. Activities of Cyclophilins in Plant Growth and Development
2.1. Nuclear Localized AtCYPs
2.2. Cytosol Localized AtCYPs
2.3. Chloroplast Localized AtCYPs
2.4. Endoplasmic Reticulum (ER) Localized AtCYPs
2.5. Mitochondria and Golgi Localized AtCYPs
3. Roles of Cyclophilins in Defense Reponses against Abiotic Stresses
4. Roles of Cyclophilins in Disease Resistance against Pathogen Infections
5. Roles of Cyclophilin at the Interface between Plant Growth and Defense; A Case Study of AtCYP20-3
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Romano, P.G.N.; Horton, P.; Gray, J.E. The Arabidopsis Cyclophilin Gene Family. Plant Physiol. 2004, 134, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heitman, J. The cyclophilins. Genome Biol. 2005, 6, 2261–2266. [Google Scholar] [CrossRef][Green Version]
- Romano, P.; Gray, J.; Horton, P.; Luan, S. Plant immunophilins: Functional versatility beyond protein maturation. New Phytol. 2005, 166, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Nigro, P.; Pompillo, G.; Capogrossi, M.C. Cyclophilin A: A key player for human diease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.B.; Hong, Y.; Dhe-Paganon, S.; Yoon, H.S. FKBP family proteins: Immunophilins with versatile biological functions. Neurosignals 2008, 16, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Petersson, U.A.; Haas, B.J.; Funk, C.; Schroder, W.P.; Kieselbach, T. Proteome Map of the Chloroplast Lumen of Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 143–164. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, L.; Luan, S. Immunophilins and Parvulins. Superfamily of Peptidyl Prolyl Isomerases in Arabidopsis. Plant Physiol. 2004, 134, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.J.; Koser, P.L.; Brandt, M.; Bergsma, D.J.; Levy, M.A. A single Trp121 to Ala121 mutation in human cyclophilin alters cyclosporin A affinity and peptidyl-prolyl isomerase activity. Biochem. Biophys. Res. Commun. 1991, 176, 1142–1148. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.M.; Walsh, C.T. Human and Escherichia coli cyclophilins: Sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry 1991, 30, 2306–2310. [Google Scholar] [CrossRef] [PubMed]
- Zydowsky, L.D.; Etzkorn, F.A.; Chang, H.Y.; Ferguson, S.B.; Stolz, L.A.; Ho, S.I.; Walsh, C.T. Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci. 1992, 1, 1092–1099. [Google Scholar] [CrossRef]
- Howard, B.R.; Vajdos, F.F.; Li, S.; Sundquist, W.I.; Hill, C.P. Structural insights into the catalytic mechanism of cyclophilin A. Nat. Struct. Mol. Biol. 2003, 10, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J.; Paramanathan, R.; Bernstein, G.; MacKenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H.; et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar] [CrossRef]
- Gasser, C.S.; Gunning, D.; Budelier, K.; Brown, S.M. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc. Natl. Acad. Sci. USA 1990, 87, 9519–9523. [Google Scholar] [CrossRef]
- Hayman, G.T.; Miernyk, J.A. The nucleotide and deduced amino acid sequences of a peptidyl-prolyl cis-trans isomerase from Arabidopsis thaliana. Biochim. Biophys. Acta 1994, 536–538. [Google Scholar] [CrossRef]
- Chou, I.T.; Gasser, C.S. Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Mol. Biol. 1997, 35, 873–892. [Google Scholar] [CrossRef]
- Jackson, K.; Söll, D. Mutations in a new Arabidopsis cyclophilin disrupt its interaction with protein phosphatase 2A. Mol. Gen. Genet. 1999, 262, 830–838. [Google Scholar] [CrossRef]
- Grebe, M.; Gadea, J.; Steinmann, T.; Kientz, M.; Rahfeld, J.U.; Salchert, K.; Koncz, C.; Jürgens, G. A conserved domain of the arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. Plant Cell 2000, 12, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Lippuner, V.; Chou, I.T.; Scott, S.V.; Ettinge, W.F.; Theg, S.M.; Gasser, C.S. Cloning and characterization of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J. Biol. Chem. 1994, 269, 7863–7868. [Google Scholar]
- Peltier, J.B.; Emanuelsson, O.; Kalume, D.E.; Ytterberg, J.; Friso, G.; Rudella, A.; Liberles, D.A.; Söderberg, L.; Roepstorff, P.; von Heijne, G.; et al. Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell 2002, 14, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Berardini, T.Z.; Bollman, K.; Sun, H.; Poethig, R.S. Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 2001, 291, 2405–2407. [Google Scholar] [CrossRef]
- Klepikova, A.V.; Kaslanov, A.S.; Gerasimov, E.S.; Logacheva, M.D.; Penin, A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016, 88, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Gopalan, G.; Kumar, A.; Garcia, V.J.; Luan, S.; Swaminathan, K. Plant immunophilins: A review of their structure-function relationship. Biochim. Biophys. Acta 2015, 1850, 2145–2158. [Google Scholar] [CrossRef]
- Zhou, D.; Mei, Q.; Li, J.; He, H. Cyclophilin A and viral infections. Biochem. Biophys. Res. Commun. 2012, 424, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Koncz, C.; deJong, F.; Villacorta, N.; Szakonyi, D.; Koncz, Z. The Spliceosome-activating complex: Molecular mechanisms underlying the function of a pleiotropic regulator. Front. Plant. Sci. 2012, 3, 9. [Google Scholar] [CrossRef]
- Lin, T.Y.; Emerman, M. Determinants of cyclophilin A-dependent TRIM5α restriction against HIV-1. Virology 2008, 8, 4017–4018. [Google Scholar] [CrossRef] [PubMed]
- Philipps, D.; Celotto, A.M.; Wang, Q.Q.; Tarng, R.S.; Graveley, B.R. Arginine/serine repeats are sufficient to constitute a splicing activation domain. Nucleic Acids Res. 2003, 31, 6502–6508. [Google Scholar] [CrossRef]
- Valcárcel, J.; Green, M.R. The SR protein family: Pleiotropic functions in pre-mRNA splicing. Trends Biochem. Sci. 1996, 21, 296–301. [Google Scholar] [CrossRef]
- Bourquin, J.P.; Stagljar, I.; Meier, P.; Moosmann, P.; Silke, J.; Baechi, T.; Georgiev, O.; Schaffner, W. A serine/arginine-rich nuclear matrix cyclophilin interacts with the C-terminal domain of RNA polymerase II. Nucleic Acids Res. 1997, 25, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Mortillaro, M.J.; Berezney, R. Matrin CYP, an SR-rich cyclophilin that associates with the nuclear matrix and splicing factors. J. Biol. Chem. 1998, 273, 8183–8192. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, D.; Göthel, S.F.; Marahiel, M.A.; Reidt, U.; Ficner, R.; Lührmann, R.; Achsel, T. Two protein-protein interaction sites on the spliceosome-associated human cyclophilin CypH. Nucleic Acids Res. 2003, 31, 4791–4796. [Google Scholar] [CrossRef]
- Gullerova, M.; Andrea, B.; Lorkovic, Z. AtCyp59 is a multidomain cyclophilin from Arabidopsis thaliana that interacts with SR proteins and the C-terminal domain of the RNA polymerase II. RNA 2006, 12, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Xia, X.; Sun, Z.; Fang, Y. Depletion of Arabidopsis SC35 and SC35-like serine/arginine-rich proteins affects the transcription and splicing of a subset of genes. PLoS Genet. 2017, 13, e1006663. [Google Scholar] [CrossRef] [PubMed]
- Natalizio, B.J.; Robson-Dixon, N.D.; Garcia-Blanco, M.A. The carboxyl-terminal domain of RNA polymerase II is not sufficient to enhance the efficiency of pre-mRNA capping or splicing in the context of a different polymerase. J. Biol. Chem. 2009, 284, 8692–8702. [Google Scholar] [CrossRef]
- Brody, Y.; Neufeld, N.; Bieberstein, N.; Causse, S.Z.; Böhnlein, E.M.; Neugebauer, K.M.; Darzacq, X.; Shav-Tal, Y. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol. 2011, 9, e1000537. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, O.; Zywicki, M.; Marquez, Y.; Skrahina, T.; Kalyna, M.; Barta, A. Identification of RNA targets for the nuclear multidomain cyclophilin atCyp59 and their effect on PPIase activity. Nucleic Acids Res. 2013, 41, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Hake, S.; Smith, H.M.S.; Holtan, H.; Magnani, E.; Mele, G.; Ramirez, J. The role of KNOX genes in plant development. Annu. Rev. Cell Dev. Biol. 2004, 20, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, Z.; Lu, G.; Lee, S.C.; Alonso, J.; Ecker, J.R.; Luan, S. A WD40 domain cyclophilin interacts with histone H3 and functions in gene repression and organogenesis in Arabidopsis. Plant Cell 2007, 19, 2403–2416. [Google Scholar] [CrossRef]
- Li, H.; Luan, S. The cyclophilin AtCYP71 Interacts with CAF-1 and LHP1 and functions in multiple chromatin remodeling processes. Mol. Plant. 2011, 4, 748–758. [Google Scholar] [CrossRef]
- Smith, M.R.; Willmann, M.R.; Wu, G.; Berardini, T.Z.; Moller, B.; Weijers, D.; Poethig, R.S. Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5424–5429. [Google Scholar] [CrossRef]
- Iki, T.; Yoshikawa, M.; Meshi, T.; Ishikawa, M. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 2012, 31, 267–278. [Google Scholar] [CrossRef]
- Czech, B.; Hannon, G.J. Small RNA sorting: Matchmaking for argonautes. Nat. Rev. Genet. 2011, 12, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Iki, T.; Yoshikawa, M.; Nishikiori, M.; Jaudal, M.C.; Matsumoto-Yokoyama, E.; Mitsuhara, I.; Meshi, T.; Ishikawa, M. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell 2010, 39, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Cigan, A.M.; Saeteurn, K.; Hake, S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 2007, 39, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Gandikota, M.; Birkenbihl, R.P.; Höhmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The miRNA156/157 recognition element in the 3’ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007, 49, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, L.; Wood, D.W.; Metcalfe, T.; Liang, X.; Gordon, M.P.; Comai, L.; Nester, E.W. Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proc. Natl. Acad. Sci. USA 1998, 95, 7040–70545. [Google Scholar] [CrossRef] [PubMed]
- Coaker, G.; Falick, A.; Staskawicz, B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science 2005, 308, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Coaker, G.; Zhu, G.; Ding, Z.; Van Doren, S.R.; Staskawicz, B. Eukaryotic cyclophilin as a molecular switch for effector activation. Mol. Microbiol. 2006, 61, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Aumüller, T.; Jahreis, G.; Fischer, G.; Schiene-Fischer, C. Role of prolyl cis/trans isomers in cyclophilin-assisted Pseudomonas syringae AvrRpt2 protease activation. Biochemistry 2010, 49, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Trupkin, S.A.; Mora-García, S.; Casal, J.J. The cyclophilin ROC1 links phytochrome and cryptochrome to brassinosteroid sensitivity. Plant J. 2012, 71, 712–723. [Google Scholar] [CrossRef]
- Kovalev, N.; Nagy, P.D. Cyclophilin A binds to the viral RNA and replication proteins, resulting in inhibition of tombusviral replicase assembly. J. Virol. 2013, 87, 13330–13342. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, X.; Chiang, Y.H.; Yadeta, K.A.; Ding, P.; Dong, L.; Zhao, Y.; Li, X.; Yu, Y.; Zhang, L.; et al. Proline isomerization of the immune receptor-interacting protein RIN4 by a cyclophilin inhibits effector-triggered immunity in Arabidopsis. Cell Host Microbe 2014, 16, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, B.; Nestestog, R.; Grini, P.E.; Skrbo, N.; Berg, A.; Salehian, Z.; Mandal, A.; Aalen, R.B. Molecular analysis of Arabidopsis endosperm and embryo promoter trap lines: Reporter-gene expression can result from T-DNA insertions in antisense orientation, in introns and in intergenic regions, in addition to sense insertion at the 5′ end of genes. J. Exp. Bot. 2005, 56, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Sirpiö, S.; Holmström, M.; Battchikova, N.; Aro, E.M. AtCYP20-2 is an auxiliary protein of the chloroplast NAD(P)H dehydrogenase complex. FEBS Lett. 2009, 583, 2355–2358. [Google Scholar] [CrossRef] [PubMed]
- Sirpiö, S.; Khrouchtchova, A.; Allahverdiyeva, Y.; Hansson, M.; Fristedt, R.; Vener, A.V.; Scheller, H.V.; Jensen, P.E.; Haldrup, A.; Aro, E.M. AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II. Plant J. 2008, 55, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Xu, Y.; Li, H.; Li, S.; Zhang, D.; Mao, Z.; Guo, S.; Yang, C.; Weng, Y.; et al. The cyclophilin CYP20-2 modulates the conformation of BRASSINAZOLE-RESISTANT1, which binds the promoter of FLOWERING LOCUS D to regulate flowering in Arabidopsis. Plant Cell 2013, 25, 2504–2521. [Google Scholar] [CrossRef] [PubMed]
- Rumeau, D.; Peltier, G.; Cournac, L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef]
- Tomašić Paić, A.; Fulgosi, H. Chloroplast immunophilins. Protoplasma 2016, 253, 249–258. [Google Scholar] [CrossRef]
- Fu, A.; He, Z.; Cho, H.S.; Lima, A.; Buchanan, B.B.; Luan, S. A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15947–15952. [Google Scholar] [CrossRef]
- Nielsen, H.; Engelbrecht, J.; Brunak, S.; von Heijne, G. Identication of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997, 10, 1–6. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Niwa, Y.; Ashida, H.; Tanaka, K.; Kawamukai, M.; Matsuda, H.; Nakagawa, T. Expression of a gene for cyclophilin which contains an amino-terminal endoplasmic reticulum-targeting signal. Plant Cell Physiol. 1999, 40, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Anders, N.; Wolters, H.; Beckmann, H.; Thomann, A.; Heinrich, R.; Schrader, J.; Singh, M.K.; Geldner, N.; Mayer, U.; et al. Role of the GNOM gene in Arabidopsis apical-basal patterning—From mutant phenotype to cellular mechanism of protein action. Eur. J. Cell Biol. 2010, 89, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kamauchi, S.; Nakatani, H.; Nakano, C.; Urade, R. Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 2005, 272, 3461–3476. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Durian, G.; Rahikainen, M.; Alegre, S.; Brosché, M.; Kangasjärvi, S. Protein phosphatase 2A in the regulatory network underlying biotic stress resistance in plants. Front. Plant. Sci. 2016, 7, 812. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Garshott, D.M.; Brownell, A.L.; Yoo, G.H.; Lin, H.S.; Freeburg, T.L.; Yoo, N.G.; Kaufman, R.J.; Callaghan, M.U.; Fribley, A.M. Cantharidins induce ER stress and a terminal unfolded protein response in OSCC. J. Dent. Res. 2015, 94, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Srivastava, R.; Howell, S.H. Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int. J. Mol. Sci. 2013, 14, 8188–8212. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, A.; Lee, S.S.; An, D.J.; Moon, K.B.; Ahn, J.C.; Kim, H.S.; Cho, H.S. Overexpression of golgi protein CYP21-4s improves crop productivity in potato and rice by increasing the abundance of mannosidic glycoproteins. Front. Plant. Sci. 2017, 8, 1250. [Google Scholar] [CrossRef] [PubMed]
- Friso, G.; van Wijk, K.J. Posttranslational protein modifications in plant metabolism. Plant Physiol. 2015, 169, 1469–1487. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res 2017, 6, 2050. [Google Scholar] [CrossRef]
- von Schaewen, A.; Frank, J.; Koiwa, H. Role of complex N-glycans in plant stress tolerance. Plant Signal. Behav. 2008, 3, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Nguema-Ona, E.; Vicré-Gibouin, M.; Gotté, M.; Plancot, B.; Lerouge, P.; Bardor, M.; Driouich, A. Cell wall O-glycoproteins and N-glycoproteins: Aspects of biosynthesis and function. Front. Plant. Sci. 2014, 5, 499. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, H.J.; Jung, W.Y.; Lee, A.; Yoon, D.H.; You, Y.N.; Kim, H.S.; Kim, B.G.; Ahn, J.C.; Cho, H.S. OsCYP21-4, a novel golgi-resident cyclophilin, increases oxidative stress tolerance in rice. Front. Plant. Sci. 2015, 6, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 5, 127–149. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. CRC. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Sunkar, R.; Wobus, U.; Strickert, M. Array platforms and bioinformatics tools for the analysis of plant transcriptome in response to abiotic stress. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2010; Volume 639, pp. 71–93. [Google Scholar]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Yadav, S.; Vaid, N.; Tuteja, N. Genome wide analysis of cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signal. Behav. 2012, 7, 1653–1666. [Google Scholar] [CrossRef]
- Dominguez-Solis, J.R.; He, Z.; Lima, A.; Ting, J.; Buchanan, B.B.; Luan, S. A cyclophilin links redox and light signals to cysteine biosynthesis and stress responses in chloroplasts. Proc. Natl. Acad. Sci. USA 2008, 105, 16386–16391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nguyen, N.H.; Nguyen, N.T.; Hong, S.W.; Lee, H. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis. Plant Cell Rep. 2013, 32, 1843–1853. [Google Scholar] [CrossRef]
- Pogorelko, G.V.; Mokryakova, M.; Fursova, O.V.; Abdeeva, I.; Piruzian, E.S.; Bruskin, S.A. Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene 2014, 538, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Mokryakova, M.V.; Pogorelko, G.V.; Bruskin, S.A.; Piruzian, E.S.; Abdeeva, I.A. The role of peptidyl-prolyl cis/trans isomerase genes of Arabidopsis thaliana in plant defense during the course of Xanthomonas campestris infection. Russ. J. Genet. 2014, 50, 140–148. [Google Scholar] [CrossRef]
- Abdeeva, I.A.; Pogorelko, G.V.; Maloshenok, L.G.; Mokrykova, M.V.; Fursova, O.V.; Bruskin, S.A. Serch for partner proteins of A. thaliana immunophilins involved in the control of Plant immunity. Molecules 2018, 23, 953. [Google Scholar] [CrossRef] [PubMed]
- Tadege, M.; Bucher, M.; Stahli, W.; Suter, M.; Dupuis, I.; Kuhlemeier, C. Activation of plant defense responses and sugar efflux by expression of pyruvate decarboxylase in potato leaves. Plant J. 1998, 16, 661–671. [Google Scholar] [CrossRef]
- Den Boon, J.A.; Diaz, A.; Ahlquist, P. Cytoplasmic viral replication complexes. Cell Host Microbe 2010, 8, 77–85. [Google Scholar] [CrossRef]
- Nagy, P.D.; Pogany, J. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 2011, 10, 137. [Google Scholar] [CrossRef]
- Bako, L.; Umeda, M.; Tiburcio, A.F.; Schell, J.; Koncz, C. The VirD2 pilot protein of Agrobacterium-transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10108–10113. [Google Scholar] [CrossRef]
- Gelvin, S.B. Traversing the Cell: Agrobacterium T-DNA’s journey to the host genome. Front. Plant. Sci. 2012, 3, 52. [Google Scholar] [CrossRef]
- van Kregten, M.; Lindhout, B.I.; Hooykaas, P.J.J.; van der Zaal, B.J. Agrobacterium-mediated T-DNA transfer and integration by minimal VirD2 consisting of the relaxase domain and a type IV secretion system translocation signal. Mol. Plant Microbe Interact. 2009, 22, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Li, W.; Viehhauser, A.; He, B.; Kim, S.; Nilsson, A.K.; Andersson, M.X.; Kittle, J.D.; Ambavaram, M.M.; Luan, S.; et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 9559–9564. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.; Barbosa dos Santos, I.; Liu, W.; Gosse, H.N.; Park, S.W. Cyclophilin 20-3 is positioned as a regulatory hub between light-dependent redox and 12-oxo-phytodienoic acid signaling. Plant Signal. Behav. 2017, 12, e1362520. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Acosta, I.F.; Farmer, E.E. Jasmonates. Arabidopsis Book 2009, 8, e0129. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Hell, R. Functional analysis of the cysteine synthase protein complex from plants: Structural, biochemical and regulatory properties. J. Plant Physiol. 2006, 163, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.H.; Tholl, D.; Park, S.W. (Auburn University, Auburn, AL, USA). Personal Communication, 2018.
- Liebthal, M.; Strüve, M.; Li, X.; Hertle, Y.; Maynard, D.; Hellweg, T.; Viehhauser, A.; Dietz, K.J. Redox-dependent conformational dynamics of decameric 2-cysteine peroxiredoxin and its interaction with cyclophilin 20-3. Plant Cell Physiol. 2016, 57, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; König, J.; Dietz, K.J.; Kandlbinder, A. Role of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20-3 in peptidyl-prolyl cis-trans isomerase and redox-related functions. Biochem. J. 2007, 401, 287–297. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; Seidel, T.; Laxa, M.; Nunes De Miranda, S.M.; Gärtner, F.; Ströher, E.; Kandlbinder, A.; Dietz, K.J. Multiple redox and non-redox interactions define 2-cys peroxiredoxin as a regulatory hub in the chloroplast. Mol. Plant. 2009, 2, 1273–1288. [Google Scholar] [CrossRef]
- Jensen, P.E.; Bassi, R.; Boekema, E.J.; Dekker, J.P.; Jansson, S.; Leister, D.; Robinson, C.; Scheller, H.V. Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta 2007, 1767, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.P. Thioredoxins and Glutaredoxins: Unifying Elements in Redox Biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef] [PubMed]
- Serrato, A.J.; Fernández-Trijueque, J.; Barajas-López, J.-D.; Chueca, A.; Sahrawy, M. Plastid thioredoxins: A “one-for-all” redox-signaling system in plants. Front. Plant. Sci. 2013, 4, 463. [Google Scholar] [CrossRef] [PubMed]
- Nikkanen, L.; Rintamäki, E. Thioredoxin-dependent regulatory networks in chloroplasts under fluctuating light conditions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130224. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Kondoh, A.; Stumpp, M.T.; Hisabori, T. Comprehensive survey of proteins targeted by chloroplast thioredoxin. Proc. Natl. Acad. Sci. USA 2001, 98, 11224–11229. [Google Scholar] [CrossRef]
- Peltier, J.; Cai, Y.; Sun, Q.; Zabrouskov, V.; Giacomelli, L.; Ytterberg, A.J.; Rutschow, H.; van Wijk, K.J. The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol. Cell. Proteom. 2005, 5, 114–133. [Google Scholar] [CrossRef]
- Caporaletti, D.; D’Alessio, A.C.; Rodriguez-Suarez, R.J.; Senn, A.M.; Duek, P.D.; Wolosiuk, R.A. Non-reductive modulation of chloroplast fructose-1,6-bisphosphatase by 2-Cys peroxiredoxin. Biochem. Biophys. Res. Commun. 2007, 355, 722–727. [Google Scholar] [CrossRef]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; De Miranda, S.M.N.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arabidopsis Book 2011, 9, e0142. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Riemann, M.; Muller, A.; Korte, A.; Furuya, M.; Weiler, E.W.; Nick, P. Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol. 2003, 133, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011, 62, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa dos Santos, I.; Park, S.-W. Versatility of Cyclophilins in Plant Growth and Survival: A Case Study in Arabidopsis. Biomolecules 2019, 9, 20. https://doi.org/10.3390/biom9010020
Barbosa dos Santos I, Park S-W. Versatility of Cyclophilins in Plant Growth and Survival: A Case Study in Arabidopsis. Biomolecules. 2019; 9(1):20. https://doi.org/10.3390/biom9010020
Chicago/Turabian StyleBarbosa dos Santos, Izailda, and Sang-Wook Park. 2019. "Versatility of Cyclophilins in Plant Growth and Survival: A Case Study in Arabidopsis" Biomolecules 9, no. 1: 20. https://doi.org/10.3390/biom9010020
APA StyleBarbosa dos Santos, I., & Park, S.-W. (2019). Versatility of Cyclophilins in Plant Growth and Survival: A Case Study in Arabidopsis. Biomolecules, 9(1), 20. https://doi.org/10.3390/biom9010020



