Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression?
Abstract
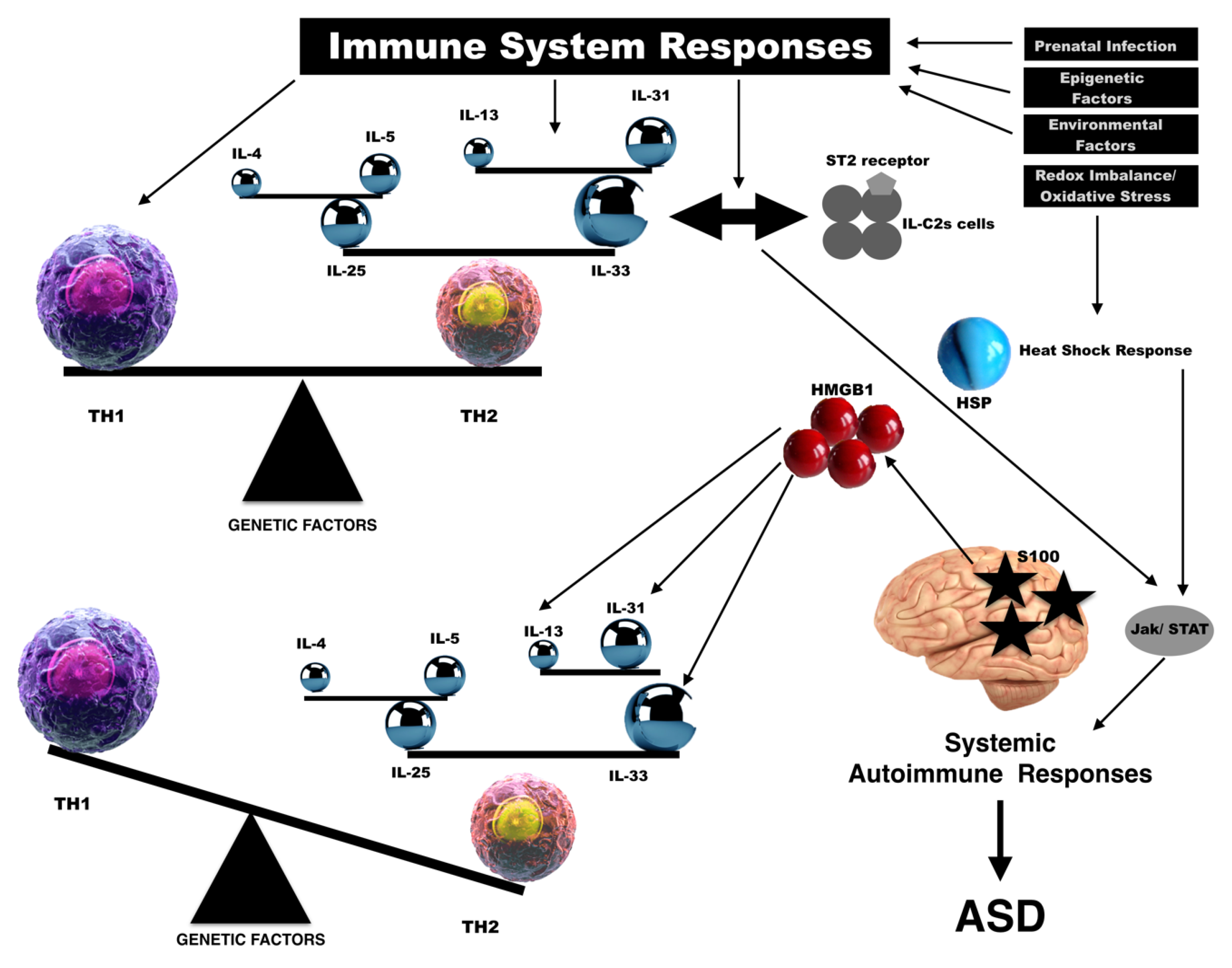
1. Background
2. Interleukin-33
3. High-Mobility Group Box 1
4. Heat-Shock Proteins
5. S100 Protein
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Asperger, H. Die „Autistischen Psychopathen” im Kindesalter. Arch. Psychiatr. Nervenkr. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Ruta, L.; Tartarisco, G.; Pioggia, G.; Gangemi, S. A systematic review of the association between allergic asthma and autism. Minerva Pediatr. 2017, 69, 538–550. [Google Scholar] [PubMed]
- Babinska, K.; Bucova, M.; Durmanova, V.; Lakatosova, S.; Janosikova, D.; Bakos, J.; Hlavata, A.; Ostatnikova, D. Increased plasma levels of the high mobility group box 1 protein (HMGB1) are associated with a higher score of gastrointestinal dysfunction in individuals with autism. Physiol. Res. 2014, 63 (Suppl. 4), S613–S618. [Google Scholar] [PubMed]
- Fagiolini, M.; Jensen, C.L.; Champagne, F.A. Epigenetic influences on brain development and plasticity. Curr. Opin. Neurobiol. 2009, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.S.; Roth, T.L. Insight from animal models of environmentally driven epigenetic changes in the developing and adult brain. Dev. Psychopathol. 2016, 28, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, V.; Schneider, J. Post-translational histone modifications and their interaction with sex influence normal brain development and elaboration of neuropsychiatric disorders. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Cirillo, A.; Bradstreet, J.J.; Antonucci, N. Epigenetic findings in autism: New perspectives for therapy. Int. J. Environ. Res. Public Health 2013, 10, 4261–4273. [Google Scholar] [CrossRef]
- Horvath, K.; Perman, J.A. Autism and gastrointestinal symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Cushing-Ruby, A.; Quraishi, H. Evaluation of atopy and immune functions in children with autism spectrum disorders (ASD): Identification of an ASD subset with distinct clinical and immunological findings. Fed. Am. Soc. Exp. Biol. 2008. [CrossRef]
- Mostafa, G.A.; Hamza, R.T.; El-Shahawi, H.H. Allergic manifestations in autistic children: Relation to disease severity. J. Pediatr. Neurol. 2008, 6, 115–123. [Google Scholar]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Magalhães, E.S.; Pinto-Mariz, F.; Bastos-Pinto, S.; Pontes, A.T.; Prado, E.A. Immune allergic response in Asperger syndrome. J. Neuroimmunol. 2009, 216, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015, 46, 232–236. [Google Scholar] [CrossRef]
- Croen, L.A.; Qian, Y.; Ashwood, P.; Daniels, J.L.; Fallin, D.; Schendel, D.; Schieve, L.A.; Singer, A.B.; Zerbo, O. Family history of immune conditions and autism spectrum and developmental disorders: Findings from the study to explore early development. Autism Res. 2018. [CrossRef] [PubMed]
- Hsiao, E.Y. Immune dysregulation in autism spectrum disorder. In International review of neurobiology. Int. Rev. Neurobiol. 2013, 113, 269–302. [Google Scholar]
- Atladóttir, H.Ó.; Henriksen, T.B.; Schendel, D.E.; Parner, E.T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 2012, 130, peds-2012. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Manfredi, A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007, 220, 35–46. [Google Scholar] [CrossRef]
- Barbosa, I.G.; Rodrigues, D.H.; Rocha, N.P.; Sousa, L.F.; Vieira, E.L.; Simoes-e-Silva, A.C.; Kummer, A.; Teixeira, A.L. Plasma levels of alarmin IL-33 are unchanged in autism spectrum disorder: A preliminary study. J. Neuroimmunol. 2015, 278, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Piancone, F.; Marventano, I.; Zoppis, M.; Hernis, A.; Zanette, M.; Trabattoni, D.; Chiappedi, M.; Ghezzo, A.; Canevini, M.P.; et al. Multiple inflammasome complexes are activated in autistic spectrum disorders. Brain Behav. Immun. 2016, 57, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Boso, M.; Brondino, N.; Pietra, S.; Barale, F.; Ucelli di Nemi, S.; Politi, P. Increased serum levels of high mobility group box 1 protein in patients with autistic disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J. Decreased Epidermal Growth Factor (EGF) Associated with HMGB1 and Increased Hyperactivity in Children with Autism. Biomark. Insights 2013, 8, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J. Increased epidermal growth factor receptor (EGFR) associated with hepatocyte growth factor (HGF) and symptom severity in children with autism spectrum disorders (ASDs). J. Cent. Nerv. Syst. Dis. 2014, 6, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Bazargan, M.; Vojdani, E.; Samadi, J.; Nourian, A.A.; Eghbalieh, N.; Cooper, E.L. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin. Diagn. Lab. Immunol. 2004, 11, 515–524. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2012, 9, 265. [Google Scholar] [CrossRef]
- Ahlsen, G.; Rosengren, L.; Belfrage, M.; Palm, A.; Haglid, K.; Hamberger, A.; Gillberg, C. Glial fibrillary acidic protein in the cerebrospinal fluid of children with autism and other neuropsychiatric disorders. Biol. Psychiatry 1993, 33, 734–743. [Google Scholar] [CrossRef]
- Boso, M.; Emanuele, E.; Minoretti, P.; Arra, M.; Politi, P.; Ucelli di Nemi, S.; Barale, F. Alterations of circulating endogenous secretory RAGE and S100A9 levels indicating dysfunction of the AGE-RAGE axis in autism. Neurosci. Lett. 2006, 410, 169–173. [Google Scholar] [CrossRef]
- Coffin, C.M.; Lowichik, A.; Putnam, A. Lipoblastoma (LPB): A clinicopathologic and immunohistochemical analysis of 59 cases. Am. J. Surg. Pathol. 2009, 33, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayadhi, L.Y.; Mostafa, G.A. A lack of association between elevated serum levels of S100B protein and autoimmunity in autistic children. J. Neuroinflamm. 2012, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Esnafoglu, E.; Ayyildiz, S.N.; Cirrik, S.; Erturk, E.Y.; Erdil, A.; Dagli, A.; Noyan, T. Evaluation of serum Neuron-specific enolase, S100B, myelin basic protein and glial fibrilliary acidic protein as brain specific proteins in children with autism spectrum disorder. Int. J. Dev. Neurosci. 2017, 61, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Guloksuz, S.A.; Abali, O.; Aktas Cetin, E.; Bilgic Gazioglu, S.; Deniz, G.; Yildirim, A.; Kawikova, I.; Guloksuz, S.; Leckman, J.F. Elevated plasma concentrations of S100 calcium-binding protein B and tumor necrosis factor alpha in children with autism spectrum disorders. Rev. Bras. Psiquiatr. 2017, 39, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kamekura, R.; Kojima, T.; Takano, K.; Go, M.; Sawada, N.; Himi, T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin. Exp. Allergy 2012, 42, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Wang, L.-C.; Yu, H.-H.; Lin, Y.-T.; Yang, Y.-H.; Chiang, B.-L. Type I IL-1 receptor (IL-1RI) as potential new therapeutic target for bronchial asthma. Mediat. Inflamm. 2010, 2010, 567351. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediat. Inflamm. 2018, 2018, 3858032. [Google Scholar] [CrossRef]
- Spooner, C.J.; Lesch, J.; Yan, D.; Khan, A.A.; Abbas, A.; Ramirez-Carrozzi, V.; Zhou, M.; Soriano, R.; Eastham-Anderson, J.; Diehl, L. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat. Immunol. 2013, 14, 1229–1236. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N. Development and function of group 2 innate lymphoid cells. Curr. Opin. Immunol. 2013, 25, 148–155. [Google Scholar] [CrossRef]
- Long, A.; Dominguez, D.; Qin, L.; Chen, S.; Fan, J.; Zhang, M.; Fang, D.; Zhang, Y.; Kuzel, T.M.; Zhang, B. Type 2 Innate Lymphoid Cells Impede IL-33–Mediated Tumor Suppression. J. Immunol. 2018, 201, 3456–3464. [Google Scholar] [CrossRef]
- Krabbendam, L.; Bal, S.M.; Spits, H.; Golebski, K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol. Rev. 2018, 286, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.; Feuerbach, D. Microglia M2A polarization as potential link between food allergy and autism spectrum disorders. Pharmaceuticals 2017, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Al-Ayadhi, L.Y. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J. Neuroimmunol. 2013, 261, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Ruta, L.; Pioggia, G.; Gangemi, S. Association between atopic dermatitis and autism spectrum disorders: A systematic review. Am. J. Clin. Dermatol. 2015, 16, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Quintana, D.; Glozier, N.; Lloyd, A.; Hickie, I.; Guastella, A. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef]
- Goodwin, G.; Johns, E. Are the high mobility group non-histone chromosomal proteins associated with ‘active’ chromatin? Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1978, 519, 279–284. [Google Scholar] [CrossRef]
- Romani, M.; Rodman, T.C.; Vidali, G.; Bustin, M. Serological analysis of species specificity in the high mobility group chromosomal proteins. J. Biol. Chem. 1979, 254, 2918–2922. [Google Scholar]
- Gangemi, S.; Casciaro, M.; Trapani, G.; Quartuccio, S.; Navarra, M.; Pioggia, G.; Imbalzano, E. Association between HMGB1 and COPD: A systematic review. Mediat. Inflamm. 2015, 2015, 164913. [Google Scholar] [CrossRef]
- Imbalzano, E.; Quartuccio, S.; Di Salvo, E.; Crea, T.; Casciaro, M.; Gangemi, S. Association between HMGB1 and asthma: A literature review. Clin. Mol. Allergy 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2010, 1799, 149–156. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cutrupi, M.C.; Colavita, L.; Manti, S.; Cuppari, C.; Salpietro, C. Neuroinflammation in Autism Spectrum Disorders: Role of High Mobility Group Box 1 Protein. Int. J. Mol. Cell. Med. 2017, 6, 148–155. [Google Scholar] [PubMed]
- Johnston, C.L.; Marzano, N.R.; van Oijen, A.M.; Ecroyd, H. Using single-molecule approaches to understand the molecular mechanisms of heat-shock protein chaperone function. J. Mol. Biol. 2018, 430, 4525–4546. [Google Scholar] [CrossRef] [PubMed]
- Brehme, M.; Voisine, C.; Rolland, T.; Wachi, S.; Soper, J.H.; Zhu, Y.; Orton, K.; Villella, A.; Garza, D.; Vidal, M. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014, 9, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Patterson, P.H. Alteration of neurodevelopment and behavior by maternal immune activation. In The Neuroimmunological Basis of Behavior and Mental Disorders; Springer: Boston, MA, USA, 2009; pp. 111–130. [Google Scholar]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Deng, M.Y.; Lam, S.; Meyer, U.; Feldon, J.; Li, Q.; Wei, R.; Luk, L.; Chua, S.E.; Sham, P.; Wang, Y. Frontal-subcortical protein expression following prenatal exposure to maternal inflammation. PLoS ONE 2011, 6, e16638. [Google Scholar] [CrossRef]
- Turturici, G.; Sconzo, G.; Geraci, F. Hsp70 and its molecular role in nervous system diseases. Biochem. Res. Int. 2011, 2011, 618127. [Google Scholar] [CrossRef]
- Zhao, D.; Mokhtari, R.; Pedrosa, E.; Birnbaum, R.; Zheng, D.; Lachman, H.M. Transcriptome analysis of microglia in a mouse model of Rett syndrome: Differential expression of genes associated with microglia/macrophage activation and cellular stress. Mol. Autism 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Abib, R.T.; Leite, M.C.; Bobermin, D.; Bambini-Junior, V.; Goncalves, C.A.; Riesgo, R.; Gottfried, C. Effect of the atypical neuroleptic risperidone on morphology and S100B secretion in C6 astroglial lineage cells. Moll. Cell. Biochem. 2008, 314, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Halder, S.K.; Hampson, D.R. Expression of fragile X mental retardation protein in neurons and glia of the developing and adult mouse brain. Brain Res. 2015, 1596, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Dreyfus, C.; DiCicco-Bloom, E. Valproic acid stimulates proliferation of glial precursors during cortical gliogenesis in developing rat. Dev. Neurobiol. 2016, 76, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 2012, 130, S160–S168. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.; Leader, G. Gastrointestinal symptoms in autism spectrum disorder: A. literature review. Rev. J. Autism Dev. Disord. 2014, 1, 11–17. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Lombardi, V.C.; De Meirleir, K.L.; Subramanian, K.; Nourani, S.M.; Dagda, R.K.; Delaney, S.L.; Palotás, A. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J. Nutritional Biochem. 2018, 61, 1–16. [Google Scholar] [CrossRef]
- Malik, A.; Sharma, D.; Zhu, Q.; Karki, R.; Guy, C.S.; Vogel, P. Kanneganti T-D: IL-33 regulates the IgA-microbiota axis to restrain IL-1α–dependent colitis and tumorigenesis. J. Clin. Investig. 2016, 126, 4469–4481. [Google Scholar] [CrossRef]
- Hodzic, Z.; Schill, E.M.; Bolock, A.M.; Good, M. IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine 2017, 61, 1–16. [Google Scholar] [CrossRef]
- Rivas, M.N.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Vocca, L.; Di Sano, C.; Uasuf, C.G.; Sala, A.; Riccobono, L.; Gangemi, S.; Albano, G.D.; Bonanno, A.; Gagliardo, R.; Profita, M. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology 2015, 220, 954–963. [Google Scholar] [CrossRef]
- Spadaro, A.; Scrivo, R.; Bombardieri, M.; Riccieri, V.; Rinaldi, T.; Taccari, E.; Valesini, G. Relationship of interleukin-12 and interleukin-13 imbalance with class-specific rheumatoid factors and anticardiolipin antibodies in systemic lupus erythematosus. Clin. Rheumatol. 2003, 22, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.P.; Gold, D.R.; Tzianabos, A.O.; Weiss, S.T.; Celedon, J.C. Cytokines, allergy, and asthma. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S.; Singer, S.M.; McDevitt, H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 1995, 16, 34–38. [Google Scholar] [CrossRef]
- Molloy, C.A.; Morrow, A.L.; Meinzen-Derr, J.; Schleifer, K.; Dienger, K.; Manning-Courtney, P.; Altaye, M.; Wills-Karp, M. Elevated cytokine levels in children with autism spectrum disorder. J. Neuroimmunol. 2006, 172, 198–205. [Google Scholar] [CrossRef]
- Itzhaky, D.; Amital, D.; Gorden, K.; Bogomolni, A.; Arnson, Y.; Amital, H. Low serum vitamin D concentrations in patients with schizophrenia. Isr. Med. Assoc. J. 2012, 14, 88–89. [Google Scholar]
- Ucuz, I.I.; Dursun, O.B.; Aydin, N. The effects of vitamin D3 on brain development and autism. Klinik Psikofarmakoloji Bülteni-Bull. Clin. Psychopharmacol. 2015, 25, 302–311. [Google Scholar] [CrossRef]
- Eyles, D.; Burne, T.; McGrath, J. Vitamin D in fetal brain development. Semin. Cell Dev. Biol. 2011, 2, 629–636. [Google Scholar] [CrossRef]
- Harms, L.R.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Gangemi, S.; La Grutta, S.; Malizia, V.; Riccobono, L.; Colombo, P.; Cibella, F.; Profita, M. 25-Hydroxyvitamin, D.; IL-31, and IL-33 in children with allergic disease of the airways. Mediat. Inflamm. 2014, 2014, 520241. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.; Karras, S.; März, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Kurutaş, E.B.; Şahin, N.; Güngör, O.; Fındıklı, E. The levels of vitamin D, vitamin D receptor, homocysteine and complex B vitamin in children with autism spectrum disorders. Clin. Psychopharmacol. Neurosci. 2018, 16, 383. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Feng, J.; Craddock, N.; Jones, I.R.; Cook, E.H., Jr.; Goldman, D.; Heston, L.L.; Chen, J.; Burkhart, P.; Li, W.; et al. Vitamin D receptor variants in 192 patients with schizophrenia and other psychiatric diseases. Neurosci. Lett. 2005, 380, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, S.; Şimşek, Ş.; Camkurt, M.A.; Çim, A.; Çelik, S.B. Association of polymorphisms in the vitamin D receptor gene and serum 25-hydroxyvitamin D levels in children with autism spectrum disorder. Gene 2016, 588, 109–114. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | No. of Patients | Age | Tissue | Alarmin | Correlation |
|---|---|---|---|---|---|---|
| Barbosa et al. [21] | 2015 | 30 | Adults | Blood | IL-33 | No differences |
| Tsilioni et al. [22] | 2015 | 40 | Children | Blood | IL-33 | No differences |
| Saresella et al. [23] | 2016 | 25 | Children | Blood | IL-33 | − |
| Emanuele et al. [24] | 2010 | 22 | Adults | Blood | HMGB1 | + |
| Russo [25] | 2013 | 38 | Children | Blood | HMGB1 | + |
| Russo [26] | 2014 | 33 | Children | Blood | HMGB1 | + |
| Babinská et al [4] | 2014 | 31 | Children/Adults | Plasma | HMGB1 | + |
| Vojdani et al. [27] | 2004 | 50 | Children | Blood | Hsp-60 | + |
| El-Ansary et al. [28] | 2012 | 20 | Adults | Blood | Hsp-70 | + |
| Ahlsen et al [29] | 1993 | 47 | Children | Cerebrospinal fluid | Glial S100 | No differences |
| Boso et al. [30] | 2006 | 18 | Adults | Blood | S100A9 | + |
| Coffin et al. [31] | 2009 | 1 | Child | Lipoblastoma | S100 | + |
| Al-Ayadhi et al. [32] | 2012 | 64 | Children | Blood | S100B | + |
| Esnafoglu et al. [33] | 2017 | 35 | Children | Blood | S100B | No differences |
| Guloksuz et al. [34] | 2017 | 40 | Children | Blood | S100B | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Salvo, E.; Casciaro, M.; Quartuccio, S.; Genovese, L.; Gangemi, S. Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression? Biomolecules 2019, 9, 2. https://doi.org/10.3390/biom9010002
Di Salvo E, Casciaro M, Quartuccio S, Genovese L, Gangemi S. Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression? Biomolecules. 2019; 9(1):2. https://doi.org/10.3390/biom9010002
Chicago/Turabian StyleDi Salvo, Eleonora, Marco Casciaro, Sebastiano Quartuccio, Lucrezia Genovese, and Sebastiano Gangemi. 2019. "Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression?" Biomolecules 9, no. 1: 2. https://doi.org/10.3390/biom9010002
APA StyleDi Salvo, E., Casciaro, M., Quartuccio, S., Genovese, L., & Gangemi, S. (2019). Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression? Biomolecules, 9(1), 2. https://doi.org/10.3390/biom9010002




