Molecular and Computational Studies Reveal That Per- and Polyfluoroalkyl Substances Can Impair Protamine–DNA Interaction, Potentially Inducing DNA Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
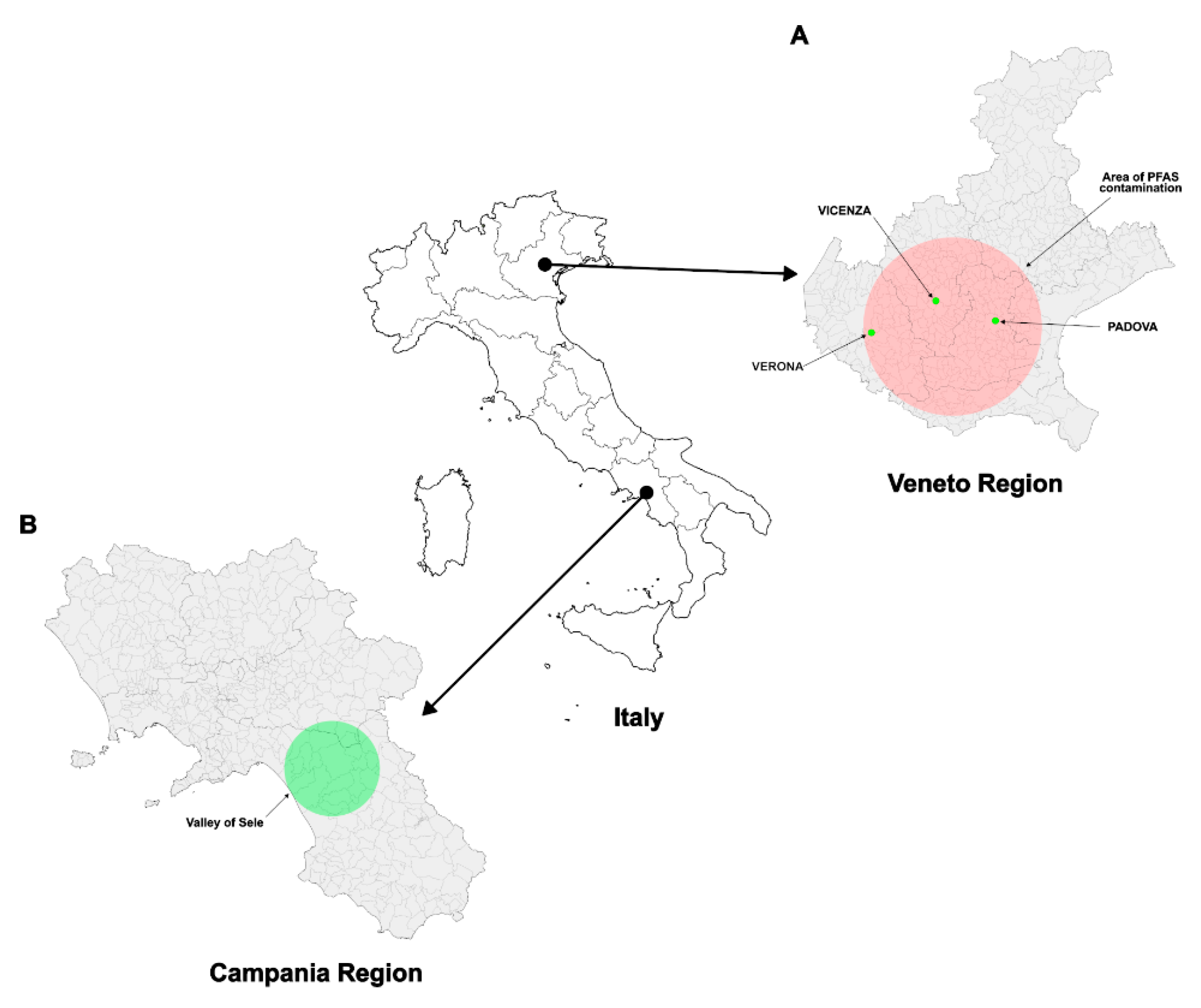
2.2. Recruitment
2.3. Inclusion, Exclusion and Confounding Criteria
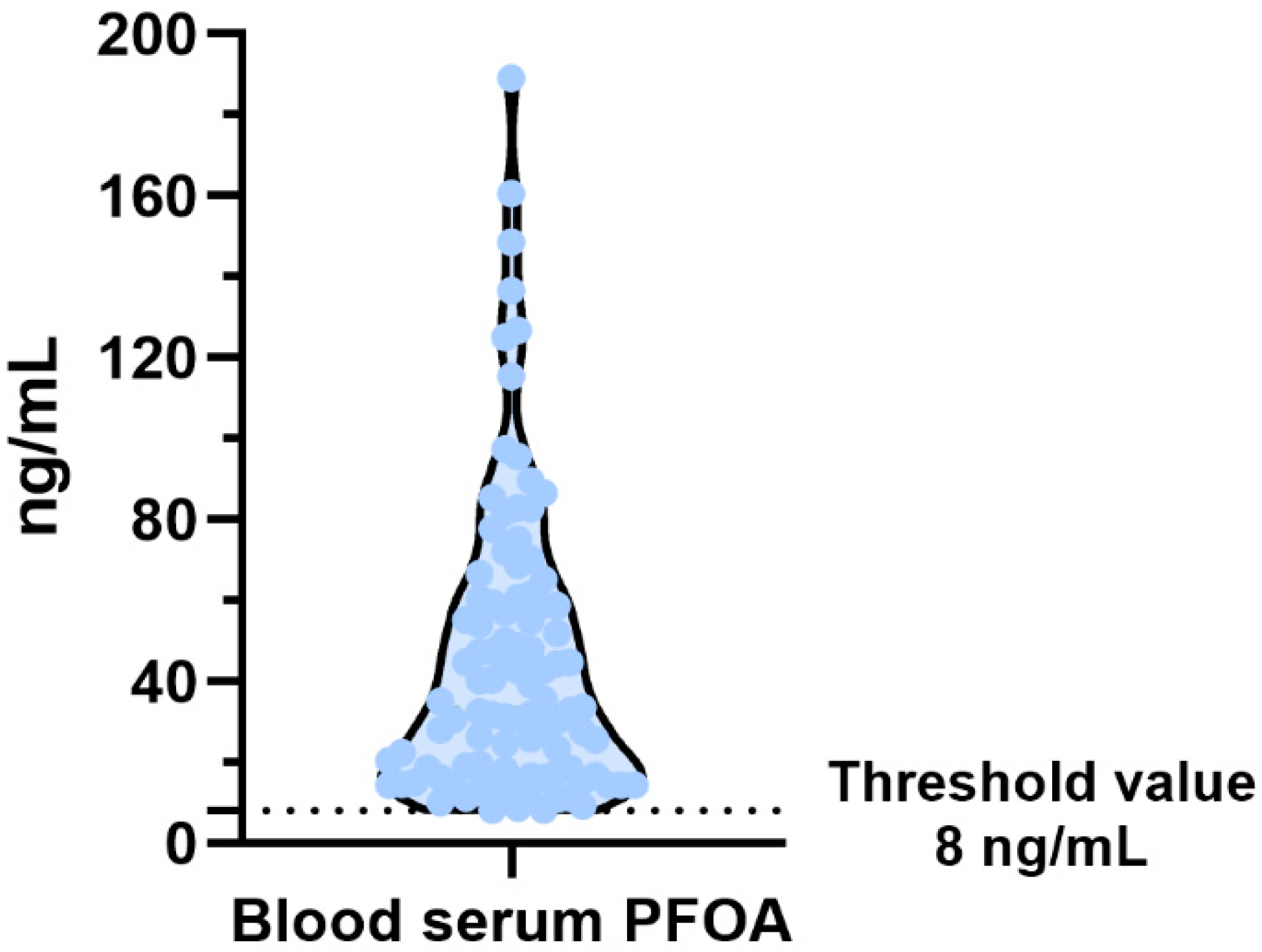
2.4. PFAS’s Determination
2.5. Extraction of Sperm Nuclear Basic Proteins (SNBP) from Spermatozoa
2.6. Electrophoresis of SNBP in AU-PAGE
2.7. Plasmid DNA Extraction
2.8. DNA Protection Assay
2.9. Computational Approach for Biomolecular Modeling
- Rigid-body docking, involving randomization of ligand orientations and energy minimization (1000 structures generated);
- Semi-flexible refinement, in which the interface regions are treated as flexible and refined using simulated annealing in torsion angle space (200 structures generated);
- Final refinement, consisting of energy minimization in an explicit solvent model (200 structures generated).
3. Results
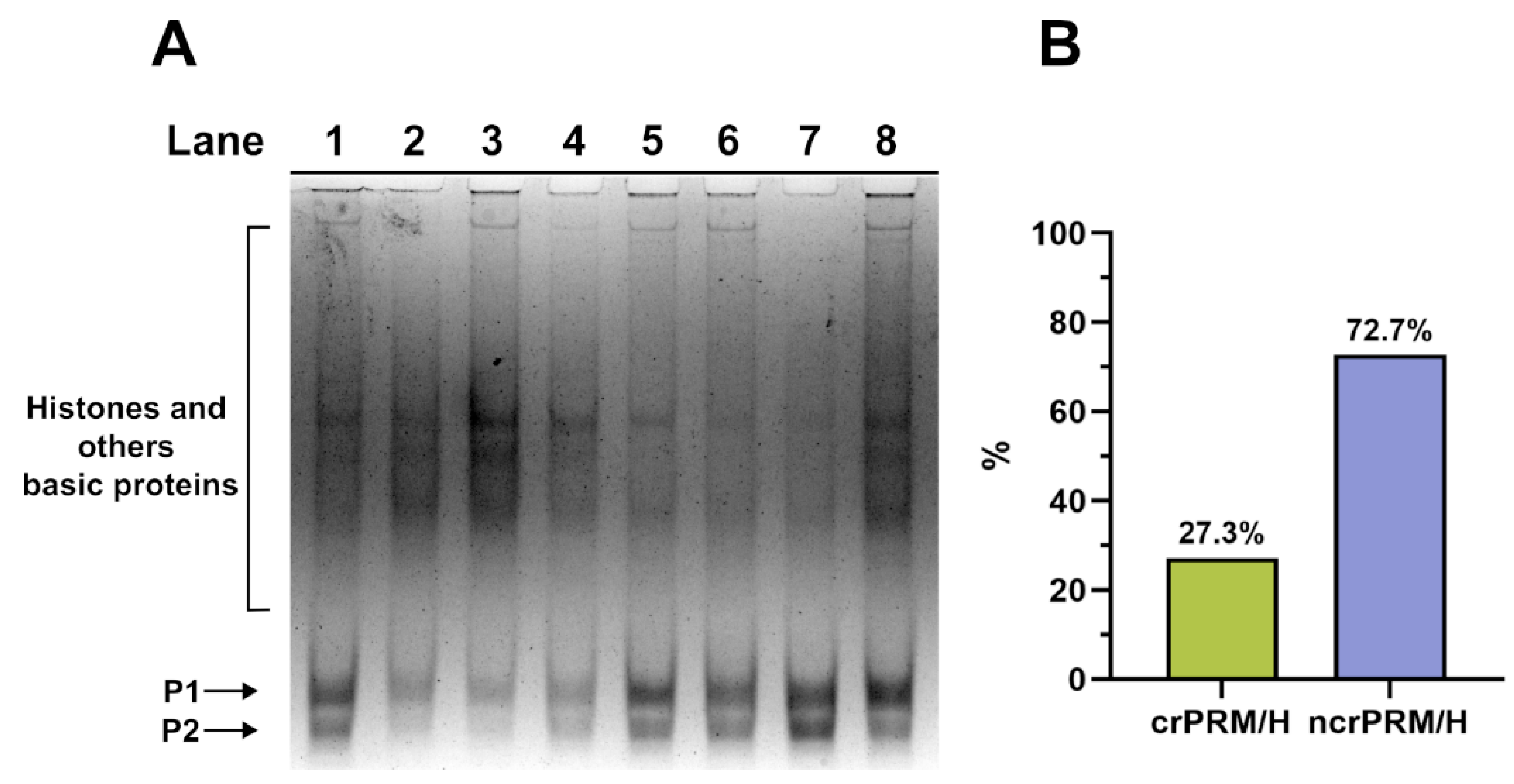
3.1. Analysis of the SNBP
3.2. Analysis of the Potential Involvment of SNBP in DNA Oxidative Damage
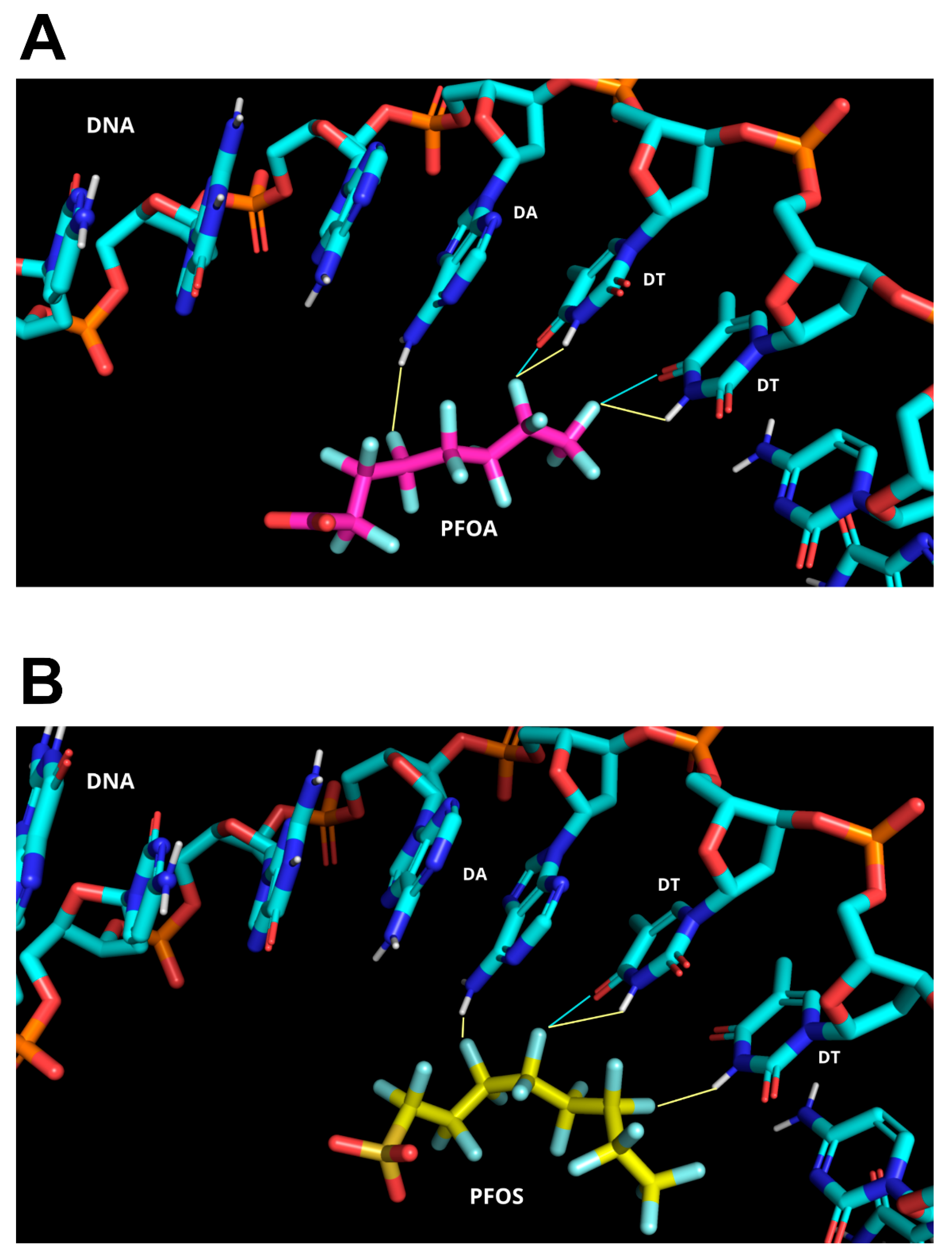
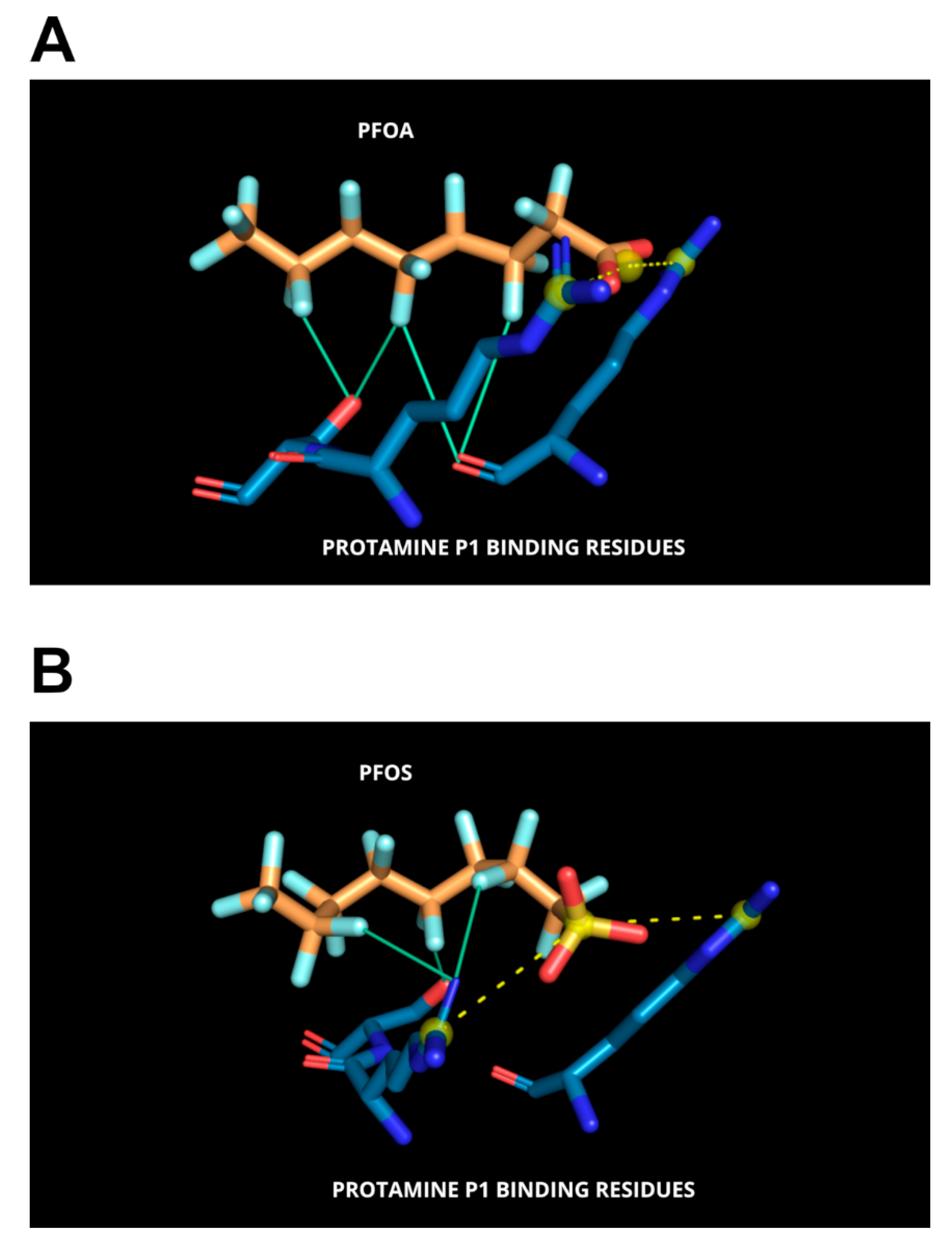
3.3. Molecular Docking Analyses
4. Discussion
5. Conclusions
6. Limitation and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fioretti, F.M.; Febbraio, F.; Carbone, A.; Branno, M.; Carratore, V.; Fucci, L.; Ausió, J.; Piscopo, M. A Sperm Nuclear Basic Protein from the Sperm of the Marine Worm Chaetopterus variopedatus with Sequence Similarity to the Arginine-Rich C-Termini of Chordate Protamine-Likes. DNA Cell Biol. 2012, 31, 1392–1402. [Google Scholar] [CrossRef]
- Moritz, L.; Hammoud, S.S. The Art of Packaging the Sperm Genome: Molecular and Structural Basis of the Histone-To-Protamine Exchange. Front. Endocrinol. 2022, 13, 895502. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Phillips, S.; Courchesne, A.; Boman, J.M.; Baazeem, A.; Bissonnette, F.; Kadoch, I.J.; Gabriel, M.S. Sperm Head Morphology Is Related to High Deoxyribonucleic Acid Stainability Assessed by Sperm Chromatin Structure Assay. Fertil. Steril. 2009, 91, 2495–2500. [Google Scholar] [CrossRef]
- Cho, C.-L.; Agarwal, A. Role of Sperm DNA Fragmentation in Male Factor Infertility: A Systematic Review. Arab. J. Urol. 2017, 16, 21–34. [Google Scholar] [CrossRef]
- Zurera-Egea, C.; Mateo, S.; Novo, S.; Asensio, M.; Boada, M.; Antich, M.; Rovira, S.; Sarrate, Z.; Blanco, J.; Anton, E. The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes. Int. J. Mol. Sci. 2025, 26, 6314. [Google Scholar] [CrossRef]
- de la Iglesia, A.; Jodar, M.; Oliva, R.; Castillo, J. Insights into the Sperm Chromatin and Implications for Male Infertility from a Protein Perspective. WIREs Mech. Dis. 2023, 15, e1588. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y. Sperm Chromatin Structure: Insights from In Vitro to In Situ Experiments. Curr. Opin. Cell Biol. 2022, 75, 102075. [Google Scholar] [CrossRef]
- Lewis, J.D.; Saperas, N.; Song, Y.; Zamora, M.J.; Chiva, M.; Ausió, J. Histone H1 and the Origin of Protamines. Proc. Natl. Acad. Sci. USA 2004, 101, 4148–4152. [Google Scholar] [CrossRef] [PubMed]
- Balhorn, R. The Protamine Family of Sperm Nuclear Proteins. Genome Biol. 2007, 8, 227. [Google Scholar] [CrossRef]
- Hærvig, K.K.; Petersen, K.U.; Hougaard, K.S.; Lindh, C.; Ramlau-Hansen, C.H.; Toft, G.; Giwercman, A.; Høyer, B.B.; Flachs, E.M.; Bonde, J.P.; et al. Maternal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Male Reproductive Function in Young Adulthood: Combined Exposure to Seven PFAS. Environ. Health Perspect 2022, 130, 107001. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Kandel, S.; Lewis, N.; D’Auvergne, O.; Rosby, R.; Hossain, E. Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health. Int. J. Environ. Res. Public Health 2025, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Farkouh, A.; Parekh, N.; Zini, A.; Arafa, M.; Kandil, H.; Tadros, N.; Busetto, G.M.; Ambar, R.; Parekattil, S.; et al. Sperm DNA Fragmentation: A Critical Assessment of Clinical Practice Guidelines. World J. Men’s Health 2022, 40, 30–37. [Google Scholar] [CrossRef]
- Siqueira, M.H.B. Sperm DNA Fragmentation: A Narrative Review. Hum. Reprod. Arch. 2025, 39, e000423. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Fernandez-Encinas, A.; Amengual, M.J.; Prada, E.; Cortés, P.; Navarro, J.; Benet, J. Double Stranded Sperm DNA Breaks, Measured by Comet Assay, Are Associated with Unexplained Recurrent Miscarriage in Couples without a Female Factor. PLoS ONE 2012, 7, e44679. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Fernández-Encinas, A.; García-Peiró, A.; Prada, E.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Human Semen Cryopreservation: A Sperm DNA Fragmentation Study with Alkaline and Neutral Comet Assay. Andrology 2014, 2, 83–87. [Google Scholar] [CrossRef]
- Derijck, A.; van der Heijden, G.; Giele, M.; Philippens, M.; de Boer, P. DNA Double-Strand Break Repair in Parental Chromatin of Mouse Zygotes, the First Cell Cycle as an Origin of de Novo Mutation. Hum. Mol. Genet. 2008, 17, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Carusone, N.; Notariale, R.; Prisco, M.; Ambrosino, A.; Perrella, S.; Manna, C.; Piscopo, M. Morphological, Gene, and Hormonal Changes in Gonads and In-Creased Micrococcal Nuclease Accessibility of Sperm Chromatin Induced by Mercury. Biomolecules 2022, 12, 87. [Google Scholar] [CrossRef]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Ferrero, G.; Festa, R.; Follia, L.; Lettieri, G.; Tarallo, S.; Notari, T.; Giarra, A.; Marinaro, C.; Pardini, B.; Marano, A.; et al. Small Noncoding RNAs and Sperm Nuclear Basic Proteins Reflect the Environmental Impact on Germ Cells. Mol. Med. 2024, 30, 12. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the Molecular and Toxicological Mechanism Associated with Interactions between Heavy Metals and the Reproductive System of Mytilus Galloprovincialis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 275, 109778. [Google Scholar] [CrossRef]
- Kumaresan, A.; Yadav, P.; Sinha, M.K.; Nag, P.; John Peter, E.S.K.; Mishra, J.S.; Kumar, S. Male Infertility and Perfluoroalkyl and Poly-Fluoroalkyl Substances: Evidence for Alterations in Phosphorylation of Proteins and Fertility-Related Functional Attributes in Bull Spermatozoa†. Biol. Reprod. 2024, 111, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Liew, F.F.; Dhawan, V.; Das, B.; Mottola, F.; Slama, P.; Rocco, L.; Roychoudhury, S. Environmental and Genetic Traffic in the Journey from Sperm to Offspring. Biomolecules 2023, 13, 1759. [Google Scholar] [CrossRef]
- Shi, L.; Tao, L.; Zong, Y.; Gao, C.; Han, B.; Gao, T.; Huang, C.; Liu, B.; Wu, H.; Tang, D.; et al. Seminal Per- and Polyfluoroalkyl Substance Exposure and Sperm Quality Impairment: From Toxic Target to Rescue. Environ. Int. 2025, 200, 109533. [Google Scholar] [CrossRef]
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, Y.; Wang, B.; Deng, S.; Zhang, F.; Fu, Z.; Yuan, Y.; Zhang, D. Toxic Effects of Per- and Polyfluoroalkyl Substances on Sperm: Epidemiological and Experimental Evidence. Front. Endocrinol. 2023, 14, 1114463. [Google Scholar] [CrossRef] [PubMed]
- Alamo, A.; La Vignera, S.; Mogioì, L.M.; Crafa, A.; Barbagallo, F.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences? J. Clin. Med. 2024, 13, 2201. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical Properties and Interactions of Perfluoroalkyl Substances (PFAS)—Challenges and Opportunities in Sensing and Remediation. Sci. Total. Environ. 2023, 905, 166764. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Dams, R.; Ameduri, B. Essential Per- and Polyfluoroalkyl Substances (PFAS) in Our Society of the Future. Molecules 2025, 30, 3220. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Tang, S.; Qiu, J.; Luo, Y.; Yang, C.; Lai, X.; Wang, Q.; Cao, H. Per- and Polyfluoroalkyl Substances (PFAS) in Consumer Products: An Overview of the Occurrence, Migration, and Exposure Assessment. Molecules 2025, 30, 994. [Google Scholar] [CrossRef]
- Fischer, F.C.; Ludtke, S.; Thackray, C.; Pickard, H.M.; Haque, F.; Dassuncao, C.; Endo, S.; Schaider, L.; Sunderland, E.M. Binding of Per- and Polyfluoroalkyl Substances (PFAS) to Serum Proteins: Implications for Toxicokinetics in Humans. Environ. Sci. Technol. 2024, 58, 1055–1063. [Google Scholar] [CrossRef]
- Frisbee, S.J.; Brooks, A.P.; Maher, A.; Flensborg, P.; Arnold, S.; Fletcher, T.; Steenland, K.; Shankar, A.; Knox, S.S.; Pollard, C.; et al. The C8 Health Project: Design, Methods, and Participants. Environ. Health Perspect. 2009, 117, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated Substances (PFAS) Affect Oxidative Stress Biomarkers In Vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Wang, W.; Hong, X.; Zhao, F.; Wu, J.; Wang, B. The Effects of Perfluoroalkyl and Polyfluoroalkyl Substances on Female Fertility: A Systematic Review and Meta-Analysis. Environ. Res. 2023, 216, 114718. [Google Scholar] [CrossRef]
- Specht, I.O.; Hougaard, K.S.; Spanò, M.; Bizzaro, D.; Manicardi, G.C.; Lindh, C.H.; Toft, G.; Jönsson, B.A.G.; Giwercman, A.; Bonde, J.P.E. Sperm DNA Integrity in Relation to Exposure to Environmental Perfluoroalkyl Substances—A Study of Spouses of Pregnant Women in Three Geographical Regions. Reprod. Toxicol. 2012, 33, 577–583. [Google Scholar] [CrossRef]
- Sun, F.; Lin, Y.; Pan, A.; Meng, T.-Q.; Xiong, C.-L.; Wang, Y.-X.; Liu, X.; Chen, D. Per- and Polyfluoroalkyl Substances in Semen Associated with Repeated Measures of Semen Quality in Healthy Adult Men. Environ. Sci. Technol. 2025, 59, 256–267. [Google Scholar] [CrossRef]
- Sciorio, R.; Greco, P.F.; Greco, E.; Tramontano, L.; Elshaer, F.M.; Fleming, S. Potential Effects of Environmental Toxicants on Sperm Quality and Potential Risk for Fertility in Humans. Front. Endocrinol. 2025, 16, 1545593. [Google Scholar] [CrossRef]
- Canova, C.; Barbieri, G.; Zare Jeddi, M.; Gion, M.; Fabricio, A.; Daprà, F.; Russo, F.; Fletcher, T.; Pitter, G. Associations between Perfluoroalkyl Substances and Lipid Profile in a Highly Exposed Young Adult Population in the Veneto Region. Environ. Int. 2020, 145, 106117. [Google Scholar] [CrossRef]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Zampaglioni, F.; Marra, V.; Miniero, R.; Valentini, S.; et al. Biomonitoring of Perfluorinated Compounds in Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy. Environ. Int. 2018, 110, 149–159. [Google Scholar] [CrossRef]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Zare Jeddi, M.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 027007. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and Poly-Fluoroalkyl Substances (PFAS) and Female Reproductive Outcomes: PFAS Elimination, Endocrine-Mediated Effects, and Disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive Oxygen Species Impact on Sperm DNA and Its Role in Male Infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Roychoudhury, S.; Nath, M.; Dutta, S. Oxidative Stress and Idiopathic Male Infertility. Adv. Exp. Med. Biol. 2022, 1358, 181–204. [Google Scholar] [CrossRef]
- Aktan, G.; Doğru-Abbasoğlu, S.; Küçükgergin, C.; Kadıoğlu, A.; Özdemirler-Erata, G.; Koçak-Toker, N. Mystery of Idiopathic Male Infertility: Is Oxidative Stress an Actual Risk? Fertil. Steril. 2013, 99, 1211–1215. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nada, E.A.; El-Tonsy, M.H.; Sharma, R.K.; Meyer, A.; Nelson, D.R.; Thomas, A.J. Negative Effects of Increased Sperm DNA Damage in Relation to Seminal Oxidative Stress in Men with Idiopathic and Male Factor Infertility. Fertil. Steril. 2003, 79, 1597–1605. [Google Scholar] [CrossRef]
- Marinaro, C.; Bianchi, A.R.; Guerretti, V.; Barricelli, G.; Berman, B.; Bertola, F.; Micali, S.; Busardò, F.P.; Di Giorgi, A.; De Maio, A.; et al. Molecular Alterations in Semen of Per-And Polyfluoroalkyl Substance Exposed Subjects: Association Between DNA Integrity, Antioxidant Capacity and Lipoperoxides. Antioxidants 2025, 14, 792. [Google Scholar] [CrossRef]
- Piscopo, M. Seasonal Dependence of Cadmium Molecular Effects on Mytilus galloprovincialis (Lamarck, 1819) Protamine-like Protein Properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef]
- Lettieri, G.; Maione, M.; Ranauda, M.A.; Mele, E.; Piscopo, M. Molecular Effects on Spermatozoa of Mytilus galloprovincialis Exposed to Hyposaline Conditions. Mol. Reprod. Dev. 2019, 86, 650–660. [Google Scholar] [CrossRef]
- Carbone, A.; Fioretti, F.M.; Fucci, L.; Ausió, J.; Piscopo, M. High Efficiency Method to Obtain Supercoiled DNA with a Commercial Plasmid Purification Kit. Acta Biochim. Pol. 2012, 59, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Kabier, M.; Gambacorta, N.; Trisciuzzi, D.; Kumar, S.; Nicolotti, O.; Mathew, B. MzDOCK: A Free Ready-to-Use GUI-Based Pipeline for Molecular Docking Simulations. J. Comput. Chem. 2024, 45, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.R.; Bianco, P.R. Laminar Flow Cells for Single-Molecule Studies of DNA-Protein Interactions. Nat. Methods 2008, 5, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Crisalli, A.M.; Cai, A.; Cho, B.P. Probing the Interactions of Perfluorocarboxylic Acids of Various Chain Lengths with Human Serum Albumin: Calorimetric and Spectroscopic Investigations. Chem. Res. Toxicol. 2023, 36, 703–713. [Google Scholar] [CrossRef]
- Stoccoro, A.; Coppedè, F. Exposure to Metals, Pesticides, and Air Pollutants: Focus on Resulting DNA Methylation Changes in Neurodegenerative Diseases. Biomolecules 2024, 14, 1366. [Google Scholar] [CrossRef]
- Passaro, D.; Rana, G.; Piscopo, M.; Viggiano, E.; De Luca, B.; Fucci, L. Epigenetic Chromatin Modifications in the Cortical Spreading Depression. Brain Res. 2010, 1329, 1–9. [Google Scholar] [CrossRef]
- Rana, G.; Donizetti, A.; Virelli, G.; Piscopo, M.; Viggiano, E.; De Luca, B.; Fucci, L. Cortical Spreading Depression Differentially Affects Lysine Methylation of H3 Histone at Neuroprotective Genes and Retrotransposon Sequences. Brain Res. 2012, 1467, 113–119. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Aghaei, M.; Bashardoust, P.; Rezvani Ghalhari, M.; Nayeri, D.; Malekpoor, M.; Sheikhi, S.; Shi, Z. An Insight into the Environmental and Human Health Impacts of Per- and Polyfluoroalkyl Substances (PFAS): Exploring Exposure Pathways and Their Implications. Environ. Sci. Eur. 2025, 37, 81. [Google Scholar] [CrossRef]
- Boston, C.; Keck, S.; Naperala, A.; Collins, J. The Evolution of PFAS Epidemiology: New Scientific Developments Call into Question Alleged “Probable Links” between PFOA and Kidney Cancer and Thyroid Disease. Front. Public Health 2025, 13, 1532277. [Google Scholar] [CrossRef]
- Mahoney, H.; Xie, Y.; Brinkmann, M.; Giesy, J.P. Next Generation Per- and Poly-Fluoroalkyl Substances: Status and Trends, Aquatic Toxicity, and Risk Assessment. Eco-Environ. Health 2022, 1, 117–131. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Raymer, J.H.; Michael, L.C.; Studabaker, W.B.; Olsen, G.W.; Sloan, C.S.; Wilcosky, T.; Walmer, D.K. Concentrations of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) and Their Associations with Human Semen Quality Measurements. Reprod. Toxicol. 2012, 33, 419–427. [Google Scholar] [CrossRef]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebaek, N.E.; Jørgensen, N. Do Perfluoroalkyl Compounds Impair Human Semen Quality? Environ. Health Perspect. 2009, 117, 923–927. [Google Scholar] [CrossRef]
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Støvring, H.; Kristensen, S.L.; Halldorsson, T.I.; Rantakokko, P.; Kiviranta, H.; Ernst, E.H.; et al. In Utero Exposure to Persistent Organochlorine Pollutants and Reproductive Health in the Human Male. Reproduction 2014, 148, 635–646. [Google Scholar] [CrossRef]
- Qin, C.; Xiang, L.; Wang, Y.-Z.; Yu, P.-F.; Meng, C.; Li, Y.-W.; Zhao, H.-M.; Hu, X.; Gao, Y.; Mo, C.-H. Binding Interaction of Environmental DNA with Typical Emerging Perfluoroalkyl Acids and Its Impact on Bioavailability. Sci. Total. Environ. 2024, 906, 167392. [Google Scholar] [CrossRef]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the Binding Model of Per- and Polyfluoroalkyl Substances to Proteins and Membranes. Environ. Int. 2023, 175, 107951. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of Arginine Residues in Cu(II)-Induced DNA Breakage and Proteinase K Resistance of H1 Histones. Sci. Rep. 2018, 8, 7414. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Conte, M.; Di Paola, F.; Conforti, S.; Rana, G.; De Petrocellis, L.; Fucci, L.; Geraci, G. Relevance of Arginines in the Mode of Binding of H1 Histones to DNA. DNA Cell Biol. 2010, 29, 339–347. [Google Scholar] [CrossRef] [PubMed]
- DeRouchey, J.; Hoover, B.; Rau, D.C. A Comparison of DNA Compaction by Arginine and Lysine Peptides: A Physical Basis for Arginine Rich Protamines. Biochemistry 2013, 52, 3000–3009. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musella, F.; Guarnieri, M.G.; Amore, S.; Montano, L.; Bertola, F.; Micali, S.; Busardò, F.P.; Di Giovanni, C.; Lettieri, G.; Piscopo, M. Molecular and Computational Studies Reveal That Per- and Polyfluoroalkyl Substances Can Impair Protamine–DNA Interaction, Potentially Inducing DNA Damage. Biomolecules 2025, 15, 1279. https://doi.org/10.3390/biom15091279
Musella F, Guarnieri MG, Amore S, Montano L, Bertola F, Micali S, Busardò FP, Di Giovanni C, Lettieri G, Piscopo M. Molecular and Computational Studies Reveal That Per- and Polyfluoroalkyl Substances Can Impair Protamine–DNA Interaction, Potentially Inducing DNA Damage. Biomolecules. 2025; 15(9):1279. https://doi.org/10.3390/biom15091279
Chicago/Turabian StyleMusella, Federica, Maria Grazia Guarnieri, Simona Amore, Luigi Montano, Francesco Bertola, Salvatore Micali, Francesco Paolo Busardò, Carmen Di Giovanni, Gennaro Lettieri, and Marina Piscopo. 2025. "Molecular and Computational Studies Reveal That Per- and Polyfluoroalkyl Substances Can Impair Protamine–DNA Interaction, Potentially Inducing DNA Damage" Biomolecules 15, no. 9: 1279. https://doi.org/10.3390/biom15091279
APA StyleMusella, F., Guarnieri, M. G., Amore, S., Montano, L., Bertola, F., Micali, S., Busardò, F. P., Di Giovanni, C., Lettieri, G., & Piscopo, M. (2025). Molecular and Computational Studies Reveal That Per- and Polyfluoroalkyl Substances Can Impair Protamine–DNA Interaction, Potentially Inducing DNA Damage. Biomolecules, 15(9), 1279. https://doi.org/10.3390/biom15091279








