Beyond the BMI Paradox: Unraveling the Cellular and Molecular Determinants of Metabolic Health in Obesity
Abstract
1. Introduction
2. Definitions and Diagnostic Criteria
2.1. Traditional Criteria of MHO
- The absence of metabolic syndrome components according to various definitions (e.g., NCEP ATP III, and IDF);
- Homeostasis model assessment of insulin resistance (HOMA-IR) below specific thresholds;
- Cardiometabolic risk factor clustering (including blood pressure, lipids, and glucose parameters);
- The absence of specific complications such as hypertension, dysglycemia, or dyslipidemia.
| Criterion | Meigs (2006) [15] | Karelis (2004/2008) [4,16] | Wildman (2008) [17] | Aguilar-Salinas (2008) [18] | Lynch (2009) [19] | BioSHaRE-EU (2014) Less Strict [20] | BioSHaRE-EU (2014) Strict [20] | Zembic et al. (2021) [14] | Wang et al. (2023) [21] |
|---|---|---|---|---|---|---|---|---|---|
| Insulin sensitivity/resistance | |||||||||
| HOMA-IR | ≤2.5 | ≤1.95 | ≤5.13 | - | - | - | - | - | - |
| Matsuda index | - | ≥7.2 | - | - | - | - | - | - | - |
| Blood pressure (mmHg) | |||||||||
| Systolic | <130 | <140 | <130 | <140 | <130 | <140 | <130 | <130 | <140 |
| Diastolic | <85 | <90 | <85 | <90 | <85 | <90 | <85 | - | <90 |
| Anti-hypertensive medication | No | No | No | No | No | No | No | No | No |
| Glucose metabolism | |||||||||
| Fasting glucose (mg/dL) | <100 | <100 | <100 | <100 | <100 | <110 | <100 | - | <100 |
| 2h glucose (mg/dL) | <140 | - | - | - | - | - | - | - | - |
| HbA1c (%) | <5.7 | - | - | - | - | - | - | - | - |
| Diabetes diagnosis | No | No | No | No | No | No | No | No | No |
| Glucose-lowering medication | No | No | No | No | No | No | No | - | No |
| Lipids | |||||||||
| Total or LDL cholesterol (mg/dL) | Total < 200 | Total < 200 | LDL < 130 | Total < 200 | Total < 200 | - | - | - | Total < 240 |
| HDL cholesterol (mg/dL) | >40 M, >50 F | >50 | >40 M, >50 F | >40 | >40 M, >50 F | >40 M, >50 F | >60 M, >70 F | - | >40 M, >50 F |
| Triglycerides (mg/dL) | <150 | <150 | <150 | <150 | <150 | <150 | <100 | - | <150 |
| Lipid-lowering medication | No | No | No | No | No | No | No | - | No |
| Anthropometric measures | |||||||||
| Waist circumference (cm) | <102 M, <88 F | - | <102 M, <88 F | - | <102 M, <88 F | <102 M, <88 F | <94 M, <80 F | - | <90 M, <85 F |
| Waist-to-hip ratio | - | - | - | - | - | - | - | <0.95 F, <1.03 M | - |
| Other biomarkers/indices | |||||||||
| C-reactive protein (mg/L) | - | - | <3.0 | - | - | - | - | - | - |
| Number of criteria required | All | ≥4 | ≤1 | All | All | ≤2 | All | All | All |
2.2. Metabolic Dysfunction-Associated Steatotic Liver Disease as an Emerging Criterion in MHO Definition
3. Global Prevalence and Demographic Characteristics
- Sex: Most studies report a higher MHO prevalence among women compared to men, potentially reflecting sex differences in body fat distribution and adipose tissue function [6]. Recent discoveries highlight significant sex-specific mechanisms, including the identification of CTRP10’s female-specific role in maintaining metabolic health during obesity [31]. A 2024 study demonstrated that adipose tissue insulin resistance is more pronounced in men than women, with men showing 10-fold lower insulin sensitivity and decreased adipose expression of insulin receptor substrate 1 (IRS1) [32]. Analysis of 9631 Saudi adults found females had significantly higher age-adjusted prevalence of MHO than males (OR = 1.22, 95% CI 1.1–1.4, p = 0.009) [33]. These sex-specific differences extend to therapeutic responses, with emerging evidence suggesting that women and men may respond differently to interventions aimed at maintaining or restoring metabolic health in obesity.
- Age: MHO is more common in younger individuals and decreases with advancing age, suggesting that metabolic health may deteriorate over time despite stable weight [6]. A longitudinal study of 9809 individuals found that genetic predisposition to higher BMI may protect against MHO conversion, though lifestyle factors did not predict conversion over 4 years [8].
- Ethnicity: Significant ethnic differences exist in MHO prevalence. Studies from Europe and North America have documented higher rates in populations of European descent compared to those of African, Hispanic, or South Asian ancestry [6]. A study examining 2350 Asian individuals with a BMI ≥ 25 kg/m2 reported that 13.3% met MHO criteria [34]. Recent analysis of Arab populations revealed leptin resistance as central to MHO-to-MUO progression, with significantly higher leptin levels in metabolically unhealthy groups [35].
- BMI: The prevalence of MHO typically decreases with increasing severity of obesity. Grade 1 obesity (BMI 30–34.9 kg/m2) shows approximately twice the prevalence of MHO compared to morbid obesity (BMI ≥ 40 kg/m2) [6].
4. Clinical Characteristics and Health Outcomes
4.1. Type 2 Diabetes Risk
4.2. Cardiovascular Disease Risk
4.3. Chronic Kidney Disease Risk
4.4. Cancer Risk
5. Pathophysiological Mechanisms Underlying MHO
5.1. Adipose Tissue Distribution Patterns
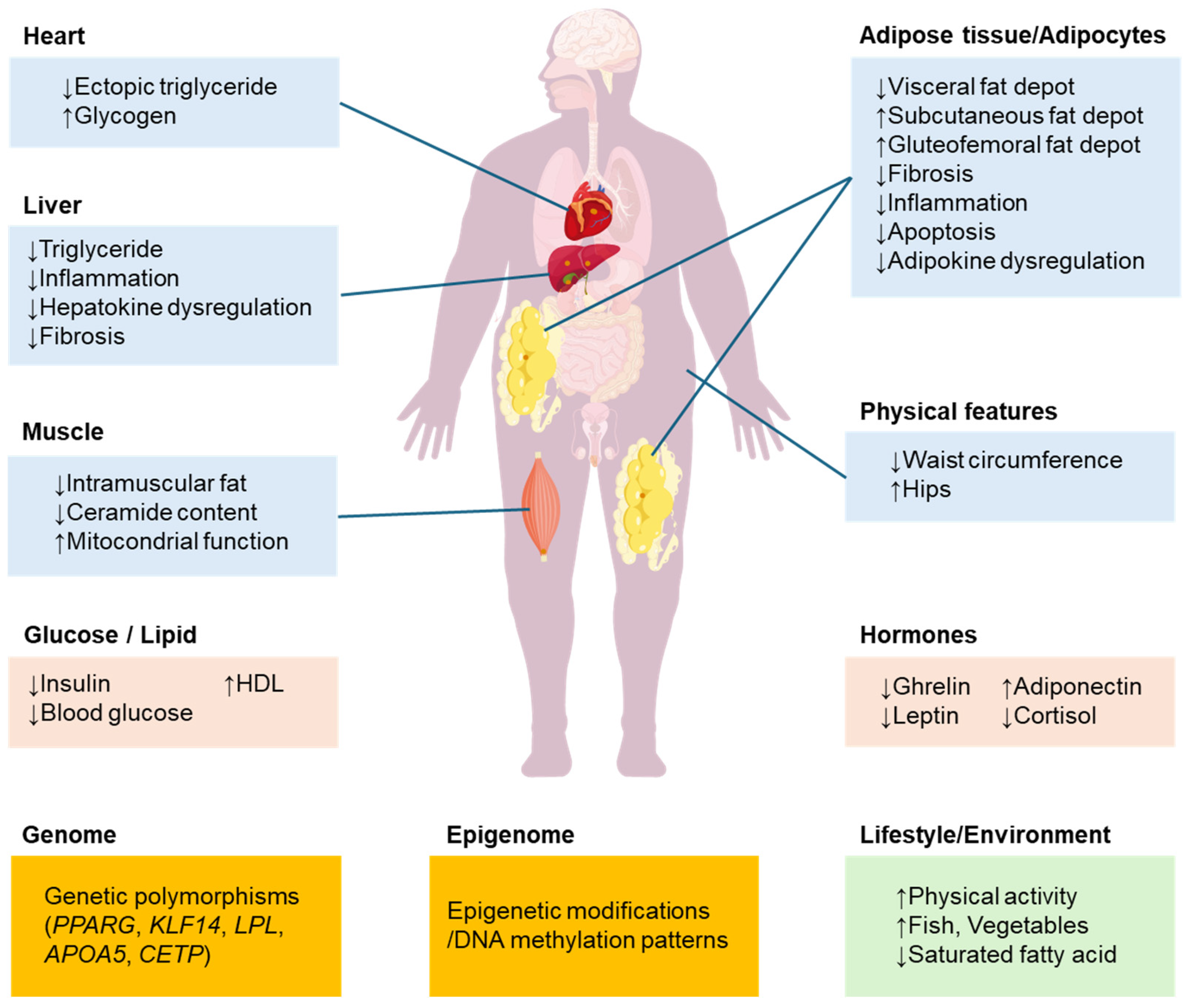
5.2. Adipose Tissue and Adipocyte Function
5.2.1. Lipogenic Capacity
5.2.2. Adipose Tissue Inflammation
5.2.3. Healthy Versus Unhealthy Adipose Expansion
5.2.4. Adipocyte-Specific Gene-Modified Animals
5.3. Genetic and Epigenetic Factors
5.3.1. Key Single-Nucleotide Polymorphisms
5.3.2. Population-Specific Genetic Associations
5.3.3. Epigenetic Contributions
5.3.4. Gene–Lifestyle Interactions
5.3.5. Epigenetic Memory of Obesity
5.4. Skeletal Muscle Characteristics
- Enhanced Mitochondrial Function: Muscle biopsies from subjects with MHO show higher mitochondrial content, enhanced oxidative enzyme activity, and more efficient respiratory chain function compared to individuals with MUO [38]. These differences correlate with improved insulin sensitivity and reduced intramyocellular lipid accumulation.
- Reduced Ectopic Lipid Deposition: Despite obesity, individuals with MHO maintain lower intramyocellular lipid content and altered lipid composition, with reduced ceramide and diacylglycerol species known to interfere with insulin signaling [38].
- Preserved Insulin Signaling: Molecular analyses of skeletal muscle from individuals with MHO demonstrate preserved insulin receptor substrate (IRS) phosphorylation and downstream Akt/PKB activation compared to subjects with MUO [52].
5.5. Liver Fat Content and Function
5.6. Lifestyle Factors
Nutritional Status and Targeted Dietary Interventions in MHO
- Replace saturated fats with PUFA-rich options (e.g., canola/soy/sunflower oils and fatty fish) to support adipose tissue expandability and insulin sensitivity mechanistically via PPARγ–LPCAT3 [93];
- Physical Activity: Individuals with MHO typically report higher levels of both leisure time and total physical activity compared to subjects with MUO [95]. A meta-analysis of 15 studies found that individuals with MHO engaged in approximately 30% more moderate-to-vigorous physical activity than their metabolically unhealthy counterparts. Meta-analysis of physical activity studies found that individuals MHO demonstrate higher physical activity levels, reduced sedentary behavior, and superior cardiorespiratory fitness compared to metabolically unhealthy obesity individuals, with higher fitness levels potentially preventing transition to metabolically unhealthy states [94]. Recent studies examining the effects of exercise on adipose tissue have revealed that “exercise is a potent behavioral intervention for preventing and reducing obesity and other metabolic diseases”: exercise appears to impose unique physiological stimuli that can alter angiogenesis and mitochondrial remodeling in adipose tissues, potentially promoting healthy adipogenesis. Studies in mice using 2H labeling techniques suggest that exercise may inhibit the generation of new adipocytes and extend the lifespan of existing adipocytes, potentially contributing to MHO [66].
- Cardiorespiratory Fitness: Independent of self-reported physical activity, measured cardiorespiratory fitness is significantly higher in individuals with MHO versus MUO. The HERITAGE Family Study demonstrated that higher baseline fitness and greater fitness improvements with exercise training predicted transition from MUO to MHO status.
- Dietary Patterns: Specific nutritional biomarkers (particularly carotenoids and vitamin D) are reportedly useful characteristics of MHO [90]. Some studies report that individuals with MHO consume more fish and vegetables and fewer sugar-sweetened beverages and saturated fatty acids [91], though others find no clear differences in total energy intake and nutrient intake between MHO and MUO [92]. Recent molecular insights have revealed how specific dietary lipid composition influences adipose tissue function. The PPARγ-LPCAT3 pathway demonstrates that dietary n-6 PUFA intake directly modulates adipose tissue expandability through membrane lipid remodeling [93]. This finding suggests that the quality of dietary fats, particularly the omega-6 PUFA content, may be as important as total fat intake in determining metabolic health outcomes in obesity. While this area requires further research in human populations, it provides a mechanistic basis for understanding how dietary composition influences the MHO phenotype.
- Sleep Quality: Some evidence suggests that individuals with MHO maintain more favorable sleep patterns, with lower prevalence of sleep disorders and more consistent sleep duration compared to subjects with MUO.
6. Temporal Stability and Transition Patterns
6.1. Transition Rates and Patterns
6.2. Predictors of Transition
- Age: Older individuals have significantly higher transition rates, with higher age associated with increased transition risk [97].
- BMI: Higher baseline BMI predicts greater likelihood of transitioning to MUO [97].
- Epigenetic Factors: Recent evidence shows that specific DNA methylation patterns predict transition, with 26 CpG sites differentially methylated between stable and unstable MHO [83].
6.3. Health Consequences of Transition
7. Pharmacological and Non-Pharmacological Interventions
7.1. Lifestyle Interventions
- Weight Loss: The impact of weight loss interventions in MHO remains somewhat controversial. Some studies suggest that weight reduction in individuals with MHO leads to improvements in inflammatory markers, liver fat content, and insulin sensitivity [99]. However, other studies have reported minimal metabolic benefits or even paradoxical worsening of certain parameters following weight loss in subjects with MHO [4].
- The HERITAGE Family Study found that individuals with MHO showed smaller improvements in insulin sensitivity and lipid profiles following weight loss compared to subjects with MUO, suggesting potentially different response patterns [92]. This has raised questions about the risk-benefit ratio of aggressive weight loss interventions in all individuals with MHO.
- Physical Activity: Exercise interventions appear particularly beneficial for individuals with MHO, often producing metabolic improvements independent of significant weight loss. A randomized controlled trial demonstrated that six months of moderate-intensity exercise in subjects with MHO led to significant reductions in visceral fat, liver fat, and systemic inflammation despite minimal changes in body weight [92]. A meta-analysis of seven intervention studies found that energy-restricted diet interventions combined with exercise effectively improved metabolic profiles for individuals with MHO [30].
7.2. Pharmacological Approaches
- Thiazolidinediones: These PPARγ agonists promote subcutaneous adipocyte differentiation and reduce ectopic fat deposition. Clinical trials have shown that pioglitazone and rosiglitazone can induce an “MHO-like” phenotype, characterized by increased subcutaneous fat but reduced liver fat and improved insulin sensitivity [102,103].
- SGLT2 Inhibitors: Emerging evidence from both animal models and clinical studies suggests that sodium-glucose cotransporter-2 (SGLT2) inhibitors may promote “healthy adipose expansion” while reducing ectopic fat deposition and improving metabolic parameters [104,105,106]. In metabolic dysfunction-associated steatohepatitis models, SGLT2 inhibitors have been shown to attenuate hepatic steatosis, inflammation, and fibrosis despite minimal weight reduction or even adipose tissue expansion [105].
- GLP-1 Receptor Agonists and GIP/GLP-1 Dual Agonists: The pharmaceutical landscape for obesity treatment is experiencing an unprecedented transformation. These agents appear to induce favorable changes in body composition beyond simple weight reduction. Recent studies with tirzepatide, a GIP/GLP-1 receptor dual agonist, have demonstrated preferential reduction in visceral fat compared to subcutaneous fat, potentially promoting a more metabolically favorable fat distribution pattern [107]. A comprehensive 2024 review highlights tirzepatide achieving up to 22.5% weight loss, with emerging triple agonists like retatrutide showing even greater efficacy [108]. The dual GIP/GLP-1 agonist could reduce cardiovascular risk and prevent conversion to metabolically unhealthy phenotype while maintaining metabolic health during weight loss [109].
- Novel Adipokine-Based Therapies: Emerging approaches targeting adipose tissue function through adipokine supplementation or receptor modulation are under investigation. Recombinant adiponectin, adiponectin receptor agonists, and agents targeting the newly discovered adipokine family of C1q/TNF-related proteins (CTRPs) have shown promising metabolic effects in preclinical studies.
8. Clinical Implications and Risk Stratification
8.1. Risk Stratification Tools
- Simplified MHO Criteria: Zembic et al. proposed a simplified clinical definition of MHO (systolic blood pressure < 130 mmHg, waist–hip ratio < 0.95 for women or <1.03 for men, and absence of diabetes) that outperformed traditional metabolic syndrome criteria in predicting cardiovascular outcomes [14]. This definition has been validated in multiple cohorts including the UK Biobank, Flemengho, and Hortega studies.
- Metabolic-BMI: This composite measure incorporates both BMI and metabolic factors into a single risk score, potentially providing more nuanced risk assessment than either measure alone.
- Liver Fat Indices: Recent developments in 2024 include enhanced diagnostic accuracy through combination approaches. Non-invasive assessments, including vibration-controlled transient elastography (VCTE), magnetic resonance elastography (MRE), and serum biomarkers, have high accuracy to diagnose advanced fibrosis and cirrhosis [111]. The newly developed LiverPRO algorithm, which obtained European CE approval in 2024, reliably identifies clinically significant liver fibrosis and elevated liver stiffness, predicting the risk of liver-related events in primary care [112]. AI-powered models utilizing non-contrast MRI, including T1WI and T2FS, accurately stage liver fibrosis [113], and AI enhances diagnostic accuracy and efficiency, aiding clinicians in making more informed treatment decisions [114].
- Using multiparametric MRI to quantify liver fat content has been suggested as a more precise method for risk stratification when available [6]. This approach provides a direct measurement of a key determinant of metabolic health in obesity.
- Triglyceride–Glucose Index: This simple index combining fasting triglycerides and glucose levels has demonstrated utility for identifying insulin resistance and predicting transition from MHO to MUO [110].
- Novel Biomarkers: ITLN1 (Omentin-1), produced by specific mesothelial cell populations, has been identified as significantly higher in individuals with MHO compared to MUO [58]. This adipokine was exclusively expressed by mesothelial cells within visceral adipose tissue and was not present in subcutaneous adipose tissue. Plasma Omentin-1 levels were significantly higher in MHO compared to MUO, establishing it as a promising marker for visceral adipose tissue functionality. Additional biomarkers include circulating microRNAs (miR-122-5p, miR-151a-3p, miR-126-5p, and miR-21-5p) and point-of-care technologies integrating miR-34a-5p, YKL-40, and comprehensive metabolomic panels [25].
8.2. Clinical Monitoring Strategies
- Comprehensive metabolic panel including liver enzymes;
- Lipid profile including triglyceride/HDL ratio;
- Anthropometric measurements including waist-to-hip ratio;
- Blood pressure assessment;
- Screening for metabolic dysfunction-associated steatotic liver disease (ultrasonography or biomarkers);
- Assessment of inflammatory markers (e.g., hsCRP) when available [115].
8.3. Personalized Intervention Approaches
- MHO Maintenance: For metabolically healthy individuals with mild obesity and no other clinical indications for weight loss, interventions focused on maintaining metabolic health rather than aggressive weight reduction might be appropriate. Physical activity promotion, Mediterranean-style dietary patterns, and monitoring for transition to MUO could be emphasized.
- Targeting Specific Metabolic Parameters: For individuals with MHO with emerging metabolic abnormalities in specific domains (e.g., borderline elevated blood pressure or glucose), targeted interventions addressing these specific parameters might be prioritized over general weight loss.
- Aggressive Approach for High-Risk MHO: For individuals with MHO with significant risk factors for transition to MUO (e.g., elevated liver fat, family history of diabetes, and advancing age), more aggressive lifestyle and potentially pharmacological interventions may be warranted despite current metabolic health.
- A personalized treatment algorithm based on stratification of obesity phenotypes has been proposed [6]. While all individuals with obesity should receive basic lifestyle interventions, additional pharmacological treatments should be tailored to specific metabolic risk profiles, with particular attention to those showing early signs of transition from MHO to MUO.
9. Future Directions
9.1. Standardized Definition and Classification
- Non-invasive assessments of ectopic fat deposition (liver, pancreas, and heart);
- Markers of adipose tissue function and expandability;
- Novel biomarkers reflecting inflammatory status;
- Genetic and metabolomic profiles predicting long-term metabolic resilience.
9.2. Mechanistic Insights
- Systems biology approaches integrating genomic, transcriptomic, proteomic, and metabolomic data;
- Advanced adipose tissue phenotyping techniques examining depot-specific function;
- Investigation of gut microbiome contributions to metabolic health in obesity;
- Exploration of brain–adipose tissue communication pathways;
- Elucidation of sex-specific mechanisms explaining the higher prevalence of MHO in women.
9.3. Longitudinal Natural History Studies
- Predictors of maintained metabolic health versus transition to MUO;
- Critical time windows for intervention to prevent metabolic deterioration;
- Impact of life-stage transitions (puberty, pregnancy, and menopause) on MHO stability;
- Influence of aging on metabolic protection mechanisms.
9.4. Intervention Studies
- Randomized trials comparing different lifestyle intervention strategies in MHO;
- Evaluation of pharmacological approaches targeting specific mechanisms underlying MHO;
- Studies examining the impact of various weight loss approaches on long-term outcomes in MHO;
- Determination of optimal monitoring and intervention thresholds for preventing transition to MUO.
9.5. Novel Therapeutic Targets
- Approaches promoting adipose tissue expandability and healthy remodeling;
- Therapies targeting ectopic fat redistribution rather than total weight reduction;
- Interventions modulating adipose tissue immunometabolism;
- Treatments targeting specific adipokine signaling pathways identified in MHO.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| DOI | Digital object identifier |
| IRS | Insulin receptor substrate |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MHNW | Metabolically healthy normal weight |
| MHO | Metabolically healthy obesity |
| MUO | Metabolically unhealthy obesity |
| NAFLD | Non-alcoholic fatty liver disease |
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023. NCHS Data Brief. 2024, 508, 10-15620. [Google Scholar] [CrossRef]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D. Metabolically healthy but obese individuals. Lancet 2008, 372, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Schulze, M.B.; Stefan, N. Metabolically healthy obesity: From epidemiology and mechanisms to clinical implications. Nat. Rev. Endocrinol. 2024, 20, 633–646. [Google Scholar] [CrossRef]
- Eckel, N.; Li, Y.; Kuxhaus, O.; Stefan, N.; Hu, F.B.; Schulze, M.B. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 714–724. [Google Scholar] [CrossRef]
- Ojalehto Lindfors, E.; De Oliveira, T.L.; Reynolds, C.A.; Zhan, Y.; Dahl Aslan, A.K.; Jylhava, J.; Sjolander, A.; Karlsson, I.K. Genetic influences, lifestyle and psychosocial aspects in relation to metabolically healthy obesity and conversion to a metabolically unhealthy state. Diabetes Obes. Metab. 2025, 27, 207–214. [Google Scholar] [CrossRef]
- Tanriover, C.; Copur, S.; Gaipov, A.; Ozlusen, B.; Akcan, R.E.; Kuwabara, M.; Hornum, M.; Van Raalte, D.H.; Kanbay, M. Metabolically healthy obesity: Misleading phrase or healthy phenotype? Eur. J. Intern. Med. 2023, 111, 5–20. [Google Scholar] [CrossRef]
- Zhou, Z.; Macpherson, J.; Gray, S.R.; Gill, J.M.R.; Welsh, P.; Celis-Morales, C.; Sattar, N.; Pell, J.P.; Ho, F.K. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK Biobank participants. Diabetologia 2021, 64, 1963–1972. [Google Scholar] [CrossRef]
- Hinnouho, G.M.; Czernichow, S.; Dugravot, A.; Batty, G.D.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and risk of mortality: Does the definition of metabolic health matter? Diabetes Care 2013, 36, 2294–2300. [Google Scholar] [CrossRef]
- Lind, L.; Riserus, U.; Arnlov, J. Impact of the Definition of Metabolically Healthy Obesity on the Association with Incident Cardiovascular Disease. Metab. Syndr. Relat. Disord. 2020, 18, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Zembic, A.; Eckel, N.; Stefan, N.; Baudry, J.; Schulze, M.B. An Empirically Derived Definition of Metabolically Healthy Obesity Based on Risk of Cardiovascular and Total Mortality. JAMA Netw. Open 2021, 4, e218505. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’aGostino, R.B. Body Mass Index, Metabolic Syndrome, and Risk of Type 2 Diabetes or Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Karelis, A.; Brochu, M.; Rabasa-Lhoret, R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004, 30, 569–572. [Google Scholar] [CrossRef]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The Obese Without Cardiometabolic Risk Factor Clustering and the Normal Weight with Cardiometabolic Risk Factor Clustering Prevalence and Correlates of 2 Phenotypes Among the US Population (NHANES 1999–2004). Obstet. Gynecol. Surv. 2008, 63, 783–784. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; García, E.G.; Robles, L.; Riaño, D.; Ruiz-Gomez, D.G.; García-Ulloa, A.C.; Melgarejo, M.A.; Zamora, M.; Guillen-Pineda, L.E.; Mehta, R.; et al. High Adiponectin Concentrations Are Associated with the Metabolically Healthy Obese Phenotype. J. Clin. Endocrinol. Metab. 2008, 93, 4075–4079. [Google Scholar] [CrossRef]
- Lynch, L.A.; O’COnnell, J.M.; Kwasnik, A.K.; Cawood, T.J.; O’FArrelly, C.; O’SHea, D.B. Are Natural Killer Cells Protecting the Metabolically Healthy Obese Patient? Obesity 2009, 17, 601–605. [Google Scholar] [CrossRef]
- van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef]
- Wang, P.; Liu, M.; Zhuang, X.; Guo, Y.; Xiong, Z.; He, L.; Cai, X.; Chen, Z.; Peng, L.; Liao, X. Association of metabolically healthy obesity in young adulthood with myocardial structure and function. Int. J. Obes. 2023, 47, 399–405. [Google Scholar] [CrossRef]
- Koliaki, C.; Dalamaga, M.; Kakounis, K.; Liatis, S. Metabolically Healthy Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Navigating the Controversies in Disease Development and Progression. Curr. Obes. Rep. 2025, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Kantartzis, K.; Machann, J.; Schick, F.; Thamer, C.; Rittig, K.; Balletshofer, B.; Machicao, F.; Fritsche, A.; Haring, H.U. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008, 168, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Yoshino, J.; Yoshino, M.; Magkos, F.; Tiemann Luecking, C.; Samovski, D.; Fraterrigo, G.; Okunade, A.L.; Patterson, B.W.; Klein, S. Metabolically normal obese people are protected from adverse effects following weight gain. J. Clin. Investig. 2015, 125, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Tobaruela-Resola, A.L.; Milagro, F.I.; Elorz, M.; Benito-Boillos, A.; Herrero, J.I.; Mogna-Pelaez, P.; Tur, J.A.; Martinez, J.A.; Abete, I.; Zulet, M.A. Circulating miR-122-5p, miR-151a-3p, miR-126-5p and miR-21-5p as potential predictive biomarkers for Metabolic Dysfunction-Associated Steatotic Liver Disease assessment. J. Physiol. Biochem. 2024, 1–14. [Google Scholar] [CrossRef]
- Verschuren, L.; Mak, A.L.; van Koppen, A.; Ozsezen, S.; Difrancesco, S.; Caspers, M.P.M.; Snabel, J.; van der Meer, D.; van Dijk, A.M.; Rashu, E.B.; et al. Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD. Nat. Commun. 2024, 15, 4564. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Tanaka, M.; Obora, A.; Kojima, T.; Fukui, M. Metabolically healthy obesity without fatty liver and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Res. Clin. Pract. 2018, 12, 4–15. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Magnanensi, J.; Hajji, Y.; Caron, A.; Majd, Z.; Rosenquist, C.; Hum, D.W.; Staels, B.; Connelly, M.A.; Loomba, R.; et al. Impact of age on NIS2+ and other non-invasive blood tests for the evaluation of liver disease and detection of at-risk MASH. JHEP Rep. 2024, 6, 101011. [Google Scholar] [CrossRef]
- Bluher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zheng, R.; Zheng, Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: A systematic review and meta-analysis: A PRISMA-compliant article. Medicine 2017, 96, e8838. [Google Scholar] [CrossRef]
- Chen, F.; Sarver, D.C.; Saqib, M.; Velez, L.M.; Aja, S.; Seldin, M.M.; Wong, G.W. Loss of CTRP10 results in female obesity with preserved metabolic health. Elife 2025, 13, RP93373. [Google Scholar] [CrossRef]
- Arner, P.; Viguerie, N.; Massier, L.; Ryden, M.; Astrup, A.; Blaak, E.; Langin, D.; Andersson, D.P. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int. J. Obes. 2024, 48, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; Kumar, B.; Al-Daghri, N.M.; Sabico, S. Trends and characteristics of the metabolically healthy obese phenotype in an Arab population. Front. Public Health 2024, 12, 1371359. [Google Scholar] [CrossRef] [PubMed]
- Geetha, L.; Deepa, M.; Anjana, R.M.; Mohan, V. Prevalence and clinical profile of metabolic obesity and phenotypic obesity in Asian Indians. J. Diabetes Sci. Technol. 2011, 5, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; Emam, O.; Kumar, B.; Al-Daghri, N.M.; Sabico, S. Exploring inflammation and adipose tissue dysfunction in metabolically healthy versus unhealthy obesity among Arab adults. Diabetol. Metab. Syndr. 2025, 17, 244. [Google Scholar] [CrossRef]
- Bell, J.A.; Kivimaki, M.; Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2014, 15, 504–515. [Google Scholar] [CrossRef]
- Tajik, S.; Mirzababaei, A.; Ghaedi, E.; Kord-Varkaneh, H.; Mirzaei, K. Risk of type 2 diabetes in metabolically healthy people in different categories of body mass index: An updated network meta-analysis of prospective cohort studies. J. Cardiovasc. Thorac. Res. 2019, 11, 254–263. [Google Scholar] [CrossRef]
- Petersen, M.C.; Smith, G.I.; Palacios, H.H.; Farabi, S.S.; Yoshino, M.; Yoshino, J.; Cho, K.; Davila-Roman, V.G.; Shankaran, M.; Barve, R.A.; et al. Cardiometabolic characteristics of people with metabolically healthy and unhealthy obesity. Cell Metab. 2024, 36, 745–761.e5. [Google Scholar] [CrossRef]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef]
- van der A, D.L.; Nooyens, A.C.; van Duijnhoven, F.J.; Verschuren, M.M.; Boer, J.M. All-cause mortality risk of metabolically healthy abdominal obese individuals: The EPIC-MORGEN study. Obesity 2014, 22, 557–564. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009, 32, 2297–2299. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, R.; Jiang, M.; Wang, W.; Chai, Y.; Liu, Q.; Zhang, W.; Han, Y.; Yan, F.; Lu, Q.; et al. Myocardial Tissue-Level Characteristics of Adults with Metabolically Healthy Obesity. JACC Cardiovasc. Imaging 2023, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ling, J.; Wu, Y.; Zhao, H.; Hu, Y.; Yan, Z.; Zhu, W.; Yu, P.; Wang, J.; Zhang, Y.; et al. Association between metabolically healthy obesity and atrial fibrillation: A systematic review and meta-analysis of longitudinal studies. Diabetes Metab. Syndr. 2025, 19, 103228. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Kaneko, H.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Morita, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Metabolically Healthy Obesity and the Risk of Cardiovascular Disease in the General Population—Analysis of a Nationwide Epidemiological Database. Circ. J. 2021, 85, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ryu, S.; Choi, Y.; Zhang, Y.; Cho, J.; Kwon, M.J.; Hyun, Y.Y.; Lee, K.B.; Kim, H.; Jung, H.S.; et al. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann. Intern. Med. 2016, 164, 305–312. [Google Scholar] [CrossRef]
- Khalili, S.; Safavi-Naini, S.A.A.; Zarand, P.; Masoumi, S.; Farsi, Y.; Hosseinpanah, F.; Azizi, F. Metabolic health’s central role in chronic kidney disease progression: A 20-year study of obesity-metabolic phenotype transitions. Sci. Rep. 2024, 14, 5244. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Siriopol, D.; Yildiz, A.B.; Berkkan, M.; Tuttle, K.R.; Zoccali, C. The risk for chronic kidney disease in metabolically healthy obese patients: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13878. [Google Scholar] [CrossRef]
- Sun, M.; Fritz, J.; Haggstrom, C.; Bjorge, T.; Nagel, G.; Manjer, J.; Engeland, A.; Zitt, E.; van Guelpen, B.; Stattin, P.; et al. Metabolically (un)healthy obesity and risk of obesity-related cancers: A pooled study. J. Natl. Cancer Inst. 2023, 115, 456–467. [Google Scholar] [CrossRef]
- Lin, C.J.; Chang, Y.C.; Cheng, T.Y.; Lo, K.; Liu, S.J.; Yeh, T.L. The association between metabolically healthy obesity and risk of cancer: A systematic review and meta-analysis of prospective cohort studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e13049. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Kim, L.J.; Nalls, M.A.; Eiriksdottir, G.; Sigurdsson, S.; Launer, L.J.; Koster, A.; Chaves, P.H.; Jonsdottir, B.; Garcia, M.; Gudnason, V.; et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: The AGES-Reykjavik study. Obesity 2011, 19, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schon, M.R.; Kern, M.; Stumvoll, M.; Bluher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Brochu, M.; Tchernof, A.; Dionne, I.J.; Sites, C.K.; Eltabbakh, G.H.; Sims, E.A.; Poehlman, E.T. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J. Clin. Endocrinol. Metab. 2001, 86, 1020–1025. [Google Scholar] [PubMed]
- Agrawal, S.; Wang, M.; Klarqvist, M.D.R.; Smith, K.; Shin, J.; Dashti, H.; Diamant, N.; Choi, S.H.; Jurgens, S.J.; Ellinor, P.T.; et al. Inherited basis of visceral, abdominal subcutaneous and gluteofemoral fat depots. Nat. Commun. 2022, 13, 3771. [Google Scholar] [CrossRef]
- Lotta, L.A.; Wittemans, L.B.L.; Zuber, V.; Stewart, I.D.; Sharp, S.J.; Luan, J.; Day, F.R.; Li, C.; Bowker, N.; Cai, L.; et al. Association of Genetic Variants Related to Gluteofemoral vs Abdominal Fat Distribution with Type 2 Diabetes, Coronary Disease, and Cardiovascular Risk Factors. JAMA 2018, 320, 2553–2563. [Google Scholar] [CrossRef]
- Hansen, G.T.; Sobreira, D.R.; Weber, Z.T.; Thornburg, A.G.; Aneas, I.; Zhang, L.; Sakabe, N.J.; Joslin, A.C.; Haddad, G.A.; Strobel, S.M.; et al. Genetics of sexually dimorphic adipose distribution in humans. Nat. Genet. 2023, 55, 461–470. [Google Scholar] [CrossRef]
- Jo, J.; Ha, N.; Ji, Y.; Do, A.; Seo, J.H.; Oh, B.; Choi, S.; Choe, E.K.; Lee, W.; Son, J.W.; et al. Genetic determinants of obesity in Korean populations: Exploring genome-wide associations and polygenic risk scores. Brief. Bioinform. 2024, 25, bbae389. [Google Scholar] [CrossRef]
- Reinisch, I.; Ghosh, A.; Noe, F.; Sun, W.; Dong, H.; Leary, P.; Dietrich, A.; Hoffmann, A.; Bluher, M.; Wolfrum, C. Unveiling adipose populations linked to metabolic health in obesity. Cell Metab. 2025, 37, 640–655.e4. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.; Huang, S.; Sun, X.; Ye, Y.; Sun, H.; Chu, X.; Shan, X.; Yuan, Y.; Shen, L.; et al. Single-cell analysis reveals a subpopulation of adipose progenitor cells that impairs glucose homeostasis. Nat. Commun. 2024, 15, 4827. [Google Scholar] [CrossRef]
- Ferrero, R.; Rainer, P.Y.; Rumpler, M.; Russeil, J.; Zachara, M.; Pezoldt, J.; van Mierlo, G.; Gardeux, V.; Saelens, W.; Alpern, D.; et al. A human omentum-specific mesothelial-like stromal population inhibits adipogenesis through IGFBP2 secretion. Cell Metab. 2024, 36, 1566–1585.e9. [Google Scholar] [CrossRef]
- Esser, N.; L’Homme, L.; De Roover, A.; Kohnen, L.; Scheen, A.J.; Moutschen, M.; Piette, J.; Legrand-Poels, S.; Paquot, N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013, 56, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Cella, M.; McCartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374.e3. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.B.; Ferraro, A.A.; Sananez, I.; Gancedo, M.C.; Baz, P.; Billordo, L.A.; Fainboim, L.; Arruvito, L. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J. Immunol. 2016, 196, 3287–3296. [Google Scholar] [CrossRef]
- McLaughlin, T.; Liu, L.F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef]
- Meister, B.M.; Hong, S.G.; Shin, J.; Rath, M.; Sayoc, J.; Park, J.Y. Healthy versus Unhealthy Adipose Tissue Expansion: The Role of Exercise. J. Obes. Metab. Syndr. 2022, 31, 37–50. [Google Scholar] [CrossRef]
- Allerton, T.D.; Savoie, J.J.; Fitch, M.D.; Hellerstein, M.K.; Stephens, J.M.; White, U. Exercise reduced the formation of new adipocytes in the adipose tissue of mice in vivo. PLoS ONE 2021, 16, e0244804. [Google Scholar] [CrossRef]
- Lentejas, J.P.R.; Sandoval, M.A.S.; Ples Evangelista, T.J.; Buenaluz-Sedurante, M.D.; Velayo, C.L. The Effect of Resistance, Aerobic, and Concurrent Aerobic and Resistance Exercises on Inflammatory Markers of Metabolically Healthy Overweight or Obese Adults: A Systematic Review and Meta-analysis. Acta Med. Philipp. 2024, 58, 90–105. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Hayashi, R.; Okuno, Y.; Mukai, K.; Kitamura, T.; Hayakawa, T.; Onodera, T.; Murata, M.; Fukuhara, A.; Imamura, R.; Miyagawa, Y.; et al. Adipocyte GR Inhibits Healthy Adipose Expansion Through Multiple Mechanisms in Cushing Syndrome. Endocrinology 2019, 160, 504–521. [Google Scholar] [CrossRef]
- Morley, T.S.; Xia, J.Y.; Scherer, P.E. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat. Commun. 2015, 6, 7906. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Gnudi, L.; Tozzo, E.; Yang, H.; Leach, F.; Kahn, B.B. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 1993, 268, 22243–22246. [Google Scholar] [CrossRef]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; Lin, Y.; Askew, G.R.; Simcox, J.A.; McClain, D.A.; Li, C.; et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Senol-Cosar, O.; Flach, R.J.; DiStefano, M.; Chawla, A.; Nicoloro, S.; Straubhaar, J.; Hardy, O.T.; Noh, H.L.; Kim, J.K.; Wabitsch, M.; et al. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat. Commun. 2016, 7, 10686. [Google Scholar] [CrossRef] [PubMed]
- Berezina, A.; Belyaeva, O.; Berkovich, O.; Baranova, E.; Karonova, T.; Bazhenova, E.; Brovin, D.; Grineva, E.; Shlyakhto, E. Prevalence, Risk Factors, and Genetic Traits in Metabolically Healthy and Unhealthy Obese Individuals. Biomed. Res. Int. 2015, 2015, 548734. [Google Scholar] [CrossRef]
- Piko, P.; Llanaj, E.; Nagy, K.; Adany, R. Genetic Background of Metabolically Healthy and Unhealthy Obesity Phenotypes in Hungarian Adult Sample Population. Int. J. Mol. Sci. 2023, 24, 5209. [Google Scholar] [CrossRef]
- Han, F.; Zhu, S.; Kong, X.; Wang, W.; Wu, Y. Integrated genetic and epigenetic analyses uncovered GLP1R association with metabolically healthy obesity. Int. J. Obes. 2024, 48, 324–329. [Google Scholar] [CrossRef]
- Small, K.S.; Todorcevic, M.; Civelek, M.; El-Sayed Moustafa, J.S.; Wang, X.; Simon, M.M.; Fernandez-Tajes, J.; Mahajan, A.; Horikoshi, M.; Hugill, A.; et al. Regulatory variants at KLF14 influence type 2 diabetes risk via a female-specific effect on adipocyte size and body composition. Nat. Genet. 2018, 50, 572–580. [Google Scholar] [CrossRef]
- Li, L.; Yin, J.; Cheng, H.; Wang, Y.; Gao, S.; Li, M.; Grant, S.F.; Li, C.; Mi, J.; Li, M. Identification of Genetic and Environmental Factors Predicting Metabolically Healthy Obesity in Children: Data From the BCAMS Study. J. Clin. Endocrinol. Metab. 2016, 101, 1816–1825. [Google Scholar] [CrossRef]
- Sedaghati-Khayat, B.; Barzin, M.; Akbarzadeh, M.; Guity, K.; Fallah, M.S.; Pourhassan, H.; Azizi, F.; Daneshpour, M.S. Lack of association between FTO gene variations and metabolic healthy obese (MHO) phenotype: Tehran Cardio-metabolic Genetic Study (TCGS). Eat. Weight Disord. 2020, 25, 25–35. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Yang, H.; Pan, H.; Gong, F.; Zhu, H. MC4R Single Nucleotide Polymorphisms Were Associated with Metabolically Healthy and Unhealthy Obesity in Chinese Northern Han Populations. Int. J. Endocrinol. 2019, 2019, 4328909. [Google Scholar] [CrossRef]
- Park, Y.J.; Moon, S.; Choi, J.; Kim, J.; Kim, H.J.; Son, H.Y.; Im, S.W.; Kim, J.I. Genome-wide association study for metabolic syndrome reveals APOA5 single nucleotide polymorphisms with multilayered effects in Koreans. Lipids Health Dis. 2024, 23, 272. [Google Scholar] [CrossRef]
- Gutierrez-Repiso, C.; Linares-Pineda, T.M.; Gonzalez-Jimenez, A.; Aguilar-Lineros, F.; Valdes, S.; Soriguer, F.; Rojo-Martinez, G.; Tinahones, F.J.; Morcillo, S. Epigenetic Biomarkers of Transition from Metabolically Healthy Obesity to Metabolically Unhealthy Obesity Phenotype: A Prospective Study. Int. J. Mol. Sci. 2021, 22, 10417. [Google Scholar] [CrossRef] [PubMed]
- Linares-Pineda, T.M.; Boughanem, H.; Gutierrez-Repiso, C.; Macias-Gonzalez, M.; Andres-Leon, E.; Rojo-Martinez, G.; Valdes, S.; Tinahones, F.J.; Morcillo, S. Epigenetic changes in the metabolically healthy obese: A case-control versus a prospective study. Eur. J. Clin. Investig. 2022, 52, e13783. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Shim, I.; Fahed, A.C.; Do, R.; Park, W.Y.; Natarajan, P.; Khera, A.V.; Won, H.H. Association of genetic risk, lifestyle, and their interaction with obesity and obesity-related morbidities. Cell Metab. 2024, 36, 1494–1503.e3. [Google Scholar] [CrossRef] [PubMed]
- Hinte, L.C.; Castellano-Castillo, D.; Ghosh, A.; Melrose, K.; Gasser, E.; Noe, F.; Massier, L.; Dong, H.; Sun, W.; Hoffmann, A.; et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 2024, 636, 457–465. [Google Scholar] [CrossRef]
- Lallukka, S.; Sadevirta, S.; Kallio, M.T.; Luukkonen, P.K.; Zhou, Y.; Hakkarainen, A.; Lundbom, N.; Orho-Melander, M.; Yki-Jarvinen, H. Predictors of Liver Fat and Stiffness in Non-Alcoholic Fatty Liver Disease (NAFLD)—An 11-Year Prospective Study. Sci. Rep. 2017, 7, 14561. [Google Scholar] [CrossRef]
- Flamini, F.; Spagnolo, N.; Viggianiello, N.; Crespi, A.; Osellame, R.; Sciarrino, F. Benchmarking integrated linear-optical architectures for quantum information processing. Sci. Rep. 2017, 7, 15133. [Google Scholar] [CrossRef]
- Beyoglu, D.; Popov, Y.V.; Idle, J.R. Metabolomic Hallmarks of Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2024, 25, 12809. [Google Scholar] [CrossRef]
- Perreault, M.; Zulyniak, M.A.; Badoud, F.; Stephenson, S.; Badawi, A.; Buchholz, A.; Mutch, D.M. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS ONE 2014, 9, e88539. [Google Scholar] [CrossRef]
- Jurado-Fasoli, L.; De-la, O.A.; Castillo, M.J.; Amaro-Gahete, F.J. Dietary differences between metabolically healthy overweight-obese and metabolically unhealthy overweight-obese adults. Br. J. Nutr. 2019, 122, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Dillon, C.; Harrington, J.M.; McCarthy, V.J.; Kearney, P.M.; Fitzgerald, A.P.; Perry, I.J. Defining metabolically healthy obesity: Role of dietary and lifestyle factors. PLoS ONE 2013, 8, e76188. [Google Scholar] [CrossRef] [PubMed]
- Tol, M.J.; Shimanaka, Y.; Bedard, A.H.; Sapia, J.; Cui, L.; Colaco-Gaspar, M.; Hofer, P.; Ferrari, A.; Qian, K.; Kennelly, J.P.; et al. Dietary control of peripheral adipose storage capacity through membrane lipid remodelling. Nat. Metab. 2025, 7, 1424–1442. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Cadenas-Sanchez, C.; Migueles, J.H.; Labayen, I.; Ruiz, J.R.; Sui, X.; Blair, S.N.; Martinez-Vizcaino, V.; Lavie, C.J. Role of Physical Activity and Fitness in the Characterization and Prognosis of the Metabolically Healthy Obesity Phenotype: A Systematic Review and Meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 190–205. [Google Scholar] [CrossRef]
- Pelekanou, C.; Anastasiou, C.A.; Mavrogianni, C.; Cardon, G.; Liatis, S.; Lindstrom, J.; Moreno, L.A.; Hilal, S.; Rurik, I.; Wikstrom, K.; et al. Physical activity in relation to metabolic health and obesity: The Feel4Diabetes study. Diabetes Obes. Metab. 2024, 26, 3705–3714. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.M.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, X.; Hu, D.; Li, G.; Song, G. Transition patterns of metabolism-weight phenotypes over time: A longitudinal study using the multistate Markov model in China. Front. Public. Health 2022, 10, 1026751. [Google Scholar] [CrossRef]
- Abiri, B.; Koohi, F.; Ebadinejad, A.; Valizadeh, M.; Hosseinpanah, F. Transition from metabolically healthy to unhealthy overweight/obesity and risk of cardiovascular disease incidence: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2041–2051. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Martinez-Gomez, D.; Ortega, F.B.; Hamer, M.; Lopez-Garcia, E.; Struijk, E.; Sadarangani, K.P.; Lavie, C.J.; Rodriguez-Artalejo, F. Physical Activity and Risk of Metabolic Phenotypes of Obesity: A Prospective Taiwanese Cohort Study in More Than 200,000 Adults. Mayo Clin. Proc. 2019, 94, 2209–2219. [Google Scholar] [CrossRef]
- Kruger, H.S.; Ricci, C.; Pieters, M.; Botha-le Roux, S.; Moss, S.J.; Kruger, I.M.; van Zyl, T.; Schutte, A.E. Lifestyle factors associated with the transition from healthy to unhealthy adiposity among black South African adults over 10 years. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2023–2032. [Google Scholar] [CrossRef]
- Aithal, G.P.; Thomas, J.A.; Kaye, P.V.; Lawson, A.; Ryder, S.D.; Spendlove, I.; Austin, A.S.; Freeman, J.G.; Morgan, L.; Webber, J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008, 135, 1176–1184. [Google Scholar] [CrossRef]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef]
- Miyachi, Y.; Tsuchiya, K.; Shiba, K.; Mori, K.; Komiya, C.; Ogasawara, N.; Ogawa, Y. A reduced M1-like/M2-like ratio of macrophages in healthy adipose tissue expansion during SGLT2 inhibition. Sci. Rep. 2018, 8, 16113. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Tsuchiya, K.; Komiya, C.; Miyachi, Y.; Mori, K.; Shimazu, N.; Yamaguchi, S.; Ogasawara, N.; Katoh, M.; Itoh, M.; et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci. Rep. 2018, 8, 2362. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Linge, J.; Neeland, I.J.; Dahlqvist Leinhard, O.; Petersson, M.; Fernandez Lando, L.; Bray, R.; Rodriguez, A. Effect of tirzepatide on body fat distribution pattern in people with type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T. Future Medications for Obesity and Clinical Implications. Diabetes Spectr. 2024, 37, 325–334. [Google Scholar] [CrossRef]
- Copur, S.; Tanriover, C.; Yavuz, F.; Tuttle, K.R.; Kanbay, M. Tirzepatide and potential use for metabolically healthy obesity. Eur. J. Intern. Med. 2023, 113, 1–5. [Google Scholar] [CrossRef]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Leiva Sisnieguez, C.E.; Balbin, E.; Dulbecco, C.A.; Aizpurua, M.; Marillet, A.G.; Reaven, G.M. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am. J. Cardiol. 2012, 109, 1749–1753. [Google Scholar] [CrossRef]
- Lai, J.C.; Liang, L.Y.; Wong, G.L. Noninvasive tests for liver fibrosis in 2024: Are there different scales for different diseases? Gastroenterol. Rep. 2024, 12, goae024. [Google Scholar] [CrossRef]
- Lindvig, K.P.; Thorhauge, K.H.; Hansen, J.K.; Kjaergaard, M.; Hansen, C.D.; Johansen, S.; Lyngbeck, E.; Israelsen, M.; Andersen, P.; Bech, K.T.; et al. Development, validation, and prognostic evaluation of LiverPRO for the prediction of significant liver fibrosis in primary care: A prospective cohort study. Lancet Gastroenterol. Hepatol. 2025, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Bai, R.; Zhao, Z.; Li, W.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, Q.; Su, N.; et al. Development of fully automated models for staging liver fibrosis using non-contrast MRI and artificial intelligence: A retrospective multicenter study. EClinicalMedicine 2024, 77, 102881. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, H.; Du, J.; Zhu, Y.; Zhu, H.; Yue, H. Artificial intelligence in imaging for liver disease diagnosis. Front. Med. 2025, 12, 1591523. [Google Scholar] [CrossRef]
- Iglesias Molli, A.E.; Penas Steinhardt, A.; Lopez, A.P.; Gonzalez, C.D.; Vilarino, J.; Frechtel, G.D.; Cerrone, G.E. Metabolically healthy obese individuals present similar chronic inflammation level but less insulin-resistance than obese individuals with metabolic syndrome. PLoS ONE 2017, 12, e0190528. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Pincu, Y.; Yoel, U.; Haim, Y.; Makarenkov, N.; Maixner, N.; Shaco-Levy, R.; Bashan, N.; Dicker, D.; Rudich, A. Assessing Obesity-Related Adipose Tissue Disease (OrAD) to Improve Precision Medicine for Patients Living with Obesity. Front. Endocrinol. 2022, 13, 860799. [Google Scholar] [CrossRef]
- Sacoto, D.; Hurtado, M.D.; Acosta, A. Precision Medicine and Obesity. Handb. Exp. Pharmacol. 2022, 274, 467–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchiya, K.; Tsutsumi, T. Beyond the BMI Paradox: Unraveling the Cellular and Molecular Determinants of Metabolic Health in Obesity. Biomolecules 2025, 15, 1278. https://doi.org/10.3390/biom15091278
Tsuchiya K, Tsutsumi T. Beyond the BMI Paradox: Unraveling the Cellular and Molecular Determinants of Metabolic Health in Obesity. Biomolecules. 2025; 15(9):1278. https://doi.org/10.3390/biom15091278
Chicago/Turabian StyleTsuchiya, Kyoichiro, and Takahiro Tsutsumi. 2025. "Beyond the BMI Paradox: Unraveling the Cellular and Molecular Determinants of Metabolic Health in Obesity" Biomolecules 15, no. 9: 1278. https://doi.org/10.3390/biom15091278
APA StyleTsuchiya, K., & Tsutsumi, T. (2025). Beyond the BMI Paradox: Unraveling the Cellular and Molecular Determinants of Metabolic Health in Obesity. Biomolecules, 15(9), 1278. https://doi.org/10.3390/biom15091278






