Global Fibrosis Burden and a Transcriptional Biomarker-Based Strategy for Early Detection in Resource-Limited Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Global Burden Analysis Based on GBD 2021
2.1.1. Inequality Analysis
2.1.2. Frontier Analysis
2.2. VGLL3 Antigen Design, Expression, and Purification
2.3. Chicken Immunization, Avian Antibody Preparation, and Antibody Validation
2.4. Antibody Binding Analysis by ic-ELISA
2.5. Myocardial Infarction Model and Immunohistochemistry
2.6. VGLL3 Detection in Human Plasma Samples
3. Results
3.1. Temporal Trends in the Proportion of Global Fibrosis-Related DALYs
3.2. Regional Disparities and Temporal Trends in Fibrosis-Related Burden Attributable to Neoplasms and COPD
3.3. Inequality Analysis Reveals Persistent Socioeconomic and Regional Disparities in Fibrosis-Related Burden
3.4. Development and Validation of a VGLL3-Targeted Avian Antibody for Fibrosis Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBD | global burden of disease |
| DALYs | disability-adjusted life years |
| ASR | age-standardized rate |
| SDI | socio-demographic index |
| VGLL3 | vestigial-like family member 3 |
| COPD | chronic obstructive pulmonary disease |
| ic-ELISA | indirect competitive enzyme-linked immunosorbent assay |
| ECM | extracellular matrix |
| IDRs | intrinsically disordered regions |
References
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Nagy, L.E.; Maher, T.M.; Distler, J.H.; Kramann, R.; Hinz, B.; Prunotto, M. Fibrosis: Cross-organ biology and pathways to development of innovative drugs. Nat. Rev. Drug Discov. 2025, 24, 543–569. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C.J. Global, regional, national, and subnational big data to inform health equity research: Perspectives from the Global Burden of Disease Study 2017. Ethn. Dis. 2019, 29 (Suppl. S1), 159. [Google Scholar] [CrossRef]
- Zimmermann, E.; Mukherjee, S.S.; Falahkheirkhah, K.; Gryka, M.C.; Kajdacsy-Balla, A.; Hasan, W.; Giraud, G.; Tibayan, F.; Raman, J.; Bhargava, R. Detection and quantification of myocardial fibrosis using stain-free infrared spectroscopic imaging. Arch. Pathol. Lab. Med. 2021, 145, 1526–1535. [Google Scholar] [CrossRef]
- Horii, Y.; Matsuda, S.; Toyota, C.; Morinaga, T.; Nakaya, T.; Tsuchiya, S.; Ohmuraya, M.; Hironaka, T.; Yoshiki, R.; Kasai, K. VGLL3 is a mechanosensitive protein that promotes cardiac fibrosis through liquid-liquid phase separation. Nat. Commun. 2023, 14, 550. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Lin, N.; Liu, Y.; Chen, H. Highly expressed VGLL3 in keloid fibroblasts promotes glycolysis and collagen production via the activation of Wnt/β-catenin signaling. Cell. Signal. 2025, 127, 111604. [Google Scholar] [CrossRef]
- Takakura, Y.; Hori, N.; Terada, N.; Machida, M.; Yamaguchi, N.; Takano, H.; Yamaguchi, N. VGLL3 activates inflammatory responses by inducing interleukin-1α secretion. FASEB J. 2021, 35, e21996. [Google Scholar] [CrossRef]
- Eriksson, M.; Larsson, A. Avian Antibodies as Potential Therapeutic Tools. Antibodies 2025, 14, 18. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: A review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Polson, A.; von Wechmar, M.B.; Van Regenmortel, M. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 1980, 9, 475–493. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Yang, R.-S.; Jiang, J.-Q.; Chang, X.-Y.; Chen, J.-J.; Qi, Y.-H.; Wu, S.-X.; Yang, X.-F. Development of a class-specific polyclonal antibody-based indirect competitive ELISA for detecting fluoroquinolone residues in milk. J. Zhejiang Univ. Sci. B 2012, 13, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.Y.; Harmouche, R.; Ross, J.C.; Diaz, A.A.; Rahaghi, F.N.; Vegas Sanchez-Ferrero, G.; Putman, R.K.; Hunninghake, G.M.; Onieva Onieva, J.; Martinez, F.J. Interstitial features at chest CT enhance the deleterious effects of emphysema in the COPDGene cohort. Radiology 2018, 288, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Löffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; Bossuyt, P.M. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef]
- Lewindon, P.J.; Shepherd, R.W.; Walsh, M.J.; Greer, R.M.; Williamson, R.; Pereira, T.N.; Frawley, K.; Bell, S.C.; Smith, J.L.; Ramm, G.A. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology 2011, 53, 193–201. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Cavalli, F.; Matera, M.G.; Cazzola, M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 2016, 40, 95–103. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in hepatic stellate cell activation and liver fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Lancaster, L.H.; de Andrade, J.A.; Zibrak, J.D.; Padilla, M.L.; Albera, C.; Nathan, S.D.; Wijsenbeek, M.S.; Stauffer, J.L.; Kirchgaessler, K.-U.; Costabel, U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 146. [Google Scholar] [CrossRef]
- Corte, T.; Bonella, F.; Crestani, B.; Demedts, M.G.; Richeldi, L.; Coeck, C.; Pelling, K.; Quaresma, M.; Lasky, J.A. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 116. [Google Scholar] [CrossRef]
- Hori, N.; Okada, K.; Takakura, Y.; Takano, H.; Yamaguchi, N.; Yamaguchi, N. Vestigial-like family member 3 (VGLL3), a cofactor for TEAD transcription factors, promotes cancer cell proliferation by activating the Hippo pathway. J. Biol. Chem. 2020, 295, 8798–8807. [Google Scholar] [CrossRef]
- Figeac, N.; Mohamed, A.D.; Sun, C.; Schönfelder, M.; Matallanas, D.; Garcia-Munoz, A.; Missiaglia, E.; Collie-Duguid, E.; De Mello, V.; Pobbati, A.V. VGLL3 operates via TEAD1, TEAD3 and TEAD4 to influence myogenesis in skeletal muscle. J. Cell Sci. 2019, 132, jcs225946. [Google Scholar] [CrossRef]
- Wu, W.; Fan, Z.; Fu, H.; Ma, X.; Wang, D.; Liu, H.; Zhang, C.; Zheng, H.; Yang, Y.; Wu, H. VGLL3 modulates chemosensitivity through promoting DNA double-strand break repair. Sci. Adv. 2024, 10, eadr2643. [Google Scholar] [CrossRef]
- Guo-Qiu, W.; Nai-Feng, L.; Xiao-Bo, F.; Linxian, L.; Chen, Z.; Lixia, G.; Zhao, L. The level of connective tissue growth factor in sera of patients with hepatitis B virus strongly correlates with stage of hepatic fibrosis. Viral Immunol. 2010, 23, 71–78. [Google Scholar] [CrossRef]
- Veidal, S.S.; Karsdal, M.A.; Vassiliadis, E.; Nawrocki, A.; Larsen, M.R.; Nguyen, Q.H.T.; Hägglund, P.; Luo, Y.; Zheng, Q.; Vainer, B. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis–identification and validation of a novel biochemical marker assay. PLoS ONE 2011, 6, e24753. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Nezafat, N.; Eskandari, S. The potential of intrinsically disordered regions in vaccine development. Expert Rev. Vaccines 2022, 21, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tsoi, L.C.; Xing, X.; Beamer, M.A.; Swindell, W.R.; Sarkar, M.K.; Berthier, C.C.; Stuart, P.E.; Harms, P.W.; Nair, R.P. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat. Immunol. 2017, 18, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kennicott, K.; Liang, Y. The immunometabolic function of VGLL3 and female-biased autoimmunity. Immunometabolism 2024, 6, e00041. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Vassilakopoulou, V.; Livaniou, E. IgY technology: Methods for developing and evaluating avian immunoglobulins for the in vitro detection of biomolecules. World J. Methodol. 2021, 11, 243. [Google Scholar] [CrossRef]
- Thirumalai, D.; Ambi, S.V.; Vieira-Pires, R.S.; Xiaoying, Z.; Sekaran, S.; Krishnan, U. Chicken egg yolk antibody (IgY) as diagnostics and therapeutics in parasitic infections—A review. Int. J. Biol. Macromol. 2019, 136, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-Z.; Ma, J.; Dou, J.-Z. The role of TDP-43 in neurodegenerative disease. Mol. Neurobiol. 2022, 59, 4223–4241. [Google Scholar] [CrossRef]
- Korobeynikov, V.A.; Lyashchenko, A.K.; Blanco-Redondo, B.; Jafar-Nejad, P.; Shneider, N.A. Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in amyotrophic lateral sclerosis. Nat. Med. 2022, 28, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xu, Y.; Qin, X.; Tao, M.; Gu, X.; Shen, L.; Chen, Y.; Zheng, M.; Qin, S.; Wu, G. RUNX1, FUS, and ELAVL1-induced circPTPN22 promote gastric cancer cell proliferation, migration, and invasion through miR-6788-5p/PAK1 axis-mediated autophagy. Cell. Mol. Biol. Lett. 2024, 29, 95. [Google Scholar] [CrossRef] [PubMed]

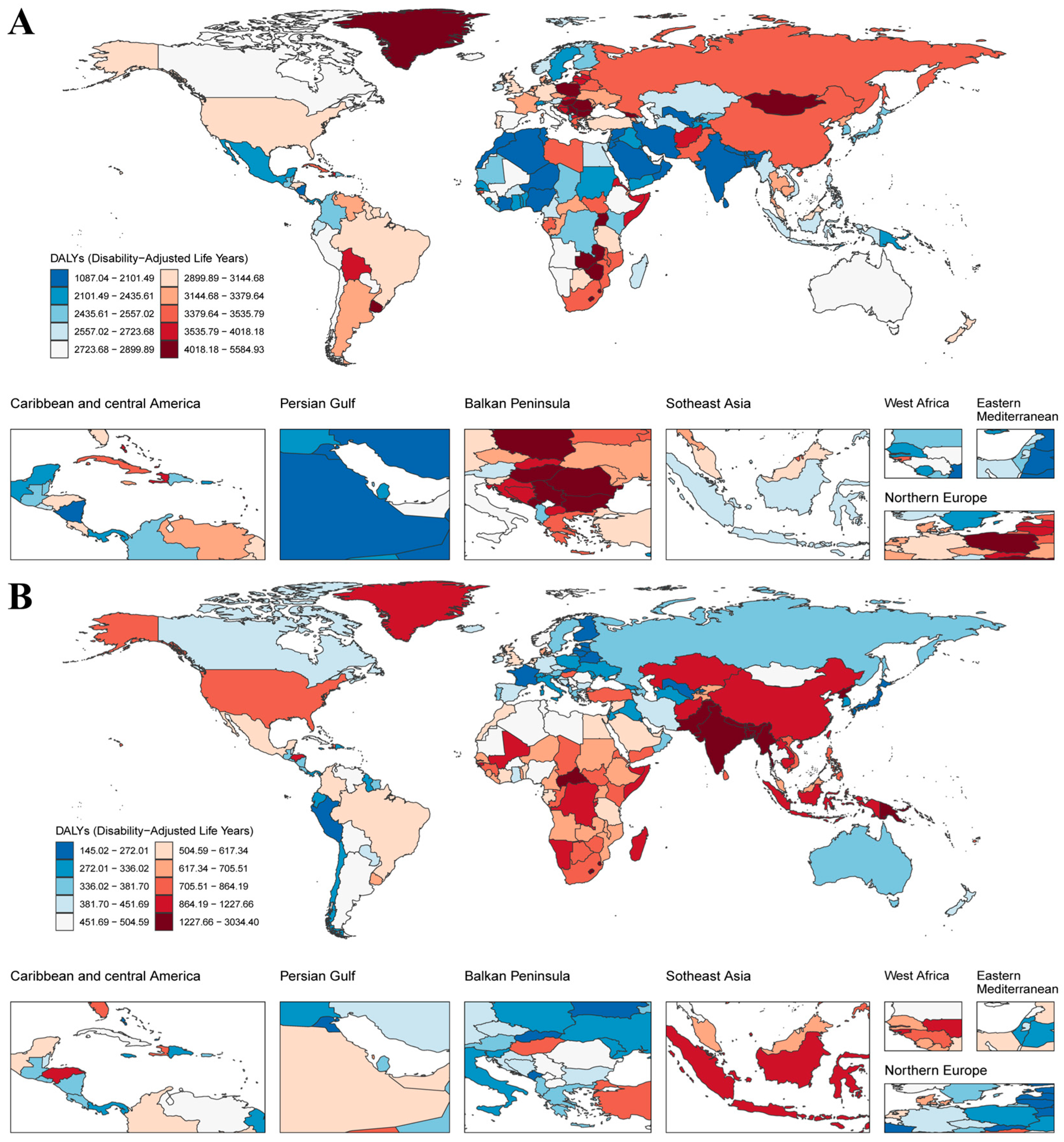


| 1990 | 2021 | |||
|---|---|---|---|---|
| Characteristics | ASDR per 100,000 (95% UI) | ASR of DALYs per 100,000 (95% UI) | ASDR per 100,000 (95% UI) | ASR of DALYs per 100,000 (95% UI) |
| neoplasms | ||||
| Global | 148.24 (140.18, 154.39) | 3969.21 (3792.05, 4135.06) | 116.49 (107.28, 124.69) | 2953.59 (2769.24, 3154.03) |
| High SDI | 170.96 (162.88, 175.00) | 4341.14 (4215.00, 4430.83) | 123.22 (113.06, 128.96) | 2920.60 (2751.48, 3031.34) |
| High-middle SDI | 173.65 (163.76, 182.46) | 4809.50 (4539.00, 5055.08) | 134.01 (121.72, 147.09) | 3388.27 (3078.37, 3728.81) |
| Middle SDI | 135.62 (125.17, 146.36) | 3778.75 (3482.10, 4077.32) | 109.59 (99.35, 121.22) | 2852.23 (2611.28, 3158.67) |
| Low-middle SDI | 82.45 (75.29, 88.04) | 2408.70 (2227.63, 2562.93) | 85.03 (79.17, 90.89) | 2376.66 (2202.92, 2542.90) |
| Low SDI | 98.74 (87.84, 110.68) | 2864.53 (2554.22, 3184.28) | 90.64 (79.86, 102.00) | 2487.39 (2164.52, 2827.72) |
| COPD | ||||
| Global | 71.92 (64.47, 77.53) | 1492.64 (1342.46, 1609.30) | 45.22 (40.61, 49.70) | 940.66 (871.48, 1014.59) |
| High SDI | 25.53 (23.71, 26.50) | 589.80 (557.84, 616.39) | 19.44 (17.26, 20.66) | 471.22 (437.45, 498.84) |
| High-middle SDI | 79.53 (70.61, 86.61) | 1511.32 (1365.74, 1635.67) | 35.91 (30.78, 40.69) | 691.14 (621.83, 772.74) |
| Middle SDI | 123.89 (109.19, 134.80) | 2332.91 (2063.49, 2546.31) | 57.45 (49.59, 65.43) | 1076.67 (963.62, 1201.24) |
| Low-middle SDI | 92.07 (74.17, 107.46) | 1963.19 (1602.24, 2252.75) | 84.76 (75.80, 93.78) | 1707.90 (1558.88, 1865.11) |
| Low SDI | 77.67 (61.91, 91.44) | 1673.81 (1373.37, 1936.99) | 70.70 (63.35, 79.76) | 1457.94 (1318.76, 1617.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Wu, L.; Zhang, C.; Dang, M. Global Fibrosis Burden and a Transcriptional Biomarker-Based Strategy for Early Detection in Resource-Limited Settings. Biomolecules 2025, 15, 1273. https://doi.org/10.3390/biom15091273
Deng Q, Wu L, Zhang C, Dang M. Global Fibrosis Burden and a Transcriptional Biomarker-Based Strategy for Early Detection in Resource-Limited Settings. Biomolecules. 2025; 15(9):1273. https://doi.org/10.3390/biom15091273
Chicago/Turabian StyleDeng, Qinqin, Longjiang Wu, Chenlu Zhang, and Mei Dang. 2025. "Global Fibrosis Burden and a Transcriptional Biomarker-Based Strategy for Early Detection in Resource-Limited Settings" Biomolecules 15, no. 9: 1273. https://doi.org/10.3390/biom15091273
APA StyleDeng, Q., Wu, L., Zhang, C., & Dang, M. (2025). Global Fibrosis Burden and a Transcriptional Biomarker-Based Strategy for Early Detection in Resource-Limited Settings. Biomolecules, 15(9), 1273. https://doi.org/10.3390/biom15091273





