Multiple Applications of Nanomaterials in the Diagnosis and Treatment of Hemorrhagic Stroke
Abstract
1. Introduction
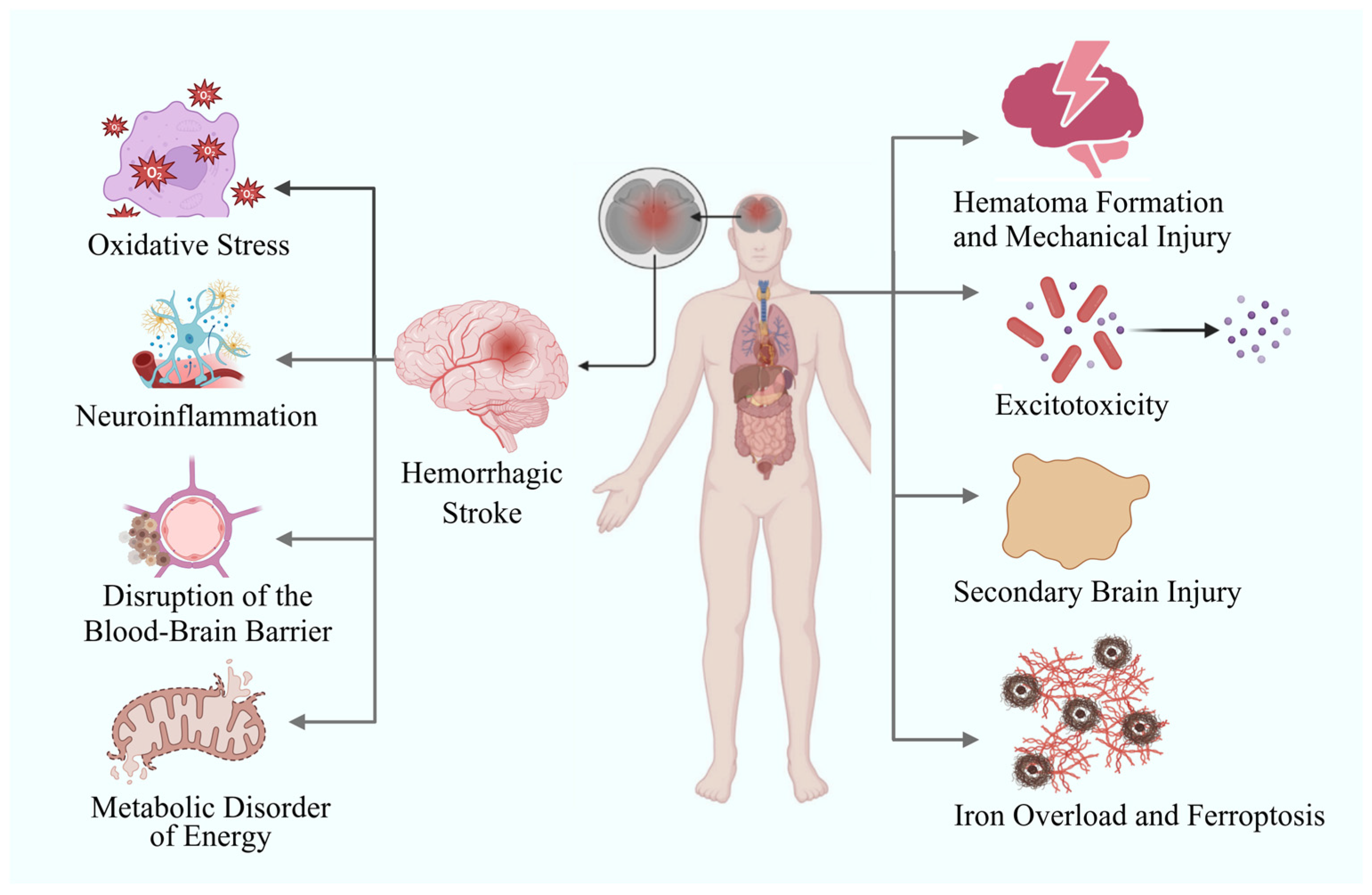
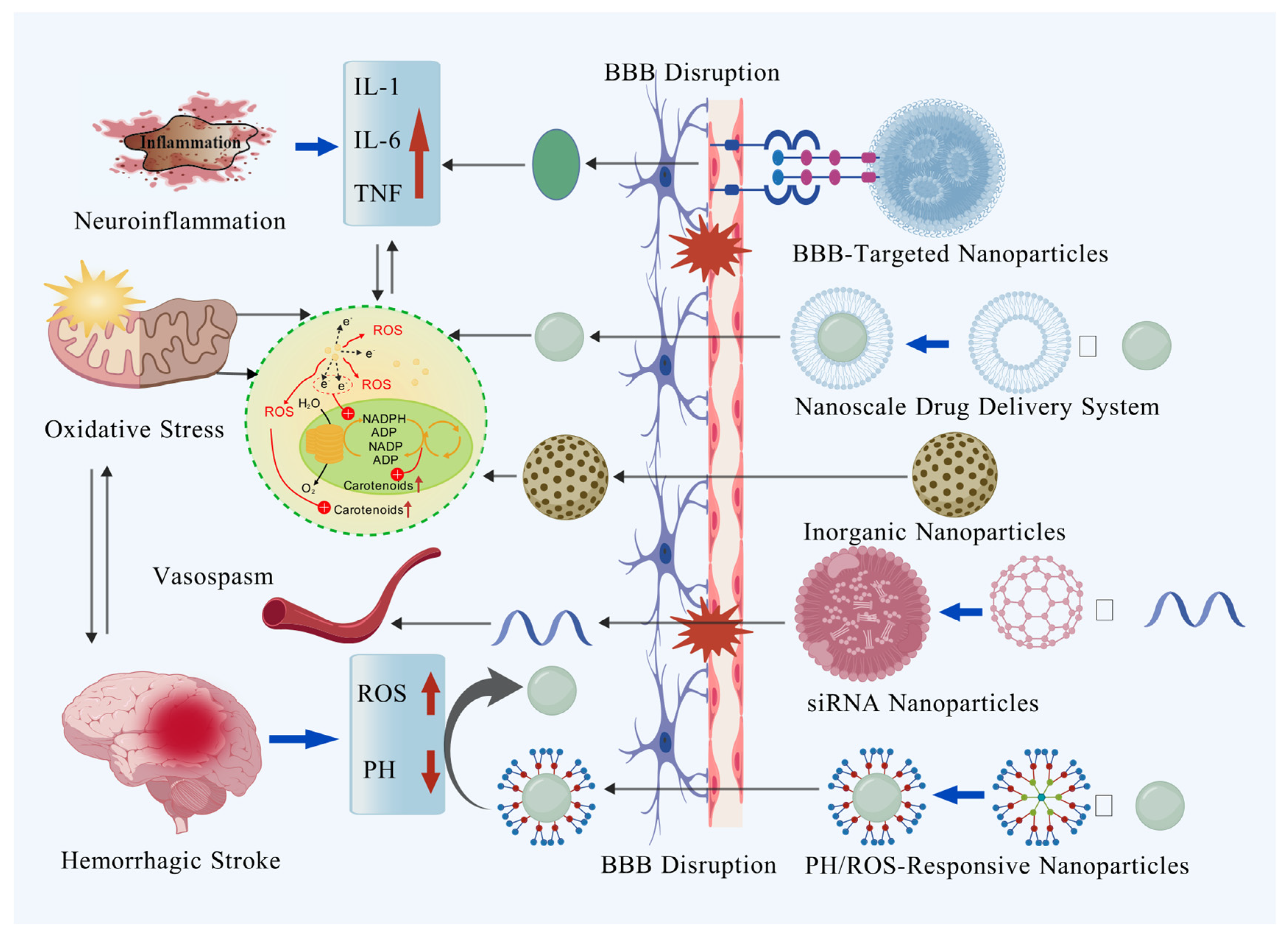
2. Role of Nanotechnology in Investigating Pathological Mechanisms and Precision Diagnosis of Hemorrhagic Stroke
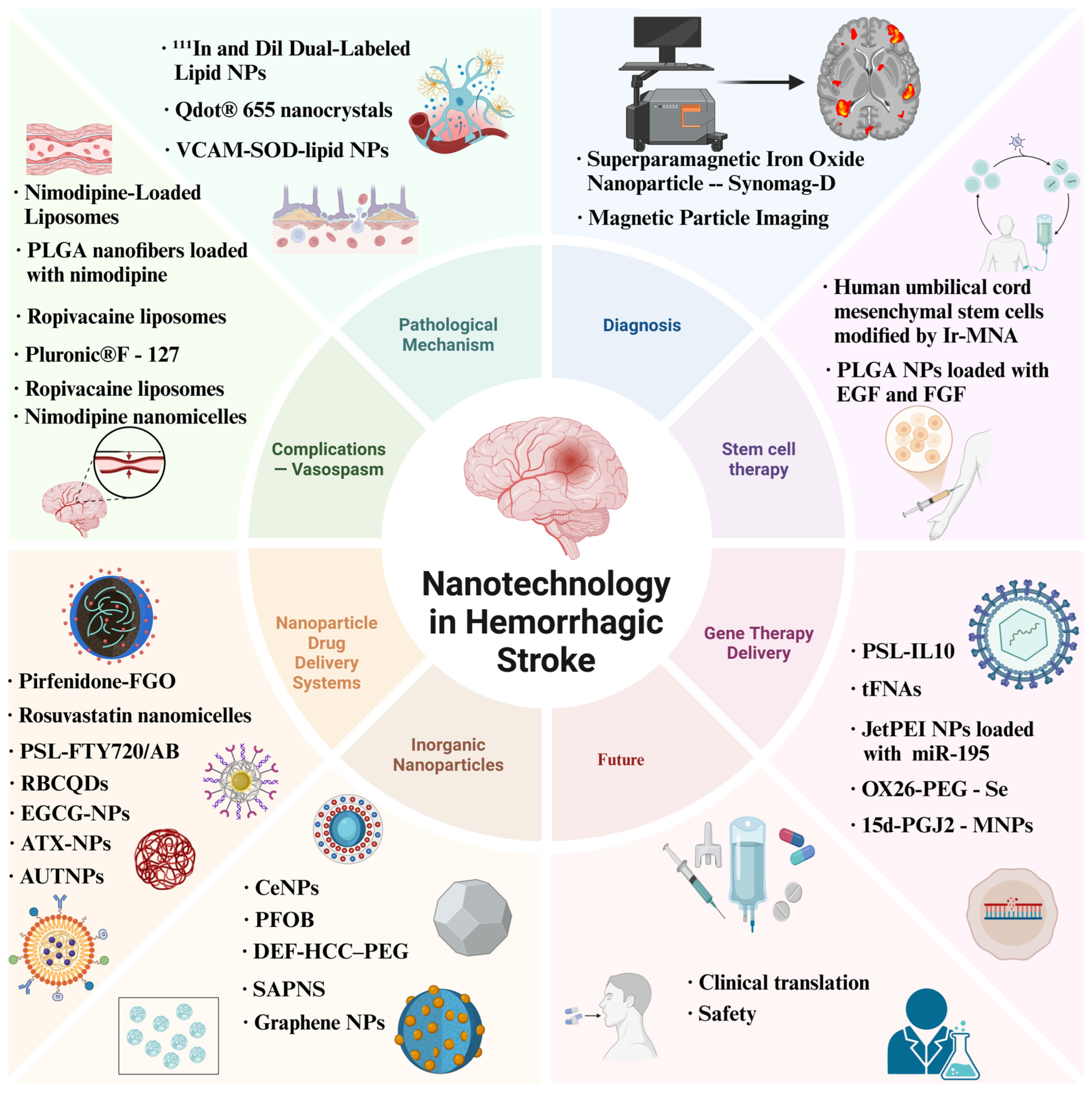
3. Nanotechnology Facilitating Hemorrhagic Stroke Treatment
3.1. Application of Nanodelivery Systems in the Treatment of Hemorrhagic Stroke Complications
3.2. Multiple Applications and Breakthroughs of the Nanometer Drug Delivery System in the Treatment of Hemorrhagic Stroke
3.3. The Intervention Efficacy of Inorganic Nanoparticles and Nanozymes in Hemorrhagic Stroke
3.4. Nanotechnology-Based Targeted Therapy for Molecular Pathway Gene Expression Post-ICH
3.5. Nanotechnology-Driven Development of Transplantation Stem Cell Therapy
3.6. The Current Limitations of Nanomaterials
4. Conclusions and Perspectives
4.1. Conclusions
4.2. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | Ammonia Borane |
| ATX | Astaxanthin |
| BBB | Blood–Brain Barrier |
| BMECs | Brain Microvascular Endothelial Cells |
| BMSC | Bone Marrow Mesenchymal Stem Cell |
| CGRP | Calcitonin Gene-Related Peptide |
| CeNPs | Cerium Nanoparticles |
| DEF | Deferoxamine |
| EGF | Epidermal Growth Factor |
| ET1 | Endothelin-1 |
| FGF | Fibroblast Growth Factor |
| FGO | Functionalized Graphene Oxide Nanosheets |
| FTY720 | Fingolimod |
| FUS | Focused Ultrasound |
| GS | Gelatin-Siloxane |
| hUC-MSCs | Human Umbilical Cord Mesenchymal Stem Cells |
| ICH | Intracerebral Hemorrhage |
| IL-10 | Interleukin-10 |
| Ir-MNAs | Iron Oxide Magnetic Nanoparticles |
| JAK2/STAT3 | Janus Kinase 2/Signal Transducer and Activator of Transcription 3 |
| JetPEI | Jet Polyethylenimine |
| Lf-NLC | Long-Circulating Nanostructured Lipid Carrier |
| LMCs | Lipid Bilayer-Coated Magnetic Mesoporous Silica Nanoparticles Doped with CeNPs and MRI Contrast-Enhancing Iron Oxide Particles |
| LNCs | Lipid Nanocapsules |
| MMP | Mitochondrial Membrane Potential |
| MNPs | Magnetic Nanoparticles |
| mPEG-PLGA | Methoxy Polyethylene Glycol-Poly(Lactic-Co-Glycolic Acid) |
| MRI | Magnetic Resonance Imaging |
| NSE | Neuron-Specific Enolase |
| OX26 | Anti-Transferrin Receptor Monoclonal Antibody OX26 |
| PDA | Polydopamine |
| PEI | Polyethylenimine |
| PEG-HCC | Poly-(Ethylene Glycol)-Conjugated Hydrophilic Carbon Clusters |
| PEG-PCL | Polyethylene Glycol-Poly(ε-Caprolactone) |
| PFC | Perfluorocarbon |
| PFOB | Perfluorooctylbromide |
| PLGA | Poly Lactic-Co-Glycolic Acid |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PS | Phosphatidylserine |
| PSL | PH-Sensitive Liposomes |
| PSL-FTY720/AB | PH-Sensitive Liposomes Encapsulating Fingolimod and Ammonia Borane |
| PSL-IL10 | Phosphatidylserine Liposome-Interleukin-10 |
| pLXSN-CGRP | Plasmid LXSN Carrying CGRP Gene |
| RbcQDs | Rb1 Carbon Quantum Dots |
| REP | RGD-Containing Elastin-Like Polypeptide |
| REP-NPs | REP Nanoparticles |
| RGD | Arg-Gly-Asp |
| Rsv@HFn | Rosuvastatin-Loaded Human H-Ferritin Nanoparticles |
| SAH | Subarachnoid Hemorrhage |
| SAPNS | Self-Assembling Peptide Nanofiber Scaffold |
| S100B | S100 Calcium-Binding Protein B |
| siRNA | Small Interfering RNA |
| SPECT/CT | Single-Photon Emission Computed Tomography/Computed Tomography |
| Tat | Tat Peptide |
| Tat-GS | Tat Peptide-Decorated Gelatin-Siloxane |
| tFNAs | Tetrahedral Framework Nucleic Acids |
| 15d-PGJ2 | 15-Deoxy-Δ12,14-Prostaglandin J2 |
References
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, T.; Li, R.; Sun, J.; Luan, X.; Wang, M. The role of NPY signaling pathway in diagnosis, prognosis and treatment of stroke. Neuropeptides 2024, 104, 102412. [Google Scholar] [CrossRef]
- Koyama, R.; Shichita, T. Glial roles in sterile inflammation after ischemic stroke. Neurosci. Res. 2023, 187, 67–71. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, T.; Ma, H.; Zhu, Y.; Wang, M. Targeting PI3K/Akt in Cerebral Ischemia Reperfusion Injury Alleviation: From Signaling Networks to Targeted Therapy. Mol. Neurobiol. 2024, 61, 7930–7949. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Nael, K.; Unal, G.; Iacoboni, M.; Liebeskind, D.S.; Bikson, M.; Saver, J.L.; TESSERACT Trial Group. High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2319231. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, X.; Qiao, S.; Huang, G.; Hermann, D.M.; Doeppner, T.R.; Zeng, M.; Liu, W.; Xu, G.; et al. A Co-Doped Fe3O4 Nanozyme Shows Enhanced Reactive Oxygen and Nitrogen Species Scavenging Activity and Ameliorates the Deleterious Effects of Ischemic Stroke. ACS Appl. Mater. Interfaces 2021, 13, 46213–46224. [Google Scholar] [CrossRef]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Ronaldson, P.T.; Davis, T.P. The role of oxidative stress in blood-brain barrier disruption during ischemic stroke: Antioxidants in clinical trials. Biochem. Pharmacol. 2024, 228, 116186. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, E.; Kim, C.H.; Song, H.T.; Lee, J.E. The role of orexin in post-stroke inflammation, cognitive decline, and depression. Mol. Brain 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Lu, Y.; Zhai, Y.; Bi, Y.; Peng, Y.; Ju, Z.; Xu, T.; Zhong, X.; Zhang, Y.; Zhong, C. Plasma neuropeptide Y and cognitive impairment after acute ischemic stroke. J. Affect. Disord. 2022, 317, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Ramos, M.; Soares, T.; Parmar, M.S. Poststroke Seizure and Epilepsy: A Review of Incidence, Risk Factors, Diagnosis, Pathophysiology, and Pharmacological Therapies. Oxidative Med. Cell. Longev. 2022, 2022, 7692215. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, W.; Qian, L.; Wang, C. Clinical practice guideline appraisal and algorithm development to identify recommendations related to nursing practice for post-stroke dysphagia. J. Clin. Nurs. 2023, 32, 6089–6100. [Google Scholar] [CrossRef]
- Miranda, J.J.; Moscoso, M.G.; Toyama, M.; Cavero, V.; Diez-Canseco, F.; Ovbiagele, B. Role of mHealth in overcoming the occurrence of post-stroke depression. Acta Neurol. Scand. 2018, 137, 12–19. [Google Scholar] [CrossRef]
- Puy, L.; Parry-Jones, A.R.; Sandset, E.C.; Dowlatshahi, D.; Ziai, W.; Cordonnier, C. Intracerebral haemorrhage. Nat. Rev. Dis. Primers 2023, 9, 14. [Google Scholar] [CrossRef]
- Garg, R.; Biller, J. Recent advances in spontaneous intracerebral hemorrhage. F1000Research 2019, 8, 302. [Google Scholar] [CrossRef]
- De Oliveira Manoel, A.L. Surgery for spontaneous intracerebral hemorrhage. Crit. Care 2020, 24, 45. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, T.; Huang, Z.; Ma, H.; Wang, M. Role of traditional Chinese medicine monomers in cerebral ischemia/reperfusion injury:a review of the mechanism. Front. Pharmacol. 2023, 14, 1220862. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Y.; Li, X.; Wang, X.; Yang, Z.; Li, W.; Cheng, W.; Yan, G. Triblock Copolymer Nanomicelles Loaded with Curcumin Attenuates Inflammation via Inhibiting the NF-κB Pathway in the Rat Model of Cerebral Ischemia. Int. J. Nanomed. 2021, 16, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, a Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, M.; Sadigh-Eteghad, S.; Farjami, A.; Salatin, S. Nanoparticle and Stem Cell Combination Therapy for the Management of Stroke. Curr. Pharm. Des. 2023, 29, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, L.; Yang, Q.; Ran, H.; Wang, J.; Hu, K.; Pu, W.; Huang, J.; Wen, L.; Zhou, L.; et al. Targeted Treatment of Ischemic Stroke by Bioactive Nanoparticle-Derived Reactive Oxygen Species Responsive and Inflammation-Resolving Nanotherapies. ACS Nano 2021, 15, 16076–16094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Hou, Y.; Tang, G.; Zhang, R.; Yang, Y.; Yan, X.; Fan, K. A Thrombin-Activated Peptide-Templated Nanozyme for Remedying Ischemic Stroke via Thrombolytic and Neuroprotective Actions. Adv. Mater. 2024, 36, e2210144. [Google Scholar] [CrossRef]
- Singh, S. Antioxidant nanozymes as next-generation therapeutics to free radical-mediated inflammatory diseases: A comprehensive review. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129374. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, W.; Zhou, L.; Wu, J.; Zhang, X.; Wei, X.; Wang, S.; Huang, Y.; Li, Y. Transferrin-Enabled Blood-Brain Barrier Crossing Manganese-Based Nanozyme for Rebalancing the Reactive Oxygen Species Level in Ischemic Stroke. Pharmaceutics 2022, 14, 1122. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.S.; Dickie, B.R.; Aldred, I.; Jasim, D.A.; Barrington, J.; Haley, M.; Lemarchand, E.; Coutts, G.; Kaur, S.; Bates, J.; et al. Selective brain entry of lipid nanoparticles in haemorrhagic stroke is linked to biphasic blood-brain barrier disruption. Theranostics 2022, 12, 4477–4497. [Google Scholar] [CrossRef]
- Reyes-Esteves, S.; Nong, J.; Glassman, P.M.; Omo-Lamai, S.; Ohashi, S.; Myerson, J.W.; Zamora, M.E.; Ma, X.; Kasner, S.E.; Sansing, L.; et al. Targeted drug delivery to the brain endothelium dominates over passivedelivery via vascular leak in experimental intracerebral hemorrhage. J. Control. Release 2023, 356, 185–195. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kajimura, M.; Morikawa, T.; Tsukada, K.; Tsuji, T.; Kusaka, G.; Tanaka, Y.; Suematsu, M. Cortical microcirculatory disturbance in the super acute phase of subarachnoid hemorrhage—In vivo analysis using two-photon laser scanning microscopy. J. Neurol. Sci. 2016, 368, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T.; Recombinant Activated Factor VII Intracerebral. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef]
- Talebloo, N.; Gudi, M.; Robertson, N.; Wang, P. Magnetic Particle Imaging: Current Applications in Biomedical Research. J. Magn. Reson. Imaging JMRI 2020, 51, 1659–1668. [Google Scholar] [CrossRef]
- Szwargulski, P.; Wilmes, M.; Javidi, E.; Thieben, F.; Graeser, M.; Koch, M.; Gruettner, C.; Adam, G.; Gerloff, C.; Magnus, T.; et al. Monitoring Intracranial Cerebral Hemorrhage Using Multicontrast Real-Time Magnetic Particle Imaging. ACS Nano 2020, 14, 13913–13923. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Ning, W.; Lv, S.; Wang, Q.; Xu, Y. The pivotal role of microglia in injury and the prognosis of subarachnoid hemorrhage. Neural. Regen. Res. 2025, 20, 1829–1848. [Google Scholar] [CrossRef]
- Aleksandrowicz, M.; Kozniewska, E. Hyponatremia as a risk factor for microvascular spasm following subarachnoid hemorrhage. Exp. Neurol. 2022, 355, 114126. [Google Scholar] [CrossRef]
- Toyota, B.D. The efficacy of an abbreviated course of nimodipine in patients with good-grade aneurysmal subarachnoid hemorrhage. J. Neurosurg. 1999, 90, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Lu, W.; Li, J.; Wang, P.; Xu, R.; Chen, T. Preparation and characterization of intravenously injectable nimodipine nanosuspension. Int. J. Pharm. 2008, 350, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, J.; Zheng, Y.; Ge, L.; Zhang, G. Preparation, characterization, and pharmacokinetics of sterically stabilized nimodipine-containing liposomes. Drug Dev. Ind. Pharm. 2006, 32, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zech, J.; Leisz, S.; Göttel, B.; Syrowatka, F.; Greiner, A.; Strauss, C.; Knolle, W.; Scheller, C.; Mäder, K. Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Eur. J. Pharm. Biopharm. 2020, 151, 116–126. [Google Scholar] [CrossRef]
- Huang, S.; Huang, Z.; Fu, Z.; Shi, Y.; Dai, Q.; Tang, S.; Gu, Y.; Xu, Y.; Chen, J.; Wu, X. A Novel Drug Delivery Carrier Comprised of Nimodipine Drug Solution and a Nanoemulsion: Preparation, Characterization, in vitro, and in vivo Studies. Int. J. Nanomed. 2020, 15, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Basalious, E.B.; Shamma, R.N. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: In vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int. J. Pharm. 2015, 493, 347–356. [Google Scholar] [CrossRef]
- Soliman, G.M.; Sharma, R.; Choi, A.O.; Varshney, S.K.; Winnik, F.M.; Kakkar, A.K.; Maysinger, D. Tailoring the efficacy of nimodipine drug delivery using nanocarriers based on A2B miktoarm star polymers. Biomaterials 2010, 31, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, J.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X.; Yang, C. Design of lactoferrin modified lipid nano-carriers for efficient brain-targeted delivery of nimodipine. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 1031–1040. [Google Scholar] [CrossRef]
- Rashed, H.M.; Shamma, R.N.; Basalious, E.B. Contribution of both olfactory and systemic pathways for brain targeting of nimodipine-loaded lipo-pluronics micelles: In vitro characterization and in vivo biodistribution study after intranasal and intravenous delivery. Drug Deliv. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Mohsen, K.; Azzazy, H.M.E.; Allam, N.K.; Basalious, E.B. Intranasal lipid nanocapsules for systemic delivery of nimodipine into the brain: In vitro optimization and in vivo pharmacokinetic study. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 116, 111236. [Google Scholar] [CrossRef]
- Ma, L.; Mao, H.; Xu, J.; Piao, J.; Piao, M. Study on the Nasal Drug Delivery System of NMD Liposomes In Situ Thermosensitive Gel. AAPS Pharmscitech 2023, 24, 234. [Google Scholar] [CrossRef]
- Döring, K.; Sperling, S.; Ninkovic, M.; Schroeder, H.; Fischer, A.; Stadelmann, C.; Streit, F.; Binder, L.; Mielke, D.; Rohde, V.; et al. Ultrasound-Induced Release of Nimodipine from Drug-Loaded Block Copolymer Micelles: In Vivo Analysis. Transl. Stroke Res. 2022, 13, 792–800. [Google Scholar] [CrossRef]
- Bai, L.P.; Zhang, Z.F. Effect of Ropivacaine Hydrochloride Nanoliposomes on Delayed Cerebral Vasospasm After Subarachnoid Hemorrhage in Rabbits. Sci. Adv. Mater. 2023, 15, 1098–1109. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Liu, W.; Qi, H. Effect of Ropivacaine nanoparticles on apoptosis of cerebral vascular endothelial cells. Mater. Express 2020, 10, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Hocking, K.M.; Kilchrist, K.V.; Wise, E.S.; Brophy, C.M.; Duvall, C.L. Endosomolytic Nano-Polyplex Platform Technology for Cytosolic Peptide Delivery to Inhibit Pathological Vasoconstriction. ACS Nano 2015, 9, 5893–5907. [Google Scholar] [CrossRef]
- Hocking, K.M.; Evans, B.C.; Komalavilas, P.; Cheung-Flynn, J.; Duvall, C.L.; Brophy, C.M. Nanotechnology Enabled Modulation of Signaling Pathways Affects Physiologic Responses in Intact Vascular Tissue. Tissue Eng. Part A 2019, 25, 416–426. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Han, H.; Yang, L.; Zhang, G.; Fan, Z. Functionalized graphene oxide as a drug carrier for loading pirfenidone in treatment of subarachnoid hemorrhage. Colloids Surf. B Biointerfaces 2015, 129, 21–29. [Google Scholar] [CrossRef]
- Zi, L.; Zhou, W.; Xu, J.; Li, J.; Li, N.; Xu, J.; You, C.; Wang, C.; Tian, M. Rosuvastatin Nanomicelles Target Neuroinflammation and Improve Neurological Deficit in a Mouse Model of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 2933–2947. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, Y.; Hayat, M.A.; Si, Y.; Ni, Y.; Zhang, J.; Qiu, Y.; Zeng, Y.; Cao, Y.; Hong, Y.; et al. Recombinant human heavy-chain ferritin nanoparticles loaded with rosuvastatin attenuates secondary brain injury in intracerebral hemorrhage. Int. J. Biol. Macromol. 2025, 302, 140542. [Google Scholar] [CrossRef]
- Gong, X.; Fan, X.; He, Y.; Wang, Y.; Zhou, F.; Yang, B. A pH-sensitive liposomal co-delivery of fingolimod and ammonia borane for treatment of intracerebral hemorrhage. Nanophotonics 2022, 11, 5133–5142. [Google Scholar] [CrossRef]
- Tang, X.; Yang, X.; Yu, Y.; Wu, M.; Li, Y.; Zhang, Z.; Jia, G.; Wang, Q.; Tu, W.; Wang, Y.; et al. Carbon quantum dots of ginsenoside Rb1 for application in a mouse model of intracerebral Hemorrhage. J. Nanobiotechnol. 2024, 22, 125. [Google Scholar] [CrossRef]
- Yang, X.; Han, M.; Wang, X.; Wang, J.; Sun, X.; Zhang, C.; Yan, S.; Huang, L.; Chen, Y. Evaluation of the synergistic effects of epigallocatechin-3-gallate-loaded PEGylated-PLGA nanoparticles with nimodipine against neuronal injury after subarachnoid hemorrhage. Front. Nutr. 2023, 9, 953326. [Google Scholar] [CrossRef]
- Galho, A.R.; Cordeiro, M.F.; Ribeiro, S.A.; Marques, M.S.; Antunes, M.F.; Luz, D.C.; Hädrich, G.; Muccillo-Baisch, A.L.; Barros, D.M.; Lima, J.V.; et al. Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebral haemorrhage in rats. Nanotechnology 2016, 27, 175101. [Google Scholar] [CrossRef] [PubMed]
- You, Z.Q.; Wu, Q.; Zhou, X.M.; Zhang, X.S.; Yuan, B.; Wen, L.L.; Xu, W.D.; Cui, S.; Tang, X.L.; Zhang, X. Receptor-Mediated Delivery of Astaxanthin-Loaded Nanoparticles to Neurons: An Enhanced Potential for Subarachnoid Hemorrhage Treatment. Front. Neurosci. 2019, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, J.; Kong, Z.; Han, G.; Xiong, Y.; Luo, T.; Chu, L.; Zhang, P.; Ma, D.; Lan, J.; et al. Catechin-Based Polyphenol Nanoparticles Ameliorated Ferroptosis to Alleviate Brain Injury after Intracerebral Hemorrhage. ACS Appl. Mater. Interfaces 2025, 17, 7424–7437. [Google Scholar] [CrossRef]
- Cai, W.; Wu, Q.; Yan, Z.Z.; He, W.Z.; Zhou, X.M.; Zhou, L.J.; Zhang, J.Y.; Zhang, X. Neuroprotective Effect of Ultrasound Triggered Astaxanthin Release Nanoparticles on Early Brain Injury After Subarachnoid Hemorrhage. Front. Chem. 2021, 9, 775274. [Google Scholar] [CrossRef]
- Marques, M.S.; Cordeiro, M.F.; Marinho, M.A.G.; Vian, C.O.; Vaz, G.R.; Alves, B.S.; Jardim, R.D.; Hort, M.A.; Dora, C.L.; Horn, A.P. Curcumin-loaded nanoemulsion improves haemorrhagic stroke recovery in wistar rats. Brain Res. 2020, 1746, 147007. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jiang, M.; Fang, J.; Yang, M.F.; Zhang, S.; Yin, Y.X.; Li, D.W.; Mao, L.L.; Fu, X.Y.; Hou, Y.J.; et al. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood-Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol. 2017, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Duan, Z.; Zhou, W.; He, S.; Wang, W.; Huang, H.; Yi, L.; Zhang, R.; Chen, J.; Zan, X.; You, C.; et al. Intranasal Delivery of Curcumin Nanoparticles Improves Neuroinflammation and Neurological Deficits in Mice with Intracerebral Hemorrhage. Small Methods 2024, 8, e2400304. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, X.; Liu, H. A facile approach for synthesis of nano-CeO2 particles loaded co-polymer matrix and their colossal role for blood-brain barrier permeability in Cerebral Ischemia. J. Photochem. Photobiol. B Biol. 2018, 187, 184–189. [Google Scholar] [CrossRef]
- Kang, D.W.; Kim, C.K.; Jeong, H.-G.; Soh, M.; Kim, T.; Choi, I.-Y.; Ki, S.-K.; Kim, D.Y.; Yang, W.; Hyeon, T.; et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017, 10, 2743–2760. [Google Scholar] [CrossRef]
- Jeong, H.G.; Cha, B.G.; Kang, D.W.; Kim, D.Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; Yang, W.; Kim, C.K.; Kim, J.; et al. Ceria Nanoparticles Synthesized with Aminocaproic Acid for the Treatment of Subarachnoid Hemorrhage. Stroke 2018, 49, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Jeong, H.-G.; Kang, D.-W.; Nam, M.-J.; Kim, C.K.; Kim, D.Y.; Choi, I.-Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; et al. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res. 2018, 11, 3582–3592. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, R.; Xie, F.; Xu, W.; Zeng, M.F.; Wang, X.; Zhu, J. Protective effects of perfluorooctyl-bromide nanoparticles on early brain injuries following subarachnoid hemorrhage in rats. Am. J. Transl. Res. 2015, 7, 1404–1416. [Google Scholar]
- Xu, W.; Xu, R.; Li, X.; Zhang, H.; Wang, X.; Zhu, J. Downregulating hypoxia-inducible factor-1α expression with perfluorooctyl-bromide nanoparticles reduces early brain injury following experimental subarachnoid hemorrhage in rats. Am. J. Transl. Res. 2016, 8, 2114–2126. [Google Scholar]
- Dharmalingam, P.; Talakatta, G.; Mitra, J.; Wang, H.; Derry, P.J.; Nilewski, L.G.; McHugh, E.A.; Fabian, R.H.; Mendoza, K.; Vasquez, V.; et al. Pervasive Genomic Damage in Experimental Intracerebral Hemorrhage: Therapeutic Potential of a Mechanistic-Based Carbon Nanoparticle. ACS Nano 2020, 14, 2827–2846. [Google Scholar] [CrossRef]
- Sang, L.Y.; Liang, Y.X.; Li, Y.; Wong, W.M.; Tay, D.K.; So, K.F.; Ellis-Behnke, R.G.; Wu, W.; Cheung, R.T. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wen, D.; Fu, W.; Xing, L.; Ma, L.; Liu, Y.; Li, H.; You, C.; Lin, Y. Treatment effect of DNA framework nucleic acids on diffuse microvascular endothelial cell injury after subarachnoid hemorrhage. Cell Prolif. 2022, 55, e13206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Wang, Y.S.; Hsu, P.Y.; Chen, C.Y.; Liao, Y.C.; Juo, S.H. miR-195 Has a Potential to Treat Ischemic and Hemorrhagic Stroke through Neurovascular Protection and Neurogenesis. Mol. Ther. Methods Clin. Dev. 2019, 13, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.H.; Wang, Z.G.; Meng, H.; Wang, Y.H.; Feng, W.; Wei, F.; Huang, Z.C.; Lin, X.N.; Ren, L. Tat peptide-decorated gelatin-siloxane nanoparticles for delivery of CGRP transgene in treatment of cerebral vasospasm. Int. J. Nanomed. 2013, 8, 865–876. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.Y.; Choi, S.K.; Kim, J.Y.; Kim, J.H.; Jeon, W.B.; Lee, J.E. Thermo-sensitive assembly of the biomaterial REP reduces hematoma volume following collagenase-induced intracerebral hemorrhage in rats. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1853–1862. [Google Scholar] [CrossRef]
- Mo, Y.; Duan, L.; Yang, Y.; Liu, W.; Zhang, Y.; Zhou, L.; Su, S.; Lo, P.C.; Cai, J.; Gao, L.; et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021, 13, 3827–3840. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Hu, H.; Xing, Z. Intra-Epidural Space Injection of OX26-PEGylated Selenium Nanoparticles Enhances Motor Function and Decrease the Risk of Neural Damage in Animal Model of Subarachnoid Hemorrhage. J. Biomed. Nanotechnol. 2022, 18, 1481–1487. [Google Scholar] [CrossRef]
- Wang, T.; Lei, H.; Li, X.; Yang, N.; Ma, C.; Li, G.; Gao, X.; Ge, J.; Liu, Z.; Cheng, L.; et al. Magnetic Targeting Nanocarriers Combined with Focusing Ultrasound for Enhanced Intracerebral Hemorrhage Therapy. Small 2023, 19, e2206982. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Lan, X.; Han, Z.; Ren, H.; Aafreen, S.; Wang, W.; Hou, Z.; Zhu, T.; Qian, A.; Han, X.; et al. Improving outcomes in intracerebral hemorrhage through microglia/macrophage-targeted IL-10 delivery with phosphatidylserine liposomes. Biomaterials 2023, 301, 122277. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Qu, Q.; Hou, Z.; Guo, T.; Xu, Y.; Qing, R.; Deng, J.; Wang, B.; Hao, S.; et al. Nanoparticle encapsulated core-shell hydrogel for on-site BMSCs delivery protects from iron overload and enhances functional recovery. J. Control. Release 2020, 320, 381–391. [Google Scholar] [CrossRef]
- Chen, K.H.; Chai, H.T.; Lin, K.C.; Chiang, J.Y.; Sung, P.H.; Chen, C.H.; Yip, H.K. Dose-dependent benefits of iron-magnetic nanoparticle-coated human umbilical-derived mesenchymal stem cell treatment in rat intracranial hemorrhage model. Stem Cell Res. Ther. 2022, 13, 265. [Google Scholar] [CrossRef]
- Rahimi Darehbagh, R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem cell therapies for neurological disorders: Current progress, challenges, and future perspectives. Eur. J. Med. Res. 2024, 29, 386. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mal, S.; Halder, A.; Das, S.; Sen, K.K.; Mukherjee, A.; Roy, P. Nano-dimensional gold synthesis for biomedical applications: Upscaling and challenges. Part. Sci. Technol. 2024, 42, 145–163. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zou, J.; Chen, Z.; He, W.; Wu, W. Current research trends of nanomedicines. Acta Pharm. Sin. B 2023, 13, 4391–4416. [Google Scholar] [CrossRef] [PubMed]
- Gällentoft, L.; Pettersson, L.M.; Danielsen, N.; Schouenborg, J.; Prinz, C.N.; Linsmeier, C.E. Impact of degradable nanowires on long-term brain tissue responses. J. Nanobiotechnol. 2016, 14, 64. [Google Scholar] [CrossRef] [PubMed]
| Nanomaterial | Disease | Main Research Conclusions | References |
|---|---|---|---|
| Indium and Dil double-labeled lipid nanoparticles | ICH | After intravenous injection, brain liposomes show a biphasic accumulation peak, with the most accumulation in the hematoma area and co-localization with activated microglia. Mechanistic studies find that in the early ICH stage, Caveolin-1-mediated transcytosis is enhanced, and in the later stage, Claudin-5 is significantly downregulated, revealing a dual-regulatory BBB permeability. | [28] |
| VCAM-SOD-lipid nanoparticles | ICH | The brain uptake of VCAM-SOD-lipid nanoparticles is significantly higher than that of SOD-lipid nanoparticles. And, the delivery efficiency of the targeted liposomes remains stable after the acute phase, while the delivery efficiency of the passive leakage pathway decays rapidly. | [29] |
| Superparamagnetic iron oxide nanoparticle-Synomag-D | ICH | Intravenous injection of superparamagnetic iron oxide nanoparticle-Synomag-D enables bedside detection within 3 min using MPI technology. MPI can clearly distinguish liquid and coagulated hematoma areas, monitor cerebral perfusion status synchronously, providing a key basis for choosing surgical timing. Moreover, it can sensitively identify complications like increased intracranial pressure and vasospasm. | [33] |
| Qdot® 655 nanocrystal | SAH | After injecting Qdot® 655 nanocrystals, it was found that within 1 min after SAH, the capillary network ruptured, and the blood flow velocity in the pre-arterioles decreased by 85%. | [30] |
| Nimodipine–lipid nanoparticles; Nimodipine nano-suspension; Nimodipine–PLGA nanofibers; Nimodipine nanoemulsion; PPPMM; Nimodipine–A2B type miktoarm polymers; lactoferrin modified long circulation nanostructured lipid carriers; 99mTc-Nimodipine-LPM; Nimodipine–lipid nanocapsules; Nimodipine-Pluronic® F-127 | SAH | These nano-formulations have improved the solubility, bioavailability, degree of vascular irritation, and concentration in the brain of nimodipine. | [38,39,40,41,42,43,44,45,46,47,48] |
| Ropivacaine–lipid nanoparticles; PLGA–ropivacaine nanoparticles | SAH | Ropivacaine nanoparticles significantly reduced the blood flow velocity of the basilar artery, the levels of nerve injury markers and endothelin-1. Meanwhile, they increased the diameter of the blood vessel and the expression of endothelial nitric oxide synthase, decreased the apoptosis of endothelial cells, and alleviated vasospasm. | [49,50] |
| HSP20 and MK2 inhibitory peptide–PPAA nanoparticles; HSP20 siRNA and HSP27 recombinant protein–PPAA nanoparticles | SAH | Nanoparticles of PPAA loaded with HSP20 and MK2 inhibitory peptides, as well as PPAA nanoparticles of HSP20 siRNA and HSP27 recombinant protein, promote the cytoplasmic delivery of HSP20, MK2 inhibitory peptides, HSP20 siRNA, and HSP27 recombinant protein through a pH-dependent endosomal escape mechanism, significantly enhancing the vasodilatory ability. | [51,52] |
| Pirfenidone–functionalized nanoscale graphene oxide nanoparticles | SAH | The efficient release of pirfenidone–FGO significantly alleviated the neuroinflammation after SAH. In addition, pirfenidone–FGO also exhibited excellent near-infrared absorption properties and could be used for photoacoustic imaging to achieve rapid and real-time monitoring of brain tissue after SAH. | [53] |
| Rosuvastatin-PEG-PCL nanomicelles | ICH | The rosuvastatin-loaded nanomicelles significantly promoted the polarization of microglia/macrophages towards the M2 phenotype, inhibited the infiltration of inflammatory cells, reduced the levels of pro-inflammatory factors, and upregulated the expression of the anti-inflammatory factor IL-10, thereby reducing neuronal degeneration, alleviating cerebral edema, and improving neurological deficits. | [54] |
| Rsv@HFn | ICH | This nano-platform enhances the ability of the drug to cross the BBB, increases its accumulation at the injury site, and improves its therapeutic effect. Rsv@HFn also promotes the translocation of Nrf-2 to the cell nucleus, increases the expression of HO-1 and CD91, facilitates the shift of M1 microglia to the M2 phenotype, and reduces neuroinflammation and oxidative stress. In addition, Rsv@HFn improves the integrity of the BBB in ICH mice, reduces cerebral edema, and alleviates neuropathological damage. | [55] |
| PSL-FTY720/AB | ICH | Compared with the ICH group, the FTY720 group, and the PSL-FTY720 group, the PSL-FTY720/AB treatment group showed the best therapeutic effects in reducing cerebral edema in the ipsilateral basal ganglia and cerebral cortex, protecting the integrity of the BBB, inhibiting neuronal apoptosis, alleviating oxidative stress and neuroinflammation, and improving neurological deficits. | [56] |
| RBCQDs | ICH | Compared with free ginsenoside Rb1, Cy5-labeled RBCQDs significantly increased the drug accumulation in brain tissue. Through mechanisms such as alleviating oxidative stress and inhibiting ferroptosis and neuronal apoptosis, it reduced cerebral edema and the water content in brain tissue, restored blood perfusion in the dura mater area, and improved the motor nerve function of mice. | [57] |
| PEGylated-PLGA EGCG nanoparticles | ICH | PEGylated-PLGA EGCG nanoparticles have remarkable sustained-release characteristics. | [58] |
| Quercetin-loaded nanoemulsion | ICH | Quercetin-loaded nanoemulsion significantly reduced the hematoma volume in rats with ICH, maintained the activity of glutathione S-transferase, and increased the content of glutathione and the total antioxidant capacity. | [59] |
| Transferrin conjugated to PEG-encapsulated ATX nanoparticles | ICH | ATX nanoparticles can be effectively internalized into the cytoplasm of primary neurons within 6 h, and upregulate the expression of Bcl-2 12 h after exposure to oxyhemoglobin, while downregulating the expressions of Bax and caspase-3. | [60] |
| Catechin-based polyphenol nanoparticles surface-modified by thiol-terminated poly(ethylene glycol) | ICH | CNPs@PEG effectively maintained BBB integrity, reduced brain edema, significantly increased the survival rate of mice with cerebral hemorrhage and markedly improved neurological deficits after ICH. Mechanistically, CNPs@PEG accomplishes this by chelating iron, enhancing tissue antioxidant capacity, reducing oxidative stress, and inhibiting iron deposition. | [61] |
| AUTNPs | SAH | AUTNPs respond to the phase transition of perfluorooctyl bromide induced by ultrasound, break through the shell, in situ destroy the nanostructure, and release the drug to neurons in a targeted manner, exerting antioxidant and anti-apoptotic effects. | [62] |
| Curcumin nanoemulsion; curcumin polymer-based nanoparticles; curcumin–PEG-PCL nanoparticles | ICH AND ICH | Compared with free curcumin, curcumin nanoparticles significantly increased the drug concentration in the brains of mice. They also showed stronger neuroprotective effects in aspects such as inhibiting iron deposition in the brain tissue around the hematoma, reducing the generation of reactive oxygen species, alleviating the damage of the BBB, reducing the degree of cerebral edema, and inhibiting neuronal apoptosis. | [63,64,65,66,67] |
| Cerium dioxide nanoparticles | ICH AND ICH | Cerium nanoparticles can reduce the level of oxidative stress induced by heme, decrease the content of nitrite formed by nitric oxide, alleviate cytotoxicity, and mitigate the inflammatory response. In vivo studies have found that after intravenous administration, CeNPs mainly accumulate in the hemorrhagic hemisphere. They can not only effectively reduce cerebral edema, but also inhibit the recruitment of microglia/macrophages around the bleeding focus and the expression of inflammatory proteins. Although the severity of hemorrhage in the cerium dioxide nanoparticle group was comparable to that in the normal saline group, cerium dioxide nanoparticles significantly reduced neuronal death, macrophage infiltration, and cerebral edema after SAH, effectively improving the survival rate of mice and the prognosis of neurological function. | [69,70] |
| Lipid-coated magnetic mesoporous silica nanoparticles doped with cerium dioxide (LMC) | ICH | Intracerebral injection of LMC can directly reach the area around the hematoma and be phagocytosed by macrophages. By reducing the infiltration of inflammatory macrophages, it can significantly alleviate cerebral edema and can be clearly visualized in brain magnetic resonance imaging. | [71] |
| Perfluorooctyl-bromide nanoparticles | SAH | Compared with the SAH model group, PFOB nanoparticles can significantly reduce the degree of cerebral edema, decrease neuronal apoptosis, inhibit the activation of Caspase-3 and the expression of Bax, and increase the expression of Bcl-2. They can also inhibit the expressions of HIF-1α, VEGF, and BNIP3, significantly reduce the brain water content, decrease the extravasation of Evans blue, and lower the proportion of neuronal apoptosis in the hippocampal region in a dose-dependent manner. | [73] |
| DEF-HCC–PEG nanoparticles | ICH | When compared with treating cells with PEG-HCC or DEF alone, DEF-HCC-PEG nanoparticles can restore the viability of heme-treated cells with significantly higher efficiency. At the same time, they can significantly reduce the levels of DNA damage markers γH2AX and p-53BP1, effectively prevent the heme-induced plasmid DNA strand breaks in vitro. Moreover, DEF-HCC-PEG has the characteristic of inhibiting the sensitivity of cells to ferroptosis. In in vivo experiments featuring treatment with DEF-HCC-PEG nanoparticles, the integrity of the nuclear and mitochondrial genomes is significantly restored, and the expression levels of senescence-related factors ANKRD1, EDN1, p21, and PVRL4 are significantly decreased. | [74] |
| SAPNS | ICH | SAPNS significantly reduces the formation of the cerebral cavity after ICH, inhibits apoptosis, and improves the recovery of sensorimotor function. | [75] |
| tFNAs | SAH | tFNA in situ alleviated the damage caused by SAH, restored the number of BMECs and intercellular junctions in the endothelial layer, inhibited cell apoptosis, promoted angiogenesis, and improved neurological function. | [76] |
| JetPEI nanoparticles loaded with miR-195 | ICH | JetPEI nanoparticles loaded with miR-195 significantly reduced brain edema, lesion volume, and blood–brain barrier leakage in ICH mice and improved neurological function scores. | [77] |
| Tat-GS nanoparticles loaded with CGRP gene | SAH | The continuous expression of CGRP in endothelial cells by Tat-GS nanoparticles encapsulating pLXSN-CGRP was 1.71 times that of gelatin-siloxane nanoparticles encapsulating pLXSN-CGRP and 6.92 times that of naked pLXSN-CGRP. Moreover, the group treated with Tat-GS nanoparticles encapsulating pLXSN-CGRP had better neurological function outcomes and less vasospasm. | [78] |
| REP-mPEG-PLGA nanoparticles | ICH | Compared with the ICH group and the REP group, mPEG-PLGA nanoparticles loaded with REP significantly reduced the hematoma volume after ICH, alleviated neuronal degeneration, necrosis, and iron overload deposition, and improved the motor coordination function of ICH mice. | [79] |
| OX26-PEG-Se nanoparticles | SAH | Compared with the control group, epidural injection of OX26-PEG-Se nanoparticles could significantly reduce the levels of serum inflammatory factors NSE and S100B and the vasoconstrictor factor ET1, and upregulate the expression of the vasodilator factor NOS, thereby significantly improving the motor function of the SAH model and reducing the risk of nerve injury. | [80] |
| 15d-PGJ2-MNPs | ICH | Under magnetic targeting guidance, 15d-PGJ2-MNPs could be effectively enriched in the brains of ICH mice, and the combination with focused ultrasound (FUS) further achieved a stable and uniform distribution of the drug. Mechanistic studies showed that the diffused 15d-PGJ2-MNPs were more likely to activate PPARγ, thereby enhancing the phagocytic ability of microglia towards hematomas. Compared with the ICH control group, the hematoma volume in the 15d-PGJ2-MNPs + magnetic targeting + FUS treatment group was significantly reduced on the 3rd day, almost completely cleared on the 7th day, and the morphology of the brain tissue around the hematoma recovered better. | [82] |
| PSL-IL10 nanoparticles | ICH | PSL-IL10 significantly inhibited the activation of glial cells, enhanced the phagocytic function of microglia/macrophages, accelerated hematoma absorption, and improved neurological function. | [83] |
| PLGA nanoparticles loaded with EGF and FGF | ICH | Core–shell structured hydrogel system: The rapidly degradable low-molecular-weight keratin hydrogel serves as the shell to remove iron overload, while the high-molecular-weight keratin hydrogel encapsulating PLGA nanoparticles loaded with EGF and FGF acts as the core to support the growth of BMSCs. The core–shell structure can not only effectively reduce the intracellular iron load and protect BMSCs from hemoglobin toxicity, but also significantly alleviate brain edema and brain atrophy by efficiently removing iron deposits in hematomas, promote the differentiation of BMSCs into neurons, and ultimately improve limb dysfunction. | [85] |
| Ir-MNA-modified human umbilical cord mesenchymal stem cells | ICH | Human umbilical cord mesenchymal stem cells modified with Ir-MNA nanoparticles can be targeted and enriched in cerebral hemorrhage foci, achieving more significant brain protection effects by inhibiting neuroinflammation, reducing oxidative stress, and alleviating mitochondrial damage. | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Jiang, T.; Han, J.; Zheng, T.; Wang, M. Multiple Applications of Nanomaterials in the Diagnosis and Treatment of Hemorrhagic Stroke. Biomolecules 2025, 15, 1272. https://doi.org/10.3390/biom15091272
Yuan B, Jiang T, Han J, Zheng T, Wang M. Multiple Applications of Nanomaterials in the Diagnosis and Treatment of Hemorrhagic Stroke. Biomolecules. 2025; 15(9):1272. https://doi.org/10.3390/biom15091272
Chicago/Turabian StyleYuan, Boyao, Taotao Jiang, Jingjing Han, Ting Zheng, and Manxia Wang. 2025. "Multiple Applications of Nanomaterials in the Diagnosis and Treatment of Hemorrhagic Stroke" Biomolecules 15, no. 9: 1272. https://doi.org/10.3390/biom15091272
APA StyleYuan, B., Jiang, T., Han, J., Zheng, T., & Wang, M. (2025). Multiple Applications of Nanomaterials in the Diagnosis and Treatment of Hemorrhagic Stroke. Biomolecules, 15(9), 1272. https://doi.org/10.3390/biom15091272







