Dual Regulation of Mitochondrial Complexes by H2S via S-Sulfhydration Controls Respiration in Type 1 Diabetic Hearts
Abstract
1. Introduction
2. Materials and Methods
2.1. Type 1 Diabetes Model
2.2. Oral Glucose Tolerance Test (OGTT)
2.3. Isolation of Mitochondria from Cardiac Tissue
2.4. ROS Determination in the Mitochondria
2.5. Determination of MMP
2.6. Determination of Mitochondrial Oxygen Consumption Rate (OCR)
2.7. Determination of S-Sulfhydration Using Modified Biotin Switch Assay
2.8. Determination of Mitochondrial Complex Activity with ELISA Kits
2.9. Determination of Mitochondrial CBS Activity
2.10. H&E and TUNEL Staining
2.11. Langendorff Perfusion and Mapping
2.12. Western Blotting Detecting Protein Expression
2.13. RNA Extraction and Quantitative Real-Time PCR
2.14. Quantification and Statistical Analysis
3. Results
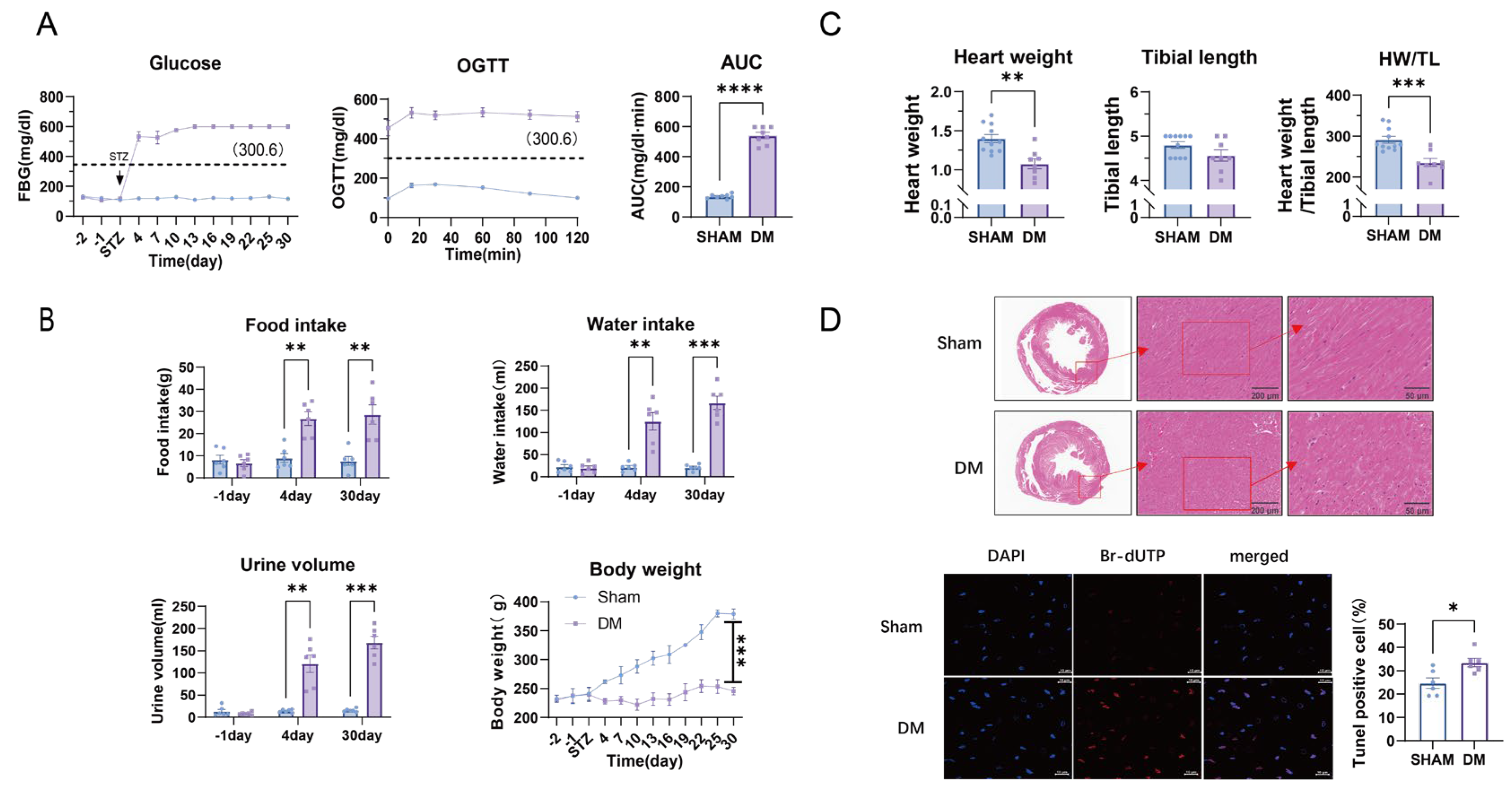
3.1. Metabolic Disturbances and Myocardial Injury in Diabetic Rats
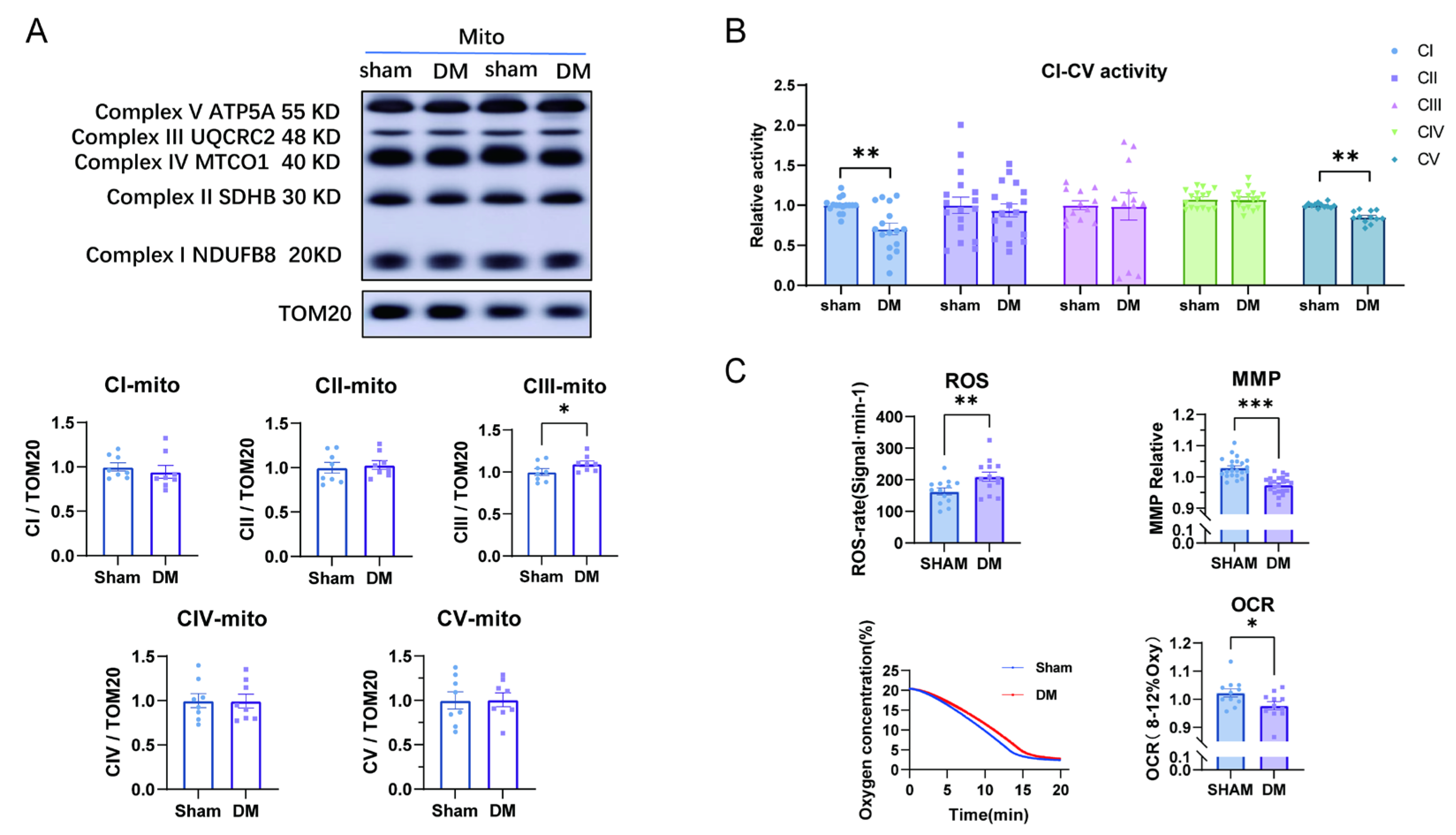
3.2. Reduced Complex I and V Activities Were Accompanied by Mitochondrial Respiratory Dysfunction in Diabetic Myocardium
3.3. Plasma H2S Levels, Cardiac Mitochondrial MPST Protein Expression, and CBS Enzyme Activity Are Decreased in Diabetes
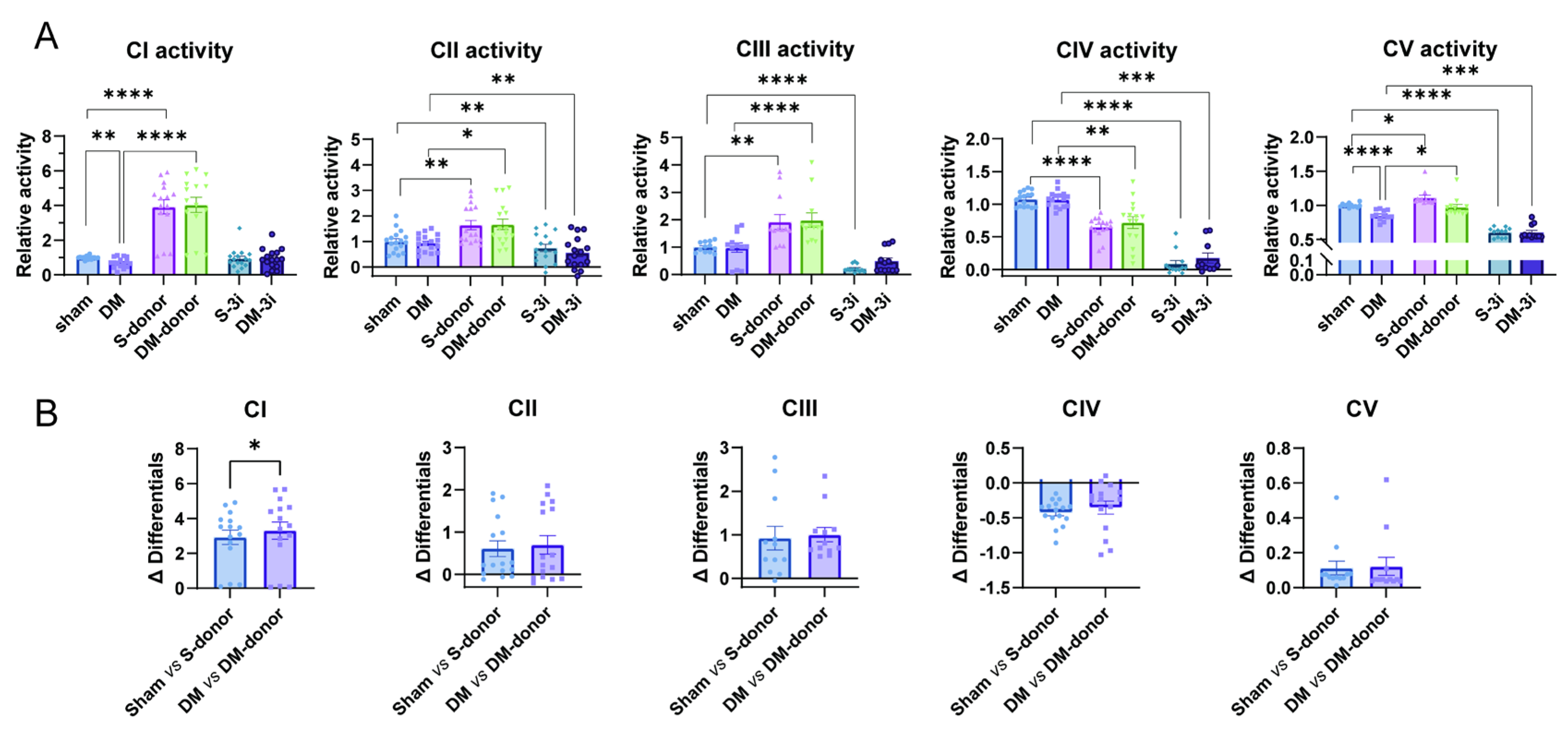
3.4. H2S Reverses the Decreased Mitochondrial Complex I and V Activity in Diabetic Hearts
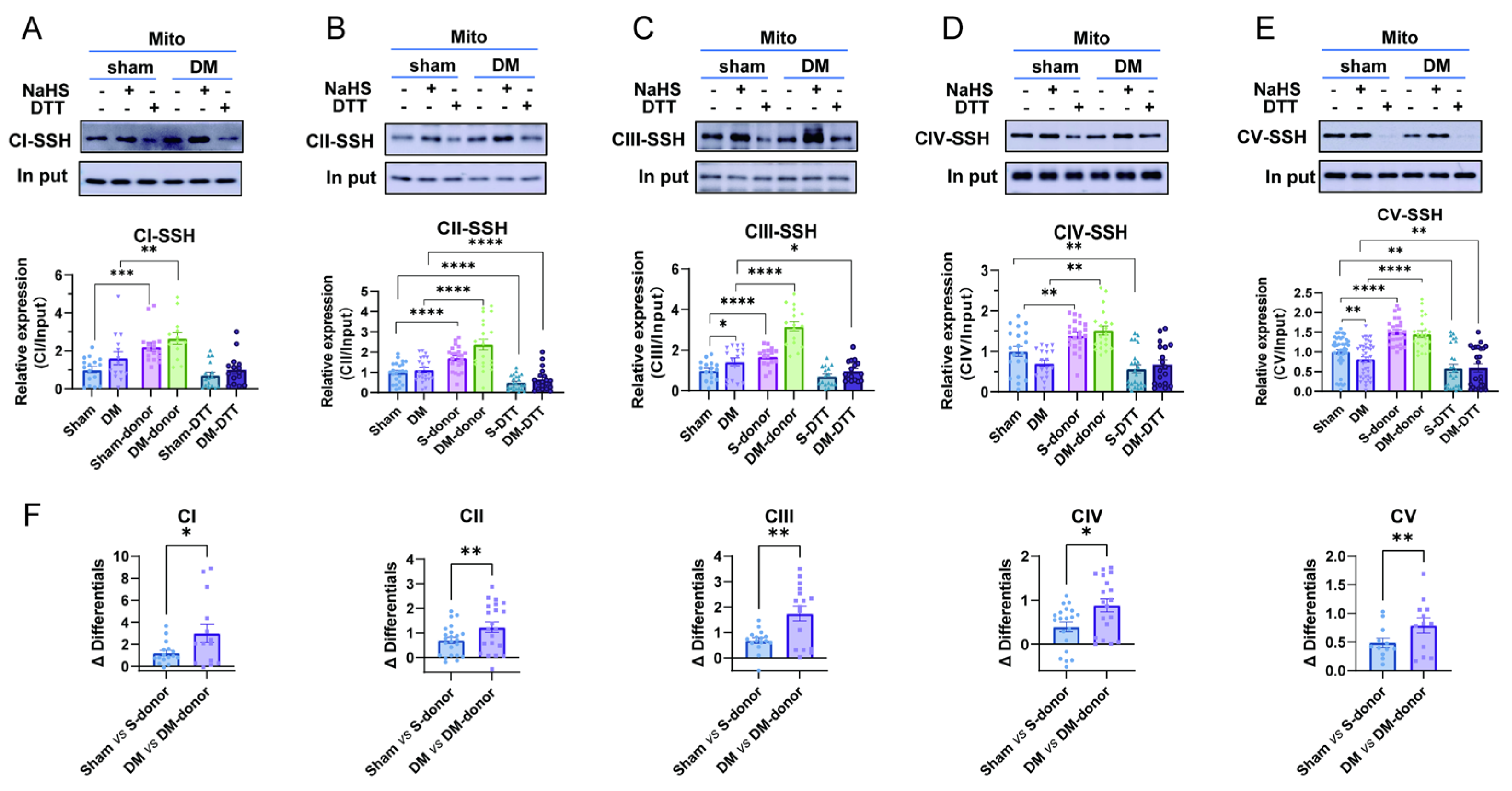
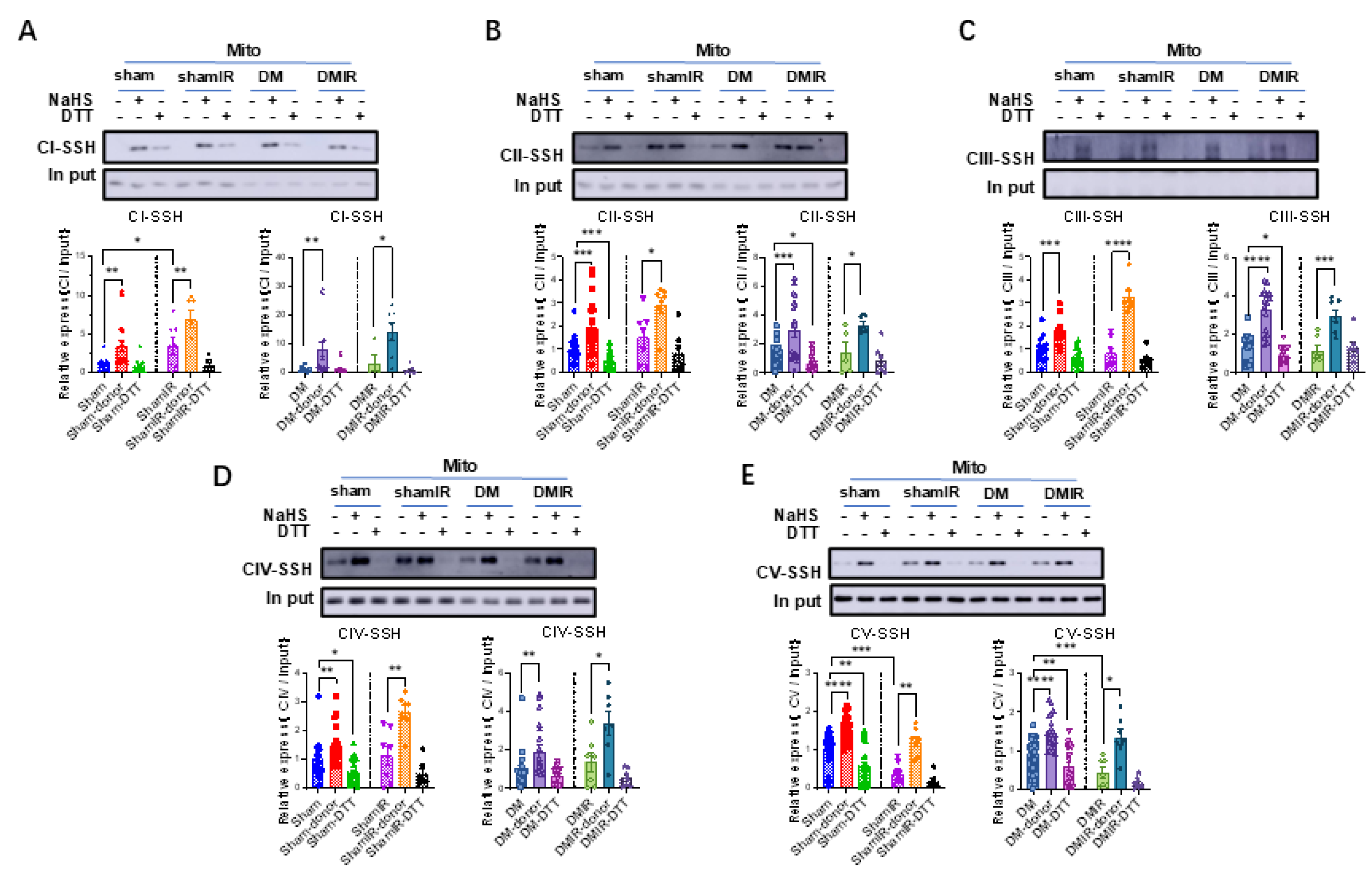
3.5. H2S Enhances Cardiac Mitochondrial Complex Activity by Promoting S-Sulfhydration, with a Greater Effect Observed in Diabetic Rat CardiacMitochondria
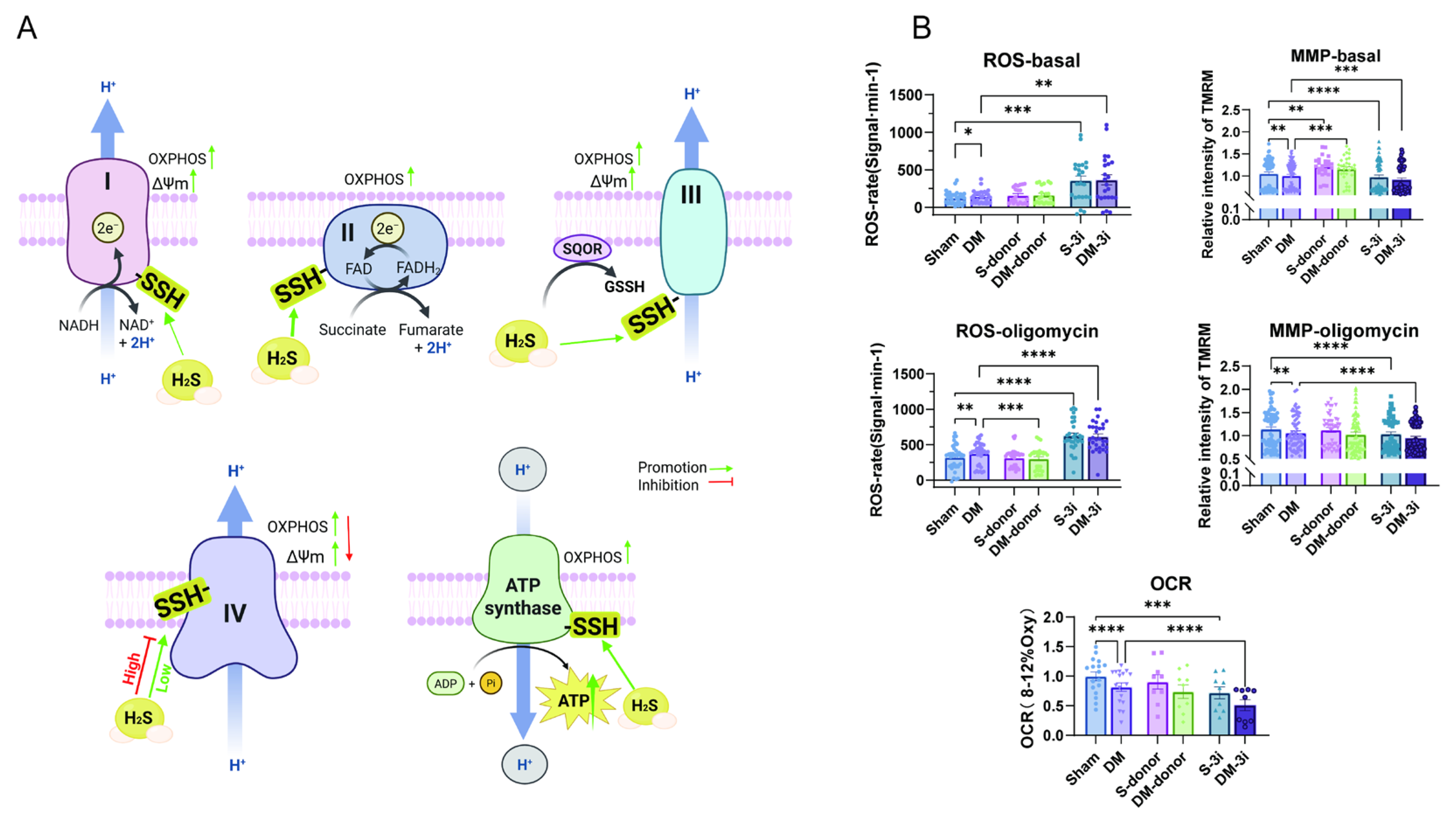
3.6. H2S Enhances Cardiac Mitochondrial Respiration Under Both Basal Conditions and CV Inhibition by Oligomycin
3.7. Persulfidation of Cardiac Mitochondrial Complexes Remains Responsive to H2S After Ischemia-Reperfusion
3.8. Ischemic-Reperfusion Reduced Complex V Responsiveness to H2S in DM Heart and Attenuated Mitochondrial Respiration
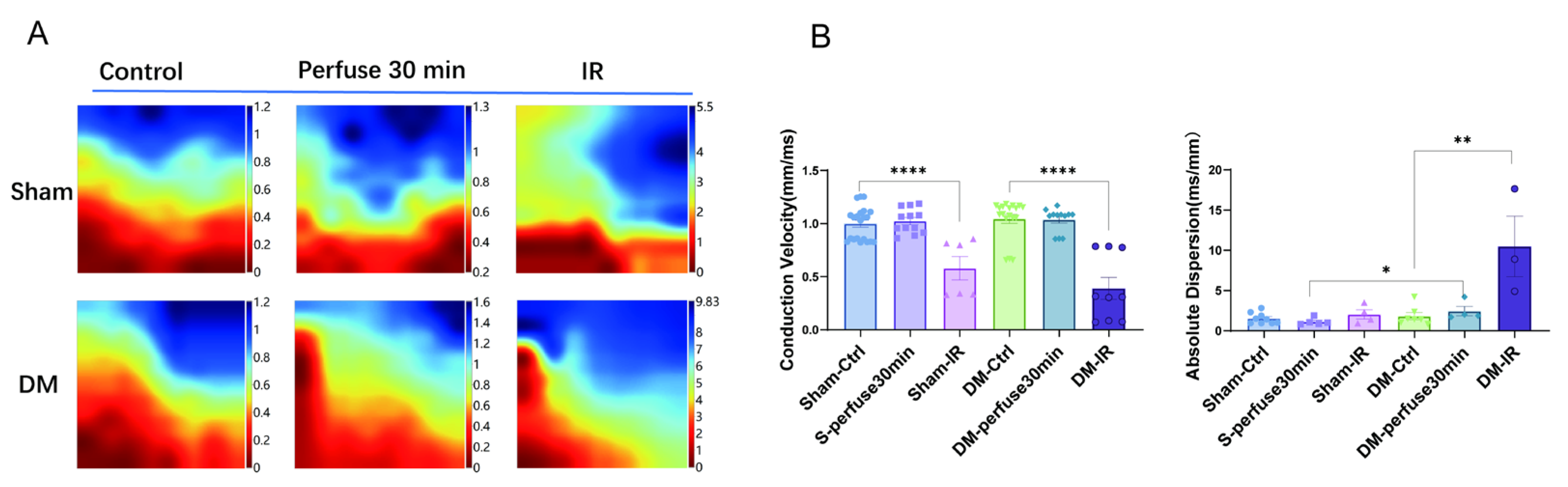
3.9. Conduction Velocity and Diffusion Rate of Electrical Activity Was Impaired After Ischemia-Reperfusion in Sham and DM Hearts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| T1DM | Type 1 diabetes mellitus |
| H2S | Hydrogen sulfide |
| STZ | Streptozotocin |
| BW | Body weight |
| FI | Food intake |
| WI | Water intake |
| UV | Urine volume |
| FBG | Fasting blood glucose |
| OGTT | Oral glucose tolerance test |
| OCR | Oxygen consumption rate |
| ROS | Reactive oxygen species |
| MMP | Mitochondrial membrane potential |
| HWI | Heart mass index |
| TL | Tibial length |
| MPST | Mercaptopyruvate thiotransferase |
| CBS | Cystathionine-beta-synthase |
| CSE | Cystathionine-gamma-lyase |
| NaHS | Sodium hydrosulfide |
| DTT | Dithiothreitol |
| HA | Hydroxylamine |
| PAG | DL-Propargylglycine |
| MMTS | S-Methyl methanethiosulfonate |
| DDW | Double-distilled water |
| HE | Hematoxylin-eosin staining |
| ELISA | Enzyme-linked immunosorbent assay |
Appendix A
| Protein Target | Manufacturer | Catalogue Number | Concentration Used |
|---|---|---|---|
| OXPHOS | Abcam (Cambridge, UK) | Ab110413 | 6.0 μg/mL |
| PINK1 | Novus (Chesterfield, MI, USA) | BC100-494 | 1:2000 |
| BNIP3L | Thermo Fisher (Pittsburgh, PA, USA) | PA5-114914 | 1:1000 |
| DRP1 | Cell signaling (Boston, MA, USA) | 8570S | 1:1000 |
| FIS1 | Thermo Fisher (Pittsburgh, PA, USA) | Ab42364 | 1:1000 |
| OPA1 | Abcam (Cambridge, UK) | Ab42364 | 1:1000 |
| MFN1 | Thermo Fisher (Pittsburgh, PA, USA) | PA5-38042 | 1:1000 |
| MFN2 | Abcam (Cambridge, UK) | Ab124773 | 1:1000 |
| β-actin | Santa Cruz (Paso Robles, CA, USA) | Sc-47778 | 1:1000 |
| TOMM20 | Santa Cruz (Paso Robles, CA, USA) | Sc-17764 | 1:1000 |
| MPST | Santa Cruz (Paso Robles, CA, USA) | Sc-376168 | 1:1000 |
| CBS | Santa Cruz (Paso Robles, CA, USA) | Sc-133154 | 1:1000 |
| CSE | Santa Cruz (Paso Robles, CA, USA) | Sc-365381 | 1:1000 |
| Primer Name | Primer Sequence | Primer Length |
|---|---|---|
| 18s RNA-F | CGCGGTTCTATTTTGTTGGT | 20 |
| 18s RNA-R | AGTCGGCATCGTTTATGGTC | 20 |
| MPST-F | CCGAGCTCTGGTATCTGCTC | 20 |
| MPST-R | ACCAGGACGCATCCAGTAAC | 20 |
| CSE-F | CTCTGGAAATCCGACGAGGA | 20 |
| CSE-R | AGGCGAAGGTCAAACAGTGC | 20 |
| CBS-F | CAATACCGCAACAATGGCGT | 20 |
| CBS-R | TGCCACGAAGTTTAGCAGGT | 20 |
References
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Feng, H.; Li, S.; Meng, G.; Liu, S.; Tang, X.; Ma, Y.; Han, Y.; Xiao, Y.; Gu, Y.; et al. SIRT3 Mediates the Antioxidant Effect of Hydrogen Sulfide in Endothelial Cells. Antioxid. Redox Signal. 2016, 24, 329–343. [Google Scholar] [CrossRef]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Zivanovic, J.; Kouroussis, E.; Kohl, J.B.; Adhikari, B.; Bursac, B.; Schott-Roux, S.; Petrovic, D.; Miljkovic, J.L.; Thomas-Lopez, D.; Jung, Y.; et al. Selective Persulfide Detection Reveals Evolutionarily Conserved Antiaging Effects of S-Sulfhydration. Cell Metab. 2019, 30, 1152–1170.e13. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H.; et al. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Gupta, R.; Sahu, M.; Tripathi, R.; Ambasta, R.K.; Kumar, P. Protein S-sulfhydration: Unraveling the prospective of hydrogen sulfide in the brain, vasculature and neurological manifestations. Ageing Res. Rev. 2022, 76, 101579. [Google Scholar] [CrossRef]
- Wu, X.; Xu, M.; Geng, M.; Chen, S.; Little, P.J.; Xu, S.; Weng, J. Targeting protein modifications in metabolic diseases: Molecular mechanisms and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 220. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Tan, B.; Rose, P.; Zhu, D.; Zhu, Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca2+/calmodulin-dependent protein kinase II. Redox. Biol. 2018, 19, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, A.A.; Fu, M.; Modis, K.; Wang, R.; Ju, Y.; Wu, L. Stimulatory effect of CSE-generated H2S on hepatic mitochondrial biogenesis and the underlying mechanisms. Nitric Oxide 2016, 58, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, M.; Li, H.; Li, Q.; Liu, N.; Dong, S.; Zhao, Y.; Pang, K.; Huang, J.; Ren, C.; et al. Exogenous H2S promotes ubiquitin-mediated degradation of SREBP1 to alleviate diabetic cardiomyopathy via SYVN1 S-sulfhydration. J. Cachexia Sarcopenia Muscle 2023, 14, 2719–2732. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, L.; Li, L.; Yang, J.; Liu, J.; Chen, Y.; Chen, Y.; Zhang, J.; Lu, Y.; Yuan, Y.; et al. S-sulfhydration of SIRT3 combats BMSC senescence and ameliorates osteoporosis via stabilizing heterochromatic and mitochondrial homeostasis. Pharmacol. Res. 2023, 192, 106788. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox. Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Zhang, M.; Huang, C.; Song, Y.; Xu, F.; Zhang, J.; Li, J.; He, M.; Li, Y.; et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020, 6, eaaz5752. [Google Scholar] [CrossRef]
- Lagoutte, E.; Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Blachier, F.; Bouillaud, F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta (BBA)—Bioenerg. 2010, 1797, 1500–1511. [Google Scholar] [CrossRef]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, G. Complexities of complex II: Sulfide metabolism in vivo. J. Biol. Chem. 2022, 298, 101661. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Landry, A.P.; Guha, A.; Vitvitsky, V.; Lee, H.J.; Seike, K.; Reddy, P.; Lyssiotis, C.A.; Banerjee, R. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 2022, 298, 101435. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. Chembiochem 2021, 22, 949–960. [Google Scholar] [CrossRef]
- Panagaki, T.; Randi, E.B.; Augsburger, F.; Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 18769–18771. [Google Scholar] [CrossRef]
- Modis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox. Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Modis, K.; Bos, E.M.; Calzia, E.; van Goor, H.; Coletta, C.; Papapetropoulos, A.; Hellmich, M.R.; Radermacher, P.; Bouillaud, F.; Szabo, C. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br. J. Pharmacol. 2014, 171, 2123–2146. [Google Scholar] [CrossRef]
- Duncan, J.G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim. Biophys. Acta (BBA)—Bioenerg. 2011, 1813, 1351–1359. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial Dysfunction in Diabetic Cardiomyopathy: The Possible Therapeutic Roles of Phenolic Acids. Int. J. Mol. Sci. 2020, 21, 6043. [Google Scholar] [CrossRef]
- Zhou, X.; An, G.; Lu, X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. 2015, 128, 325–335. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Zhu, S.C.; Chen, C.; Wu, Y.N.; Ahmed, M.; Kitmitto, A.; Greenstein, A.S.; Kim, S.J.; Shao, Y.F.; Zhang, Y.H. Cardiac complex II activity is enhanced by fat and mediates greater mitochondrial oxygen consumption following hypoxic re-oxygenation. Pflügers Arch.–Eur. J. Physiol. 2020, 472, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, J.; Zhang, S.; Pang, K.; Zhao, Y.; Liu, N.; Huang, J.; Kang, J.; Dong, S.; Li, H.; et al. Exogenous H2S initiating Nrf2/GPx4/GSH pathway through promoting Syvn1-Keap1 interaction in diabetic hearts. Cell Death Discov. 2023, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Lefer, D.J. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br. J. Pharmacol. 2008, 155, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Wang, X.Y.; Bian, J.S. An Updated Insight Into Molecular Mechanism of Hydrogen Sulfide in Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury Under Diabetes. Front. Pharmacol. 2021, 12, 651884. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sasakura, K.; Suwanai, Y.; Toma-Fukai, S.; Shimamoto, K.; Takano, Y.; Shibuya, N.; Terai, T.; Komatsu, T.; Ueno, T.; et al. Discovery and Mechanistic Characterization of Selective Inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide. Sci. Rep. 2017, 7, 40227. [Google Scholar] [CrossRef]
- Yang, G.; Tang, G.; Zhang, L.; Wu, L.; Wang, R. The pathogenic role of cystathionine gamma-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am. J. Pathol. 2011, 179, 869–879. [Google Scholar] [CrossRef]
- Ng, H.H.; Yildiz, G.S.; Ku, J.M.; Miller, A.A.; Woodman, O.L.; Hart, J.L. Chronic NaHS treatment decreases oxidative stress and improves endothelial function in diabetic mice. Diab. Vasc. Dis. Res. 2017, 14, 246–253. [Google Scholar] [CrossRef]
- Slade, L.; Deane, C.S.; Szewczyk, N.J.; Etheridge, T.; Whiteman, M. Hydrogen sulfide supplementation as a potential treatment for primary mitochondrial diseases. Pharmacol. Res. 2024, 203, 107180. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Preza, F.I.; de la Cruz, S.H.; Santiago-Castaneda, C.; Silva-Velasco, D.L.; Beltran-Ornelas, J.H.; Tapia-Martinez, J.; Sánchez-López, A.; Rocha, L.; Centurión, D. Hydrogen sulfide prevents the vascular dysfunction induced by severe traumatic brain injury in rats by reducing reactive oxygen species and modulating eNOS and H2S-synthesizing enzyme expression. Life Sci. 2023, 312, 121218. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Impact of Hydrogen Sulfide on Mitochondrial and Bacterial Bioenergetics. Int. J. Mol. Sci. 2021, 22, 12688. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113 Pt A, 186–198. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Yi, J.; Hu, H.; Lai, Q.; Nie, L.; Liu, M.; Chu, C.; Yang, J. AP39 through AMPK-ULK1-FUNDC1 pathway regulates mitophagy, inhibits pyroptosis, and improves doxorubicin-induced myocardial fibrosis. iScience. 2024, 27, 109321. [Google Scholar] [CrossRef]
- De Giusti, C.J.; Palomeque, J.; Mattiazzi, A. Ca2+ mishandling and mitochondrial dysfunction: A converging road to prediabetic and diabetic cardiomyopathy. Pflügers Arch.-Eur. J. Physiol. 2022, 474, 33–61. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Li, X.; Wei, X.; Wang, H. Hydrogen Sulfide Plays an Important Role in Diabetic Cardiomyopathy. Front. Cell Dev. Biol. 2021, 9, 627336. [Google Scholar] [CrossRef]
- Chen, P.; Poddar, R.; Tipa, E.V.; Dibello, P.M.; Moravec, C.D.; Robinson, K.; Green, R.; Kruger, W.D.; Garrow, T.A.; Jacobsen, D.W. Homocysteine metabolism in cardiovascular cells and tissues: Implications for hyperhomocysteinemia and cardiovascular disease. Adv. Enzyme. Regul. 1999, 39, 93–109. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.; Zhu, L.H.; Liu, J.X.; Jin, L.Y.; Cui, H.; Yu, L.; Zhang, Y.H. Dual Regulation of Mitochondrial Complexes by H2S via S-Sulfhydration Controls Respiration in Type 1 Diabetic Hearts. Biomolecules 2025, 15, 1197. https://doi.org/10.3390/biom15081197
Su T, Zhu LH, Liu JX, Jin LY, Cui H, Yu L, Zhang YH. Dual Regulation of Mitochondrial Complexes by H2S via S-Sulfhydration Controls Respiration in Type 1 Diabetic Hearts. Biomolecules. 2025; 15(8):1197. https://doi.org/10.3390/biom15081197
Chicago/Turabian StyleSu, Tong, Li Han Zhu, Jun Xian Liu, Li Yuan Jin, Huixing Cui, Longhao Yu, and Yin Hua Zhang. 2025. "Dual Regulation of Mitochondrial Complexes by H2S via S-Sulfhydration Controls Respiration in Type 1 Diabetic Hearts" Biomolecules 15, no. 8: 1197. https://doi.org/10.3390/biom15081197
APA StyleSu, T., Zhu, L. H., Liu, J. X., Jin, L. Y., Cui, H., Yu, L., & Zhang, Y. H. (2025). Dual Regulation of Mitochondrial Complexes by H2S via S-Sulfhydration Controls Respiration in Type 1 Diabetic Hearts. Biomolecules, 15(8), 1197. https://doi.org/10.3390/biom15081197








