A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases
Abstract
1. Introduction
1.1. Oxidative Stress as a Common Pathogenic Feature of Cardiovascular and Metabolic Diseases
1.2. ROS-Sensitive Calcium Channels Relevant to Cardiovascular and Metabolic Diseases
1.3. ROS Activation of TRPM2
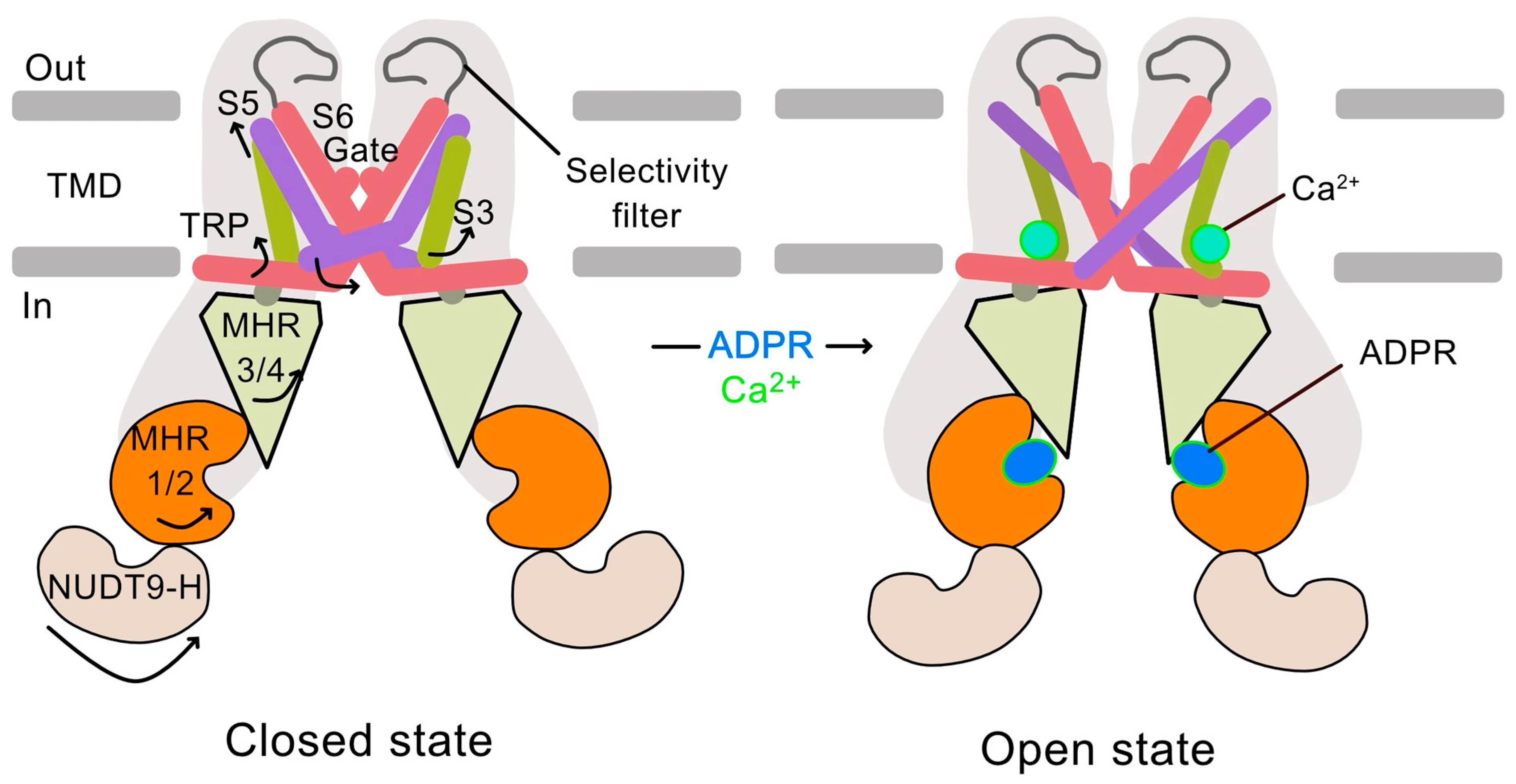
1.4. The TRPM2 Calcium Channel: Structure, Activation, and Function
1.5. Sources of ROS Involved in CVDs and Metabolic Diseases
1.5.1. The NOX Family
1.5.2. Mitochondria
1.6. Antioxidants
1.7. TRPM2-Mediated Ca2+ Influx Triggers a Self-Perpetuating ROS Amplification Cycle
- Stress activates the TRPM2 channel at the plasma membrane, increasing Ca2+ influx.
- The resulting Ca2+ overload leads to lysosomal impairment and redistribution of lysosomal Zn2+ to mitochondria.
- The rise in mitochondrial Zn2+ leads to mitochondrial membrane depolarization, breakdown of the branched network, and excessive ROS (mtROS) generation.
- The mtROS stimulates PARP1 activation in the nucleus, generating ADPR.
- ADPR feeds back to the plasma membrane TRPM2, perpetuating the cycle and exacerbating progressive organelle damage and cell dysfunction.
1.7.1. Step 1: Activation of TRPM2 Channels by External Stressors
1.7.2. Step 2: TRPM2-Mediated Rise in Cytoplasmic Ca2+ Targets Lysosomes
Ca2+ First Targets Lysosomes
Decline in Lysosomal Numbers, ROS Production and Cell Death
Lysosomal Dyshomeostasis in CVDs and Metabolic Diseases
1.7.3. Step 3: A Paradigm Shift–Mitochondrial Damage Is Driven by Lysosomal Zn2+, Not Directly by Ca2+
Ca2+-Induced Rise in Mitochondrial Zn2+ as the Primary Driver of Mitochondrial Damage
Zn2+ Targets Mitochondrial Complexes, Primarily Complex III, to Cause Loss of ΔΨmt and Exacerbate ROS Production
Zn2+ Plays a Major Role in Mitochondrial Fragmentation
Therapeutic Potential of TRPM2-Ca2+-Zn2+-Mediated Mechanism
1.7.4. Step 4: Mitochondrial ROS Generates ADPR from the Nucleus for Feedback Activation of TRPM2, Perpetuating the Cycle
2. Outstanding Questions
| Cardiovascular Diseases | |||||
|---|---|---|---|---|---|
| Disease | TRPM2 | NOX | Zinc Involvement | Mitochondrial ROS | PARP1 |
| Ischaemia- Reperfusion: Stroke | Chemical inhibition or genetic KO of TRPM2 in male mice subjected to I/R injury: ↓Neuronal cell death ↓Infarct size ↓Memory loss [100, 138]. | NOX2 KO in mice subjected to I/R injury: Slows the progression of infarct development but does not prevent overall brain damage [139]. | ↑Zn2+ levels in the brain in TBI, but not in TRPM2 KO mice [100]. Zn2+ chelation (TPEN, Ca-EDTA) in rodent model: ↓Infarct size [104,140,141]. | I/R injury mouse model: ↑Mitochondrial ROS in hippocampus. Scavenging mito-ROS with MitoQ: ↓Hippocampal damage [142] | PARP1 KO in mice: Protects against I/R injury [143]. |
| Ischaemia- Reperfusion: Heart attack | Chemical inhibition or genetic KO of TRPM2 in male mice subjected to I/R injury: ↓Infarct size ↓Inflammation ↑Cardiac outcome [144]. | NOX2 KO in mice subjected to I/R injury: ↓Infarct size [145]. | Zn2+ chelation (TPEN) in rat: ↓Infarct area in rat hearts during I/R injury [146]. ↑Myocardial recovery in isolated hearts, reducing tissue damage during ischemia (ex vivo model) [147]. | Scavenging mito-ROS with MitoQ in I/R injury rat model: ↓Heart dysfunction ↓Mitochondrial damage ↓Cell death [148]. | Chemical inhibition of PARP1 in mice subjected to I/R injury: ↓Infarct size ↓Inflammation ↑Cardiac function [149]. |
| Atherosclerosis | TRPM2 KO in Apoe-/- mice slows AS progression [150]. TRPM2 KO and KD in EC: ↓Mitochondrial dysfunction and damage [60]. | NOX2 KO in Apoe/-e mice: ↓Plaque formation due to NOX2 depletion in macrophages and vessel wall cells [151]. | Zn2+ levels elevated in advanced human atherosclerotic lesions [152]. Excess mitochondrial Zn2+ causes its fragmentation in EC [60]. | Scavenging ROS with MitoQ in Apoe/-e mice: ↓Plaques [153]. | PARP1 chemical inhibition or KO in Apoe/-e mice: ↓Plaque formation ↓ Progression of AS [154]. |
| Hypertension | Patient-derived VSMC: TRPM2 inhibition (siRNA/chemical) reduced Ang II-induced Ca2+ influx. Hypertensive LinA3 mouse model: TRPM2 inhibitors reverse hypertension-associated hypercontractility of mesenteric arteries [155]. TRPM2 activation in EC: ↑Endothelial barrier dysfunction [156]. ↑EC dysfunction [157]. | Rodent models: Ang II-induced nitric oxide production rescued by NOX inhibition [158]. NOX1 KO in mice: ↓Ang II-induced hypertension ↓Vascular ROS and remodelling [159]. | Endothelial cells: High glucose causes Zn2+ dependent mitochondrial damage/dysfunction [60], decreasing NO bioavailability [66]. | Ang II-induced and DOCA salt hypertension mouse models: Mito-ROS scavenger (Mito-TEMPO) caused ↑NO bioavailability ↓Blood pressure [160]. | Patient-derived VSMC: PARP1 upregulated by Ang II [155]. PARP1 activation in EC: ↑Cell death [161]. |
| Metabolic Diseases | |||||
| Disease | TRPM2 | NOX | Zinc | Mitochondrial ROS | PARP1 |
| Type 2 diabetes | TRPM2 KO in mice: ↑Insulin sensitivity ↑Resistance to diet-induced obesity ↑Glucose metabolism ↓Obesity-mediated inflammation [162]. Chemical inhibition or RNAi silencing of TRPM2 in pancreatic β-cells prevents FFA induced: ROS increase, Mitochondrial damage, and Cell death [62]. | NOX2 KO in mice: ↑GSIS ↓ROS production [163]. Pancreatic β-cells/islets exposed to FFA: ↓ROS [62]. | Zn2+ chelation (TPEN): ↓FFA-induced β-cell death [62]. Overexpression of hZnT8: ↑Pancreatic Zn2+ ↓Insulin and glucose tolerance [164]. | Excess nutrition: ↑mtROS production ↑Insulin resistance ↑β-cell dysfunction [165]. Complex III KO mice: Early hyperglycaemia ↑Glucose intolerance ↓GSIS [119]. | PARP1 inhibition in diabetic models: ↑Insulin sensitivity ↓Vascular damage [166]. PARP1 inhibitor (PJ34): ↓Pancreatic β-cell death [62]. |
| Insulin resistance | TRPM2 KO in obese mice: ↓Insulin resistance in EC [157], skeletal muscle, adipose, heart [162]. | NOX2 KO in obese mice: ↓Insulin resistance ↓Superoxide [167]. NOX2 inhibition (chemical and siRNA) in IR -/- mice: ↓Superoxide ↑Vascular function [168]. | Chronic high-dose zinc in mice: ↓Glucose tolerance ↑Insulin resistance [169]. | Azoxystrobin inhibition of Complex III-generated mtROS production in HFD mice: ↑Glucose tolerance ↑Insulin sensitivity ↓Body weight ↓Liver fat ↑Mitochondrial function [170]. | PARP-1 inhibition or KO In HFD mice: ↑Glucose tolerance ↑Insulin sensitivity ↓Weight gain [171]. |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef]
- Nugent, R.; Fottrell, E. Non-communicable diseases and climate change: Linked global emergencies. Lancet 2019, 394, 622–623. [Google Scholar] [CrossRef]
- Tamargo, J.; Kjeldsen, K.P.; Delpon, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; et al. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Heal. 2014, 3, 6. [Google Scholar] [CrossRef]
- Brand, M.D. Riding the tiger—Physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 592–661. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Shitaw, E.E.; AlAhmad, M.; Sivaprasadarao, A. Inter-Organelle Crosstalk in Oxidative Distress: A Unified TRPM2-NOX2 Mediated Vicious Cycle Involving Ca2+, Zn2+, and ROS Amplification. Antioxidants 2025, 14, 776. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V. The TRP channels, a remarkably functional family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef]
- Beech, D.J. Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ. J. 2013, 77, 570–579. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef]
- Bon, R.S.; Wright, D.J.; Beech, D.J.; Sukumar, P. Pharmacology of TRPC Channels and Its Potential in Cardiovascular and Metabolic Medicine. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W. Store-Operated Calcium Entry: An Historical Overview. Adv. Exp. Med. Biol. 2017, 981, 205–214. [Google Scholar] [CrossRef]
- Bovo, E.; Mazurek, S.R.; Zima, A.V. Oxidation of ryanodine receptor after ischemia-reperfusion increases propensity of Ca2+ waves during beta-adrenergic receptor stimulation. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1032–H1040. [Google Scholar] [CrossRef]
- Gyorke, S.; Terentyev, D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 2008, 77, 245–255. [Google Scholar] [CrossRef]
- Fujii, S.; Ushioda, R.; Nagata, K. Redox states in the endoplasmic reticulum directly regulate the activity of calcium channel, inositol 1,4,5-trisphosphate receptors. Proc. Natl. Acad. Sci. USA 2023, 120, e2216857120. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Xu, S.Z.; Sukumar, P.; Zeng, F.; Li, J.; Jairaman, A.; English, A.; Naylor, J.; Ciurtin, C.; Majeed, Y.; Milligan, C.J.; et al. TRPC channel activation by extracellular thioredoxin. Nature 2008, 451, 69–72. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. TRPV(1) channels in cardiovascular system: A double edged sword? Int. J. Cardiol. 2017, 228, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kozai, D.; Kobayashi, R.; Ebert, M.; Mori, Y. Roles of TRPM2 in oxidative stress. Cell Calcium 2011, 50, 279–287. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S.; Mori, Y. Involvement of TRPM2 channel in amplification of reactive oxygen species-induced signaling and chronic inflammation. Nihon Yakurigaku Zasshi (Folia Pharmacol. Jpn.) 2009, 134, 122–126. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhai, Y.; Liang, S.; Mori, Y.; Han, R.; Sutterwala, F.S.; Qiao, L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 2013, 4, 1611. [Google Scholar] [CrossRef]
- Belrose, J.C.; Jackson, M.F. TRPM2: A candidate therapeutic target for treating neurological diseases. Acta Pharmacol. Sin. 2018, 39, 722–732. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S. Targeting TRPM2 in ROS-Coupled Diseases. Pharmaceuticals 2016, 9, 57. [Google Scholar] [CrossRef]
- Perraud, A.L.; Fleig, A.; Dunn, C.A.; Bagley, L.A.; Launay, P.; Schmitz, C.; Stokes, A.J.; Zhu, Q.; Bessman, M.J.; Penner, R.; et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001, 411, 595–599. [Google Scholar] [CrossRef]
- McHugh, D.; Flemming, R.; Xu, S.Z.; Perraud, A.L.; Beech, D.J. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J. Biol. Chem. 2003, 278, 11002–11006. [Google Scholar] [CrossRef]
- Wang, L.; Fu, T.M.; Zhou, Y.; Xia, S.; Greka, A.; Wu, H. Structures and gating mechanism of human TRPM2. Science 2018, 362, eaav4809. [Google Scholar] [CrossRef]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Destée, A.; Gressier, B.; Chartier-Harlin, M.C. NADPH oxidases in Parkinson’s disease: A systematic review. Mol. Neurodegener. 2017, 12, 84. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Noreng, S.; Ota, N.; Sun, Y.; Ho, H.; Johnson, M.; Arthur, C.P.; Schneider, K.; Lehoux, I.; Davies, C.W.; Mortara, K.; et al. Structure of the core human NADPH oxidase NOX2. Nat. Commun. 2022, 13, 6079. [Google Scholar] [CrossRef]
- Block, K.; Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]
- Bleier, L.; Drose, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003, 278, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; Perez-Perez, R.; Lobo-Jarne, T.; Harbour, M.E.; Ding, S.; Penas, A.; Diaz, F.; Moraes, C.T.; Fearnley, I.M.; Zeviani, M.; et al. Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. EMBO J. 2020, 39, e102817. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6, 206. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Zhang, Y.; Wang, Y.; Qiao, X.; Sun, C.; Chen, Y.; Guo, M.; Han, W.; Ye, A.; et al. Precision Redox: The Key for Antioxidant Pharmacology. Antioxid. Redox Signal. 2021, 34, 1069–1082. [Google Scholar] [CrossRef]
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463. [Google Scholar] [CrossRef]
- Abuarab, N.; Munsey, T.; Jiang, L.; Li, J.; Sivaprasadarao, A. High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2+-mediated mitochondrial fission. Sci. Signal. 2017, 10, eaal4161. [Google Scholar] [CrossRef]
- AlAhmad, M.; Isbea, H.; Shitaw, E.; Li, F.; Sivaprasadarao, A. NOX2-TRPM2 coupling promotes Zn2+ inhibition of complex III to exacerbate ROS production in a cellular model of Parkinson’s disease. Sci. Rep. 2024, 14, 18431. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Munsey, T.S.; Sivaprasadarao, A. TRPM2-mediated rise in mitochondrial Zn2+ promotes palmitate-induced mitochondrial fission and pancreatic beta-cell death in rodents. Cell Death Differ. 2017, 24, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Cheung, J.Y. TRPM2 protects against tissue damage following oxidative stress and ischaemia-reperfusion. J. Physiol. 2016, 594, 4181–4191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, L.H. Multiple molecular mechanisms form a positive feedback loop driving amyloid beta42 peptide-induced neurotoxicity via activation of the TRPM2 channel in hippocampal neurons. Cell Death Dis. 2018, 9, 195. [Google Scholar] [CrossRef]
- Manna, P.T.; Munsey, T.S.; Abuarab, N.; Li, F.; Asipu, A.; Howell, G.; Sedo, A.; Yang, W.; Naylor, J.; Beech, D.J.; et al. TRPM2 mediated intracellular Zn2+ release triggers pancreatic beta cell death. Biochem. J. 2015, 466, 537–546. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Yoon, Y.; Galloway, C.A.; Jhun, B.S.; Yu, T. Mitochondrial dynamics in diabetes. Antioxid. Redox Signal. 2011, 14, 439–457. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasadarao, A.; Abuarab, N.; Li, F. TRPM2 channels in mitochondrial dynamics and cancer. Oncotarget 2017, 8, 84620–84621. [Google Scholar] [CrossRef] [PubMed]
- Kondadi, A.K.; Reichert, A.S. Mitochondrial Dynamics at Different Levels: From Cristae Dynamics to Interorganellar Cross Talk. Annu. Rev. Biophys. 2024, 53, 147–168. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Cao, M.; Luo, X.; Wu, K.; He, X. Targeting lysosomes in human disease: From basic research to clinical applications. Signal Transduct. Target. Ther. 2021, 6, 379. [Google Scholar] [CrossRef]
- Tan, J.X.; Finkel, T. Lysosomes in senescence and aging. EMBO Rep. 2023, 24, e57265. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Cang, C.; Aranda, K.; Seo, Y.J.; Gasnier, B.; Ren, D. TMEM175 Is an Organelle K+ Channel Regulating Lysosomal Function. Cell 2015, 162, 1101–1112. [Google Scholar] [CrossRef]
- Jinn, S.; Drolet, R.E.; Cramer, P.E.; Wong, A.H.; Toolan, D.M.; Gretzula, C.A.; Voleti, B.; Vassileva, G.; Disa, J.; Tadin-Strapps, M.; et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 2389–2394. [Google Scholar] [CrossRef]
- Miner, G.E.; Rivera-Kohr, D.A.; Zhang, C.; Sullivan, K.D.; Guo, A.; Fratti, R.A. Reciprocal regulation of vacuolar calcium transport and V-ATPase activity, and the effects of Phosphatidylinositol 3,5-bisphosphate. BioRxiv 2020. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Yang, S.; Li, M.; Wu, Y.; Hu, S.; Wang, W.; Jiang, A.; Zhang, Q.; Zhang, J.; et al. The HEAT repeat protein HPO-27 is a lysosome fission factor. Nature 2024, 628, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, S.; Scotto-Rosato, A.; Medina, D.L. TRPML1: The Ca2+ retaker of the lysosome. Cell Calcium 2018, 69, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, L.; Lotfi, P.; Pal, R.; Ronza, A.D.; Sharma, J.; Sardiello, M. Lysosome biogenesis in health and disease. J. Neurochem. 2019, 148, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Todkar, K.; Ilamathi, H.S.; Germain, M. Mitochondria and Lysosomes: Discovering Bonds. Front. Cell Dev. Biol. 2017, 5, 106. [Google Scholar] [CrossRef]
- Bourdenx, M.; Dehay, B. What lysosomes actually tell us about Parkinson’s disease? Ageing Res. Rev. 2016, 32, 140–149. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Hughes, A.L.; Madeo, F.; Ruckenstuhl, C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 2016, 32, 2–12. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- Nixon, R.A. New perspectives on lysosomes in ageing and neurodegenerative disease. Ageing Res. Rev. 2016, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Plotegher, N.; Duchen, M.R. Crosstalk between Lysosomes and Mitochondria in Parkinson’s Disease. Front. Cell Dev. Biol. 2017, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Udayar, V.; Chen, Y.; Sidransky, E.; Jagasia, R. Lysosomal dysfunction in neurodegeneration: Emerging concepts and methods. Trends Neurosci. 2022, 45, 184–199. [Google Scholar] [CrossRef]
- Ischia, J.; Bolton, D.M.; Patel, O. Why is it worth testing the ability of zinc to protect against ischaemia reperfusion injury for human application. Metallomics 2019, 11, 1330–1343. [Google Scholar] [CrossRef]
- Ji, S.G.; Medvedeva, Y.V.; Wang, H.L.; Yin, H.Z.; Weiss, J.H. Mitochondrial Zn2+ Accumulation: A Potential Trigger of Hippocampal Ischemic Injury. Neuroscientist 2019, 25, 126–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, F.; Yin, J.; Pan, R.; Shi, W.; Qi, Z.; Fang, Y.; Huang, Y.; Li, S.; Luo, Y.; et al. Synergistic Interaction Between Zinc and Reactive Oxygen Species Amplifies Ischemic Brain Injury in Rats. Stroke 2018, 49, 2200–2210. [Google Scholar] [CrossRef]
- Ye, M.; Yang, W.; Ainscough, J.F.; Hu, X.P.; Li, X.; Sedo, A.; Zhang, X.H.; Zhang, X.; Chen, Z.; Li, X.M.; et al. TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis. 2014, 5, e1541. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gale, J.R.; Reynolds, I.J.; Weiss, J.H.; Aizenman, E. The Multifaceted Roles of Zinc in Neuronal Mitochondrial Dysfunction. Biomedicines 2021, 9, 489. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Lin, B.; Shuttleworth, C.W.; Weiss, J.H. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 1105–1114. [Google Scholar] [CrossRef]
- Shuttleworth, C.W.; Weiss, J.H. Zinc: New clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011, 32, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.; McClanahan, T.; Ji, S.G.; Weiss, J.H. Mechanisms of rapid reactive oxygen species generation in response to cytosolic Ca2+ or Zn2+ loads in cortical neurons. PLoS ONE 2013, 8, e83347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, R.; Li, S.; Luo, Y.; Yan, F.; Yin, J.; Qi, Z.; Yan, Y.; Ji, X.; Liu, K.J. Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke 2014, 45, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Abuarab, N.; Sivaprasadarao, A. Reciprocal regulation of actin cytoskeleton remodelling and cell migration by Ca2+ and Zn2+: Role of TRPM2 channels. J. Cell Sci. 2016, 129, 2016–2029. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Dineley, K.E.; Richards, L.L.; Votyakova, T.V.; Reynolds, I.J. Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria. Mitochondrion 2005, 5, 55–65. [Google Scholar] [CrossRef]
- Deus, C.M.; Yambire, K.F.; Oliveira, P.J.; Raimundo, N. Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends Mol. Med. 2020, 26, 71–88. [Google Scholar] [CrossRef]
- Kiraly, S.; Stanley, J.; Eden, E.R. Lysosome-Mitochondrial Crosstalk in Cellular Stress and Disease. Antioxidants 2025, 14, 125. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef]
- Park, J.S.; Koentjoro, B.; Veivers, D.; Mackay-Sim, A.; Sue, C.M. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 2014, 23, 2802–2815. [Google Scholar] [CrossRef]
- Tsunemi, T.; Krainc, D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet. 2014, 23, 2791–2801. [Google Scholar] [CrossRef]
- Link, T.A.; von Jagow, G. Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group. J. Biol. Chem. 1995, 270, 25001–25006. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, M.S.; Hirst, J. The inhibition of mitochondrial complex I (NADH:ubiquinone oxidoreductase) by Zn2+. J. Biol. Chem. 2006, 281, 34803–34809. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kowada, T.; Du, Y.; Amagai, Y.; Matsui, T.; Inaba, K.; Mizukami, S. Organelle-Level Labile Zn. ACS Sens. 2022, 7, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.L.; Vargas, L.; Turk, C.N.; Baaten, J.E.; Matzen, J.T.; Dardov, V.J.; Attle, S.J.; Li, J.; Quackenbush, D.C.; Goncalves, R.L.; et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 2015, 11, 834–836. [Google Scholar] [CrossRef]
- Diaz, F.; Garcia, S.; Padgett, K.R.; Moraes, C.T. A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum. Mol. Genet. 2012, 21, 5066–5077. [Google Scholar] [CrossRef]
- Lang, A.L.; Nissanka, N.; Louzada, R.A.; Tamayo, A.; Pereira, E.; Moraes, C.T.; Caicedo, A. A Defect in Mitochondrial Complex III but Not in Complexes I or IV Causes Early beta-Cell Dysfunction and Hyperglycemia in Mice. Diabetes 2023, 72, 1262–1276. [Google Scholar] [CrossRef]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Tabara, L.C.; Segawa, M.; Prudent, J. Molecular mechanisms of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2025, 26, 123–146. [Google Scholar] [CrossRef]
- Slupe, A.M.; Merrill, R.A.; Flippo, K.H.; Lobas, M.A.; Houtman, J.C.; Strack, S. A calcineurin docking motif (LXVP) in dynamin-related protein 1 contributes to mitochondrial fragmentation and ischemic neuronal injury. J. Biol. Chem. 2013, 288, 12353–12365. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Nhu, N.T.; Li, Q.; Liu, Y.; Xu, J.; Xiao, S.Y.; Lee, S.D. Effects of Mdivi-1 on Neural Mitochondrial Dysfunction and Mitochondria-Mediated Apoptosis in Ischemia-Reperfusion Injury After Stroke: A Systematic Review of Preclinical Studies. Front. Mol. Neurosci. 2021, 14, 778569. [Google Scholar] [CrossRef]
- Ding, M.; Dong, Q.; Liu, Z.; Liu, Z.; Qu, Y.; Li, X.; Huo, C.; Jia, X.; Fu, F.; Wang, X. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc. Diabetol. 2017, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Kugler, B.A.; Deng, W.; Duguay, A.L.; Garcia, J.P.; Anderson, M.C.; Nguyen, P.D.; Houmard, J.A.; Zou, K. Pharmacological inhibition of dynamin-related protein 1 attenuates skeletal muscle insulin resistance in obesity. Physiol. Rep. 2021, 9, e14808. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Colecraft, H.M.; Trimmer, J.S. Controlling ion channel function with renewable recombinant antibodies. J. Physiol. 2022, 600, 2023–2036. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
- Faux, N.G.; Ritchie, C.W.; Gunn, A.; Rembach, A.; Tsatsanis, A.; Bedo, J.; Harrison, J.; Lannfelt, L.; Blennow, K.; Zetterberg, H.; et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: Additional phase II analyses. J. Alzheimer’s Dis. JAD 2010, 20, 509–516. [Google Scholar] [CrossRef]
- Priel, T.; Aricha-Tamir, B.; Sekler, I. Clioquinol attenuates zinc-dependent beta-cell death and the onset of insulitis and hyperglycemia associated with experimental type I diabetes in mice. Eur. J. Pharmacol. 2007, 565, 232–239. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, W.; Xu, H.; Zhou, J.M.; Wang, Z.Y. Clioquinol inhibits zinc-triggered caspase activation in the hippocampal CA1 region of a global ischemic gerbil model. PLoS ONE 2010, 5, e11888. [Google Scholar] [CrossRef]
- Krishnakumar, R.; Kraus, W.L. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef]
- Rey, G.; Reddy, A.B. Interplay between cellular redox oscillations and circadian clocks. Diabetes Obes. Metab. 2015, 17 (Suppl. 1), 55–64. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Reinke, H.; Altmeyer, M.; Gutierrez-Arcelus, M.; Hottiger, M.O.; Schibler, U. Poly(ADP-Ribose) Polymerase 1 Participates in the Phase Entrainment of Circadian Clocks to Feeding. Cell 2010, 142, 943–953. [Google Scholar] [CrossRef]
- Jia, J.; Verma, S.; Nakayama, S.; Quillinan, N.; Grafe, M.R.; Hurn, P.D.; Herson, P.S. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011, 31, 2160–2168. [Google Scholar] [CrossRef]
- McCann, S.K.; Dusting, G.J.; Roulston, C.L. Nox2 knockout delays infarct progression and increases vascular recovery through angiogenesis in mice following ischaemic stroke with reperfusion. PLoS ONE 2014, 9, e110602. [Google Scholar] [CrossRef]
- Calderone, A.; Jover, T.; Mashiko, T.; Noh, K.M.; Tanaka, H.; Bennett, M.V.; Zukin, R.S. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 9903–9913. [Google Scholar] [CrossRef]
- Wang, W.M.; Liu, Z.; Liu, A.J.; Wang, Y.X.; Wang, H.G.; An, D.; Heng, B.; Xie, L.H.; Duan, J.L.; Liu, Y.Q. The Zinc Ion Chelating Agent TPEN Attenuates Neuronal Death/apoptosis Caused by Hypoxia/ischemia Via Mediating the Pathophysiological Cascade Including Excitotoxicity, Oxidative Stress, and Inflammation. CNS Neurosci. Ther. 2015, 21, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Abdel Mageed, S.S.; Safar, M.M.; El-Yamany, M.F.; Oraby, M.A. MitoQ alleviates hippocampal damage after cerebral ischemia: The potential role of SIRT6 in regulating mitochondrial dysfunction and neuroinflammation. Life Sci. 2023, 328, 121895. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, M.J.; Sampei, K.; Mandir, A.S.; Hurn, P.D.; Traystman, R.J.; Bao, J.; Pieper, A.; Wang, Z.Q.; Dawson, T.M.; Snyder, S.H.; et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997, 3, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Wajima, T.; Negoro, T.; Ishii, M.; Nakano, Y.; Kiuchi, Y.; Mori, Y.; Shimizu, S. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013, 97, 271–281. [Google Scholar] [CrossRef]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Ikeda, Y.; Oka, S.; Fong, G.H.; Tian, R.; Sadoshima, J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circ. Res. 2013, 112, 1135–1149. [Google Scholar] [CrossRef]
- Lin, C.L.; Tseng, H.C.; Chen, R.F.; Chen, W.P.; Su, M.J.; Fang, K.M.; Wu, M.L. Intracellular zinc release-activated ERK-dependent GSK-3beta-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ. 2011, 18, 1651–1663. [Google Scholar] [CrossRef]
- Karck, M.; Appelbaum, Y.; Schwalb, H.; Haverich, A.; Chevion, M.; Uretzky, G. TPEN, a transition metal chelator, improves myocardial protection during prolonged ischemia. J. Heart Lung Transpl. 1992, 11, 979–985. [Google Scholar]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.; Murphy, M.P.; Sammut, I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef]
- Luo, J.M.; Lin, H.B.; Weng, Y.Q.; Lin, Y.H.; Lai, L.Y.; Li, J.; Li, F.X.; Xu, S.Y.; Zhang, H.F.; Zhao, W. Inhibition of PARP1 improves cardiac function after myocardial infarction via up-regulated NLRC5. Chem. Biol. Interact. 2024, 395, 111010. [Google Scholar] [CrossRef]
- Zong, P.; Feng, J.; Yue, Z.; Yu, A.S.; Vacher, J.; Jellison, E.R.; Miller, B.; Mori, Y.; Yue, L. TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2-CD36 inflammatory axis in macrophages. Nat. Cardiovasc. Res. 2022, 1, 344–360. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Hakim, Z.S.; Madamanchi, N.R.; Rojas, M.; Madamanchi, C.; Runge, M.S. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Stadler, N.; Stanley, N.; Heeneman, S.; Vacata, V.; Daemen, M.J.; Bannon, P.G.; Waltenberger, J.; Davies, M.J. Accumulation of zinc in human atherosclerotic lesions correlates with calcium levels but does not protect against protein oxidation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1024–1030. [Google Scholar] [CrossRef]
- Mercer, J.R.; Yu, E.; Figg, N.; Cheng, K.K.; Prime, T.A.; Griffin, J.L.; Masoodi, M.; Vidal-Puig, A.; Murphy, M.P.; Bennett, M.R. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/-/ApoE-/- mice. Free Radic. Biol. Med. 2012, 52, 841–849. [Google Scholar] [CrossRef]
- Oumouna-Benachour, K.; Hans, C.P.; Suzuki, Y.; Naura, A.; Datta, R.; Belmadani, S.; Fallon, K.; Woods, C.; Boulares, A.H. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: Effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation 2007, 115, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, R.; Neves, K.B.; Anagnostopoulou, A.; Rios, F.J.; Lacchini, S.; Montezano, A.C.; Touyz, R.M. Crosstalk Between Vascular Redox and Calcium Signaling in Hypertension Involves TRPM2 (Transient Receptor Potential Melastatin 2) Cation Channel. Hypertension 2020, 75, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.M.; Malik, A.B. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 2009, 101, 619–625. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.L.; Ye, F.; Xie, J.W.; Zeng, J.W.; Qin, L.; Xue, J.; Wang, Y.T.; Guo, K.M.; Ma, M.M.; et al. Free fatty acid-induced H2O2 activates TRPM2 to aggravate endothelial insulin resistance via Ca2+-dependent PERK/ATF4/TRB3 cascade in obese mice. Free Radic. Biol. Med. 2019, 143, 288–299. [Google Scholar] [CrossRef]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef]
- Mathews, M.T.; Berk, B.C. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 711–717. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Jung, D.Y.; Ko, H.J.; Lee, Y.; Friedline, R.H.; Lee, E.; Jun, J.; Ma, Z.; Kim, F.; et al. TRPM2 Ca2+ channel regulates energy balance and glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E807–E816. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, B.; Brun, T.; Deffert-Delbouille, C.; Mahiout, Z.; Daali, Y.; Ma, X.J.; Krause, K.H.; Maechler, P. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes 2012, 61, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bai, S.; Sheline, C.T. hZnT8 (Slc30a8) Transgenic Mice That Overexpress the R325W Polymorph Have Reduced Islet Zn2+ and Proinsulin Levels, Increased Glucose Tolerance After a High-Fat Diet, and Altered Levels of Pancreatic Zinc Binding Proteins. Diabetes 2017, 66, 551–559. [Google Scholar] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys) function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of poly (ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: Endothelial dysfunction, as a common underlying theme. Antioxid. Redox Signal. 2005, 7, 1568–1580. [Google Scholar] [CrossRef]
- Souto Padron de Figueiredo, A.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef]
- Sukumar, P.; Viswambharan, H.; Imrie, H.; Cubbon, R.M.; Yuldasheva, N.; Gage, M.; Galloway, S.; Skromna, A.; Kandavelu, P.; Santos, C.X.; et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes 2013, 62, 2130–2134. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, D.; Zhu, Y.; Fang, Z.; Che, L.; Lin, Y.; Xu, S.; Li, J.; Huang, C.; Zou, Y.; et al. Chronic High Dose Zinc Supplementation Induces Visceral Adipose Tissue Hypertrophy without Altering Body Weight in Mice. Nutrients 2017, 9, 14. [Google Scholar] [CrossRef]
- Gao, A.H.; Fu, Y.Y.; Zhang, K.Z.; Zhang, M.; Jiang, H.W.; Fan, L.X.; Nan, F.J.; Yuan, C.G.; Li, J.; Zhou, Y.B.; et al. Azoxystrobin, a mitochondrial complex III Qo site inhibitor, exerts beneficial metabolic effects in vivo and in vitro. Biochim. Biophys. Acta 2014, 1840, 2212–2221. [Google Scholar] [CrossRef]
- Bai, P.; Canto, C.; Oudart, H.; Brunyanszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlAhmad, M.; Shitaw, E.E.; Sivaprasadarao, A. A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases. Biomolecules 2025, 15, 1193. https://doi.org/10.3390/biom15081193
AlAhmad M, Shitaw EE, Sivaprasadarao A. A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases. Biomolecules. 2025; 15(8):1193. https://doi.org/10.3390/biom15081193
Chicago/Turabian StyleAlAhmad, Maali, Esra Elhashmi Shitaw, and Asipu Sivaprasadarao. 2025. "A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases" Biomolecules 15, no. 8: 1193. https://doi.org/10.3390/biom15081193
APA StyleAlAhmad, M., Shitaw, E. E., & Sivaprasadarao, A. (2025). A TRPM2-Driven Signalling Cycle Orchestrates Abnormal Inter-Organelle Crosstalk in Cardiovascular and Metabolic Diseases. Biomolecules, 15(8), 1193. https://doi.org/10.3390/biom15081193






