Reconstructing the Antibiotic Pipeline: Natural Alternatives to Antibacterial Agents
Abstract
1. Introduction
2. Antibiotics and the Emergence of AMR
2.1. Efflux Pump Mechanism
2.2. Modification of Molecular Target
2.3. Enzymatic Inactivation of Antibiotics
2.4. Limiting Drug Uptake
3. The State of Global AMR
4. Natural Alternatives to Antibacterial Agents
4.1. Some Antibacterial Alternatives of Natural Origin
4.1.1. Plant Derivatives
4.1.2. Bacteriophages
| Bacteriophage | Report Type | Target Bacteria | Administration Route | Result | Reference |
|---|---|---|---|---|---|
| ɸ9184 | Case report | Enterococcus faecium | Intravenous and oral adjuvant with daptomycin and vancomycin | Clinical improvement within 24 h is indicated by bacterial growth suppression. | [119] |
| Intestine bacteriophage cocktail | Case report | MDR (P. aeruginosa, E. coli, K. pneumoniae, P. mirabilis) and vancomycin-resistant E. faecium | Transdermal using hydrogel | Successful treatment of fracture-related infections with no need for surgical revision after a year. | [120] |
| 14/10, PT07 and PNM P. aeruginosa bacteriophage | Case report | P. aeruginosa | Intravenous administration with ceftazidime–avibactam | A new P. aeruginosa infection occurred upon completion of phage therapy. Biofilm and phage-resistant mutants indicate possible antibiotic-influenced stress. | [121] |
| Phage-Muddy Muddy_HRMGD04 | Case report | Mycobacterium chelonae | Intravenous adjuvant | Host immune reaction with stable improvement of the cutaneous infection. | [122] |
| D29_HRMGD40 BPsΔ33HTH_HRM10 | Case study | Mycobacterium abscessus | Intravenous administration | Typical decline in bacterial diversity and no observed increase in resistance to phage therapy or antibiotics. | [123] |
| Different phage therapy | Review of cases | Mainly M. abscessus, S. aureus, P. aeruginosa | - | A 70% success rate among 17 patients, with 2 cases of failure. | [124] |
| TSPphg | Case study | MRSA | Topical application | Significant reduction in bacterial count at 68 µg/mL for 90 min relative to kanamycin. | [125] |
| AB-PA01 | Case report | P. aeruginosa | Nebulised and intravenous adjuvant | Successful resolution of the infection, along with the apparent elimination of pathogen colonisation. | [126] |
| Anti-K. pneumoniae phage | Case report | β-Lactamase-positive K. pneumoniae | Intravesical and oral routes. Administered with meropenem | Lytic activity of bacteriophage was established using a spot test; however, treatment was specific to a single strain. | [127] |
| vB_Ts2631 | Case study | Acinetobacter baumannii, P. aeruginosa, and members of Enterobacteriaceae | Endolysins | Electron microscopy of A. baumannii showed cell wall damage with cytoplasmic leakage accompanied by apparent signs of cell decay. | [128] |
| OMKO1 | Case report | P. aeruginosa | Administered as injectables with ceftazidime | No evidence of recurring infection was observed in the patient. Potentially, the phage can attack bacteria. | [129] |
| ɸABKT21phi3 ɸKpKT21phi1 | Case report | K. pneumoniae, A. baumannii | Intravenous adjuvant with meropenem and colistin | Graft healing and the absence of chronic pain in the patient’s bone were observed. After 8 months, complete absence of pathogens was reported. | [130] |
| Achromobacter phage cocktail | Case report | MDR Achromobacter xylosoxidans | Inhaled and oral routes. Administered with Piperacillin/Tazobactam | Improved lung function indicates the treatment of cystic fibrosis infection in a patient. | [131] |
| Staphylococcal phage Sb-1 | Case report | MRSA | Interstitial/intraosseous and later administered levofloxacin. | Long-term resolution of diabetic foot ulcer with no event of reoccurrence. | [132] |
4.1.3. Microbiome-Based Interventions
| Microbiome-Based Interventions | Type | Bacteria | Antibacterial Activity | Reference |
|---|---|---|---|---|
| Mulberry and Bacillus spp.-derived postbiotics | Postbiotics | E. faecalis, E. coli, Salmonella spp., and S. aureus | Variable antibacterial activity against the tested bacterial strains via the formation of complexes with cell walls. MICs of 30–40.5 mg/mL. | [151] |
| Ascophillum nodosum and Lithothamnium calcareum | Prebiotics | E. coli | Antibacterial activity possibly due to acidity, competition for nutrients, induction of host immune cells, and production of bacteriocins at MICs of 18 and 20 mg/mL. | [94] |
| Lacticaseibacillus rhamnosus | Probiotics | Streptococcus mutans | A decrease in the number of viable bacteria indicates antibacterial and antibiofilm potential. | [152] |
| Lactobacillus casei and L. plantarum | Postbiotics | S. mutans | At MICs of 64 µg/mL and 128 µg/mL, Lactobacillus casei and Lactobacillus plantarum inhibited bacteria. | [153] |
| Bacillus amyloliquefaciens and L. plantarum | Postbiotics | E. coli, P. aeruginosa, Salmonella spp., Clostridium spp., and S. aureus | Showed broad-spectrum antimicrobial activity against tested isolates and exhibited an immune response with an MIC of 25 mg/mL. | [154] |
| Lactobacillus paracasei ET-22 | Postbiotics | S. mutans | The living bacteria, heat-killed bacteria, and secretions of postbiotics showed antibiofilm function. | [155] |
| L. plantarum | Postbiotics | Salmonella spp. | The in vitro study showed a triggered AMP-activated protein kinase (AMPK) signalling pathway that induced autophagy with an MIC of 25 mg/mL. | [156] |
| L. plantarum EIR/IF-1 | Probiotics | Prevotella denticola, Streptococcus sanguinis, and Fusobacterium nucleatum | Inhibition of microbial growth and inhibited biofilm formation with an MIC of 12.5 mg/mL. | [157] |
| Saccharomyces cerevisiae (PTCC 5269) | Postbiotics | Salmonella typhi, Streptococcus mutans, E. coli, and Listeria monocytogenes | Reduced cell viability, suppressed cell division, and induced apoptosis in bacterial cells. | [158] |
| Lactobacillus spp. | Probiotics | E. faecalis | A cocktail mix of three species of Lactobacillus spp. showed high inhibitory activity relative to a single supernatant and a common treatment option against the bacterium with an MIC of 50 mg/mL. | [159] |
| Leuconostoc mesenteroides | Postbiotics | Vibrio spp., P. aeruginosa, and E. coli | Exerted inhibitive activity on isolates alone and in combination with an essential oil. The leakage of intracellular metabolites and DNA damage was reported at an MIC of 0.5 µg/mL. | [160] |
| Gut microbiome | FMT | ESBL (E. coli and K. pneumoniae) MDR Enterobacter aerogenes | Change in resistant microbial profile, followed by the absence of resistant species. | [161] |
| L. plantarum | Probiotics | P. aeruginosa, S. typhimurium, B. spp., E. coli, and S. aureus | Broad-spectrum bacterial inhibitory activity. | [162] |
| Phthalyl pullulan NAPs-treated L. plantarum | Synbiotics | E. coli and Listeria monocytogenes | Antibacterial activity via the production of plantarcin due to intracellular stimulation. | [163] |
4.1.4. Metals
4.1.5. Antimicrobial Peptides (AMPs)
4.1.6. Immunomodulating Agents
4.1.7. Antimicrobial Enzymes
5. Roles of Antibiotic Alternatives in Mitigation of AMR
6. Current Challenges in Implementation
7. Recent Trends in Drug Discovery, Future Directions, and Research Needs
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samal, J. A historical exploration of pandemics of some selected diseases in the world. Int. J. Health Sci. Res. 2014, 4, 165–169. [Google Scholar]
- Barbieri, R.; Signoli, M.; Chevé, D.; Costedoat, C.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. Yersinia pestis: The natural history of plague. Clin. Microbiol. Rev. 2020, 34, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; Afifi, W.M.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Rev. Infect. Dis. 1980, 2, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A. Antibiotics in contemporary medicine: Advances, obstacles, and the future. Bull. J. Multidiscip. Ilmu 2023, 2, 548–557. [Google Scholar]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Christensen, S.B. Drugs that changed society: History and current status of the early antibiotics: Salvarsan, sulfonamides, and β-lactams. Molecules 2021, 26, 6057. [Google Scholar] [CrossRef]
- Smith, W.P.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 4 June 2025).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 1 May 2025).
- Peng, J.-J.; Balasubramanian, B.; Ming, Y.-Y.; Niu, J.-L.; Yi, C.-M.; Ma, Y.; Liu, W.-C. Identification of antimicrobial resistance genes and drug resistance analysis of Escherichia coli in the animal farm environment. J. Infect. Public Health 2021, 14, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Vingino, A.; Roberts, M.C.; Wainstein, M.; West, J.; Norman, S.A.; Lambourn, D.; Lahti, J.; Ruiz, R.; D’Angeli, M.; Weissman, S.J. Surveillance for antibiotic-resistant E. coli in the Salish Sea Ecosystem. Antibiotics 2021, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Releases Report on State of Development of Antibacterials; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Scotti, R.; Casciaro, B.; Stringaro, A.; Maggi, F.; Colone, M.; Gabbianelli, R. Fighting microbial infections from Escherichia coli O157: H7: The combined use of three essential oils of the cymbopogon genus and a derivative of esculentin-1a peptide. Antibiotics 2024, 13, 86. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as antibiotic alternatives for human and animal applications. Encyclopedia 2023, 3, 561–581. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Anderson, M.; Panteli, D.; Van Kessel, R.; Ljungqvist, G.; Colombo, F.; Mossialos, E. Challenges and opportunities for incentivising antibiotic research and development in Europe. Lancet Reg. Health–Eur. 2023, 33, 100705. [Google Scholar] [CrossRef]
- Elsayad, K. What ancient Egyptian medicine can teach us. JCO Glob. Oncol. 2023, 9, e2300146. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Singh, C.K. Recent development in the sustainable remediation of antibiotics: A review. Total Environ. Res. Themes 2022, 3, 100008. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.W.; Tiong, H.L.M.; Luang-In, V.; Ma, N.L. An overview of antibiotic and antibiotic resistance. Environ. Adv. 2023, 11, 100331. [Google Scholar] [CrossRef]
- Larsson, D.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef]
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; Perez Del Molino, M.; Bou, G.; García-Contreras, R.; Wood, T.; Tomás, M. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Pérez-Varela, M.; Corral, J.; Aranda, J.; Barbé, J. Roles of efflux pumps from different superfamilies in the surface-associated motility and virulence of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2019, 63, e02190.e18. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Neu, H.M.; Gilbreath, J.J.; Michel, S.L.; Zurawski, D.V.; Merrell, D.S. Copper resistance of the emerging pathogen Acinetobacter baumannii. Appl. Environ. Microbiol. 2016, 82, 6174–6188. [Google Scholar] [CrossRef] [PubMed]
- Zack, K.M.; Sorenson, T.; Joshi, S.G. Types and mechanisms of Efflux Pump Systems and the potential of Efflux Pump Inhibitors in the restoration of Antimicrobial susceptibility, with a special reference to Acinetobacter baumannii. Pathogens 2024, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Kornelsen, V.; Kumar, A. Update on multidrug resistance efflux pumps in Acinetobacter spp. Antimicrob. Agents Chemother. 2021, 65, e0051421. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, C.K.; Kumar, M.; Singh, D.K. Whole-genome sequencing of Alcaligenes sp. strain MMA: Insight into the antibiotic and heavy metal resistant genes. Front. Pharmacol. 2023, 14, 1144561. [Google Scholar] [CrossRef]
- Sethuvel, D.P.M.; Bakthavatchalam, Y.D.; Karthik, M.; Irulappan, M.; Shrivastava, R.; Periasamy, H.; Veeraraghavan, B. β-Lactam resistance in ESKAPE pathogens mediated through modifications in penicillin-binding proteins: An overview. Infect. Dis. Ther. 2023, 12, 829–841. [Google Scholar] [CrossRef]
- Kuo, C.-J.; Ke, J.-N.; Kuo, T.; Lin, C.-Y.; Hsieh, S.-Y.; Chiu, Y.-F.; Wu, H.-Y.; Huang, M.-Z.; Bui, N.-N.; Chiu, C.-H. Multiple amino acid substitutions in penicillin-binding protein-1A confer amoxicillin resistance in refractory Helicobacter pylori infection. J. Microbiol. Immunol. Infect. 2023, 56, 40–47. [Google Scholar] [CrossRef]
- Aribisala, J.O.; S’thebe, N.W.; Sabiu, S. In silico exploration of phenolics as modulators of penicillin binding protein (PBP) 2× of Streptococcus pneumoniae. Sci. Rep. 2024, 14, 8788. [Google Scholar] [CrossRef]
- Egorov, A.; Ulyashova, M.; Rubtsova, M.Y. Bacterial enzymes and antibiotic resistance. Acta Naturae 2018, 10, 33–48. [Google Scholar] [CrossRef]
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, a025395. [Google Scholar] [CrossRef]
- Gjonbalaj, M.; Keith, J.W.; Do, M.H.; Hohl, T.M.; Pamer, E.G.; Becattini, S. Antibiotic degradation by commensal microbes shields pathogens. Infect. Immun. 2020, 88, e00012–e00020. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Rodrigues, S.C.; Duarte, F.V.; Costa, P.M.d.; Costa, P.M.d. The role of outer membrane proteins in UPEC antimicrobial resistance: A systematic review. Membranes 2022, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer membrane porins contribute to antimicrobial resistance in gram-negative bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zheng, X.; Sun, Y.; Fang, R.; Zhang, S.; Zhang, X.; Lin, J.; Cao, J.; Zhou, T. Molecular mechanisms and epidemiology of carbapenem-resistant Escherichia coli isolated from Chinese patients during 2002–2017. Infect. Drug Resist. 2020, 13, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, S.A.; Lee, S.; Housden, N.G.; Kaminska, R.; Kleanthous, C.; Bayley, H. Orientation of the OmpF porin in planar lipid bilayers. Chembiochem 2017, 18, 554–562. [Google Scholar] [CrossRef]
- Choi, U.; Lee, C.-R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The two weapons against bacterial biofilms: Detection and treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef]
- Haidar, A.; Muazzam, A.; Nadeem, A.; Atique, R.; Saeed, H.A.; Naveed, A.; Sharif, J.; Perveen, A.; Fatima, H.R.; Samad, A. Biofilm formation and antibiotic resistance in Pseudomonas aeruginosa. Microbe 2024, 3, 100078. [Google Scholar] [CrossRef]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, Y.; Li, Z.; Pang, X.; Gao, X. Quorum Sensing: Not Just a Bridge Between Bacteria. MicrobiologyOpen 2025, 14, e70016. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding quorum-sensing and biofilm forming in anaerobic bacterial communities. Int. J. Mol. Sci. 2024, 25, 12808. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, X.; Li, M.; Liu, Y.; Ma, J.; Wang, X.; Yu, Z.; Cheng, W.; Zhang, W.; Sun, H. Relationship between biofilm formation and antibiotic resistance of Klebsiella pneumoniae and updates on antibiofilm therapeutic strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1324895. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Piątkowski, J. The role of human oral microbiome in dental biofilm formation. In Microbial Biofilms—Importance and Applications; InTech: London, UK, 2016; pp. 329–382. [Google Scholar] [CrossRef]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microb. Biofilms 2015, 3(3), 287–300. [Google Scholar]
- Singh, S.; Selvakumar, S.; Swaminathan, P. Harnessing Advanced Molecular Diagnostics and Bioinformatics to Ascertain Antimicrobial Resistance in ESKAPE Organisms. Microbe 2025, 7, 100316. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial resistance: Addressing a global threat to humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- WHO. Global Health Issues to Track In 2021; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. 2021 Antibacterial Agents In Clinical and Preclinical Development: An Overview and Analysis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar]
- Sartorius, B.; Gray, A.P.; Weaver, N.D.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: A cross-country systematic analysis. Lancet Glob. Health 2024, 12, e201–e216. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (Glass) Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Magnusson, U.; Moodley, A.; Osbjer, K. Antimicrobial resistance at the livestock-human interface: Implications for Veterinary Services. Rev. Sci. Et Tech. 2021, 40, 511–521. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.; Dissanayake, R.; Klemperer, K.; Toxvaerd, F.; Sharland, M.; Center for Global Development. The Economics of Antibiotic Resistance (Report No. 682); Center for Global Development: Washington, DC, USA, 2024. [Google Scholar]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef] [PubMed]
- Baris, E.; Thiebaud, A.; Evans, T. Containing Antimicrobial Resistance is a Smart Investment in Global Public Health and Wealth. 2017. Available online: http://resistancecontrol.info/2017/containing-antimicrobial-resistance-is-a-smart-investment-in-global-public-health-and-wealth/ (accessed on 13 May 2025).
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-resistant infections: A threat to our economic future. World Bank Rep. 2017, 2, 1–132. [Google Scholar]
- Ahmed, S.A.; Barış, E.; Go, D.S.; Lofgren, H.; Osorio-Rodarte, I.; Thierfelder, K. Assessing the global economic and poverty effects of antimicrobial resistance. World Dev. 2018, 111, 148–160. [Google Scholar] [CrossRef]
- Chindelevitch, L.; Jauneikaite, E.; Wheeler, N.E.; Allel, K.; Ansiri-Asafoakaa, B.Y.; Awuah, W.A.; Bauer, D.C.; Beisken, S.; Fan, K.; Grant, G. Applying data technologies to combat AMR: Current status, challenges, and opportunities on the way forward. arXiv 2022, arXiv:2208.04683. [Google Scholar] [CrossRef]
- Frost, I.; Kapoor, G.; Craig, J.; Liu, D.; Laxminarayan, R. Status, challenges and gaps in antimicrobial resistance surveillance around the world. J. Glob. Antimicrob. Resist. 2021, 25, 222–226. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Sam, S. Importance and effectiveness of herbal medicines. J. Pharmacogn. Phytochem. 2019, 8, 354–357. [Google Scholar]
- Hardy, K. Paleomedicine and the use of plant secondary compounds in the Paleolithic and Early Neolithic. Evol. Anthropol. Issues News Rev. 2019, 28, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K. Paleomedicine and the evolutionary context of medicinal plant use. Rev. Bras. De Farmacogn. 2021, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 1–28. [Google Scholar] [CrossRef]
- Alemu, M.; Lulekal, E.; Asfaw, Z.; Warkineh, B.; Debella, A.; Abebe, A.; Degu, S.; Debebe, E. Antibacterial activity and phytochemical screening of traditional medicinal plants most preferred for treating infectious diseases in Habru District, North Wollo Zone, Amhara Region, Ethiopia. PLoS ONE 2024, 19, e0300060. [Google Scholar] [CrossRef]
- Tibaldi Bollati, M.L.; Casero, C.N.; Pigni, N.B.; Leiva González, S.; García, M.E. Quest for antibacterial alkaloids from Rauhia multiflora through bioassay complementary-guided fractionation. Nat. Prod. Res. 2024, 38, 2111–2117. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial activity of essential plant oils and their major components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef] [PubMed]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Loukili, E.H.; Bellaouchi, R.; Asehraou, A.; Douzi, Y.; Addi, M.; Salamatullah, A.M.; Nafidi, H.-A. Chemical profiling, antibacterial efficacy, and synergistic actions of Ptychotis verticillata Duby essential oil in combination with conventional antibiotics. Nat. Prod. Commun. 2024, 19, 1934578X231222785. [Google Scholar] [CrossRef]
- Assefa, A.; Belay, S.; Kloos, H. Evaluation of in-vitro antibacterial activity of extracts of Calpurina aurea, Vernonia amygdalina and Rumex nepalensis in Goba district, southeastern Ethiopia. Egypt. J. Basic Appl. Sci. 2024, 11, 69–83. [Google Scholar] [CrossRef]
- Kharal, S.; Siddique, F.; Arshad, M.; Aziz, N.; Khalid, W.; Zubair Khalid, M.; Jamal, A.; Hailu, G.G.; Ali Alharbi, S.; Ansari, M.J. Comparative analysis of phytochemical compounds, antioxidant potential, and in vitro antibacterial activity of Zahidi date parts from Pakistan. Int. J. Food Prop. 2024, 27, 799–814. [Google Scholar] [CrossRef]
- Joković, N.; Matejić, J.; Zvezdanović, J.; Stojanović-Radić, Z.; Stanković, N.; Mihajilov-Krstev, T.; Bernstein, N. Onion peel as a potential source of antioxidants and antimicrobial agents. Agronomy 2024, 14, 453. [Google Scholar] [CrossRef]
- Elkahoui, S.; Tepe, A.S.; Snoussi, M.; Alkhiyari, A.H.M.; Jamal, A.; Gzara, L.; Haddaji, N.; Abdelgadir, A.; Badraoui, R. Phytochemical characterization, antimicrobial activity, pharmacokinetic, in silico molecular docking and interaction analysis of Ajwa (Phoenix dactylifera L.) palm date seeds. Pharmacogn. Mag. 2024, 20, 817–831. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Hejna, M.; Di Dio, M.; Rossi, L. Ascophillum nodosum and Lithothamnium calcareum and their prebiotic potential on Lactobacillus strains. J. Funct. Foods 2024, 118, 106257. [Google Scholar] [CrossRef]
- Rahminiwati, M.; Aprilia, E.; Wiendarlina, I.; Affif, U.; Najwa, C.A. Papaya extract as a prebiotic strengthens the inhibitory activity of lactobacillus against the growth of pathogenic bacteria. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 6–7 December 2023; p. 012127. [Google Scholar]
- Al Noman, Z.; Anika, T.T.; Sachi, S.; Ferdous, J.; Sarker, Y.A.; Sabur, M.A.; Rahman, M.T.; Sikder, M.H. Evaluation of antibacterial efficacy of garlic (Allium sativum) and ginger (Zingiber officinale) crude extract against multidrug-resistant (MDR) poultry pathogen. J. Adv. Vet. Anim. Res. 2023, 10, 151. [Google Scholar] [CrossRef]
- Budiansyah, A.; Haroen, U.; Syafwan, S.; Kurniawan, K. Antioxidant and antibacterial activities of the rhizome extract of Curcuma zedoaria extracted using some organic solvents. J. Adv. Vet. Anim. Res. 2023, 10, 347. [Google Scholar] [CrossRef]
- Mohamed, E.A.A.; Muddathir, A.M.; Osman, M.A. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep. 2020, 10, 17148. [Google Scholar] [CrossRef]
- Egharevba, G.O.; Dosumu, O.O.; Oguntoye, S.O.; Njinga, N.S.; Dahunsi, S.O.; Hamid, A.A.; Anand, A.; Amtul, Z.; Priyanka, U. Antidiabetic, antioxidant and antimicrobial activities of extracts of Tephrosia bracteolata leaves. Heliyon 2019, 5, e02275. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, M.F.; Dhillon, H.S.; Samtiya, M.; Shama, E.; Anand, T.; Dhewa, T.; Chaudhary, V.; Chaudhary, P. Positive and negative aspects of bacteriophages and their immense role in the food chain. NPJ Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Markey, N.; Howitt, B.; El-Mansouri, I.; Schwartzenberg, C.; Kotova, O.; Meier, C. Clinical trials are becoming more complex: A machine learning analysis of data from over 16,000 trials. Sci. Rep. 2024, 14, 3514. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage procurement for therapeutic purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B. Phage therapy—Challenges, opportunities and future prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Hankin, M. The bactericidal action of the waters of the Jamuna and Ganges rivers on Cholera microbes. Ann. Inst. Pasteur 1896, 10, 511–523. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. “I will survive”: A tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes 2019, 10, 92–99. [Google Scholar] [CrossRef]
- Turner, P.E.; Azeredo, J.; Buurman, E.T.; Green, S.; Haaber, J.K.; Haggstrom, D.; Kameda de Figueiredo Carvalho, K.; Kirchhelle, C.; Gonzalez Moreno, M.; Pirnay, J.-P. Addressing the research and development gaps in modern phage therapy. Phage 2024, 5, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Lin, Y.-W.; Fabijan, A.P.; Chang, R.Y.; Rao, G.G.; Iredell, J.; Chan, H.-K.; Li, J. Pharmacokinetics/pharmacodynamics of phage therapy: A major hurdle to clinical translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.F.; Reidy, T.G.; Armbruster, C.R.; Sun, M.; Van Tyne, D.; Turner, P.E.; Koff, J.L.; Bomberger, J.M. Lytic bacteriophages induce the secretion of antiviral and proinflammatory cytokines from human respiratory epithelial cells. PLoS Biol. 2024, 22, e3002566. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Podlacha, M.; Gaffke, L.; Grabowski, Ł.; Mantej, J.; Grabski, M.; Pierzchalska, M.; Pierzynowska, K.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage DNA induces an interrupted immune response during phage therapy in a chicken model. Nat. Commun. 2024, 15, 2274. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Majewska, J.; Beta, W.; Lecion, D.; Hodyra-Stefaniak, K.; Kłopot, A.; Kaźmierczak, Z.; Miernikiewicz, P.; Piotrowicz, A.; Ciekot, J.; Owczarek, B. Oral application of T4 phage induces weak antibody production in the gut and in the blood. Viruses 2015, 7, 4783–4799. [Google Scholar] [CrossRef]
- Anand, T.; Vashisth, M.; Jaglan, A.; Nokhwal, A.; Virmani, N.; Bera, B.; Vaid, R.; Singh, R.; Tripathi, B. Phage therapy in tackling AMR: Potential and prospects. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2022, 43, 50–57. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Han, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Stellfox, M.E.; Fernandes, C.; Shields, R.K.; Haidar, G.; Hughes Kramer, K.; Dembinski, E.; Mangalea, M.R.; Arya, G.; Canfield, G.S.; Duerkop, B.A. Bacteriophage and antibiotic combination therapy for recurrent Enterococcus faecium bacteremia. MBio 2024, 15, e03396-23. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Gessner, A.; Merabishvili, M.; Hitzenbichler, F.; Mannala, G.K.; Peterhoff, D.; Walter, N.; Pirnay, J.-P.; Hiergeist, A.; Rupp, M. Case report: Local bacteriophage therapy for fracture-related infection with polymicrobial multi-resistant bacteria: Hydrogel application and postoperative phage analysis through metagenomic sequencing. Front. Med. 2024, 11, 1428432. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; López-Hernández, I.; Rodríguez-Fernández, M.; Pérez-Florido, J.; Casimiro-Soriguer, C.S.; Djebara, S.; Merabishvili, M.; Pirnay, J.-P.; Rodríguez-Baño, J.; Tomás, M. Case report: Analysis of phage therapy failure in a patient with a Pseudomonas aeruginosa prosthetic vascular graft infection. Front. Med. 2023, 10, 1199657. [Google Scholar] [CrossRef]
- Little, J.S.; Dedrick, R.M.; Freeman, K.G.; Cristinziano, M.; Smith, B.E.; Benson, C.A.; Jhaveri, T.A.; Baden, L.R.; Solomon, D.A.; Hatfull, G.F. Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection. Nat. Commun. 2022, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Dedrick, R.M.; Gray, A.L.; Vladar, E.K.; Smith, B.E.; Freeman, K.G.; Malcolm, K.C.; Epperson, L.E.; Hasan, N.A.; Hendrix, J. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022, 185, 1860–1874.e1812. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. In Open Forum Infectious Diseases; Oxford University Press USA, 2020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Ji, X.; Li, Q.; Zhang, G.; Peng, J.; Hai, J.; Zhang, Y.; Ci, B.; Li, H.; Xiong, Y. TSPphg lysin from the extremophilic thermus bacteriophage TSP4 as a potential antimicrobial agent against both gram-negative and gram-positive pathogenic bacteria. Viruses 2020, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1179–1181. [Google Scholar] [CrossRef]
- Kuipers, S.; Ruth, M.M.; Mientjes, M.; de Sévaux, R.G.; van Ingen, J. A Dutch case report of successful treatment of chronic relapsing urinary tract infection with bacteriophages in a renal transplant patient. Antimicrob. Agents Chemother. 2019, 64, e01281.e19. [Google Scholar] [CrossRef]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.-K.; Kaczorowski, T. Ts2631 endolysin from the extremophilic thermus scotoductus bacteriophage vB_Tsc2631 as an antimicrobial agent against gram-negative multidrug-resistant bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Hoyle, N.; Zhvaniya, P.; Balarjishvili, N.; Bolkvadze, D.; Nadareishvili, L.; Nizharadze, D.; Wittmann, J.; Rohde, C.; Kutateladze, M. Phage therapy against Achromobacter xylosoxidans lung infection in a patient with cystic fibrosis: A case report. Res. Microbiol. 2018, 169, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Kutter, E.; Bryan, D.; Wheat, G.; Kuhl, S. Resolving digital staphylococcal osteomyelitis using bacteriophage—A case report. Antibiotics 2018, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.J.; Khan Mirzaei, M.; Nilsson, A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016, 7, 1209. [Google Scholar] [CrossRef] [PubMed]
- Vandenheuvel, D.; Lavigne, R.; Brüssow, H. Bacteriophage therapy: Advances in formulation strategies and human clinical trials. Annu. Rev. Virol. 2015, 2, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current clinical landscape and global potential of bacteriophage therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- He, S.; Lin, F.; Hu, X.; Pan, P. Gut microbiome-based therapeutics in critically ill adult patients—A narrative review. Nutrients 2023, 15, 4734. [Google Scholar] [CrossRef]
- Yadav, S.; Kapley, A. Antibiotic resistance: Global health crisis and metagenomics. Biotechnol. Rep. 2021, 29, e00604. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, G.; Malard, F.; Rubio, M.T.; Ruggeri, A.; Mamez, A.C.; Brissot, E.; Giannotti, F.; Dulery, R.; Joly, A.C.; Baylatry, M.T. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica 2019, 104, 1682. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, G.A.; Mabrouk, A.M.; El-Ssayad, M.F.; Mehaya, F.M.; Sharaf, O.M.; Ibrahim, M.I. Properties of Postbiotics Produced by Probiotics: Antimicrobial, Antioxidant Activities and Production of Vitamins, Organic Acids, Research Square: Durham, NC, USA, 1 ed; 2024. [Google Scholar] [CrossRef]
- Meireles Piazentin, A.C.; Nobrega Mendonca, C.M.; Vallejo, M.; Mussatto, I.; de Souza Oliveira, R.P. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuter. Braz. J. Microbiol. 2022, 53, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Salva, S.; Tiscornia, I.; Gutiérrez, F.; Alvarez, S.; Bollati-Fogolín, M. Lactobacillus rhamnosus postbiotic-induced immunomodulation as safer alternative to the use of live bacteria. Cytokine 2021, 146, 155631. [Google Scholar] [CrossRef]
- de Castro Sousa, S.; da Silva, G.G.; de Sousa Moura, F.A.; Pereira, D.R.; Machado, L.P.; dos Santos Silva, L.; da Silva Delgado, F.; Bezerra, R.M.; Dourado, L.R.B. Synbiotic supplements as antibiotic alternatives in broiler diets. Semin. Ciências Agrárias 2023, 44, 1859–1878. [Google Scholar] [CrossRef]
- Mohammed, A.; Hu, J.; Murugesan, R.; Cheng, H.-W. Effects of a synbiotic as an antibiotic alternative on behavior, production performance, cecal microbial ecology, and jejunal histomorphology of broiler chickens under heat stress. PLoS ONE 2022, 17, e0274179. [Google Scholar] [CrossRef]
- Grigoryan, Z.; Shen, M.J.; Twardus, S.W.; Beuttler, M.M.; Chen, L.A.; Bateman-House, A. Fecal microbiota transplantation: Uses, questions, and ethics. Med. Microecol. 2020, 6, 100027. [Google Scholar] [CrossRef]
- Thanush, D.; Basavaraj, H.; Gowrav, M. Current regulation and initial considerations for successful development and commercialization of microbiome therapies. Adv. Gut Microbiome Res. 2023, 2023, 6657515. [Google Scholar] [CrossRef]
- Abbas, Z.; Tong, Y.; Zhang, J.; Wang, J.; Guo, H.; Cheng, Q.; Zhou, Y.; Ahmad, B.; Wei, X.; Si, D. Enhancing the antioxidant and anti-inflammatory potentials of mulberry-derived postbiotics through submerged fermentation with B. subtilis H4 and B. amyloliquefaciens LFB112. Food Biosci. 2024, 60, 104252. [Google Scholar] [CrossRef]
- Santana, G.B.; Quelemes, P.V.; da Silva Neta, E.R.; de Lima, S.G.; Vale, G.C. Chemical Characterization and Effect of a Lactobacilli-Postbiotic on Streptococcus mutans Biofilm In Vitro. Microorganisms 2024, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Ghafari, H.-A.; Bahador, A. Postbiotic mediators derived from Lactobacillus species enhance riboflavin-mediated antimicrobial photodynamic therapy for eradication of Streptococcus mutans planktonic and biofilm growth. BMC Oral Health 2024, 24, 836. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Guo, H.n.; Abbas, Z.; Zhang, J.; Wang, J.; Cheng, Q.; Peng, S.; Yang, T.; Bai, T.; Zhou, Y. Optimizing postbiotic production through solid-state fermentation with Bacillus amyloliquefaciens J and Lactiplantibacillus plantarum SN4 enhances antibacterial, antioxidant, and anti-inflammatory activities. Front. Microbiol. 2023, 14, 1229952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, J.; Sun, Z.; Fan, J.; Liu, F.; Zhao, W.; Liu, W.-H.; Zhang, M.; Hung, W.-L. Postbiotics derived from L. paracasei ET-22 inhibit the formation of S. mutans biofilms and bioactive substances: An analysis. Molecules 2023, 28, 1236. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Huang, W.; Shu, X.; Ma, S.; Yang, C.; Zhang, R.; Xiao, X.; Wu, Y. Lactiplantibacillus plantarum postbiotics suppress Salmonella infection via modulating bacterial pathogenicity, autophagy and inflammasome in mice. Animals 2023, 13, 3215. [Google Scholar] [CrossRef]
- Karaca, B.; Gursoy, M.; Kiran, F.; Loimaranta, V.; Söderling, E.; Gursoy, U.K. Postbiotics of the Lactiplantibacillus plantarum EIR/IF-1 strain show antimicrobial activity against oral microorganisms with pH adaptation capability. Microbiol. Res. 2023, 14, 1442–1456. [Google Scholar] [CrossRef]
- Hosseini, H.; Abbasi, A.; Sabahi, S.; Akrami, S.; Yousefi-Avarvand, A. Assessing the potential biological activities of postbiotics derived from saccharomyces cerevisiae: An in vitro study. Probiotics Antimicrob. Proteins 2024, 16, 1348–1364. [Google Scholar] [CrossRef]
- Shaaban, S.; Hamad, G.M.; Genena, S.; Meheissen, M.A.; Moussa, S. Evaluation of the antibacterial activity of Lactobacilli probiotics supernatants against Enterococcus faecalis (in-vitro study). BMC Oral Health 2022, 22, 407. [Google Scholar] [CrossRef]
- Toushik, S.H.; Park, J.-H.; Kim, K.; Ashrafudoulla, M.; Ulrich, M.S.I.; Mizan, M.F.R.; Roy, P.K.; Shim, W.-B.; Kim, Y.-M.; Park, S.H. Antibiofilm efficacy of Leuconostoc mesenteroides J. 27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res. Int. 2022, 156, 111163. [Google Scholar]
- Gouveia, M.A.d.C.; Lins, M.T.C.; Silva, G.A.P.d. Acute diarrhea with blood: Diagnosis and drug treatment. J. De Pediatr. 2020, 96, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, Y.; Wu, Q.; Zhang, J.; Xie, X.; Ding, Y.; Cai, S.; Ye, Q.; Chen, M.; Xue, L. Evaluation of the antibacterial activity and probiotic potential of Lactobacillus plantarum isolated from Chinese homemade pickles. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8818989. [Google Scholar] [CrossRef]
- Hong, L.; Kim, W.-S.; Lee, S.-M.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Pullulan nanoparticles as prebiotics enhance the antibacterial properties of Lactobacillus plantarum through the induction of mild stress in probiotics. Front. Microbiol. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, B. Metal-based therapy in traditional and modern medicine systems. In Biomedical Applications of Metals; Rai, M., Ingle, A., Medici, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 195–211. [Google Scholar] [CrossRef]
- Chaudhari, S.Y.; Nariya, M.B.; Galib, R.; Prajapati, P.K. Acute and subchronic toxicity study of Tamra Bhasma (incinerated copper) prepared with and without Amritikarana. J. Ayurveda Integr. Med. 2016, 7, 23–29. [Google Scholar] [CrossRef]
- Quadros, L.S.; Bangera, H.; Kotian, S.R.; Bhat, K.M. Effects of lead in various preparatory stages of Nagabhasma on function and histopathology of cornu ammonis of hippocampus. J. Clin. Diagn. Res. JCDR 2016, 10, AF01. [Google Scholar] [CrossRef]
- Mikulski, M.A.; Wichman, M.D.; Simmons, D.L.; Pham, A.N.; Clottey, V.; Fuortes, L.J. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int. J. Occup. Environ. Health 2017, 23, 187–192. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Ravikanth, V.; Olajide, O.A.; Li, C.; Wei, L.-X. Chemical compositions of metals in Bhasmas and Tibetan Zuotai are a major determinant of their therapeutic effects and toxicity. Evid. -Based Complement. Altern. Med. 2019, 2019, 1697804. [Google Scholar] [CrossRef]
- Koch, I.; Moriarty, M.; House, K.; Sui, J.; Cullen, W.R.; Saper, R.B.; Reimer, K.J. Bioaccessibility of lead and arsenic in traditional Indian medicines. Sci. Total Environ. 2011, 409, 4545–4552. [Google Scholar] [CrossRef]
- Ghdeeb, N.J.; Hussain, N.A. Antimicrobial activity of ZnO nanoparticles prepared using a green synthesis approach. Nano Biomed. Eng. 2023, 15, 14–20. [Google Scholar] [CrossRef]
- Elmehrath, S.; Ahsan, K.; Munawar, N.; Alzamly, A.; Nguyen, H.L.; Greish, Y. Antibacterial efficacy of copper-based metal–organic frameworks against Escherichia coli and Lactobacillus. RSC Adv. 2024, 14, 15821–15831. [Google Scholar] [CrossRef]
- Oetiker, N.; Salinas, D.; Lucero-Mora, J.; Orellana, R.; Quiroz-Muñoz, M.; Bravo, D.; Pérez-Donoso, J.M. Antimicrobial effect of copper nanoparticles on relevant supragingival oral bacteria. Microorganisms 2024, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Hamid, L.L.; Ali, A.Y.; Ohmayed, M.M.; Ramizy, A.; Mutter, T.Y. Antimicrobial activity of silver nanoparticles and cold plasma in the treatment of hospital wastewater. Kuwait J. Sci. 2024, 51, 100212. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Li, S.; Wang, Q. The morphologies of the semiconductor oxides and their gas-sensing properties. Sensors 2017, 17, 2779. [Google Scholar] [CrossRef] [PubMed]
- Akhil, K.; Jayakumar, J.; Gayathri, G.; Khan, S.S. Effect of various capping agents on photocatalytic, antibacterial and antibiofilm activities of ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 160, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Droepenu, E.K.; Amenyogbe, E.; Boatemaa, M.A.; Opoku, E. Study of the antimicrobial activity of zinc oxide nanostructures mediated by two morphological structures of leaf extracts of Eucalyptus radiata. Heliyon 2024, 10(4), e25590. [Google Scholar] [CrossRef]
- Taqveem, H.; Rahman, K.U.; Khan, S.; Khan, A.; Al-Ansi, W.; Fahad, S.; Nawaz, N.; Karishma, N.; Hussain, W.; Khan, L.A. Antibacterial activity of silver nanoparticles synthesized from Aloe Vera Extract. Int. J. Environ. Agric. Biotechnol. 2024, 9, 10–22161. [Google Scholar] [CrossRef]
- Lee, K.Y.; Atwill, E.R.; Li, X.; Feldmann, H.R.; Williams, D.R.; Weimer, B.C.; Aly, S.S. Impact of zinc supplementation on phenotypic antimicrobial resistance of fecal commensal bacteria from pre-weaned dairy calves. Sci. Rep. 2024, 14, 4448. [Google Scholar] [CrossRef]
- Balogun, S.A.; Abolarinwa, T.O.; Adesanya, F.A.; Ateba, C.N.; Fayemi, O.E. Spectroscopic and antibacterial activities of cobalt and nickel nanoparticles: A comparative analysis. J. Anal. Sci. Technol. 2024, 15, 33. [Google Scholar] [CrossRef]
- Karahutová, L.; Bujňáková, D. Antimicrobial and anti-biofilm efficacy of different inorganic and organic zinc forms against multidrug-resistant Escherichia, Klebsiella, Staphylococcus and Pseudomonas. Vet. Res. Commun. 2024, 48, 1899–1905. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z. Antibacterial activities of titanium dioxide (TiO2) nanotube with planar titanium silver (TiAg) to prevent orthopedic implant infection. J. Orthop. Surg. Res. 2024, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Kamali, F.; Faghihi, K.; Mirhoseini, F. High antibacterial activity of new eco-friendly and biocompatible polyurethane nanocomposites based on Fe3O4/Ag and starch moieties. Polym. Eng. Sci. 2022, 62, 1444–1462. [Google Scholar] [CrossRef]
- Attallah, N.G.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.K.; Aadim, K.A. Synthesis and study of structural properties of calcium oxide nanoparticles produced by laser-induced plasma and its effect on antibacterial activity. Sci. Technol. Indones. 2022, 7, 427–434. [Google Scholar] [CrossRef]
- Jawad, A.S.; Thewaini, Q.N.O.; Al-Musawi, S. Cytotoxicity effect and antibacterial activity of Al2O3 nanoparticles activity against streptococcus pyogenes and proteus vulgaris. J. Appl. Sci. Nanotechnol. 2021, 1, 42–50. [Google Scholar] [CrossRef]
- Azizabadi, O.; Akbarzadeh, F.; Danshina, S.; Chauhan, N.P.S.; Sargazi, G. An efficient ultrasonic assisted reverse micelle synthesis route for Fe3O4@ Cu-MOF/core-shell nanostructures and its antibacterial activities. J. Solid State Chem. 2021, 294, 121897. [Google Scholar] [CrossRef]
- Amrulloh, H.; Fatiqin, A.; Simanjuntak, W.; Afriyani, H.; Annissa, A. Antioxidant and antibacterial activities of magnesium oxide nanoparticles prepared using aqueous extract of Moringa oleifera bark as green agents. J. Multidiscip. Appl. Nat. Sci. 2021, 1(1), 44–53. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, S.M.; Rana, N.F.; Tanweer, T.; Batool, A.; Webster, T.J.; Menaa, F.; Riaz, S.; Rehman, Z.; Batool, F. Ciprofloxacin-loaded gold nanoparticles against antimicrobial resistance: An in vivo assessment. Nanomaterials 2021, 11, 3152. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021, 6, 12–25. [Google Scholar] [CrossRef]
- Abass, A.A.; Alaarage, W.K.; Abdulrudha, N.H.; Haider, J. Evaluating the antibacterial effect of cobalt nanoparticles against multi-drug resistant pathogens. J. Med. Life 2021, 14, 823. [Google Scholar] [CrossRef]
- Parwa, A.; Muhammad, M.; Ahmed, A. Synthesis and Antibacterial Activity of Cobalt (III) and Nickel (II) Complexes. Int. J. Psychosoc. Rehabil. 2020, 24, 448–456. [Google Scholar]
- Benhalima, L.; Amri, S.; Bensouilah, M.; Ouzrout, R. Antibacterial effect of copper sulfate against multi-drug resistant nosocomial pathogens isolated from clinical samples. Pak. J. Med. Sci. 2019, 35, 1322. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, E.T.; Ackah, M.; Gore, D.B. Bioaccessibility, exposure and risk assessment of potentially toxic elements and essential micronutrients in ayurvedic, traditional Chinese and Ghanaian medicines. Biometals 2023, 36, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating bacterial resistance by combination of antibiotics with antimicrobial peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial peptides: A new frontier in antifungal therapy. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Neshani, A.; Sedighian, H.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Jahangiri, A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020, 146, 104238. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, N.; Otani, T.; Inai, T. Nisin, a food preservative produced by Lactococcus lactis, affects the localization pattern of intermediate filament protein in HaCaT cells. Anat. Sci. Int. 2019, 94, 163–171. [Google Scholar] [CrossRef]
- Madhavan, M.; Sumodan, P.; Dhanya, C.; Mary, A.S.; Mustafa, S. Antimicrobial Peptides (AMPs): Current State and Future Prospects for the Treatment of Human Parasitic Diseases. In Natural Product Based Drug Discovery Against Human Parasites; Singh, A., Rathi, B., Verma, A.K., Singh, I.K., Eds.; Springer: Singapore, 2023; pp. 203–228. ISBN 978-981-19-9605-4. [Google Scholar] [CrossRef]
- Heinzinger, L.R.; Pugh, A.R.; Wagner, J.A.; Otto, M. Evaluating the translational potential of bacteriocins as an alternative treatment for Staphylococcus aureus infections in animals and humans. Antibiotics 2023, 12, 1256. [Google Scholar] [CrossRef]
- Walsh, L.; Johnson, C.N.; Hill, C.; Ross, R.P. Efficacy of phage-and bacteriocin-based therapies in combatting nosocomial MRSA infections. Front. Mol. Biosci. 2021, 8, 654038. [Google Scholar] [CrossRef]
- Pizzolato-Cezar, L.R.; Okuda-Shinagawa, N.M.; Machini, M.T. Combinatory therapy antimicrobial peptide-antibiotic to minimize the ongoing rise of resistance. Front. Microbiol. 2019, 10, 1703. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, S.; Balakrishnan, S.; Ranjith, S.; Husain, F.; Sathyan, A.; Peter, A.S.; Prahalathan, C.; Kumarasamy, A. Bacteriocin—A potential antimicrobial peptide towards disrupting and preventing biofilm formation in the clinical and environmental locales. Environ. Sci. Pollut. Res. 2020, 27, 44922–44936. [Google Scholar] [CrossRef] [PubMed]
- Kranjec, C.; Mathew, J.P.; Ovchinnikov, K.; Fadayomi, I.; Yang, Y.; Kjos, M.; Li, W.-W. A bacteriocin-based coating strategy to prevent vancomycin-resistant Enterococcus faecium biofilm formation on materials of interest for indwelling medical devices. Biofilm 2024, 8, 100211. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.K.; Omoregbe, O.; Hart, A.; Miri, T.; Eze, U.A.; Onyeaka, H. Applications of bacteriocins of lactic acid bacteria in biotechnology and food preservation: A bibliometric review. Open Microbiol. J. 2022, 16, 1874-2858/22. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Popa, L.I.; Marutescu, L.; Gheorghe, I.; Popa, M.; Czobor Barbu, I.; Cristescu, R.; Chifiriuc, M.-C. Bacteriocins in the era of antibiotic resistance: Rising to the challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2022, 50, D488–D496. [Google Scholar] [CrossRef]
- de Carvalho, G.G.; Maquera-Huacho, P.M.; Pontes, C.S.; de Annunzio, S.R.; Mendonça, C.R.F.; de Souza Rastelli, A.N.; de Oliveira, K.T.; Teughels, W.; Chorilli, M.; Zandim-Barcelos, D.L. Chlorin-e6 conjugated to the antimicrobial peptide LL-37 loaded nanoemulsion enhances photodynamic therapy against multi-species biofilms related to periodontitis. Photodiagnosis Photodyn. Ther. 2023, 43, 103725. [Google Scholar] [CrossRef]
- Chatupheeraphat, C.; Peamchai, J.; Luk-In, S.; Eiamphungporn, W. Synergistic effect and antibiofilm activity of the antimicrobial peptide K11 with conventional antibiotics against multidrug-resistant and extensively drug-resistant Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2023, 13, 1153868. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, S.; Wang, X.; Thunders, M.; Qiu, J.; Li, Y. Discovery and mechanism of action of a novel antimicrobial peptide from an earthworm. Microbiol. Spectr. 2023, 11, e03206–e03222. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Huang, Z.; Zhao, Q.; Fan, J.; Tian, Y.; Huang, A. Isolation, identification and characterization of a novel antimicrobial peptide from Moringa oleifera seeds based on affinity adsorption. Food Chem. 2023, 398, 133923. [Google Scholar] [CrossRef]
- Phuket, T.R.N.; Charoensapsri, W.; Amparyup, P.; Imjongjirak, C. Antibacterial activity and immunomodulatory role of a proline-rich antimicrobial peptide SpPR-AMP1 against Vibrio campbellii infection in shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108479. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Tang, W.; Xing, Y.; Liu, P.; Dang, X. Antibacterial mechanism of the novel antimicrobial peptide Jelleine-Ic and its efficacy in controlling Pseudomonas syringae pv. actinidiae in kiwifruit. Pest Manag. Sci. 2023, 79, 3681–3692. [Google Scholar] [CrossRef]
- Park, H.J.; Kang, H.K.; Park, E.; Kim, M.K.; Park, Y. Bactericidal activities and action mechanism of the novel antimicrobial peptide Hylin a1 and its analog peptides against Acinetobacter baumannii infection. Eur. J. Pharm. Sci. 2022, 175, 106205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lu, H.; Zhu, W.; Zhang, Y.; Huo, X.; Yang, C.; Xiao, S.; Zhang, Y.; Su, J. A novel antimicrobial peptide derived from bony fish IFN1 exerts potent antimicrobial and anti-inflammatory activity in mammals. Microbiol. Spectr. 2022, 10, e02013-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Z.; Diao, Q.; Zhou, Y.; Ao, J.; Liu, C.; Sun, Y. Antimicrobial activity and mechanisms of a derived antimicrobial peptide TroNKL-27 from golden pompano (Trachinotus ovatus) NK-lysin. Fish Shellfish Immunol. 2022, 126, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Zhang, X.; Li, M.; Zhang, Q.; Wang, Y. Antibacterial and anti-inflammatory properties of a novel antimicrobial peptide derived from LL-37. Antibiotics 2022, 11, 754. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, X.; Li, Z.; Yuan, J.; Shen, F.; Zhang, S. Synergistic effect and antibiofilm activity of an antimicrobial peptide with traditional antibiotics against multi-drug resistant bacteria. Microb. Pathog. 2021, 158, 105056. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, Y.; Guan, N.; Xia, X.; Liu, W. NKL-24: A novel antimicrobial peptide derived from zebrafish NK-lysin that inhibits bacterial growth and enhances resistance against Vibrio parahaemolyticus infection in Yesso scallop, Patinopecten yessoensis. Fish Shellfish Immunol. 2020, 106, 431–440. [Google Scholar] [CrossRef]
- Wang, H.; He, H.; Chen, X.; Zhou, M.; Wei, M.; Xi, X.; Ma, C.; Du, Q.; Chen, T.; Shaw, C. A novel antimicrobial peptide (Kassinatuerin-3) isolated from the skin secretion of the African frog, Kassina senegalensis. Biology 2020, 9, 148. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Cui, Q.; Jia, B.-Y.; Pei, Z.-H.; Odah, K.A.; Wang, Y.-M.; Dong, W.-L.; Kong, L.-C.; Ma, H.-X. Design and characterization of a novel hybrid antimicrobial peptide OM19R based on oncocin and MDAP-2. Int. J. Pept. Res. Ther. 2020, 26, 1839–1846. [Google Scholar] [CrossRef]
- Ma, B.; Guo, Y.; Fu, X.; Jin, Y. Identification and antimicrobial mechanisms of a novel peptide derived from egg white ovotransferrin hydrolysates. Lwt 2020, 131, 109720. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Q.; Wang, D.; Li, J. Characterization and antimicrobial mechanism of CF-14, a new antimicrobial peptide from the epidermal mucus of catfish. Fish Shellfish Immunol. 2019, 92, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubiani, A.S.; Maher, Y.A.; Fathi, A.; Abourehab, M.A.; Alarjah, M.; Khan, M.S.; Al-Ghamdi, S.B. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm. J. 2018, 26, 1089–1097. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Zurawski, D.V.; McLendon, M.K. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics 2020, 9, 155. [Google Scholar] [CrossRef]
- Mir, M.A.; Qadri, U. Significance of immunotherapy for human fungal diseases and antifungal drug discovery. In Human Pathogenic Microbes; MirM, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 163–186. ISBN 978-0-323-96127-1. [Google Scholar] [CrossRef]
- Cavaco, M.; Castanho, M.A.; Neves, V. The use of antibody-antibiotic conjugates to fight bacterial infections. Front. Microbiol. 2022, 13, 835677. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Semenova, E.; Shi, Y.; Rosenthal, K.; Oganesyan, V.; Warrener, P.; Stover, C.; Sellman, B.R. Alanine scanning mutagenesis of the MEDI4893 (Suvratoxumab) epitope reduces alpha toxin lytic activity in vitro and Staphylococcus aureus fitness in infection models. Antimicrob. Agents Chemother. 2018, 62, e01033.e18. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Read, A.F. Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 12878–12886. [Google Scholar] [CrossRef]
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2023, 4, e113–e125. [Google Scholar] [CrossRef]
- Baker, S.J.; Payne, D.J.; Rappuoli, R.; De Gregorio, E. Technologies to address antimicrobial resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 12887–12895. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Bagamian, K.H.; Pecenka, C.J.; Muhib, F.; Puett, C.A.; Hausdorff, W.P.; Scheele, S. Potential impact and cost-effectiveness of Shigella vaccination in 102 low-income and middle-income countries in children aged 5 years or younger: A modelling study. Lancet Glob. Health 2023, 11, e880–e891. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Alwabsi, H. An Overview of the Antimicrobial Activity of Some Microbial Enzymes. Am. J. Biochem. Biotechnol. 2024, 20, 140–150. [Google Scholar] [CrossRef]
- Shah, D.; Mital, K. The role of trypsin: Chymotrypsin in tissue repair. Adv. Ther. 2018, 35, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, J.N.; Lynch, M.D. The past, present, and future of enzyme-based therapies. Drug Discov. Today 2022, 27, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Subash, H.A.; Santhosh, K.; Kannan, K.; Pitchiah, S. Extraction of keratin degrading enzyme from marine Actinobacteria of Nocardia sp and their antibacterial potential against oral pathogens. Oral Oncol. Rep. 2024, 9, 100184. [Google Scholar] [CrossRef]
- Tarek, H.; Cho, S.S.; Nam, K.B.; Lee, J.M.; Lee, S.H.; Yoo, J.C. Mode of Action of Antimicrobial Potential Protease SH21 Derived from Bacillus siamensis. Int. J. Mol. Sci. 2024, 25, 7046. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Wang, W.; Hong, B.; Ma, Y.; Wang, J. Characterization of a cell wall hydrolase with high activity against vegetative cells, spores and biofilm of Bacillus cereus. Int. J. Food Microbiol. 2024, 414, 110617. [Google Scholar] [CrossRef]
- Gadallah, E.E.; El-Borai, A.M.; El-Aassar, S.A.; Beltagy, E.A. Purification, characterization, immobilization and applications of an enzybiotic β-1, 3-1, 4-glucanase produced from halotolerant marine Halomonas meridiana ES021. World J. Microbiol. Biotechnol. 2023, 39, 89. [Google Scholar] [CrossRef]
- Wang, S.-W.; Wang, T.-Y. Study on antibacterial activity and structure of chemically modified lysozyme. Molecules 2022, 28, 95. [Google Scholar] [CrossRef]
- Minami, M.; Akahori, S.; Ohta, M. Amylase from Streptococcus pyogenes inhibits biofilm formation in Streptococcus salivarius. GSC Adv. Res. Rev. 2023, 14, 47–53. [Google Scholar] [CrossRef]
- Al-Kadmy, I.M.; Aziz, S.N.; Suhail, A.; Abid, S.A.; Naji, E.N.; Al-Kadmy, Z.; Algammal, A.M.; Ahmed, H.R.; Khodeer, D.M.; Batiha, G.E.-S. Enhancing the anti-biofilm activity of novel keratinase isolated from Acinetobacter baumannii using Reduced Graphene oxide: A way to recycle feather waste pollution. Clean. Waste Syst. 2023, 5, 100087. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Lucky, R.; Mubeen Sultana, D.; Asgari, S. Felcy Anne Jeno: Antibacterial activity of cellulase enzyme isolated from gut microbiota of Coptotermes ceylonicus (Termite). Int. J. Zool. Investig. 2021, 7, 405–408. [Google Scholar]
- Vimal, A.; Kumar, A. Antimicrobial potency evaluation of free and immobilized l-asparaginase using chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 61, 102231. [Google Scholar] [CrossRef]
- Vaddadi Suman, D.N. Characterization of pectinase and antibacterial activity by using Nocardiopsis dasnonivelli s10 isolated from marine samples. J. Cardiovasc. Dis. Res. 2021, 12, 616–631. [Google Scholar]
- Yassein, A.S.; Hassan, M.M.; Elamary, R.B. Prevalence of lipase producer Aspergillus niger in nuts and anti-biofilm efficacy of its crude lipase against some human pathogenic bacteria. Sci. Rep. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial activity of chemically and biologically treated chitosan prepared from black soldier fly (Hermetia illucens) pupal shell waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef]
- Deepa, N.; Anuanandhi, K.; Sankar, D.; Ashok, K.; Babu, M.; Usha, R. Antibacterial activity of protease and lipase enzymes obtained from. Proteus Mirabilis 2021, 7, 592–595. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; LLuch-Senar, M. Characterization of different alginate lyases for dissolving Pseudomonas aeruginosa biofilms. Sci. Rep. 2020, 10, 9390. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L. Antibiofilm activity of acidic phospholipase isoform isolated from Bothrops erythromelas snake venom. Toxins 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Fanaei Pirlar, R.; Emaneini, M.; Beigverdi, R.; Banar, M.; van Leeuwen, W.B.; Jabalameli, F. Combinatorial effects of antibiotics and enzymes against dual-species Staphylococcus aureus and Pseudomonas aeruginosa biofilms in the wound-like medium. PLoS ONE 2020, 15, e0235093. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Okshevsky, M.; van de Ven, T.G.; Tufenkji, N. Developing antibacterial nanocrystalline cellulose using natural antibacterial agents. ACS Appl. Mater. Interfaces 2018, 10, 33827–33838. [Google Scholar] [CrossRef] [PubMed]
- Leonarta, F.; Lee, C.-K. Nanofibrous membrane with encapsulated glucose oxidase for self-sustained antimicrobial applications. Membranes 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Stepanov, N.; Frolov, G.; Efremenko, E. Combined modification of fiber materials by enzymes and metal nanoparticles for chemical and biological protection. Int. J. Mol. Sci. 2022, 23, 1359. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Schwab, M.; Naik, T.; Daudé, D.; Chabrière, E.; Elias, M. Structural and biochemical characterization of AaL, a quorum quenching lactonase with unusual kinetic properties. Sci. Rep. 2018, 8, 11262. [Google Scholar] [CrossRef]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert Rev. Anti-Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial cellulose containing combinations of antimicrobial peptides with various QQ enzymes as a prototype of an “enhanced antibacterial” dressing: In silico and in vitro data. Pharmaceutics 2020, 12, 1155. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Yang, J.; Chidambaram, S.B.; Sakharkar, M.K. Enhancing drug efficacy against mastitis pathogens—An in vitro pilot study in Staphylococcus aureus and Staphylococcus epidermidis. Animals 2020, 10, 2117. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, J.; Nakamura, K.; Nakamura, T.; Iwano, H. Fitness trade-offs between phage and antibiotic sensitivity in phage-resistant variants: Molecular action and insights into clinical applications for phage therapy. Int. J. Mol. Sci. 2023, 24, 15628. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. Efsa J. 2021, 19, e06651. [Google Scholar]
- Ardakani, Z.; Canali, M.; Aragrande, M.; Tomassone, L.; Simoes, M.; Balzani, A.; Beber, C.L. Evaluating the contribution of antimicrobial use in farmed animals to global antimicrobial resistance in humans. One Health 2023, 17, 100647. [Google Scholar] [CrossRef]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hlongwane, M.M.; Mohammed, M.; Mokgalaka, N.S.; Dakora, F.D. The potential of rhizobacteria to mitigate abiotic stress in Lessertia frutescens. Plants 2023, 12, 196. [Google Scholar] [CrossRef]
- Tshamano, N.W.; Joshua, M.; Terry, M.N.; Lee, K.S. A new era of entrepreneurship: The transformative potential of African traditional medicine. Soc. Sci. 2023, 12, 135–142. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 457104. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef]
- Barkhordari, A.; Barzegar, S.; Hekmatimoghaddam, H.; Jebali, A.; Moghadam, S.R.; Khanjani, N. The toxic effects of silver nanoparticles on blood mononuclear cells. Int. J. Occup. Environ. Med. 2014, 5, 164. [Google Scholar]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological aspects, safety assessment, and green toxicology of silver nanoparticles (AgNPs)—Critical review: State of the art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updates 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Nang, S.C.; Tang, S.S. The safety of bacteriophages in treatment of diseases caused by multidrug-resistant bacteria. Pharmaceuticals 2023, 16, 1347. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Hajam, I.A.; Caldera, J.; Liu, G.Y. Integrating complex host-pathogen immune environments into S. aureus vaccine studies. Cell Chem. Biol. 2022, 29, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-M.; Caldera, J.; Hajam, I.A.; Liu, G.Y. Toward an effective Staphylococcus vaccine: Why have candidates failed and what is the next step? Expert Rev. Vaccines 2023, 22, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Thitiananpakorn, K.; Aiba, Y.; Tan, X.; Watanabe, S.; Kiga, K.; Sato’o, Y.; Boonsiri, T.; Li, F.; Sasahara, T.; Taki, Y.; et al. Association of mprF mutations with cross-resistance to daptomycin and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2020, 10, 16107. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.M.; Martin, M.J.; Selleck, E.M.; Lebreton, F.; Sampaio, J.L.M.; Gilmore, M.S. Daptomycin resistance and tolerance due to loss of function in Staphylococcus aureus dsp1 and asp23. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Antunes, B.; Zanchi, C.; Johnston, P.R.; Maron, B.; Witzany, C.; Regoes, R.R.; Hayouka, Z.; Rolff, J. The evolution of antimicrobial peptide resistance in Pseudomonas aeruginosa is severely constrained by random peptide mixtures. PLoS Biol. 2024, 22, e3002692. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Chegini, Z.; van Belkum, A.; Mirzaii, M.; Khoramrooz, S.S.; Darban-Sarokhalil, D. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase–negative staphylococci strains: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, B.R.; Zoio, P.; Fonseca, L.P.; Calado, C.R. Technologies for high-throughput identification of antibiotic mechanism of action. Antibiotics 2021, 10, 565. [Google Scholar] [CrossRef]
- Ayon, N.J. High-throughput screening of natural product and synthetic molecule libraries for antibacterial drug discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Yüce, I.; Morlock, G.E. Nanomole-scaled high-throughput chemistry plus direct bioautography on the same chromatography plate for drug discovery. Anal. Chim. Acta 2021, 1182, 338950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lyu, W.; Zhang, W.; Chen, Y.; Luo, F.; Wang, Y.; Ji, H.; Zhang, G. Discovery of natural products capable of inducing porcine host defense peptide gene expression using cell-based high throughput screening. J. Anim. Sci. Biotechnol. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3D printing techniques and their applications to organ-on-a-chip platforms: A systematic review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef]
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Ye, L.; Zhu, L.; Yu, Q. Lactobacillus protects against S. Typhimurium–induced intestinal inflammation by determining the fate of epithelial proliferation and differentiation. Mol. Nutr. Food Res. 2020, 64, 1900655. [Google Scholar] [CrossRef]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-printing of nanoemulsified eucalypt extracts and their antimicrobial activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, T.R.; Rhomberg, P.R.; Sader, H.S.; Jones, R.N. Antimicrobial activity of omiganan pentahydrochloride tested against contemporary bacterial pathogens commonly responsible for catheter-associated infections. J. Antimicrob. Chemother. 2008, 61, 1092–1098. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Hogan, W.; Shenkman, E.; Bian, J. Applications of artificial intelligence in drug development using real-world data. Drug Discov. Today 2021, 26, 1256–1264. [Google Scholar] [CrossRef]
- Talat, A.; Khan, A.U. Artificial intelligence as a smart approach to develop antimicrobial drug molecules: A paradigm to combat drug-resistant infections. Drug Discov. Today 2023, 28, 103491. [Google Scholar] [CrossRef]
- Krentzel, D.; Shorte, S.L.; Zimmer, C. Deep learning in image-based phenotypic drug discovery. Trends Cell Biol. 2023, 33, 538–554. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Yu, D.; Fan, M.-M.; Zhang, X.; Jin, Z.-Y.; Tang, C.; Liu, X.-F. Antimicrobial resistance crisis: Could artificial intelligence be the solution? Mil. Med. Res. 2024, 11, 7. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Balasegaram, M.; Piddock, L.J. The Global Antibiotic Research and Development Partnership (GARDP) not-for-profit model of antibiotic development. ACS Infect. Dis. 2020, 6, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Gallant, K. Innovation in antimicrobial resistance: The CARB-X perspective. ACS Infect. Dis. 2020, 6, 1317–1322. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs and devices: Comparison of European and US approval processes. JACC Basic Transl. Sci. 2016, 1, 399–412. [Google Scholar] [CrossRef]
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance mechanisms to antimicrobial peptides in gram-positive bacteria. Front. Microbiol. 2020, 11, 593215. [Google Scholar] [CrossRef] [PubMed]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I. Alternatives therapeutic approaches to conventional antibiotics: Advantages, limitations and potential application in medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
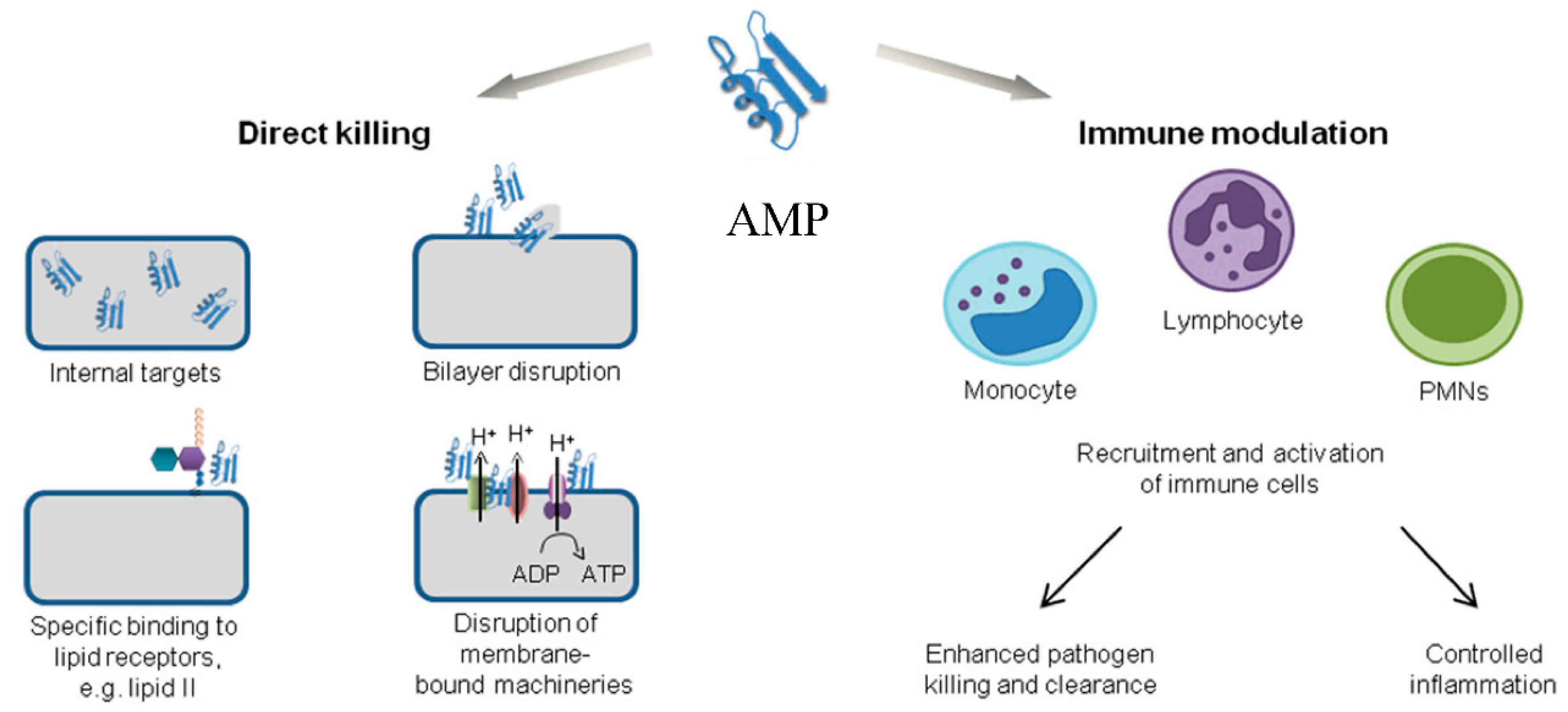
| Plant | Phytochemical Compound | Bacterial Isolates | Test MIC (mg/mL) | Positive Control MIC (mg/mL) | Reference |
|---|---|---|---|---|---|
| Withania somnifera (L.) (Ethanolic extract) | Alkaloids, tannins, steroids, and flavonoids | S. aureus | 2 | 0.625 | [86] |
| E. coli | 2 | 0.352 | |||
| Calpurina aurea (Ethanolic extract) | - | S. aureus, Pseudomonas aeruginosa, Salmonella typhi, E. coli, and Shigella dysenteriae | 1.56 | 0 | [90] |
| Phoenix dactylifera L. (Hydro-ethanolic extract) | Flavonoids and phenols | S. typhi, H. pylori, E. coli, and S. aureus | 22 | 0 | [91] |
| Allium cepa (Ethanolic extract) | Phenol and flavonoids | S. aureus, Enterobacter faecalis, E. coli., Proteus mirabilis, P. aeruginosa, Klebsiella aerogenes, Salmonella enterica, and Shigella sonnei | 0.78–12.50 | 0.008–2.50 | [92] |
| Phoenix dactylifera (Methanolic extract) | Ketones, lipids, phenols, terpenes, steroids, vitamins, acids, alcohol, etc. | Staphylococcus spp., P. aeruginosa, E. faecalis, K. pneumoniae, and Enterobacter cloacae | 0.7–1.4 | 0.312–2.5 | [93] |
| Lithothamnium calcareum, Ascophillum nodosum | Polyphenol, fatty acid, alcohol, and flavonoids | E. coli | 13 | - | [94] |
| Carica papaya | Flavonoids, alkaloids, and saponin | E. coli, S. aureus, and P. aeruginosa | - | - | [95] |
| Allium sativum (Garlic) Zingiber officinale (Ginger) | Allicin Essential oil and phenols | E. coli and Salmonella spp. | 0.312 and 0.625 | - | [96] |
| Allium sativum (n-hexane fraction) | Alkaloids, phenolics, saponins, flavonoids, coumarin, and triterpenoids | P. aeruginosa, Salmonella typhi, E. coli, S. aureus, Bacillus subtills, and S. pneumoniae | 1.562–12.5 | 7.810 | [97] |
Pulicaria crispa Pulicaria undulata (essential oil extract) | Saponins, coumarin, tannins, steroids, and triterpenoids | S. aureus, B. subtills, P. aeruginosa, and E. coli | 6.25–25 | - | [98] |
| Tephrosia bracteolate (Ethyl acetate extract) | Alkaloids, tannins, steroids, and flavonoids | S. aureus and B. subtills | 6.25 | 10 | [99] |
| Metal | Metal Complex | Bacteria | Result | Reference |
|---|---|---|---|---|
| Copper (Cu) | Cu-1,2,3-benzenetricarboxylate | E. coli and Lactobacillus spp. | Bacterial cell membrane disruption. | [171] |
| Zinc (Zn) | Zinc sulfate monohydrate (ZnSO4.H2O) | E. coli and Enterococcus spp. | Significant decreases in MIC values for E. coli with ciprofloxacin and nalidixic acid; however, no significant change was observed with Enterococcus spp. | [178] |
| Cobalt (Co) and Nickel (Ni) | CoNAPS and NiNAPS | V. cholerae, MDR E. coli, S. aureus, and S. enterica | Both NAPs showed antibacterial activity against all tested bacteria. NiNAPs showed a relatively higher activity at a low concentration. | [179] |
| Zinc (Zn) | Zn (II) sulfate monohydrate (ZnSO4.H2O), Zn (II) sulfate heptahydrate (ZnSO4.6H2O), Zn (II) chelate of protein hydrolysate, Zn (II)chelate of amino acid hydrate, and Zn (II) chelate of glycine hydrate | E. coli, S. aureus, P. aeruginosa, and Klebsiella oxytoca | Findings showed specificity and dose dependency in the action of Zn (II) sulfates and Zn (II) amino acid complexes; however, disruption of biofilm formation was also observed. Antibacterial activity was observed with all complexes. | [180] |
| Titanium (Ti) | Titanium dioxide (TiO2) and Titanium silver (TiAg) | E. coli and S. aureus | Antibacterial rates of 99.91% and 97.38% were observed for S. aureus on the first and tenth days, respectively. The observed values were noted to be 99.02% and 95.13% for E. coli. | [181] |
| Silver (Ag) | Starch-based polyurethane nanocomposites (magnetic NAPs and silver NAPs) | E. coli and S. aureus | The nanocomposite showed high antibacterial activity, which increased with an increase in concentration. | [182] |
| Silver (Ag) | Silver nanoparticles (AgNAPs) | S. aureus | A decrease in membrane integrity of 45.8% in the isolate and reduced membrane potential based on flow cytometry. In vivo, the analysis showed healing of wounds. | [183] |
| Calcium (Ca) | Calcium oxide (Ca) NAPs | K. pneumoniae and S. aureus | The different laser energies showed variations in antibacterial activity. | [184] |
| Aluminium | Aluminium oxide (AlO3) NAPs | P. vulgaris and Streptococcus pyogenes | Antibacterial activity was observed at 200 µg/mL with high lethality on P. vulgaris. | [185] |
| Iron (Fe) | Iron oxide (Fe3O4)–copper metal–organic framework (Cu-MOF) | Gram-positive and Gram-negative | Stabilises the MOF to enable persistent Cu release that kills bacteria; however, it does not show significant antibacterial activity. | [186] |
| Magnesium | Magnesium oxide (MgO) NAPs | E. faecalis, E. coli, S. aureus, and Shigella dysenteriae | Antibacterial activity against all tested bacteria with MICs of 300–550 µg/ml. | [187] |
| Gold (Au) | Ciprofloxacin-loaded gold NAPs (CIP-AuNAPs) | E. faecalis | In vitro, assessment of metal complex and ciprofloxacin showed MICs of 1 µg/mL and 2 µg/mL, with a lower bacterial load observed in mice treated with CIP-AuNAPs. | [188] |
| Titanium (Ti) | Titanium oxide (TiO2) NAPs | E. coli and S. aureus | Cellular disruption of cells by cell membrane penetration. Induces an abundance of oxidative stress proteins, which impairs biofilm formation. | [189] |
| Cobalt (Co) | Cobalt (II) sulfate heptahydrate (CoCl2.6H2O) NAPs | E. coli, S. aureus, and P. vulgaris | Variable-defined clear ZOI was observed for all tested bacteria, with the highest antibacterial activity at 100 µg/mL. | [190] |
| Cobalt (Co) and Nickel (Ni) | Cobalt (II) sulfate heptahydrate (CoCl2.6H2O) + ammonium chloride (NH4Cl) and Nikel (II) sulfate heptahydrate + hydrogen peroxide (H2O2) + Hydrochloric acid (HCL) | S. aureus | Antibacterial activity was higher with cobalt complexes. The activity based on ZOI was higher on nutrient agar with higher concentration; however, antibacterial activity was relatively lower on blood agar regardless of the concentration. | [191] |
| Copper (Cu) | Copper (II) sulfate pentahydrate (CuSO4.6H2O) | Enterobacteriaceae, Staphylococci, and Pseudomonas | Potent against 52% tested isolates with MICs between 100 and 200 µg/mL, with the greatest bactericidal effect shown at an MBC of 1600 µg/mL. | [192] |
| Peptide | Source | Bacteria | Mode of Action | Reference |
|---|---|---|---|---|
| Chlorin-e6 | Spirulina maxima | Multi-species biofilm | Significantly impacted bacterial viability. | [207] |
| K11 | Cecropin A1, melittin, and magainin 2 | K. pneumoniae | Increased potency of combined antibiotics and prevented biofilm formation. | [208] |
| EWAMP-R | Eisenia andrei | S. aureus and E. coli | The peptide binds to phospholipids of S. aureus and inserts into E. coli membrane triggering apoptosis. | [209] |
| MOp3 | Moringa oleifera | S. aureus | Interacts with DNA gyrase and dihydrofolate reductase to damage the cell membrane. | [210] |
| SpPR-AMP1 | Scylla paramamosain | Vibrio campbelli | Membrane disruption in bacterial cells and host immune system modulation in vivo. | [211] |
| Jelleine-Ic | Apis mellifera | Pseudomonas syringae | Destroyed the cell membrane, induced intracellular reactive oxygen, reduced esterase activity, and altered DNA replication. | [212] |
| Hylin a1 | Hypsiboas albopunctayus | MDR A. baumannii | Disruption of the bacterial membrane and its permeability by binding to lipopolysaccharide. | [213] |
| gcIFN-20 | Ctenopharyngodon idella | Streptococcus spp., K. pneumoniae, P. aeruginosa, S. aureus, and E. coli | Interacts with bacterial lipopolysaccharide and causes aggregation and neutralisation. Disrupts the cell membrane and inhibits protein synthesis. | [214] |
| TroNKL-27 | Trachinotus ovatus | Edwardsiella tarda, Streptococcus agalactiae, Vibrio spp., S. aureus, and E. coli | Degrade bacterial genomic DNA and alter cell integrity, causing leakage of cellular contents. | [215] |
| KR-12-3 | LL-37 derivative | Streptococcus gordonii | Disrupts the cell wall, reduces the production of inflammatory cytokines, and deregulates bacterial genes responsible for adhesion. | [216] |
| Pt5-1c | Phosvitin | K. pneumoniae, S. aureus, and E. coli | Restored the sensitivity of bacteria to the tested antibiotics. | [217] |
| NKL-24 | Danio rerio NK-lysin | Vibrio parahaemolyticus | Exhibits active membrane cell-killing, reduces bacterial movement, and downregulates transcription genes associated with bacterial virulence. | [218] |
| Kassinatuerin-3 | Kassina senegalensis | MRSA, P. aeruginosa, E. faecalis, S. aureus, and E. coli | Inactive against Gram-negative bacteria. Disruption of the cell membrane of Gram-positive bacteria. | [219] |
| OM19R | Oncocin and MDAP-2 | E. coli, K. pneumoniae, Salmonella spp., and Shigella spp. | Selectively inhibit bacterial cells without initiating cell lysis. | [220] |
| OVTp12 | Ovotransferrin | S. aureus and E. coli | Disruption of cell integrity and a significant increase in membrane permeability. | [221] |
| P5 | Cecropin A-magainin 2 hybrid | P. aeruginosa, B. subtills, A. baumannii, and S. aureus | Destruction of inner and outer bacterial membranes and increased membrane permeability. | [222] |
| CF-14 | Catfish | Shewanella putrefaciens | Cell wall penetration and accumulation in bacterial cells, with slight toxicity in red blood cells. | [223] |
| KC246043.1 | Bacillus megaterium | Micrococcus luteus, Salmonella typhi, P. aeruginosa, S. aureus, and E. coli | Broad-spectrum antibacterial activity. | [224] |
| Enzymes | Source | Bacteria Isolates | Mode of Action | Reference |
|---|---|---|---|---|
| Keratinase | Norcardia sp | Streptococcus mutans, S. typhi, and K. pneumoniae | Hydrolyses proteins on the bacterial surface | [238] |
| Protease SH21 | Bacillus siamensis | E. coli, S. aureus, and Micrococcus luteus | Disrupts the cell membrane | [239] |
| Hydrolase Lys14579 | Bacillus cereus | B. cereus | Induced cell wall degradation | [240] |
| β-1,3-1,4-Glucanase | Halomonas meridiana ES021 | B. subtillis, Streptococcus agalactiae, and Vibrio damsela | Isolate-dependent antibacterial effect | [241] |
| Lysozyme | Hen egg white | E. coli, P. aeruginosa, and B. subtilis | Lyses the bacterial cell wall | [242] |
| Amylase | Streptococcus pyogenes | Streptococcus salivarius | Inhibits biofilm formation | [243] |
| Keratinase | A. baumannii | Multi-species biofilm | Downregulation of biofilm formation genes | [244] |
| Chitosanase | Hermertia illucens | E. coli and Micrococcus flavus | Moderate to high inhibitory activity | [245] |
| Cellulase | Coptotermes ceylonicus | E. coli and B. subtills | Effective inhibition | [246] |
| L-asparaginase | Purpureocillium lilacinum | P. aeruginosa, K. pneumoniae, S. typhimurium, P. vulgaris, S. aureus, and L. monocytogenes | Chitosan NAPs increased efficacy, high ZOI | [247] |
| Pectinase | Nocardiopsis dasnonivelli | Bacillus spp., S. aureus, E. coli, and K. pneumoniae | Limited antibacterial activity | [248] |
| Lipase | Aspergillus niger | MRSA, P. mirabilis, P. aeruginosa, and E. coli, | Distortion of cell shapes indicates metabolomic disturbances | [249] |
| Chitosanase | Hermertia illucens | E. coli, S. aureus, P. aeruginosa, and B. subtilis | Similar inhibitory activity with antibiotics tested | [250] |
| Lipase and protease | P. mirabilis | S. aureus, P. aeruginosa, E. coli, and B. subtilis | Inhibitory effect evident in ZOI | [251] |
| Lyase | Flavobacterium multivorum | P. aeruginosa | Biofilm dissolution | [252] |
| Phospholipase | Bothrops erythromelas | E. coli, S. aureus, and A. baumannii | Antibacterial and antibiofilm activities | [253] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekwueme, C.T.; Anyiam, I.V.; Ekwueme, D.C.; Anumudu, C.K.; Onyeaka, H. Reconstructing the Antibiotic Pipeline: Natural Alternatives to Antibacterial Agents. Biomolecules 2025, 15, 1182. https://doi.org/10.3390/biom15081182
Ekwueme CT, Anyiam IV, Ekwueme DC, Anumudu CK, Onyeaka H. Reconstructing the Antibiotic Pipeline: Natural Alternatives to Antibacterial Agents. Biomolecules. 2025; 15(8):1182. https://doi.org/10.3390/biom15081182
Chicago/Turabian StyleEkwueme, Chiemerie T., Ifeoma V. Anyiam, David C. Ekwueme, Christian K. Anumudu, and Helen Onyeaka. 2025. "Reconstructing the Antibiotic Pipeline: Natural Alternatives to Antibacterial Agents" Biomolecules 15, no. 8: 1182. https://doi.org/10.3390/biom15081182
APA StyleEkwueme, C. T., Anyiam, I. V., Ekwueme, D. C., Anumudu, C. K., & Onyeaka, H. (2025). Reconstructing the Antibiotic Pipeline: Natural Alternatives to Antibacterial Agents. Biomolecules, 15(8), 1182. https://doi.org/10.3390/biom15081182








