Application of Prodigiosin Extracts in Textile Dyeing and Novel Printing Processes for Halochromic and Antimicrobial Wound Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Bacterial Strains and Culture Media
2.3. Testing Conditions for Prodigiosin Production
2.4. Production of Crude Prodigiosin Biopigment
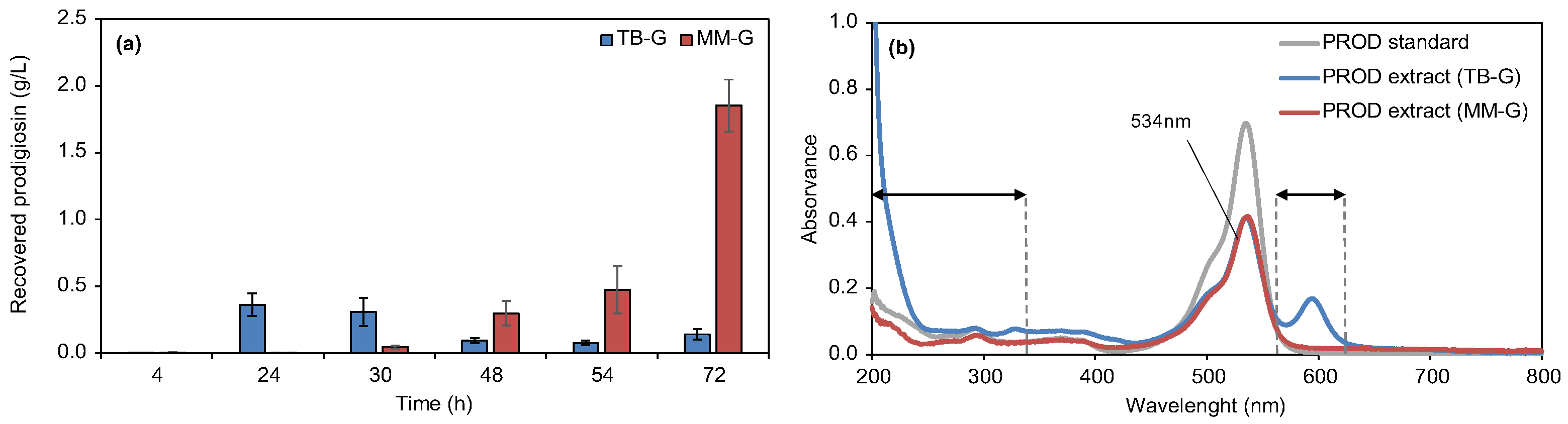
2.5. High-Performance Liquid Chromatography of Prodigiosin Crude Biopigment
2.6. UV–Vis Spectroscopy of Prodigiosin Solution
2.7. Textile Dyeing and Printing with Prodigiosin Biopigment
2.7.1. Functionalization of Textiles with Prodigiosin Extract Through Dyeing Process
- (i)
- Multifiber adjacent fabric, SK, and CO: heating at a rate of 2.0 °C/min to 80 °C, maintained at this temperature for 60 min, followed by cooling to 60 °C at a rate of 3.5 °C/min;
- (ii)
- PES: heating in stages to 60 °C (3 °C/min, maintained for 10 min), followed by 90 °C (2 °C/min, maintained for 30 min) to 130 °C (1.5 °C/min, maintained for 45 min), with further cooling in two stages: from 130 to 80 °C (2 °C/min) and from 80 to 30 °C (2.5 °C/min);
- (iii)
- PA and WO: heating at 100 °C (2 °C/min, maintained for 60 min), followed by cooling to 60 °C (2 °C/min).
2.7.2. CO and PES Printing with Prodigiosin Biopigment and Biopolymers
2.8. Preparation of Buffers for Dyeing Multifiber and Fabrics
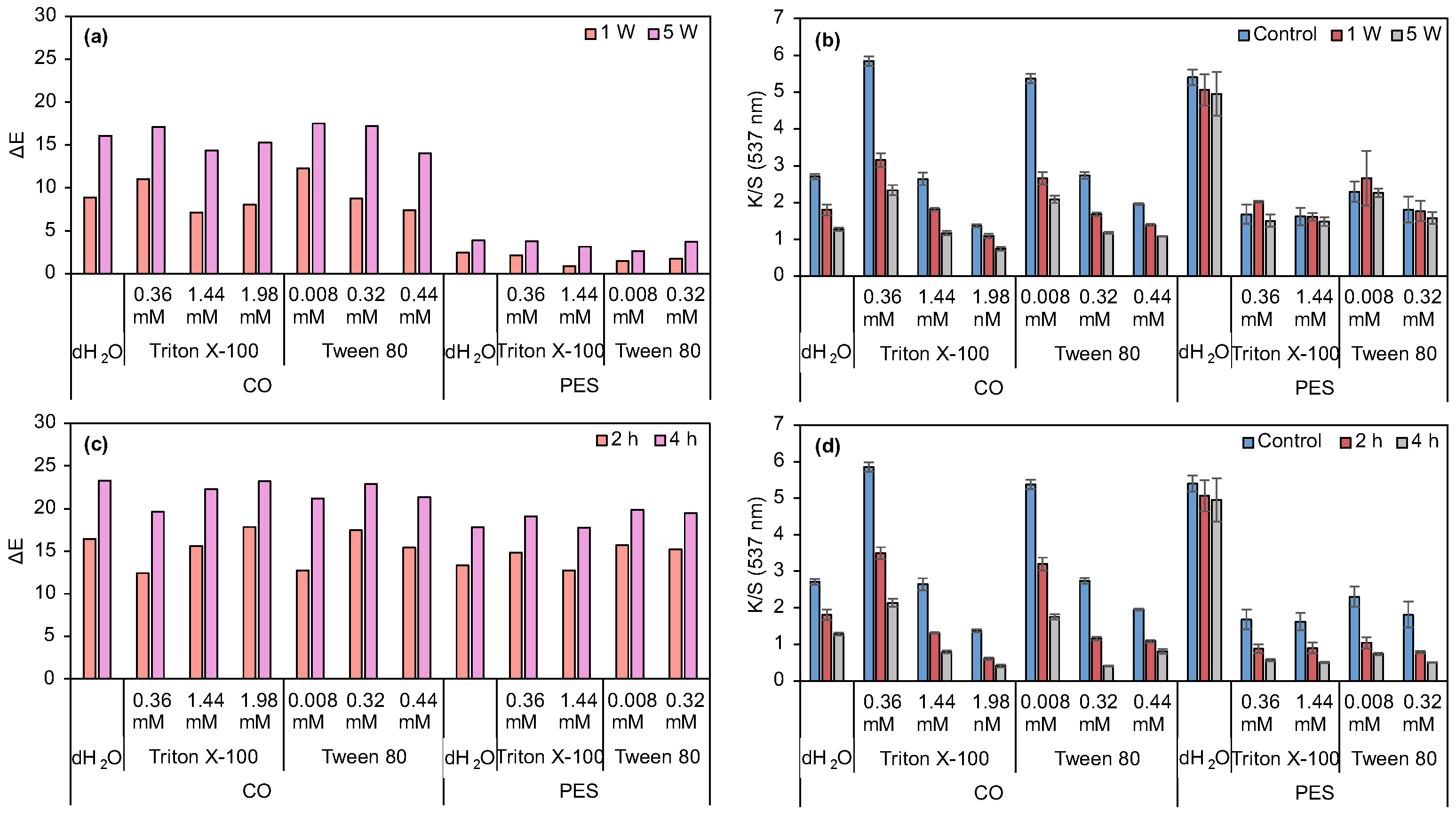
2.9. Evaluating Surfactant Effects on Dye Solubility and CO and PES Dyeing Process with Acidic Prodogiosin
2.10. Optimization of Dyeing with Non-Ionic Surfactants and Acidic Prodogiosin
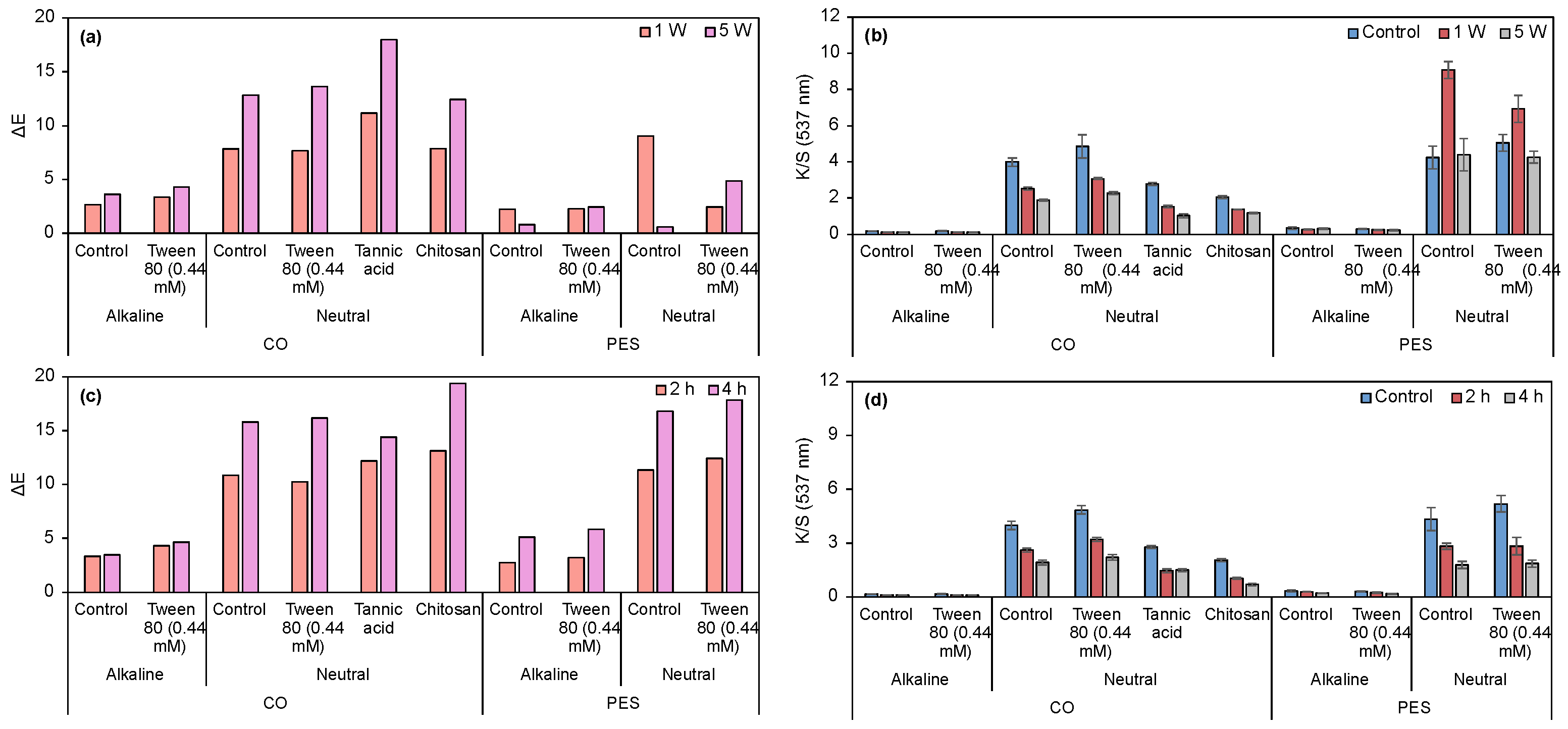
2.11. Evaluation of Dyeing Performance in CO and PES Fabrics Under Alkaline and Neutral Prodigiosin
2.12. Mordanting Process of CO Fabric Under Alkaline and Neutral Prodigiosin
2.13. Characterization of Functionalized Fabrics
2.13.1. CIELab* Coordinates and Color Strength (K/S)
2.13.2. Washing Fastness
2.13.3. Light Fastness
2.13.4. Rubbing Fastness
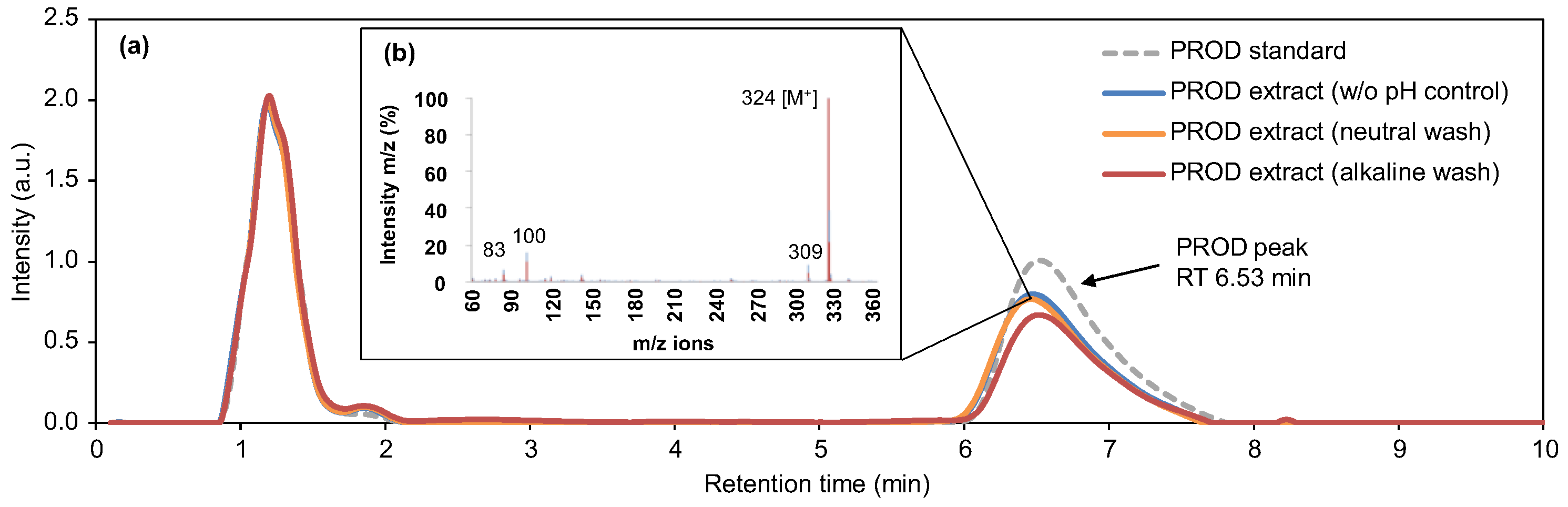
2.13.5. Halochromic Properties of the Functionalized Fabrics with Neutral Prodigiosin
2.13.6. Swelling Determination
2.13.7. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.13.8. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
2.13.9. Antimicrobial Activity
2.13.10. Statistical Analysis
2.13.11. Photographs
3. Results and Discussion
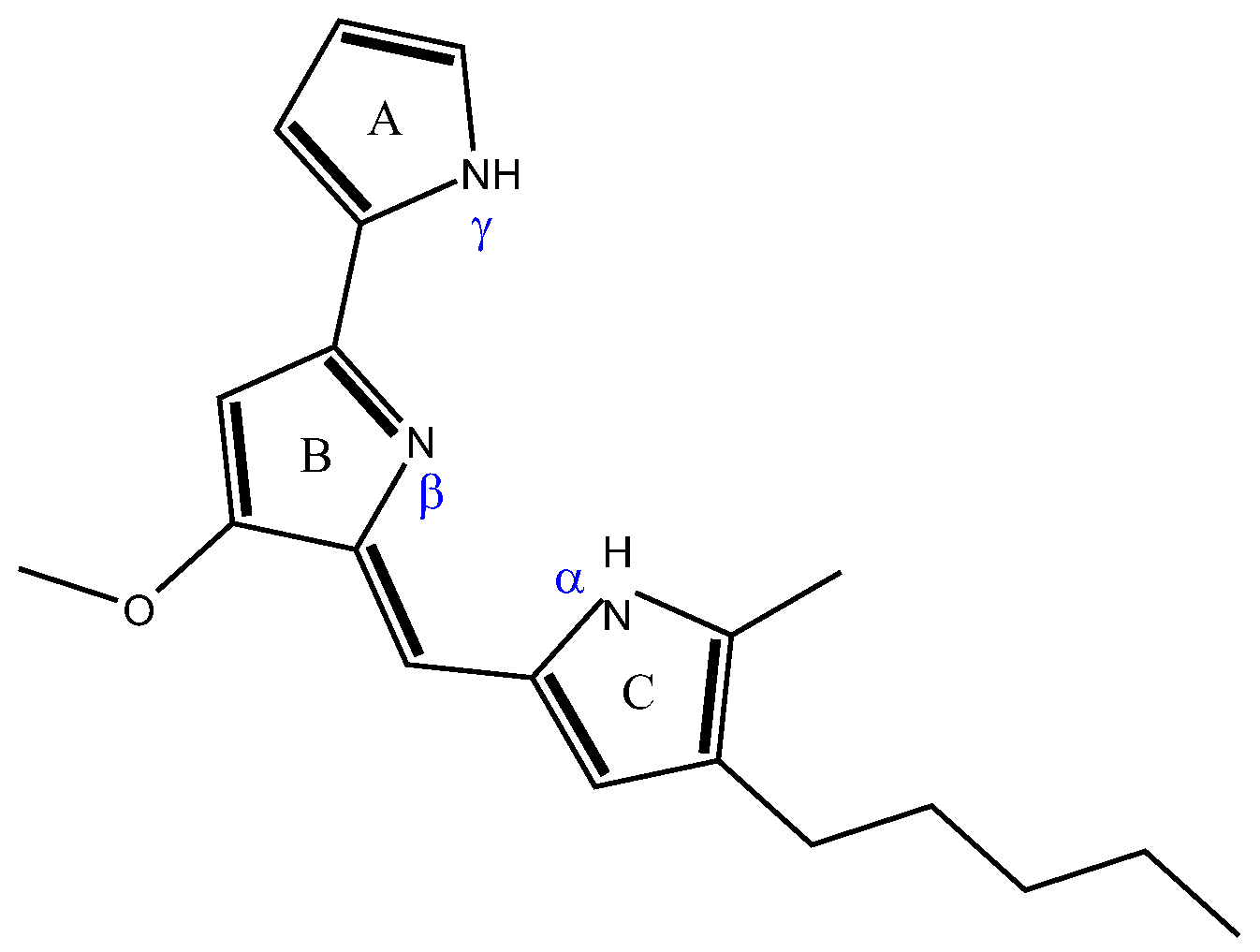
3.1. Production, Extraction, and Characterization of Prodigiosin Crude Extracts
3.2. Application and Optimization of the Biopigment and Color Fastness Assays
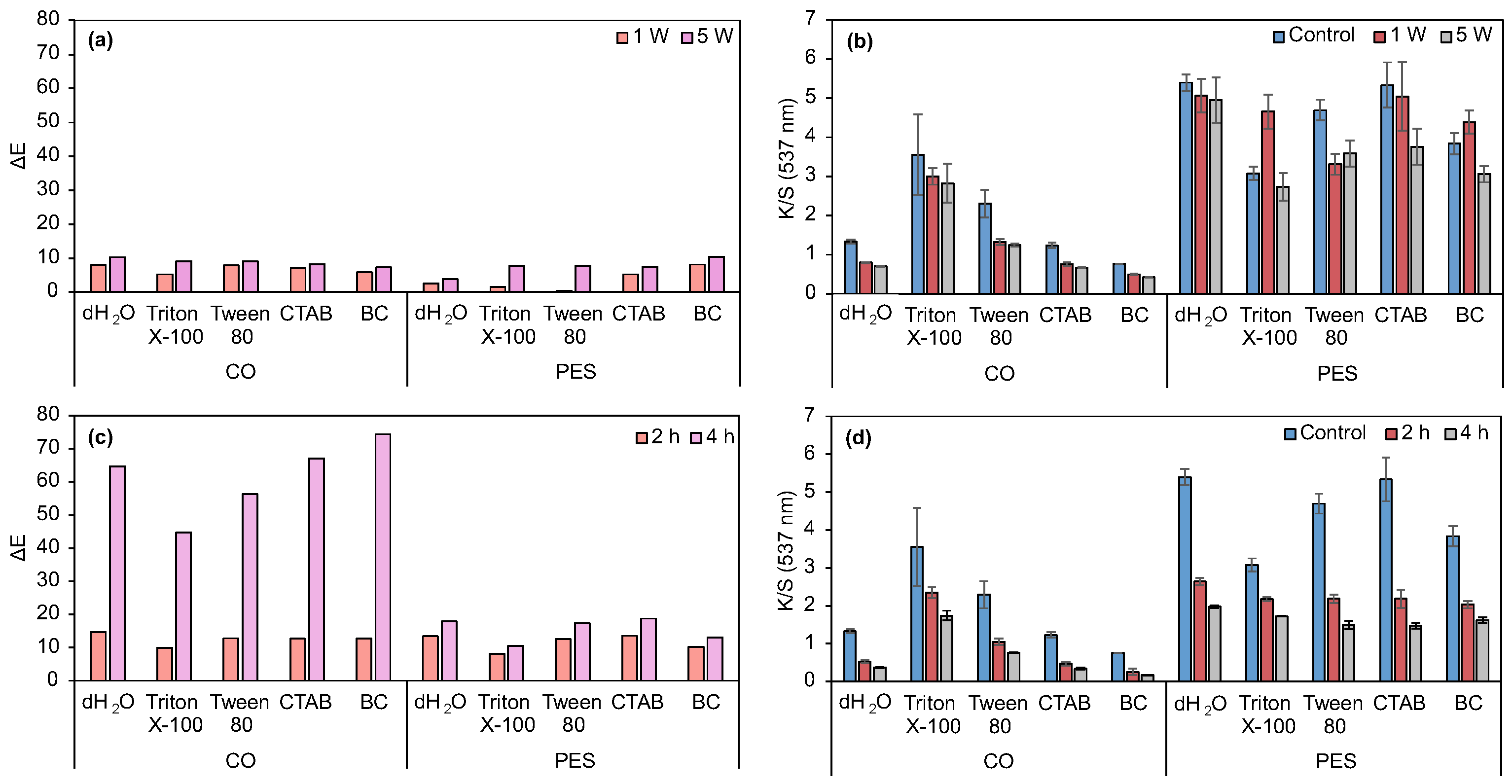
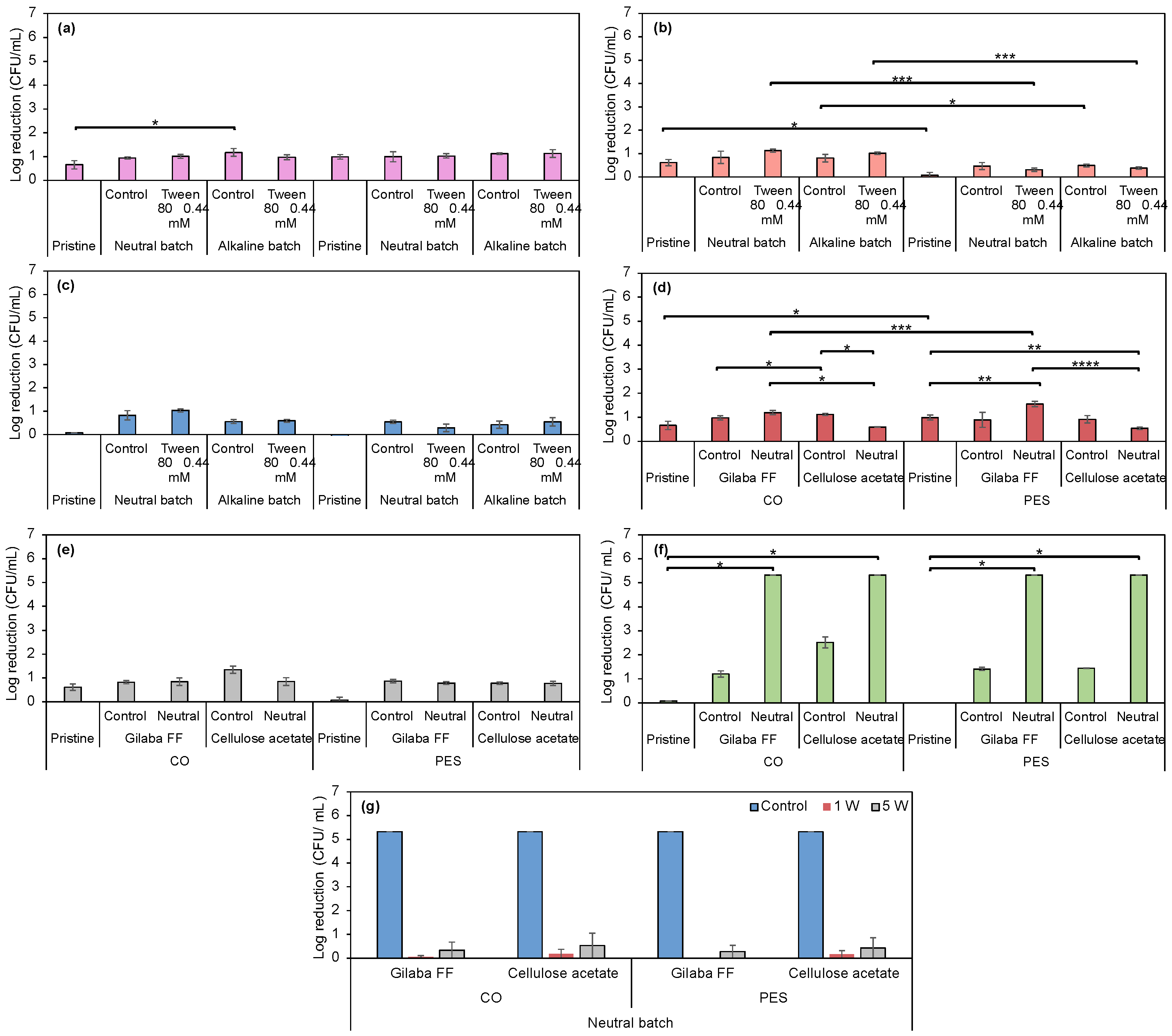
3.2.1. Evaluation of Surfactants’ Effect on Prodigiosin Solubility and CO and PES Dyeing Process Under Acidic Biopigment
3.2.2. Evaluation of Dyeing Performance in CO and PES Fabrics Under Alkaline and Neutral Prodigiosin
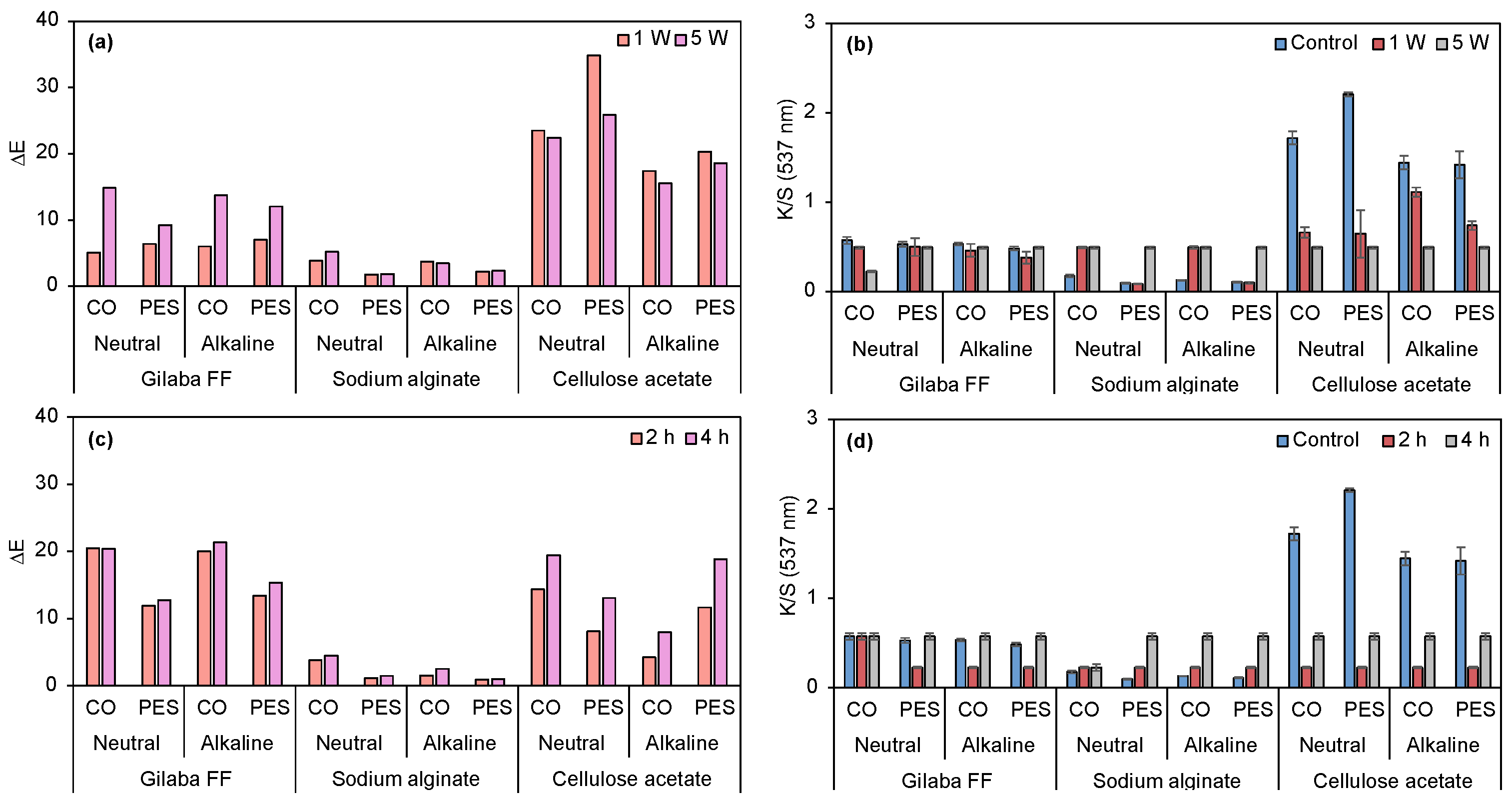
3.2.3. Application of Biopigment in Conventional Printing: A Comparative Study of Commercial Thickener and Biopolymers
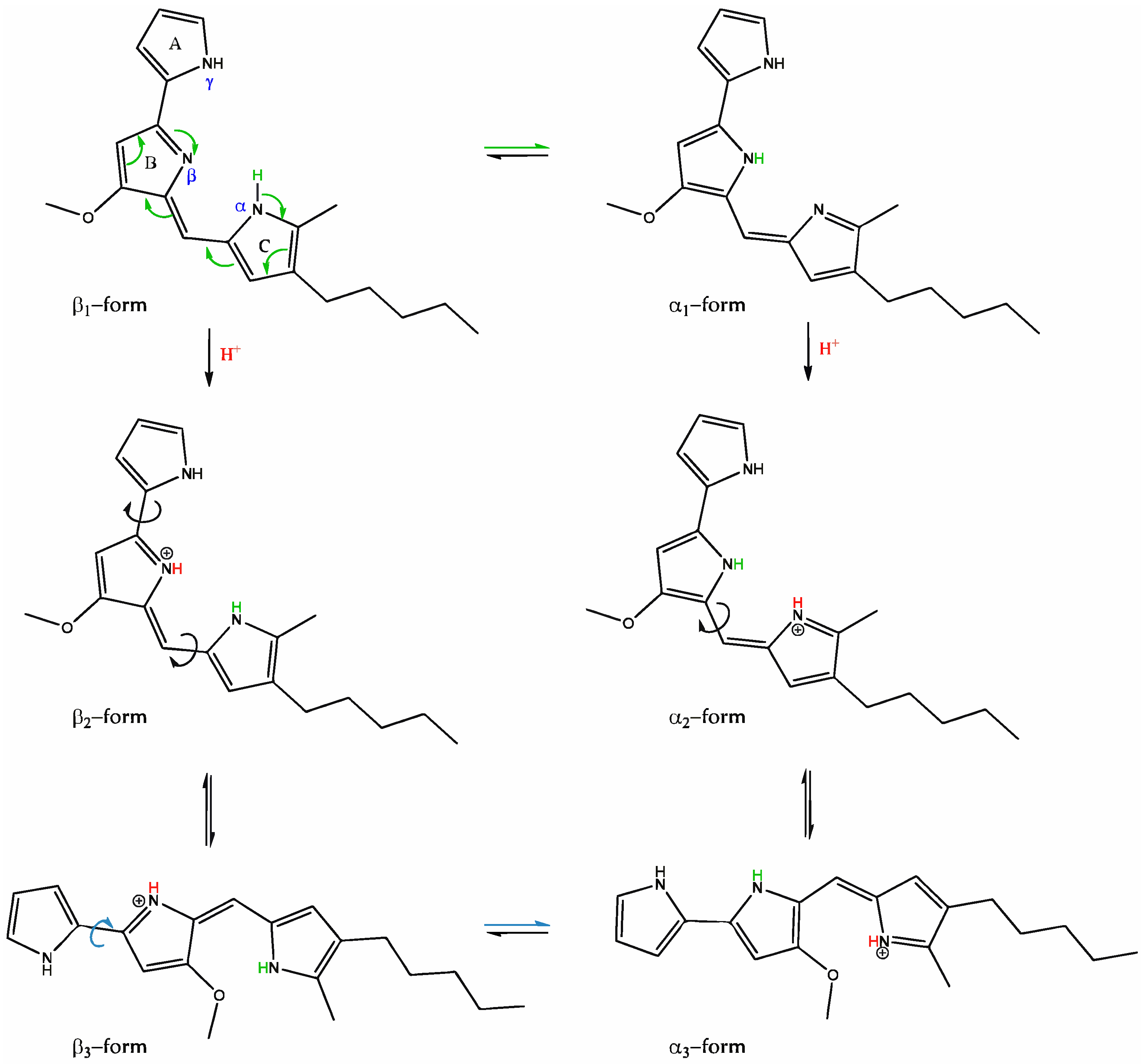
3.2.4. Halochromic Properties of Prodigiosin
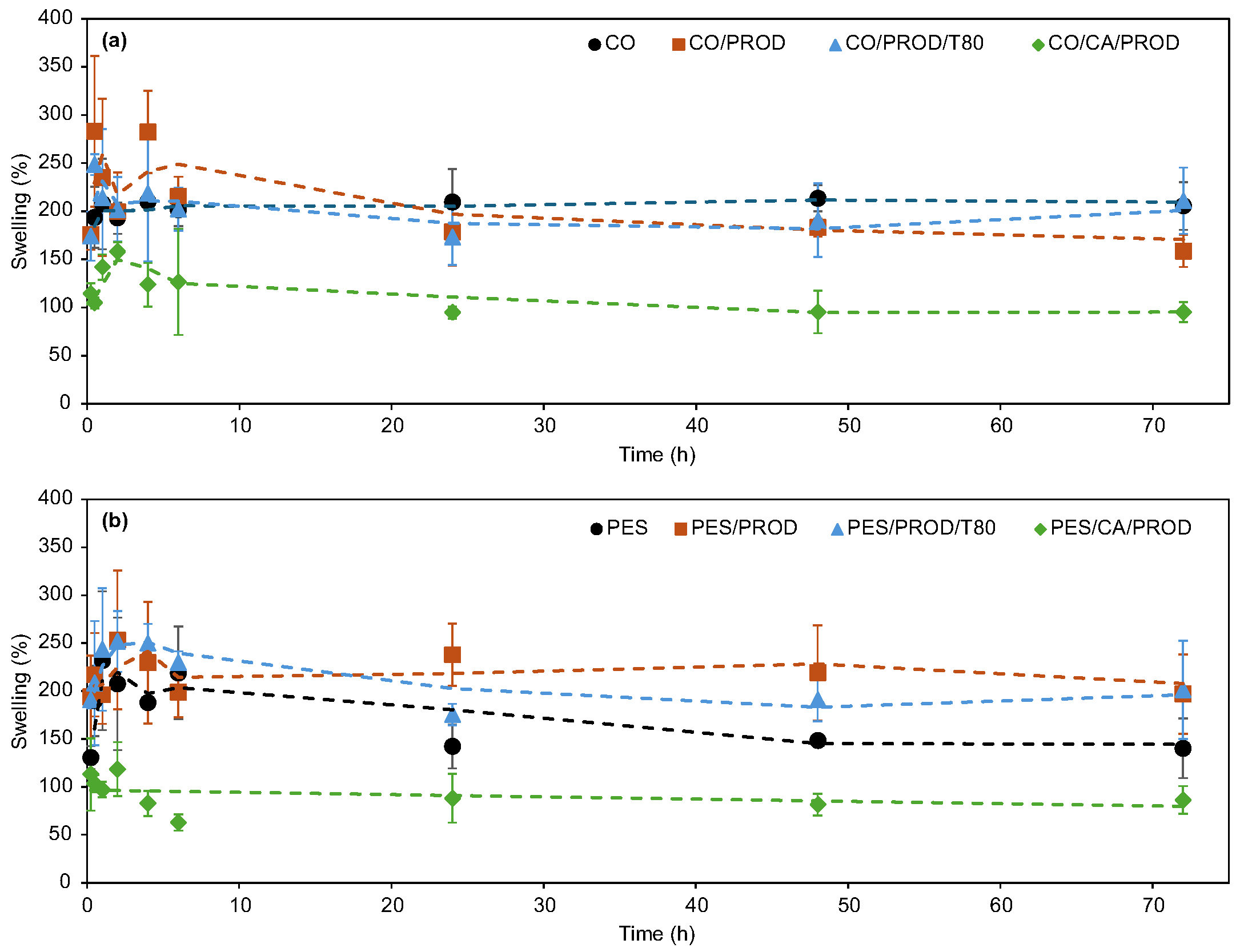
3.2.5. Swelling
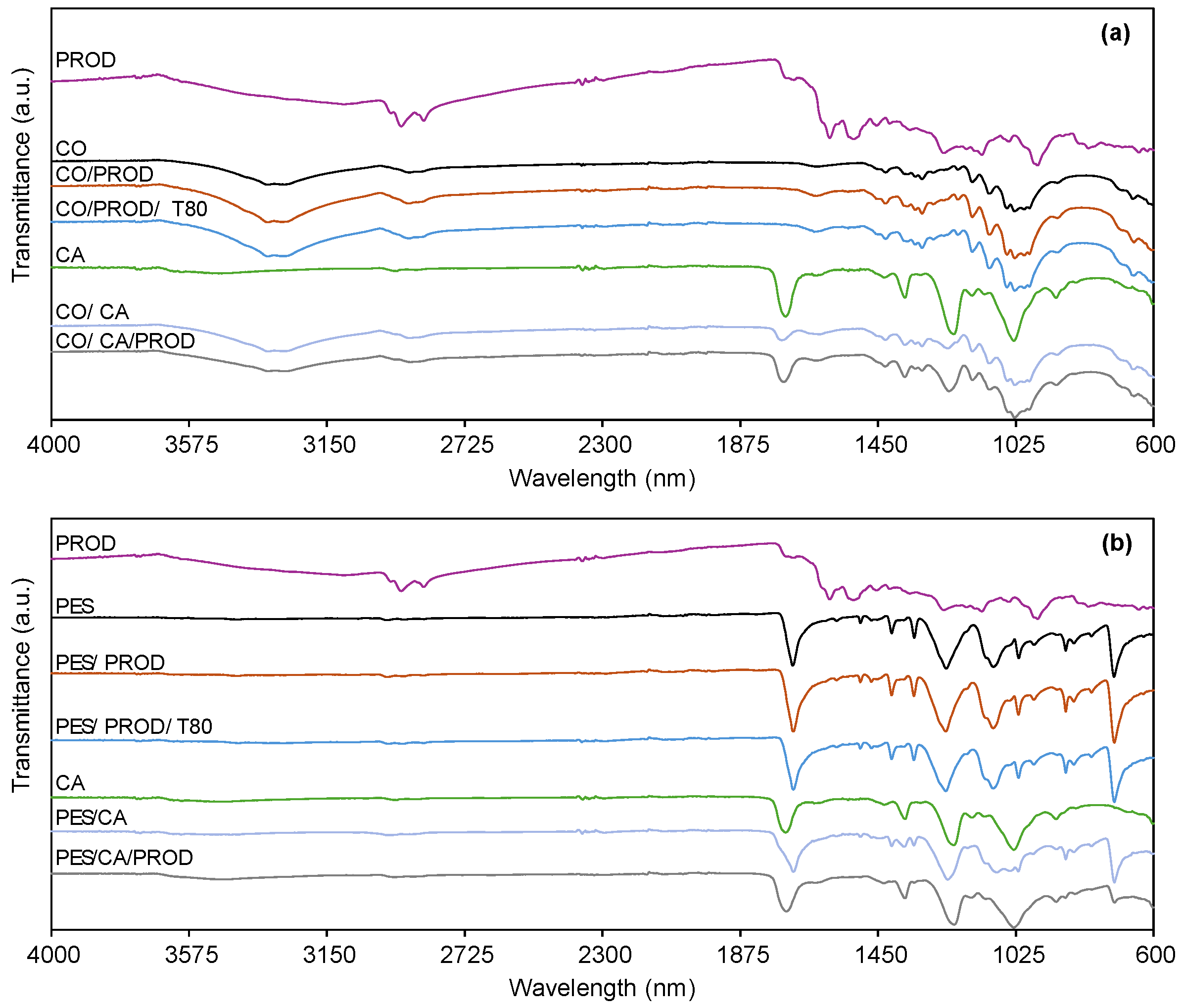
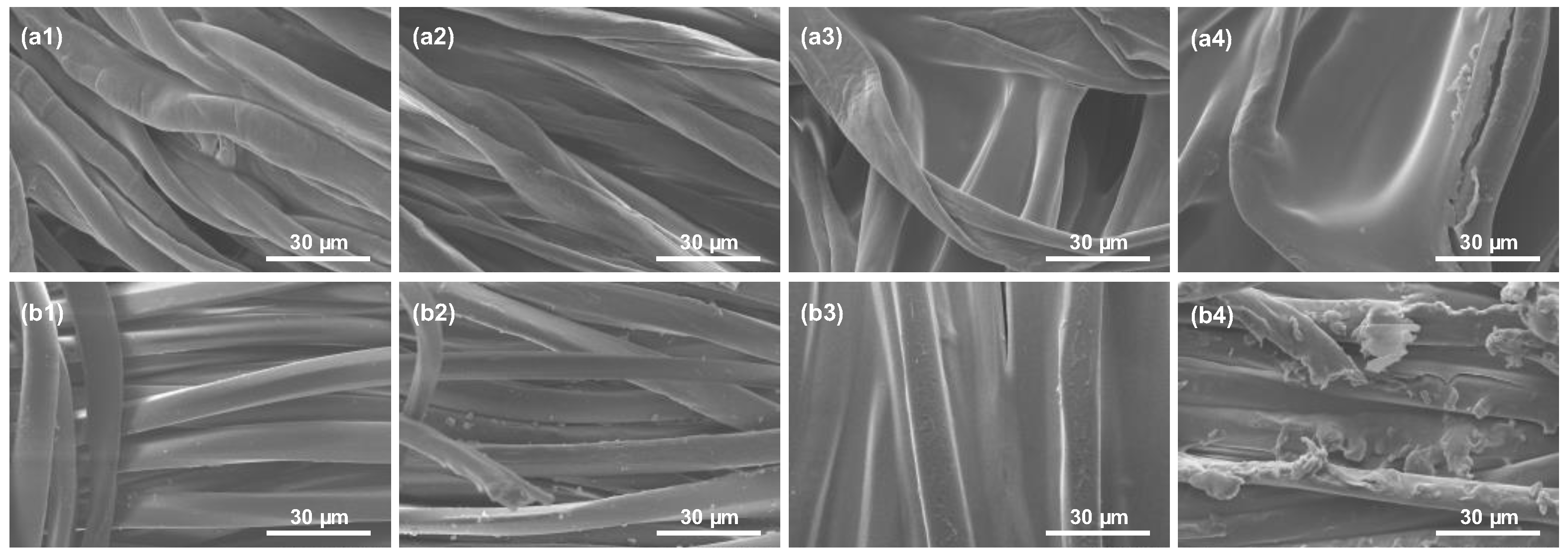
3.2.6. Chemical and Morphological Characterization by ATR-FTIR and SEM
3.2.7. Evaluation of Antimicrobial Properties in Prodigiosin-Funcionalized Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1 W | One washing cycle |
| 5 W | Five washing cycle |
| BC | Alkylbenzyldimethylammonium |
| CA | Acetate |
| CFU | Colony-forming unit |
| Ch | Chitosan |
| CIELab* | Expression of color determined by the International Commission on Illumination |
| CMC | Critical micellar concentration |
| CO | Cotton |
| CTAB | Cetyltrimethylammonium bromide |
| E. coli | Escherichia coli bacteria |
| FTIR | Fourier transform infrared spectroscopy |
| g | Centrifugal force |
| HPLC-DAD-MS | High-Performance Liquid Chromatography with Diode Array Detectors Coupled to Mass Spectrometry |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| K | Absorption coefficient |
| K/S | Color strength |
| Log10 reduction | Logarithmic reduction |
| MM-G | Mineral salts medium supplemented with glycerol and lactid acid |
| OD | Optical density |
| P. aeruginosa | Pseudomonas aeruginosa bacteria |
| P. putida | Pseudomonas putida bacteria |
| PA | Polyamide |
| PAC | Acrylic |
| PES | Polyester |
| PROD | Prodigiosin |
| R | Reflectance decimal fraction |
| RT | Retention time |
| S | Scattering coefficient |
| S. aureus | Staphylococcus aureus bacteria |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning Electron Microscopy |
| SEM-EDS | Energy-Dispersive Spectroscopy |
| SK | Silk |
| TA | Tannic acid |
| TB-G | Terrific broth supplemented with glycerol |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| UV | Ultraviolet |
| UV/Vis | Ultraviolet/visible |
| WO | Wool |
| Δa* | Red-green color difference |
| Δb* | Yellow-blue color difference |
| ΔE | Color difference |
| ΔL* | Lightness difference |
References
- Srivastava, A.; Rani, R.M.; Patle, D.S.; Kumar, S. Emerging bioremediation technologies for the treatment of textile wastewater containing synthetic dyes: A comprehensive review. J. Chem. Technol. Biotechnol. 2021, 97, 26–41. [Google Scholar] [CrossRef]
- Leal Filho, W.; Perry, P.; Heim, H.; Dinis, M.A.P.; Moda, H.; Ebhuoma, E.; Paço, A. An overview of the contribution of the textiles sector to climate change. Front. Environ. Sci. 2022, 10, 973102. [Google Scholar] [CrossRef]
- Lara, L.; Cabral, I.; Cunha, J. Ecological Approaches to Textile Dyeing: A Review. Sustainability 2022, 14, 8353. [Google Scholar] [CrossRef]
- Santiago, D.; Cunha, J.; Cabral, I. Chromatic and medicinal properties of six natural textile dyes: A review of eucalyptus, weld, madder, annatto, indigo and woad. Heliyon 2023, 9, e22013. [Google Scholar] [CrossRef] [PubMed]
- Pranta, A.D.; Rahaman, M.T. Extraction of eco-friendly natural dyes and biomordants for textile coloration: A critical review. Nano Struct. Nano Objects 2024, 39, 101243. [Google Scholar] [CrossRef]
- Alves, C.; Ribeiro, A.; Pinto, E.; Santos, J.; Soares, G. Exploring Z-Tyr-Phe-OH-based hydrogels loaded with curcumin for the development of dressings for wound healing. J. Drug Deliv. Sci. Technol. 2022, 73, 103484. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Magdi, M.; Elsisi, H. An eco-friendly method of extracting alizarin from Rubia tinctorum roots under supercritical carbon dioxide and its application to wool dyeing. Sci. Rep. 2023, 13, 30. [Google Scholar] [CrossRef]
- Schmidt-Przewozna, K.; Zajaczek, K. Influence of Flavonoid Dyes on the Color and Pro - Health Benefits of Linen Fabrics. J. Nat. Fibers 2022, 19, 11165–11180. [Google Scholar] [CrossRef]
- Fernandes, R.D.V.; Pranovich, A.; Valyukh, S.; Zille, A.; Hallberg, T.; Järrendahl, K. Iridescence Mimicking in Fabrics: A Ultraviolet/Visible Spectroscopy Study. Biomimetics 2024, 9, 71. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Li, Y.; Li, X.; Liu, G.; Hu, M.; Liu, Y.; Huang, Y.; Zhou, L.; Zhou, W.; et al. Chameleon-inspired structural coloration of textiles with non-close-packed photonic crystals for high color saturation and color fastness. Chem. Eng. J. 2024, 483, 149053. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial Secondary Metabolites as Biopigments for Textile Dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Devi, M.; Ramakrishnan, E.; Deka, S.; Parasar, D.P. Bacteria as a source of biopigments and their potential applications. J. Microbiol. Methods 2024, 219, 106907. [Google Scholar] [CrossRef]
- Alves, M.B.N.; Jorge, A.M.S.; Pereira, J.F.B. The biotechnology revolution in textile dyeing. Trends Biotechnol. 2024, 42, 1211–1214. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.; Kirubagaran, R.; Venil, C.; Dufossé, L. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef]
- Carvalho, C.; Couceiro, M.; Montagna, G.; Costa Pereira, C.; Carlos, F. Bio dyes and Bio pigments: The sustainable approach in industrial textile dyeing and printing processes. In Proceedings of the Human Systems Engineering and Design (IHSED2024) Future Trends and Applications, Pula, Croatia, 22–24 September 2024. [Google Scholar]
- Ren, Y.; Fu, R.; Fang, K.; Xie, R.; Hao, L.; Chen, W.; Shi, Z. Clean dyeing of acrylic fabric by sustainable red bacterial pigment based on nano-suspension system. J. Clean. Prod. 2021, 281, 125295. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnol. Appl. Biochem. 2021, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, S.; Mariano-Silva, G.; Leite, M.O.; Mura, F.B.; Verma, M.L.; da Silva, S.S.; Chandel, A.K. Production of fungal and bacterial pigments and their applications. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–361. [Google Scholar]
- Gomes, M.; Felgueiras, H.P.; Leite, B.R.; Soares, G.M.B. Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics 2025, 14, 520. [Google Scholar] [CrossRef]
- Simsek Geyik, M.; Efe, D.; Gormez, A. Identification of Bacteria Producing Red Pigments and Their Application in the Textile Industry. Arab. J. Sci. Eng. 2024, 50, 6221–6230. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lim, S.; Yoon, K.-h.; Lee, J.I.; Mitchell, R.J. Biotechnological Activities and Applications of Bacterial Pigments Violacein and Prodigiosin. J. Biol. Eng. 2021, 15, 10. [Google Scholar] [CrossRef]
- Paul, T.; Bandyopadhyay, T.K.; Mondal, A.; Tiwari, O.N.; Muthuraj, M.; Bhunia, B. A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Convers. Biorefinery 2020, 12, 1409–1431. [Google Scholar] [CrossRef]
- Kampers, L.F.C.; Volkers, R.J.M.; Martins dos Santos, V.A.P. Pseudomonas putida KT2440 is HV1 certified, not GRAS. Microb. Biotechnol. 2019, 12, 845–848. [Google Scholar] [CrossRef]
- Martínez-García, E.; de Lorenzo, V. Pseudomonas putida as a synthetic biology chassis and a metabolic engineering platform. Curr. Opin. Biotechnol. 2024, 85, 103025. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.B.; Jacobson, T.B.; Venkataraman, M.V.; Hofstetter, H.; Amador-Noguez, D.; Thomas, M.G.; Pfleger, B.F. Stepwise genetic engineering of Pseudomonas putida enables robust heterologous production of prodigiosin and glidobactin A. Metab. Eng. 2021, 67, 112–124. [Google Scholar] [CrossRef]
- Domröse, A.; Weihmann, R.; Thies, S.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Rapid generation of recombinant Pseudomonas putida secondary metabolite producers using yTREX. Synth. Syst. Biotechnol. 2017, 2, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kramar, A.D.; Ilic-Tomic, T.R.; Lađarević, J.M.; Nikodinovic-Runic, J.B.; Kostic, M.M. Halochromic cellulose textile obtained via dyeing with biocolorant isolated from Streptomyces sp. strain NP4. Cellulose 2021, 28, 8771–8784. [Google Scholar] [CrossRef]
- Islan, G.A.; Rodenak-Kladniew, B.; Noacco, N.; Duran, N.; Castro, G.R. Prodigiosin: A promising biomolecule with many potential biomedical applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef]
- Srilekha, V.; Krishna, G.; Sreelatha, B.; Jagadeesh Kumar, E.; Rajeshwari, K.V.N. Prodigiosin: A fascinating and the most versatile bioactive pigment with diverse applications. Syst. Microbiol. Biomanuf. 2023, 4, 66–76. [Google Scholar] [CrossRef]
- Metwally, R.A.; El Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.A.; Sabry, S.A. Antimicrobial activity of textile fabrics dyed with prodigiosin pigment extracted from marine Serratia rubidaea RAM_Alex bacteria. Egypt. J. Aquat. Res. 2021, 47, 301–305. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, R.; Zheng, Q.; Song, X.; Wu, J.; Ren, Y. Continuous mode of color and functionality construction for cotton by bacterial pigment based on nano-suspension system. Ind. Crop. Prod. 2024, 214, 118510. [Google Scholar] [CrossRef]
- Sundararajan, P.; Ramasamy, S.P. Development of sustainable, eco-friendly antimicrobial finishing of cotton fabric using prodigiosin of Serratia marcescens SP1. Prog. Org. Coat. 2024, 188, 108216. [Google Scholar] [CrossRef]
- Asitok, A.; Ekpenyong, M.; Ben, U.; Antigha, R.; Ogarekpe, N.; Rao, A.; Akpan, A.; Benson, N.; Essien, J.; Antai, S. Stochastic modeling and meta-heuristic multivariate optimization of bioprocess conditions for co-valorization of feather and waste frying oil toward prodigiosin production. Prep. Biochem. Biotechnol. 2022, 53, 690–703. [Google Scholar] [CrossRef]
- Wu, J.; Fu, R.; Xiao, M.; Zheng, Q.; Wu, L.; Fang, K.; Ren, Y. Synergetic construction of color and multifunction for sustainable lyocell fabric by microbial nano pigment. Chem. Eng. J. 2024, 481, 148453. [Google Scholar] [CrossRef]
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.U.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian Marine Bacterium, Pseudoalteromonas rubra, Produces Antimicrobial Prodiginine Pigments. ACS Omega 2020, 5, 4626–4635. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E. Bacterial Pigment Prodigiosin as Multifaceted Compound for Medical and Industrial Application. Appl. Microbiol. 2024, 4, 1702–1728. [Google Scholar] [CrossRef]
- Domröse, A.; Klein, A.S.; Hage-Hülsmann, J.; Thies, S.; Svensson, V.; Classen, T.; Pietruszka, J.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Efficient recombinant production of prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. [Google Scholar] [CrossRef]
- Hartmans, S.; Smits, J.P.; van der Werf, M.J.; Volkering, F.; de Bont, J.A.M. Metabolism of Styrene Oxide and 2-Phenylethanol in the Styrene-Degrading Xanthobacter Strain 124X. Appl. Environ. Microbiol. 1989, 55, 2850–2855. [Google Scholar] [CrossRef]
- Taghavijeloudar, M.; Kebria, D.Y.; Yaqoubnejad, P. Simultaneous harvesting and extracellular polymeric substances extrusion of microalgae using surfactant: Promoting surfactant-assisted flocculation through pH adjustment. Bioresour. Technol. 2021, 319, 124224. [Google Scholar] [CrossRef]
- Knoch, H.; Ulbrich, M.H.; Mittag, J.J.; Buske, J.; Garidel, P.; Heerklotz, H. Complex Micellization Behavior of the Polysorbates Tween 20 and Tween 80. Mol. Pharm. 2021, 18, 3147–3157. [Google Scholar] [CrossRef]
- Poghosyan, A.H.; Mamasakhlisov, Y.S. The mechanism of flip-flops in a AOT lamella: A molecular dynamics study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128681. [Google Scholar] [CrossRef]
- Abdolla, N.; El-Dossoki, F.; Hamza, O.; Gomaa, E. Measurements and Modelling of the Micellization of Alkyl Benzyl Dimethyl Ammonium Chloride and Cetyl Trimethyl Ammonium Chloride in Various Aqueous Media at 298.15 °K. Egypt. J. Chem. 2023, 66, 1415–1431. [Google Scholar] [CrossRef]
- Kaźmierczak, B.; Klimonda, A.; Kowalska, I.; Kutyłowska, M.; Piekarska, K.; Jadwiszczak, P. Separation of cationic biocide by means of ultrafiltration process. E3S Web Conf. 2018, 44, 00068. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Vieira, B.; Alves, C.; Silva, B.; Pinto, E.; Cerqueira, F.; Silva, R.; Remião, F.; Shvalya, V.; Cvelbar, U.; et al. Halochromic Silk Fabric as a Reversible pH-Sensor Based on a Novel 2-Aminoimidazole Azo Dye. Polymers 2023, 15, 1730. [Google Scholar] [CrossRef]
- Ehsani, A.; Asefnejad, A.; Sadeghianmaryan, A.; Rajabinejad, H.; Chen, X. Fabrication of Wound Dressing Cotton Nano-Composite Coated with Tragacanth/Polyvinyl Alcohol: Characterization and In Vitro Studies. ECS J. Solid State Sci. Technol. 2021, 10, 013002. [Google Scholar] [CrossRef]
- Gupta, P.; Purwar, R. Influence of cross-linkers on the properties of cotton grafted poly (acrylamide-co-acrylic acid) hydrogel composite: Swelling and drug release kinetics. Iran. Polym. J. 2021, 30, 381–391. [Google Scholar] [CrossRef]
- Vieira, B.; Padrão, J.; Alves, C.; Silva, C.; Vilaça, H.; Zille, A. Enhancing Functionalization of Health Care Textiles with Gold Nanoparticle-Loaded Hydroxyapatite Composites. Nanomaterials 2023, 13, 1752. [Google Scholar] [CrossRef]
- Liu, J.; Yang, M.; Tan, J.; Yin, Y.; Yang, Y.; Wang, C. pH-responsive discoloration silk fibroin films based on prodigiosin from microbial fermentation. Dye. Pigment. 2022, 198, 109994. [Google Scholar] [CrossRef]
- Tunca Koyun, M.; Sirin, S.; Aslim, B.; Taner, G.; Nigdelioglu Dolanbay, S. Characterization of prodigiosin pigment by Serratia marcescens and the evaluation of its bioactivities. Toxicol. In Vitro 2022, 82, 105368. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin inhibits motility and activates bacterial cell death revealing molecular biomarkers of programmed cell death. AMB Express 2016, 6, 50. [Google Scholar] [CrossRef]
- Wang, S.; Salmon, S. Progress toward Circularity of Polyester and Cotton Textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, L.; Hu, M.; Wang, L.; Feng, L. Sustainable dispersant-free dyeing of poly(lactic acid) with microbial prodigiosins in decamethylcyclopentasiloxane (D5) medium. J. Text. Inst. 2024, 115, 2596–2604. [Google Scholar] [CrossRef]
- Feng, L.; Ren, L.; Wang, L.; Zhang, H. Eco-friendly antibacterial dyeing of poly(lactic acid) with prodigiosins suspension produced by Zooshikella ganghwensis. J. Text. Inst. 2021, 113, 2435–2442. [Google Scholar] [CrossRef]
- Valdés-Díaz, G.; Rodríguez-Calvo, S.; Pérez-Gramatges, A.; Rapado-Paneque, M.; Fernandez-Lima, F.A.; Ponciano, C.R.; da Silveira, E.F. Effects of gamma radiation on phase behaviour and critical micelle concentration of Triton X-100 aqueous solutions. J. Colloid Interface Sci. 2007, 311, 253–261. [Google Scholar] [CrossRef]
- Pereira, R.F.S.; de Carvalho, C.C.C.R. Improving Bioprocess Conditions for the Production of Prodigiosin Using a Marine Serratia rubidaea Strain. Mar. Drugs 2024, 22, 142. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Z.; Zhang, Z.; Zhao, J.; Liu, X.; Meng, G.; Zhang, J.; Zhang, J. Two-Step Optimization for Improving Prodigiosin Production Using a Fermentation Medium for Serratia marcescens and an Extraction Process. Fermentation 2024, 10, 85. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent advances in reactive oxygen species scavenging nanomaterials for wound healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef] [PubMed]
- Abirami, S.; Kumar, S.D.; Murugan, A. Dyeing of Jute Fabrics with Prodigiosin Produced from Sago Waste and their Applications. J. Pure Appl. Microbiol. 2022, 16, 147–156. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Costa, R.V.; Moghtader, F.; Gouveia, I.C. The Sustainable Bioactive Dyeing of Textiles: A Novel Strategy Using Bacterial Pigments, Natural Antibacterial Ingredients, and Deep Eutectic Solvents. Gels 2023, 9, 800. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Yu, Z.; Lou, J.; Wang, F.; Zhang, J. Dyeing and functional properties of polyester fabric dyed with prodigiosins nanomicelles produced by microbial fermentation. J. Clean. Prod. 2017, 148, 375–385. [Google Scholar] [CrossRef]
- Gong, J.; Ren, Y.; Fu, R.; Li, Z.; Zhang, J. pH-Mediated Antibacterial Dyeing of Cotton with Prodigiosins Nanomicelles Produced by Microbial Fermentation. Polymers 2017, 9, 468. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Velmurugan, P.; Malathi, M.; Lakshmanaperumalsamy, P. Extraction and Application of Pigment from Serratia marcescens SB08, an Insect Enteric Gut Bacterium, for Textile Dyeing. Textiles 2021, 1, 21–36. [Google Scholar] [CrossRef]
- Haghighian, N.; Kataky, R. Rapid fingerprinting of bacterial species using nanocavities created on screen-printed electrodes modified by β-cyclodextrin. Sens. Diagn. 2023, 2, 1228–1235. [Google Scholar] [CrossRef]
- Jehlička, J.; Němec, I.; Varnali, T.; Culka, A.; Svatoš, A.; Frank, O.; Oren, A.; Edwards, H.G.M. The pink pigment prodigiosin: Vibrational spectroscopy and DFT calculations. Dye. Pigment. 2016, 134, 234–243. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Luti, K.J.K. A kinetic model for prodigiosin production by Serratia marcescens as a bio-colorant in bioreactor. In Proceedings of the 2nd International Conference On Materials Engineering & Science (IConMEAS 2019), Baghdad, Iraq, 25–26 September 2019. [Google Scholar]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Swathi, E.; Pius, A. Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current status on the biodegradability of acrylic polymers: Microorganisms, enzymes and metabolic pathways involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Nikdel, M.; Rajabinejad, H.; Yaghoubi, H.; Mikaeiliagah, E.; Cella, M.A.; Sadeghianmaryan, A.; Ahmadi, A. Fabrication of Cellulosic Nonwoven Material Coated with Polyvinyl Alcohol and Zinc Oxide/Mesoporous Silica Nanoparticles for Wound Dressing Purposes with Cephalexin Delivery. ECS J. Solid State Sci. Technol. 2021, 10, 057003. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Shen, Y.; Liu, F.; Zhou, Y.; Wu, H.; Liu, Q.; Deng, B. Preparation of Centella asiatica loaded gelatin/chitosan/nonwoven fabric composite hydrogel wound dressing with antibacterial property. Int. J. Biol. Macromol. 2021, 192, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Abou Elmaaty, T.M.; Elsisi, H.; Elsayad, G.; Elhadad, H.; Plutino, M.R. Recent Advances in Functionalization of Cotton Fabrics with Nanotechnology. Polymers 2022, 14, 4273. [Google Scholar] [CrossRef]
- Jardak, M.; Atoissi, A.; Msalbi, D.; Atoui, D.; Bouizgarne, B.; Rigane, G.; Ben Salem, R.; Aifa, S.; Mnif, S. Antibacterial, antibiofilm and cytotoxic properties of prodigiosin produced by a newly isolated Serratia sp. C6LB from a milk collection center. Microb. Pathog. 2022, 164, 105449. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Archangi, B.; Zolgharnein, H.; Zamani, I. Biocolorant “prodigiosin” interferes with the growth of biofouling bacteria: An in vitro and in silico approach. Pigment Resin Technol. 2021, 51, 24–32. [Google Scholar] [CrossRef]
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers 2019, 11, 1769. [Google Scholar] [CrossRef]
- Kramar, A.D.; Obradović, B.M.; Vesel, A.; Kuraica, M.M.; Kostić, M.M. Surface cleaning of raw cotton fibers with atmospheric pressure air plasma. Cellulose 2018, 25, 4199–4209. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Rana, J.; Goindi, G.; Kaur, N.; Sahu, O. Optimization and synthesis of cellulose acetate based grafted gel and study of swelling characteristics. Mater. Today Proc. 2022, 48, 1614–1619. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Mouro, C.; Riool, M.; Gouveia, I.C. Antimicrobial Food Packaging Based on Prodigiosin-Incorporated Double-Layered Bacterial Cellulose and Chitosan Composites. Polymers 2022, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Guyett, P.C.; Chew, D.; Azevedo, V.; Blennerhassett, L.C.; Rosca, C.; Tomlinson, E. Optimizing SEM-EDX for fast, high-quality and non-destructive elemental analysis of glass. J. Anal. At. Spectrom. 2024, 39, 2565–2579. [Google Scholar] [CrossRef]
- Morita, H.; Marks, S.; Anderson, I. The Recent Resolution and Detection Limit Improvement of EDS and EBSD with SEM: Invited Paper. In Proceedings of the 2019 IEEE International Meeting for Future of Electron Devices, Kansai (IMFEDK), San Francisco, CA, USA, 7–11 December 2019; pp. 51–54. [Google Scholar]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- López-R, M.; Barrios, Y.; Perez, L.D.; Soto, C.Y.; Sierra, C. Metal-Organic Framework (MOFs) tethered to cotton fibers display antimicrobial activity against relevant nosocomial bacteria. Inorganica Chim. Acta 2022, 537, 120955. [Google Scholar] [CrossRef]
- Fakhkhari, P.; Tajeddin, E.; Azimirad, M.; Salmanzadeh-Ahrabi, S.; Abdi-Ali, A.; Nikmanesh, B.; Eshrati, B.; Gouya, M.M.; Owlia, P.; Zali, M.R.; et al. Involvement of Pseudomonas aeruginosa in the occurrence of community and hospital acquired diarrhea, and its virulence diversity among the stool and the environmental samples. Int. J. Environ. Health Res. 2020, 32, 61–71. [Google Scholar] [CrossRef]
- Visvalingam, J.; Yakandawala, N.; Regmi, S.; Adeniji, A.; Sharma, P.; Sailer, M. Wound Gel Formulations Containing Poloxamer 407 and Polyhexanide Have In Vitro Antimicrobial and Antibiofilm Activity Against Wound-Associated Microbial Pathogens. Microorganisms 2024, 12, 2362. [Google Scholar] [CrossRef]
- Ali, M.A.; Aly, N.M.; Abou El-Kheir, A.A. Imparting antibacterial activity, UV-protection properties and enhancing performance of casein modified polyester fabrics. J. Text. Inst. 2020, 112, 1436–1448. [Google Scholar] [CrossRef]
- Novi, V.T.; Gonzalez, A.; Brockgreitens, J.; Abbas, A. Highly efficient and durable antimicrobial nanocomposite textiles. Sci. Rep. 2022, 12, 17332. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Patel, M.; Shruti; Dwivedi, N.; Singh, S.; Kumar, P.; Chouhan, M.; Yadav, A.K.; Mondal, D.P.; et al. Development of copper impregnated bio-inspired hydrophobic antibacterial nanocoatings for textiles. Colloids Surf. B Biointerfaces 2022, 220, 112913. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Giełdowska, M.; Król, P.; Sobańska, Z. Preparation of Cotton–Zinc Composites by Magnetron Sputtering Metallization and Evaluation of their Antimicrobial Properties and Cytotoxicity. Materials 2022, 15, 2746. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Ju, K.S.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, A.; Rastegari, B.; Sharifi, H.; Khajeh-Zadeh, H.; Moayedi, J. The effectiveness of antimicrobial photodynamic therapy with prodigiosin against reference strains of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Lasers Med. Sci. 2022, 37, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Gong, J.; Fu, R.; Zhang, J.; Fang, K.; Liu, X. Antibacterial dyeing of silk with prodigiosins suspention produced by liquid fermentation. J. Clean. Prod. 2018, 201, 648–656. [Google Scholar] [CrossRef]
- Hazarika, D.J.; Kakoti, M.; Kalita, R.; Gautom, T.; Goswami, G.; Barooah, M.; Boro, R.C. Prodigiosin from an Endofungal Bacterium Serratia marcescens D1 Inhibits Biofilm Formation in Gram-Positive Bacteria. Microbiology 2021, 90, 829–838. [Google Scholar] [CrossRef]
| Properties/ Fabrics | Cotton | Polyester | Silk | Wool | Polyamide |
|---|---|---|---|---|---|
| Substrate | Fabric | Fabric | Fabric | Fabric | Fabric |
| Composition | 100% CO | 100% PES | 100% SK | 100% WO | 100% PA |
| Fabric construction | Plaine-weave | Plaine-weave | Plaine-weave | Plaine-weave | Plaine-weave |
 |  |  |  |  | |
| Density in yarns/cm (warp × weft) | 33 × 30 | 32 × 26 | 140 × 50 | 16 × 14 | 46 × 34 |
| Mass per unit area (g/m2) | 137 | 100 | 57 | 212 | 115 |
| Thickness (mm) | 0.27 | 0.26 | 0.20 | 0.47 | 0.27 |
| Acidic Batch (from Table S4) | Neutral Batch | Alkaline Batch | ||||
|---|---|---|---|---|---|---|
| Multifiber Composition | Without Surfactant | Tween 80 | Without Surfactant | Tween 80 | Without Surfactant | Tween 80 |
| CA |  |  |  |  |  |  |
| CO | ||||||
| PA | ||||||
| PES | ||||||
| PAC | ||||||
| WO | ||||||
| Control (Neutral Batch) | 1 Cycle | 10 Cycles | ||||
|---|---|---|---|---|---|---|
| NaOH | HCl | NaOH | HCl | |||
| Dyed samples | CO/Prodigiosin |  |  |  |  |  |
| CO/Prodigiosin/ Tween 80 |  |  |  |  |  | |
| PES/Prodigiosin |  |  |  |  |  | |
| PES/Prodigiosin/ Tween 80 |  |  |  |  |  | |
| Printed samples | CO/Cellulose acetate/ Prodigiosin |  |  |  |  |  |
| CO/Gilaba FF/ Prodigiosin |  |  |  |  |  | |
| PES/Cellulose acetate/ Prodigiosin |  |  |  |  |  | |
| PES/Gilaba FF/ Prodigiosin |  |  |  |  |  | |
| Elemental Composition (Mass, %) | |||
|---|---|---|---|
| O | C | ||
| Control | CO | 54.76 | 45.24 |
| PES | 59.88 | 40.12 | |
| Dyed samples | CO/Prodigiosin/Tween 80 | 54.48 | 45.52 |
| PES/Prodigiosin/Tween 80 | 58.99 | 41.01 | |
| Printed samples | CO/Cellulose acetate | 54.13 | 45.87 |
| CO/Cellulose acetate/Prodigiosin | 53.98 | 46.02 | |
| PES/Cellulose acetate | 53.89 | 46.11 | |
| PES/Cellulose acetate/Prodigiosin | 55.18 | 44.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, C.; Soares-Castro, P.; Fernandes, R.D.V.; Pereira, A.; Rodrigues, R.; Fonseca, A.R.; Santos, N.C.; Zille, A. Application of Prodigiosin Extracts in Textile Dyeing and Novel Printing Processes for Halochromic and Antimicrobial Wound Dressings. Biomolecules 2025, 15, 1113. https://doi.org/10.3390/biom15081113
Alves C, Soares-Castro P, Fernandes RDV, Pereira A, Rodrigues R, Fonseca AR, Santos NC, Zille A. Application of Prodigiosin Extracts in Textile Dyeing and Novel Printing Processes for Halochromic and Antimicrobial Wound Dressings. Biomolecules. 2025; 15(8):1113. https://doi.org/10.3390/biom15081113
Chicago/Turabian StyleAlves, Cátia, Pedro Soares-Castro, Rui D. V. Fernandes, Adriana Pereira, Rui Rodrigues, Ana Rita Fonseca, Nuno C. Santos, and Andrea Zille. 2025. "Application of Prodigiosin Extracts in Textile Dyeing and Novel Printing Processes for Halochromic and Antimicrobial Wound Dressings" Biomolecules 15, no. 8: 1113. https://doi.org/10.3390/biom15081113
APA StyleAlves, C., Soares-Castro, P., Fernandes, R. D. V., Pereira, A., Rodrigues, R., Fonseca, A. R., Santos, N. C., & Zille, A. (2025). Application of Prodigiosin Extracts in Textile Dyeing and Novel Printing Processes for Halochromic and Antimicrobial Wound Dressings. Biomolecules, 15(8), 1113. https://doi.org/10.3390/biom15081113








