Membrane-Type 5 Matrix Metalloproteinase (MT5-MMP): Background and Proposed Roles in Normal Physiology and Disease
Abstract
1. Background
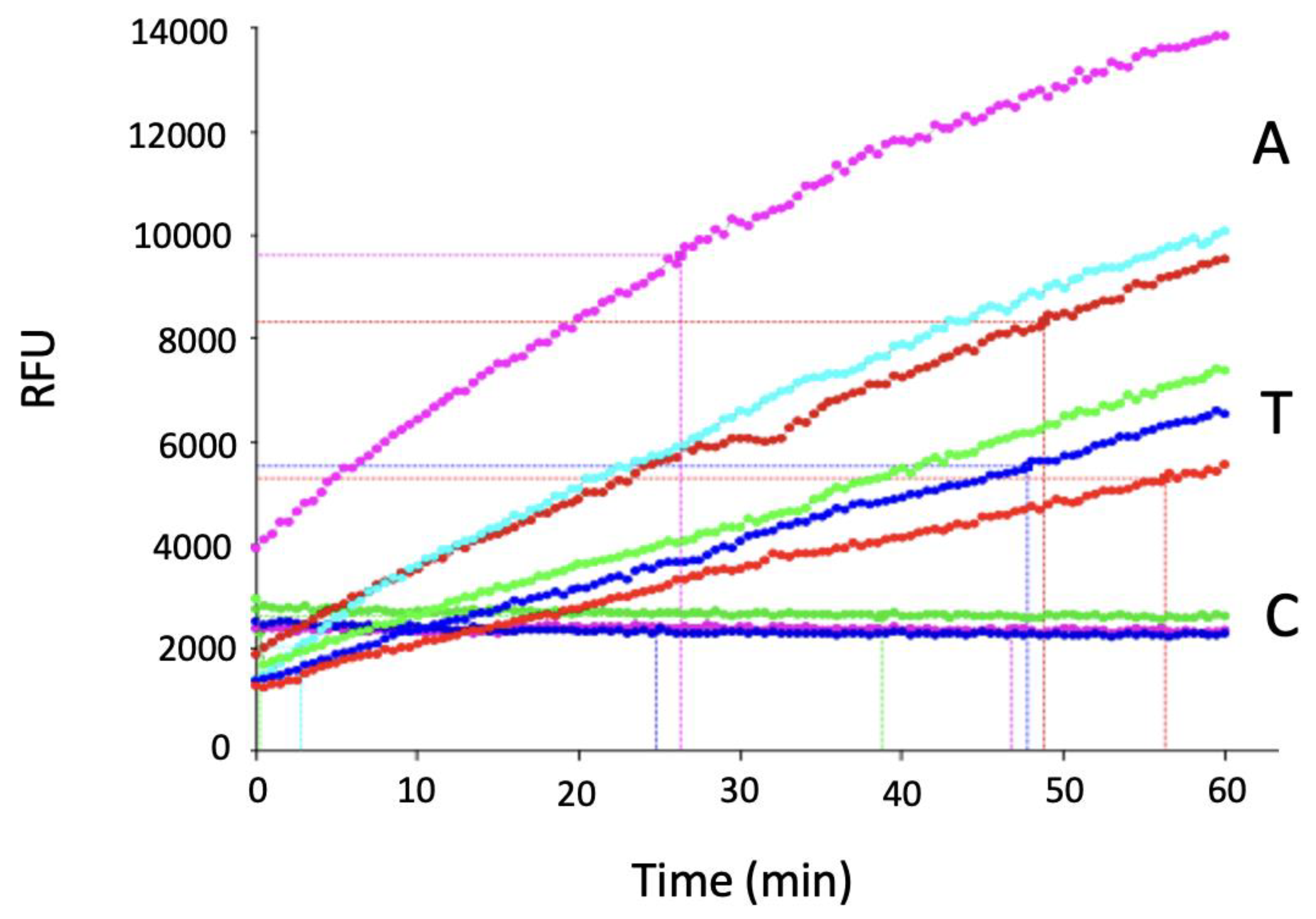
2. MT5-MMP Substrates
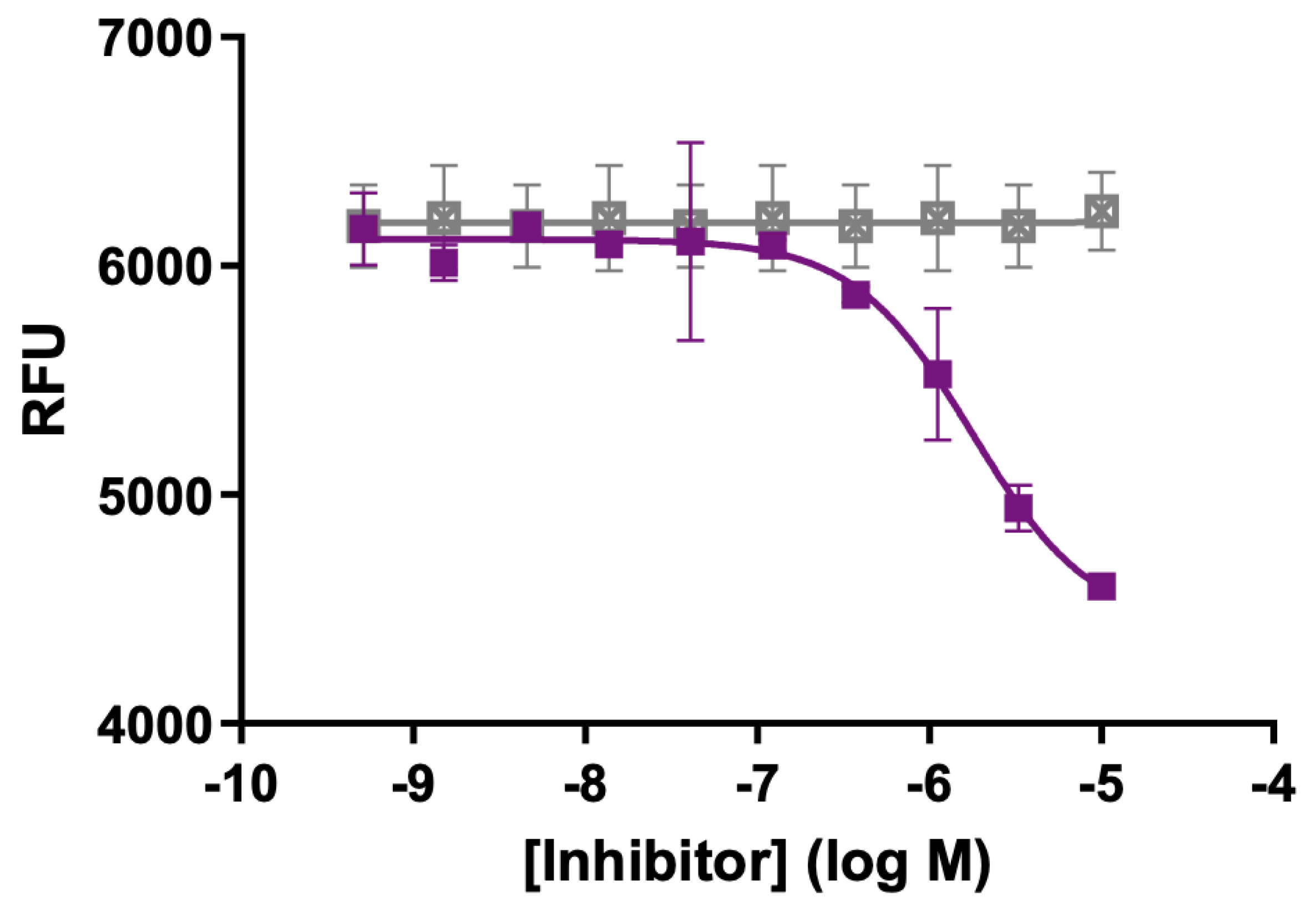
3. MT5-MMP Inhibition
4. MT5-MMP Roles in Cancer
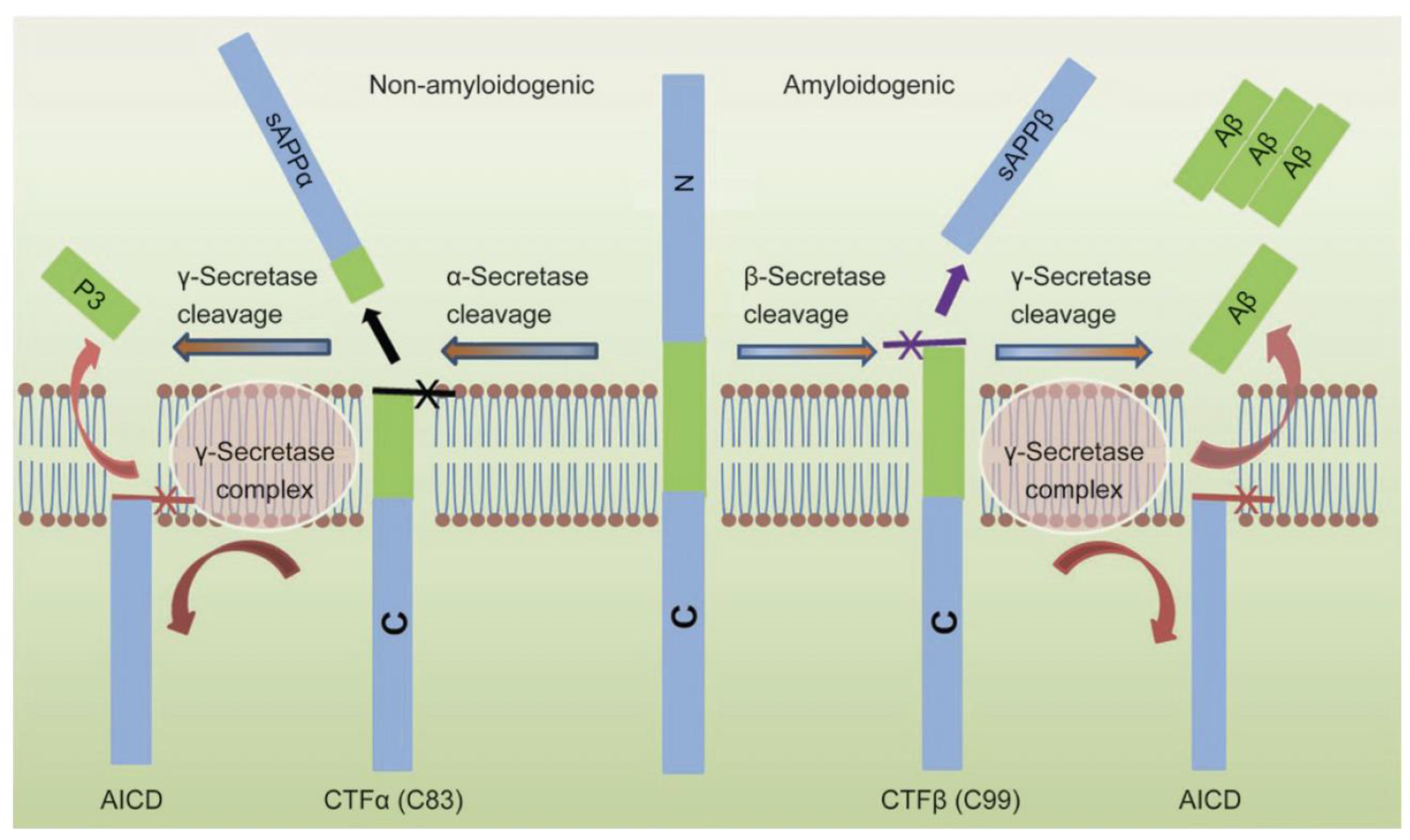
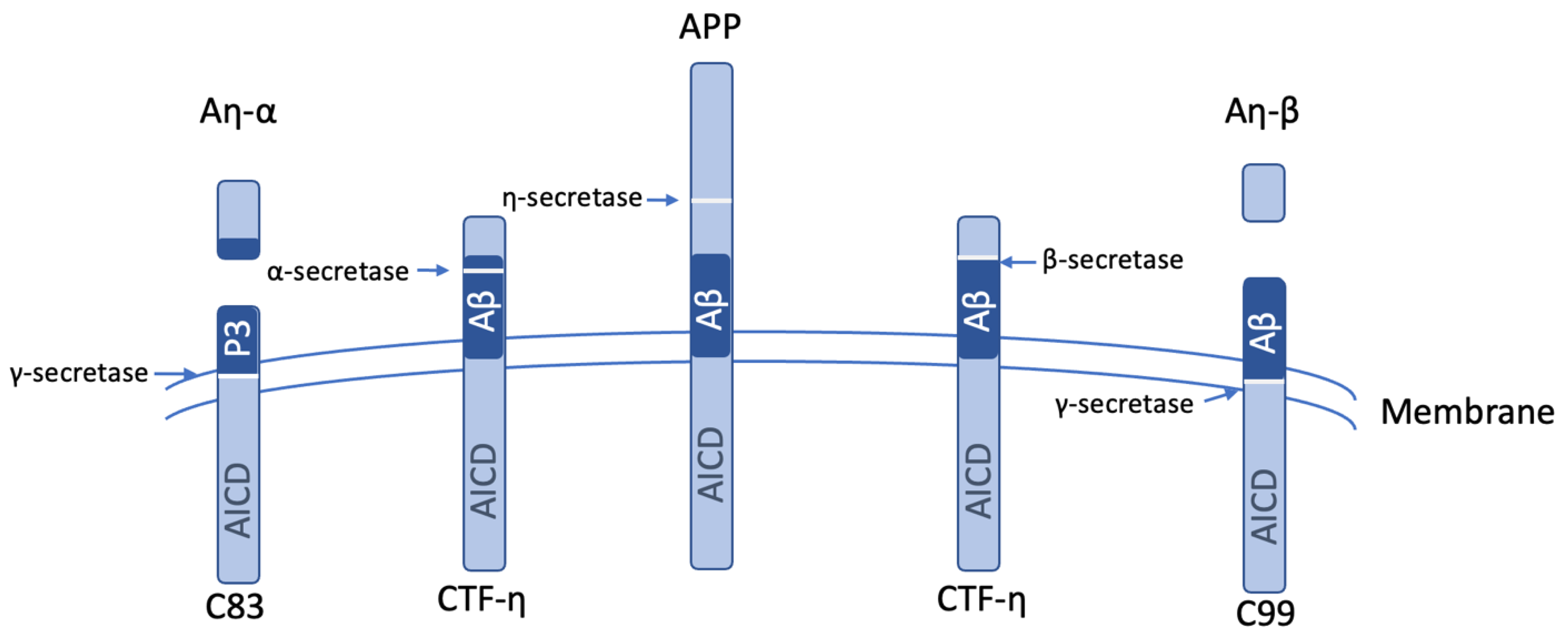
5. MT5-MMP Roles in Alzheimer’s Disease
6. MT5-MMP Roles in Other Pathologies
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llano, E.; Pendás, A.M.; Freije, J.P.; Nakano, A.; Knäuper, V.; Murphy, G.; López-Otin, C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase A overexpressed in brain tumors. Cancer Res. 1999, 59, 2570–2576. [Google Scholar] [PubMed]
- Pei, D. 141. Membrane-type matrix metalloproteinase 5. In Handbook of Proteolytic Enzymes, 2nd ed.; Barrett, A.J., Rawlings, N.D., Woessner, J.F., Eds.; Elsevier Academic Press: London, UK, 2004; Volume 1, pp. 555–557. [Google Scholar]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.K.; Chang, B.; Niedzwiecki, L.; Stein, R.L. Mechanistic studies on the human matrix metalloproteinase stromelysin. Biochemistry 1992, 31, 10757–10762. [Google Scholar] [CrossRef]
- Pelmenschikov, V.; Siegbahn, P.E.M. Catalytic Mechanism of Matrix Metalloproteinases: Two-Layered ONIOM Study. Inorg. Chem. 2002, 41, 5659–5666. [Google Scholar] [CrossRef]
- Manzetti, S.; McCulloch, D.R.; Herington, A.C.; van der Spoel, D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J. Comput. Aided Mol. Des. 2003, 17, 551–565. [Google Scholar] [CrossRef]
- Evans, M.J.; Cravatt, B.F. Mechanism-based profiling of enzyme families. Chem. Rev. 2006, 106, 3279–3301. [Google Scholar] [CrossRef]
- Bertini, I.; Fragai, M.; Luchinat, C.; Melikian, M.; Toccafondi, M.; Lauer, J.L.; Fields, G.B. Structural Basis for Matrix Metalloproteinase 1-Catalyzed Collagenolysis. J. Am. Chem. Soc. 2012, 134, 2100–2110. [Google Scholar] [CrossRef]
- Cerofolini, L.; Fields, G.B.; Fragai, M.; Geraldes, C.F.G.C.; Luchinat, C.; Parigi, G.; Ravera, E.; Svergun, D.I.; Teixeira, J.M.C. Examination of matrix metalloproteinase-1 (MMP-1) in solution: A preference for the pre-collagenolysis state. J. Biol. Chem. 2013, 288, 30659–30671. [Google Scholar] [CrossRef] [PubMed]
- Strouhalova, K.; Tolde, O.; Rosel, D.; Brábek, J. Cytoplasmic tail of MT1-MMP: A hub of MT1-MMP regulation and function. Int. J. Mol. Sci. 2023, 24, 5068. [Google Scholar] [CrossRef]
- Singh, C.; Frank, J.; Martin, K.; Gopi, N.; Tokmina-Roszyk, D.; Fields, G.B. Synthesis of Variants of the Matrix Metalloproteinase 14 Cytoplasmic Domain and Evaluation of Methods for Quantification of Intracellular Matrix Metalloproteinase 14. Int. J. Pept. Res. Ther. 2025, 31, 23. [Google Scholar] [CrossRef]
- Pei, D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J. Biol. Chem. 1999, 274, 8925–8932. [Google Scholar] [CrossRef]
- Romanic, A.M.; Burns-Kurtis, C.L.; Ao, Z.; Arleth, A.J.; Ohlstein, E.H. Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. Am. J. Physiol. Ren. Physiol. 2001, 281, F309–F317. [Google Scholar] [CrossRef]
- Hayashita-Kinoh, H.; Kinoh, H.; Okada, A.; Komori, K.; Itoh, Y.; Chiba, T.; Kajita, M.; Yana, I.; Seiki, M. Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differ. 2001, 12, 573–580. [Google Scholar]
- Sekine-Aizawa, Y.; Hama, E.; Watanabe, K.; Tsubuki, S.; Kanai-Azuma, M.; Kanai, Y.; Arai, H.; Aizawa, H.; Iwata, N.; Saido, T.C. Matrix metalloproteinase (MMP) system in brain: Identification and characterization of brain-specific MMP highly expressed in cerebellum. Eur. J. Neurosci. 2001, 13, 935–948. [Google Scholar] [CrossRef]
- Luo, J. The role of matrix metalloproteinases in the morphogenesis of the cerebellar cortex. Cerebellum 2005, 4, 239–245. [Google Scholar] [CrossRef]
- Monea, S.; Jordan, B.A.; Srivastava, S.; DeSouza, S.; Ziff, E.B. Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J. Neurosci. 2006, 26, 2300–2312. [Google Scholar] [CrossRef]
- Folgueras, A.R.; Valdes-Sanchez, T.; Llano, E.; Menendez, L.; Baamonde, A.; Denlinger, B.L.; Belmonte, C.; Juarez, L.; Lastra, A.; Garcia-Suarez, O.; et al. Metalloproteinase MT5-MMP is an essential modulator of neuro-immune interactions in thermal pain stimulation. Proc. Natl. Acad. Sci. USA 2009, 106, 16451–16456. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Nonaka, T.; Okada, A.; Kinoh, H.; Hayashita-Kinoh, H.; Yoshida, N.; Yana, I.; Seiki, M. Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett. 2004, 557, 125–128. [Google Scholar] [CrossRef]
- Jaworski, D.M. Developmental regulation of membrane type-5 matrix metalloproteinase (MT5-MMP) expression in the rat nervous system. Brain Res. 2000, 860, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, J.; Xia, L.; Du, Y.; Wu, B.; Shi, X.; Tian, N.; Pang, Y.; Yi, L.; Chen, M.; et al. PCSK6 exacerbates Alzheimer’s disease pathogenesis by promoting MT5-MMP maturation. Exp. Neurol. 2024, 374, 114688. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, D. Shedding of membrane type matrix metalloproteinase 5 by a furin-type convertase: A potential mechanism for down-regulation. J. Biol. Chem. 2001, 276, 35953–35960. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Pei, D. Mint-3 regulates the retrieval of the internalized membrane-type matrix metalloproteinase, MT5-MMP, to the plasma membrane by binding to its carboxyl end motif EWV. J. Biol. Chem. 2004, 279, 20461–20470. [Google Scholar] [CrossRef]
- Wang, H.; Lakshmana, M.K.; Fields, G.B. Identification of binding partners that facilitate membrane-type 5 matrix metalloproteinase (MT5-MMP) processing of amyloid precursor protein. J. Cell. Physiol. 2024, 239, e31218. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Koshikawa, N.; Miyamori, H.; Tanaka, M.; Sasaki, T.; Okada, Y.; Seiki, M.; Sato, H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene 2003, 22, 4617–4626. [Google Scholar] [CrossRef]
- Gaetje, R.; Holtrich, U.; Engels, K.; Kourtis, K.; Cikrit, E.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Expression of membrane-type 5 matrix metalloproteinase in human endometrium and endometriosis. Gynecol. Endocrinol. 2007, 23, 567–573. [Google Scholar] [CrossRef]
- Pilat, D.; Paumier, J.-M.; Louis, L.; Manrique, C.; García-González, L.; Stephan, D.; Bernard, A.; Pardossi-Piquard, R.; Checler, F.; Khrestchatisky, M.; et al. Suppression of MT5-MMP Reveals Early Modulation of Alzheimer’s Pathogenic Events in Primary Neuronal Cultures of 5xFAD Mice. Biomolecules 2024, 14, 1645. [Google Scholar] [CrossRef]
- Porlan, E.; Martí-Prado, B.; Morante-Redolat, J.M.; Consiglio, A.; Delgado, A.C.; Kypta, R.; López-Otín, C.; Kirstein, M.; Fariñas, I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat. Cell Biol. 2014, 16, 629–638. [Google Scholar] [CrossRef]
- Wang, X.; Yi, J.; Lei, J.; Pei, D. Expression, purification and characterization of recombinant mouse MT5-MMP protein products. FEBS Lett. 1999, 462, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.B.; Morrison, C.J.; Overall, C.M.; Strittmatter, S.M.; Fournier, A.E. Membrane-type matrix metalloproteinase-3 regulates neuronal responsiveness to myelin through Nogo-66 receptor 1 cleavage. J. Biol. Chem. 2011, 286, 31418–31424. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Takino, T.; Miyamori, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of amyloid-beta precursor protein (APP) by membrane-type matrix metalloproteinases. J. Biochem. 2006, 139, 517–526. [Google Scholar] [CrossRef]
- Willem, M.; Tahirovic, S.; Busche, M.A.; Ovsepian, S.V.; Chafai, M.; Kootar, S.; Hornburg, D.; Evans, L.D.; Moore, S.; Daria, A.; et al. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 2015, 526, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Remacle, A.G.; Savinov, A.Y.; Chernov, A.V.; Cieplak, P.; Radichev, I.A.; Williams, R.; Shiryaeva, T.N.; Gawlik, K.; Postnova, T.I.; et al. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J. Biol. Chem. 2009, 284, 30615–30626. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Savinov, A.Y.; Cieplak, P.; Ratnikov, B.I.; Motamedchaboki, K.; Smith, J.W.; Strongin, A.Y. Matrix metalloproteinase proteolysis of the myelin basic protein isoforms is a source of immunogenic peptides in autoimmune multiple sclerosis. PLoS ONE 2009, 4, e4952. [Google Scholar] [CrossRef]
- Ito, K.; Okamoto, I.; Araki, N.; Kawano, Y.; Nakao, M.; Fujiyama, S.; Tomita, K.; Mimori, T.; Saya, H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene 1999, 18, 7080–7090. [Google Scholar] [CrossRef]
- Marambaud, P.; Shioi, J.; Serban, G.; Georgakopoulos, A.; Sarner, S.; Nagy, V.; Baki, L.; Wen, P.; Efthimiopoulos, S.; Shao, Z.; et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002, 21, 1948–1956. [Google Scholar] [CrossRef]
- Medrano-González, P.A.; Rivera-Ramírez, O.; Montaño, L.F.; Rendón-Huerta, E.P. Proteolytic Processing of CD44 and Its Implications in Cancer. Stem Cells Int. 2021, 2021, 6667735. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Itoh, Y.; Chiba, T.; Mori, H.; Okada, A.; Kinoh, H.; Seiki, M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001, 153, 893–904. [Google Scholar] [CrossRef]
- Suenaga, N.; Mori, H.; Itoh, Y.; Seiki, M. CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene 2005, 24, 859–868. [Google Scholar] [CrossRef]
- Stawikowski, M.; Knapinska, A.M.; Fields, G.B. Determining the Substrate Specificity of Matrix Metalloproteases using Fluorogenic Peptide Substrates. In Methods in Molecular Biology: Matrix Metalloproteinases Methods and Protocols; Galea, C.A., Ed.; Humana Press: Totowa, NJ, USA, 2017; Volume 1579, pp. 137–183. [Google Scholar]
- Devy, L.; Pieters, H.; Frans, N.; Naa, L.; Kuang, G.; Van Gool, R.; Ladner, R.C.; Brepoels, E.; Nixon, A.; Hoet, R.; et al. Metalloproteinase Binding Proteins. World Intellectual Property Organization WO2007/079218A2, 2007. [Google Scholar]
- Zipfel, P.; Rochais, C.; Baranger, K.; Rivera, S.; Dallemagne, P. Matrix Metalloproteinases as New Targets in Alzheimer’s Disease: Opportunities and Challenges. J. Med. Chem. 2020, 63, 10705–10725. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Chalmers, M.J.; Busby, S.A.; Minond, D.; Griffin, P.R.; Fields, G.B. Identification of specific hemopexin-like domain residues that facilitate matrix metalloproteinase collagenolytic activity. J. Biol. Chem. 2009, 284, 24017–24024. [Google Scholar] [CrossRef]
- Pahwa, S.; Bhowmick, M.; Amar, S.; Cao, J.; Strongin, A.Y.; Fridman, R.; Weiss, S.J.; Fields, G.B. Characterization and regulation of MT1-MMP cell surface-associated activity. Chem. Biol. Drug Des. 2019, 93, 1251–1264. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Drotleff, G.; Chai, C.; Twohill, D.; Ernce, A.; Tokmina-Roszyk, D.; Grande, I.; Rodriguez, M.; Larson, B.; Fields, G.B. Screening MT1-MMP Activity and Inhibition in Three-Dimensional Tumor Spheroids. Biomedicines 2023, 11, 562. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Fields, G.B. The expanding role of MT1-MMP in cancer progression. Pharmaceuticals 2019, 12, 77. [Google Scholar] [CrossRef]
- Palmulli, R.; Jackson, H.K.; Edgar, J.R. Tethered Exosomes Containing the Matrix Metalloproteinase MT1-MMP Contribute to Extracellular Matrix Degradation. J. Extracell. Vesicles 2025, 14, e70122. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, L.; Cho, H.; Xie, X.; Eliceiri, G.L. Additional MDA-MB-231 breast cancer cell matrix metalloproteinases promote invasiveness. J. Cell. Physiol. 2008, 216, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef]
- Benson, C.S.; Babu, S.D.; Radhakrishna, S.; Selvamurugan, N.; Ravi Sankar, B. Expression of matrix metalloproteinases in human breast cancer tissues. Dis. Markers 2013, 34, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.P.; Gunsalus, K.T.W.; Schoenike, B.; Richardson, A.L.; Friedl, A.; Roopra, A. The transcription factor REST is lost in aggressive breast cancer. PLoS Genet. 2010, 6, e1000979. [Google Scholar] [CrossRef]
- Cloud, A.S.; Vargheese, A.M.; Gunewardena, S.; Shimak, R.M.; Ganeshkumar, S.; Kumaraswamy, E.; Jensen, R.A.; Chennathukuzhi, V.M. Loss of REST in breast cancer promotes tumor progression through estrogen sensitization, MMP24 and CEMIP overexpression. BMC Cancer 2022, 22, 180. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar]
- Sugimoto, W.; Itoh, K.; Hirata, H.; Abe, Y.; Torii, T.; Mitsui, Y.; Budirahardja, Y.; Tanaka, N.; Kawauchi, K. MMP24 as a Target of YAP is a Potential Prognostic Factor in Cancer Patients. Bioengineering 2020, 7, 18. [Google Scholar] [CrossRef]
- Okimoto, R.A.; Breitenbuecher, F.; Olivas, V.R.; Wu, W.; Gini, B.; Hofree, M.; Asthana, S.; Hrustanovic, G.; Flanagan, J.; Tulpule, A.; et al. Inactivation of Capicua drives cancer metastasis. Nat. Genet. 2017, 49, 87–96. [Google Scholar] [CrossRef]
- Luo, Y.P.; Zhong, M.; Wang, L.P.; Zhong, M.; Sun, G.Q.; Li, J. Inhibitory effects of RNA interference on MMP-24 expression and invasiveness of ovarian cancer SKOV(3) cells. J. Southern Med. Univ. 2009, 29, 781–784. [Google Scholar]
- Baren, J.P.; Stewart, G.D.; Stokes, A.; Gray, K.; Pennington, C.J.; O’Neill, R.; Deans, D.A.C.; Paterson-Brown, S.; Riddick, A.C.P.; Edwards, D.R.; et al. mRNA profiling of the cancer degradome in oesophago-gastric adenocarcinoma. Br. J. Cancer 2012, 107, 143–149. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, S.; Sampieri, C.L.; Ochoa-Lara, M.; León-Córdoba, K.; Remes-Troche, J.M. Expression of the matrix metalloproteases 2, 14, 24, and 25 and tissue inhibitor 3 as potential molecular markers in advanced human gastric cancer. Dis. Markers 2014, 2014, 285906. [Google Scholar] [CrossRef] [PubMed]
- Aitchison, E.E.; Dimesa, A.M.; Shoari, A. Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets. BioTech 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, T.E.; Broaddus, W.C.; Rooprai, H.K.; Pilkington, G.J.; Fillmore, H.L. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro-oncology 2004, 6, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.J.; Kellett, K.A.B.; Thinakaran, G.; Hooper, N.M. A Greek tragedy: The growing complexity of Alzheimer amyloid precursor protein proteolysis. J. Biol. Chem. 2016, 291, 19235–19244. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Pilat, D.; Baranger, K.; Rivera, S. Emerging Alternative Proteinases in APP Metabolism and Alzheimer’s Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP. Front. Aging Neurosci. 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Baranger, K.; Marchalant, Y.; Bonnet, A.E.; Crouzin, N.; Carrete, A.; Paumier, J.M.; Py, N.A.; Bernard, A.; Bauer, C.; Charrat, E.; et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell. Mol. Life Sci. 2016, 73, 217–236. [Google Scholar] [CrossRef]
- Pilat, D.; Paumier, J.-M.; García-González, L.; Louis, L.; Stephan, D.; Manrique, C.; Khrestchatisky, M.; Di Pasquale, E.; Baranger, K.; Rivera, S. MT5-MMP promotes neuroinflammation, neuronal excitability and Aβ production in primary neuron/astrocyte cultures from the 5xFAD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 65. [Google Scholar] [CrossRef]
- Afram, E.; Lauritzen, I.; Bourgeois, A.; El Manaa, W.; Duplan, E.; Chami, M.; Valverde, A.; Charlotte, B.; Pardossi-Piquard, R.; Checler, F. The η-secretase-derived APP fragment ηCTF is localized in Golgi, endosomes and extracellular vesicles and contributes to Aβ production. Cell. Mol. Life Sci. 2023, 80, 97. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, D.; Radosinska, J. The Link Between Matrix Metalloproteinases and Alzheimer’s Disease Pathophysiology. Mol. Neurobiol. 2025, 62, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Mensch, M.; Dunot, J.; Yishan, S.M.; Harris, S.S.; Blistein, A.; Avdiu, A.; Pousinha, P.A.; Giudici, C.; Busche, M.A.; Jedlicka, P.; et al. Aη-α and Aη-β peptides impair LTP ex vivo within the low nanomolar range and impact neuronal activity in vivo. Alzheimer’s Res. Ther. 2021, 13, 125. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Rice, H.C.; de Malmazet, D.; Schreurs, A.; Frere, S.; Van Molle, I.; Volkov, A.N.; Creemers, E.; Vertkin, I.; Nys, J.; Ranaivoson, F.M.; et al. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science 2019, 363, eaao4827. [Google Scholar] [CrossRef] [PubMed]
- Baranger, K.; Bonnet, A.E.; Girard, S.D.; Paumier, J.M.; Garcia-Gonzalez, L.; Elmanaa, W.; Bernard, A.; Charrat, E.; Stephan, D.; Bauer, C.; et al. MT5-MMP Promotes Alzheimer’s Pathogenesis in the Frontal Cortex of 5xFAD Mice and APP Trafficking in vitro. Front. Mol. Neurosci. 2017, 9, 163. [Google Scholar] [CrossRef]
- Baranger, K.; Khrestchatisky, M.; Rivera, S. MT5-MMP, just a new APP processing proteinase in Alzheimer’s disease? J. Neuroinflamm. 2016, 13, 167. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Paumier, J.M.; Louis, L.; Pilat, D.; Bernard, A.; Stephan, D.; Jullien, N.; Checler, F.; Nivet, E.; Khrestchatisky, M.; et al. MT5-MMP controls APP and beta-CTF/C99 metabolism through proteolytic-dependent and -independent mechanisms relevant for Alzheimer’s disease. FASEB J. 2021, 35, e21727. [Google Scholar] [CrossRef]
- Fourriere, L.; Gleeson, P.A. Amyloid beta production along the neuronal secretory pathway: Dangerous liaisons in the Golgi? Traffic 2021, 22, 319–327. [Google Scholar] [CrossRef]
- Bianchi, F.T.; Camera, P.; Ala, U.; Imperiale, D.; Migheli, A.; Boda, E.; Tempia, F.; Berto, G.; Bosio, Y.; Oddo, S.; et al. The collagen chaperone HSP47 is a new interactor of APP that affects the levels of extracellular beta-amyloid peptides. PLoS ONE 2011, 6, e22370. [Google Scholar] [CrossRef]
- Zhou, L.; Xue, X.; Yang, K.; Feng, Z.; Liu, M.; Pastor-Pareja, J.C. Convergence of secretory, endosomal, and autophagic routes in trans-Golgi-associated lysosomes. J. Cell Biol. 2023, 222, e202203045. [Google Scholar] [CrossRef]
- Jansen, J.C.; Timal, S.; van Scherpenzeel, M.; Michelakakis, H.; Vicogne, D.; Ashikov, A.; Moraitou, M.; Hoischen, A.; Huijben, K.; Steenbergen, G.; et al. TMEM199 Deficiency Is a Disorder of Golgi Homeostasis Characterized by Elevated Aminotransferases, Alkaline Phosphatase, and Cholesterol and Abnormal Glycosylation. Am. J. Hum. Genet. 2016, 98, 322–330. [Google Scholar] [CrossRef]
- Li, B.; Clohisey, S.M.; Chia, B.S.; Wang, B.; Cui, A.; Eisenhaure, T.; Schweitzer, L.D.; Hoover, P.; Parkinson, N.J.; Nachshon, A.; et al. Genome-wide CRISPR screen identifies host dependency factors for influenza A virus infection. Nat. Commun. 2020, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, D.S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Feng, J.; Broaddus, R.R. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis. 2010, 1, e32. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Saha, O.; Landeira, B.S.; de Farias, A.R.M.; Hermant, X.; Carrier, A.; Pelletier, A.; Gadaut, J.; Davoine, L.; Dupont, C.; et al. The Alzheimer susceptibility gene BIN1 induces isoform-dependent neurotoxicity through early endosome defects. Acta Neuropathol. Commun. 2022, 10, 4. [Google Scholar] [CrossRef]
- Keren-Kaplan, T.; Sarić, A.; Ghosh, S.; Williamson, C.D.; Jia, R.; Li, Y.; Bonifacino, J.S. RUFY3 and RUFY4 are ARL8 effectors that promote coupling of endolysosomes to dynein-dynactin. Nat. Commun. 2022, 13, 1506. [Google Scholar] [CrossRef]
- Grau, S.; Baldi, A.; Bussani, R.; Tian, X.; Stefanescu, R.; Przybylski, M.; Richards, P.; Jones, S.A.; Shridhar, V.; Clausen, T.; et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 2005, 102, 6021–6026. [Google Scholar] [CrossRef]
- Haugstetter, J.; Maurer, M.A.; Blicher, T.; Pagac, M.; Wider, G.; Ellgaard, L. Structure-function analysis of the endoplasmic reticulum oxidoreductase TMX3 reveals interdomain stabilization of the N-terminal redox-active domain. J. Biol. Chem. 2007, 282, 33859–33867. [Google Scholar] [CrossRef]
- Medinas, D.B.; Rozas, P.; Hetz, C. Critical roles of protein disulfide isomerases in balancing proteostasis in the nervous system. J. Biol. Chem. 2022, 298, 102087. [Google Scholar] [CrossRef]
- Madar, I.H.; Sultan, G.; Tayubi, I.A.; Hasan, A.N.; Pahi, B.; Rai, A.; Sivanandan, P.K.; Loganathan, T.; Begum, M.; Rai, S. Identification of marker genes in Alzheimer’s disease using a machine-learning model. Bioinformation 2021, 17, 348–355. [Google Scholar] [CrossRef]
- Hoe, H.-S.; Cooper, M.J.; Burns, M.P.; Lewis, P.A.; van der Brug, M.; Chakraborty, G.; Cartagena, C.M.; Pak, D.T.S.; Cookson, M.R.; Rebeck, G.W. The metalloprotease inhibitor TIMP-3 regulates amyloid precursor protein and apolipoprotein E receptor proteolysis. J. Neurosci. 2007, 27, 10895–10905. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, S.J.; Jo, C.; Park, M.H.; Han, C.; Kim, E.J.; Huh, G.Y.; Koh, Y.H. Altered TIMP-3 Levels in the Cerebrospinal Fluid and Plasma of Patients with Alzheimer’s Disease. J. Pers. Med. 2022, 12, 827. [Google Scholar] [CrossRef]
- Warren, K.M.; Reeves, T.M.; Phillips, L.L. MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J. Neurotrauma 2012, 29, 1922–1940. [Google Scholar] [CrossRef]
- Dong, Z.; Katar, M.; Alousi, S.; Berk, R.S. Expression of membrane-type matrix metalloproteinases 4, 5, and 6 in mouse corneas infected with P. aeruginosa. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3223–3227. [Google Scholar]
- Fortunato, S.J.; Menon, R. Screening of novel matrix metalloproteinases (MMPs) in human fetal membranes. J. Assist. Reprod. Genet. 2002, 19, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, L.; Selkoe, D. Verubecestat for Prodromal Alzheimer’s Disease. N. Engl. J. Med. 2019, 381, 388. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, D.; Knapinska, A.M.; Wang, H.; Fields, G.B. Membrane-Type 5 Matrix Metalloproteinase (MT5-MMP): Background and Proposed Roles in Normal Physiology and Disease. Biomolecules 2025, 15, 1114. https://doi.org/10.3390/biom15081114
Jadhav D, Knapinska AM, Wang H, Fields GB. Membrane-Type 5 Matrix Metalloproteinase (MT5-MMP): Background and Proposed Roles in Normal Physiology and Disease. Biomolecules. 2025; 15(8):1114. https://doi.org/10.3390/biom15081114
Chicago/Turabian StyleJadhav, Deepak, Anna M. Knapinska, Hongjie Wang, and Gregg B. Fields. 2025. "Membrane-Type 5 Matrix Metalloproteinase (MT5-MMP): Background and Proposed Roles in Normal Physiology and Disease" Biomolecules 15, no. 8: 1114. https://doi.org/10.3390/biom15081114
APA StyleJadhav, D., Knapinska, A. M., Wang, H., & Fields, G. B. (2025). Membrane-Type 5 Matrix Metalloproteinase (MT5-MMP): Background and Proposed Roles in Normal Physiology and Disease. Biomolecules, 15(8), 1114. https://doi.org/10.3390/biom15081114







