Influence of Monosodium Glutamate on Astroglia of Rat Habenula
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemical Analyses
2.2. Morphological and Morphometric Analyses
2.3. Statistical Analyses
3. Results
3.1. Morphological Analyses of GFAP-IR Astrocytes
3.2. Morphological Analyses of S100β-IR Astrocytes
3.3. Morphometric and Statistical Analyses
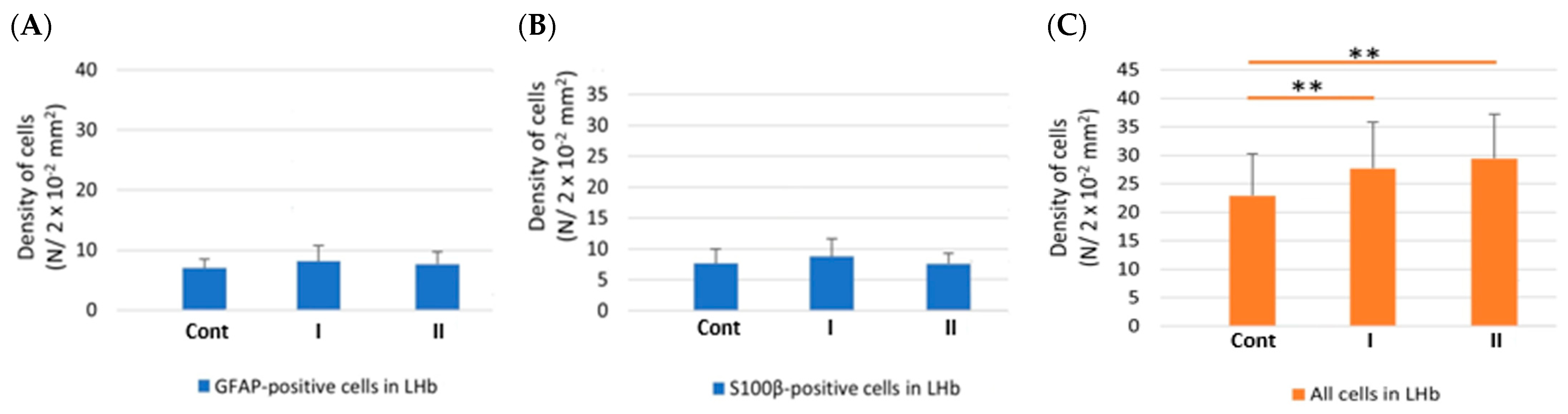
3.3.1. Density of Cells
3.3.2. Morphometric Parameters of GFAP-IR Astrocytes
3.3.3. Morphometric Parameters of S100β-IR Astrocytes
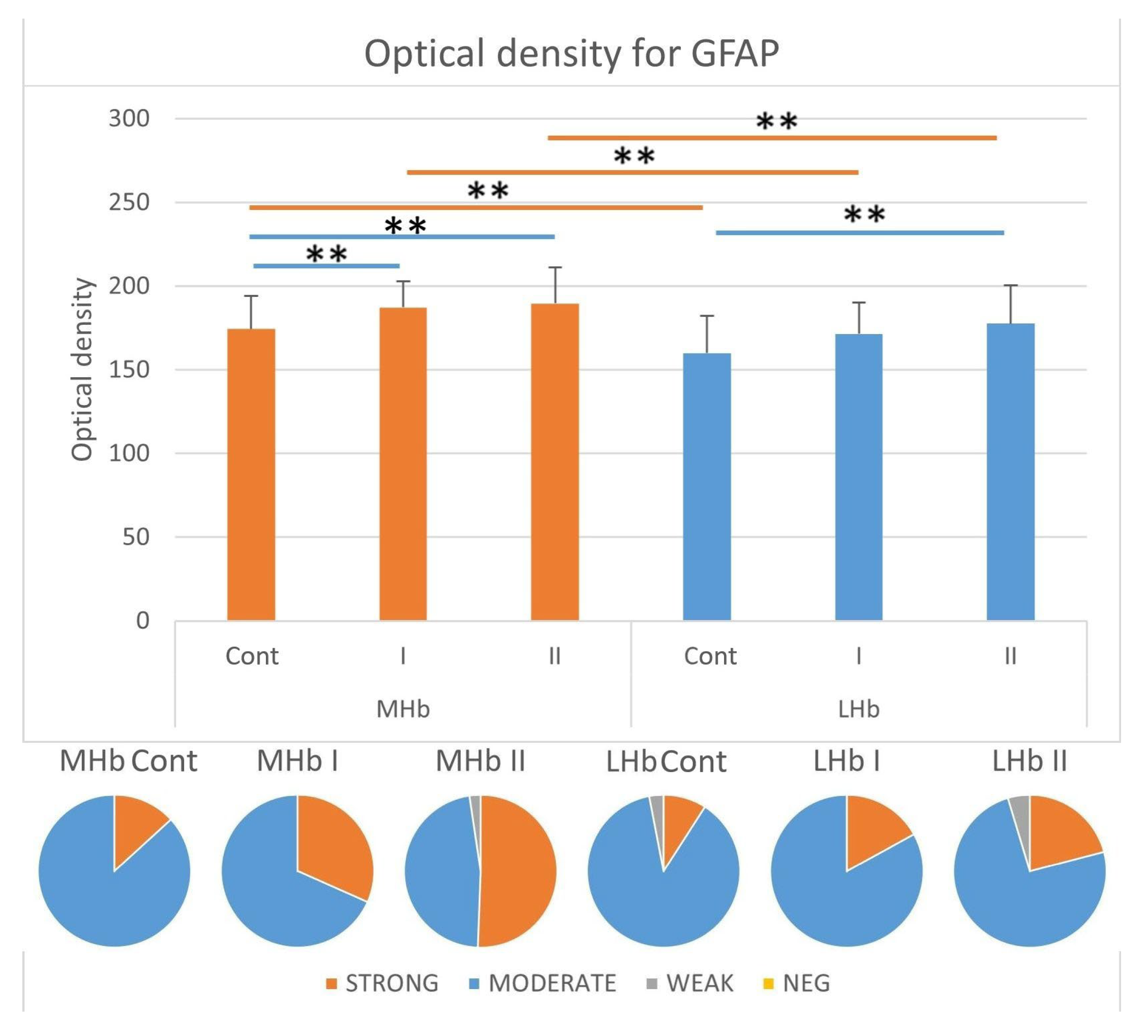
3.3.4. Immunostaining Intensity of GFAP-IR Cells
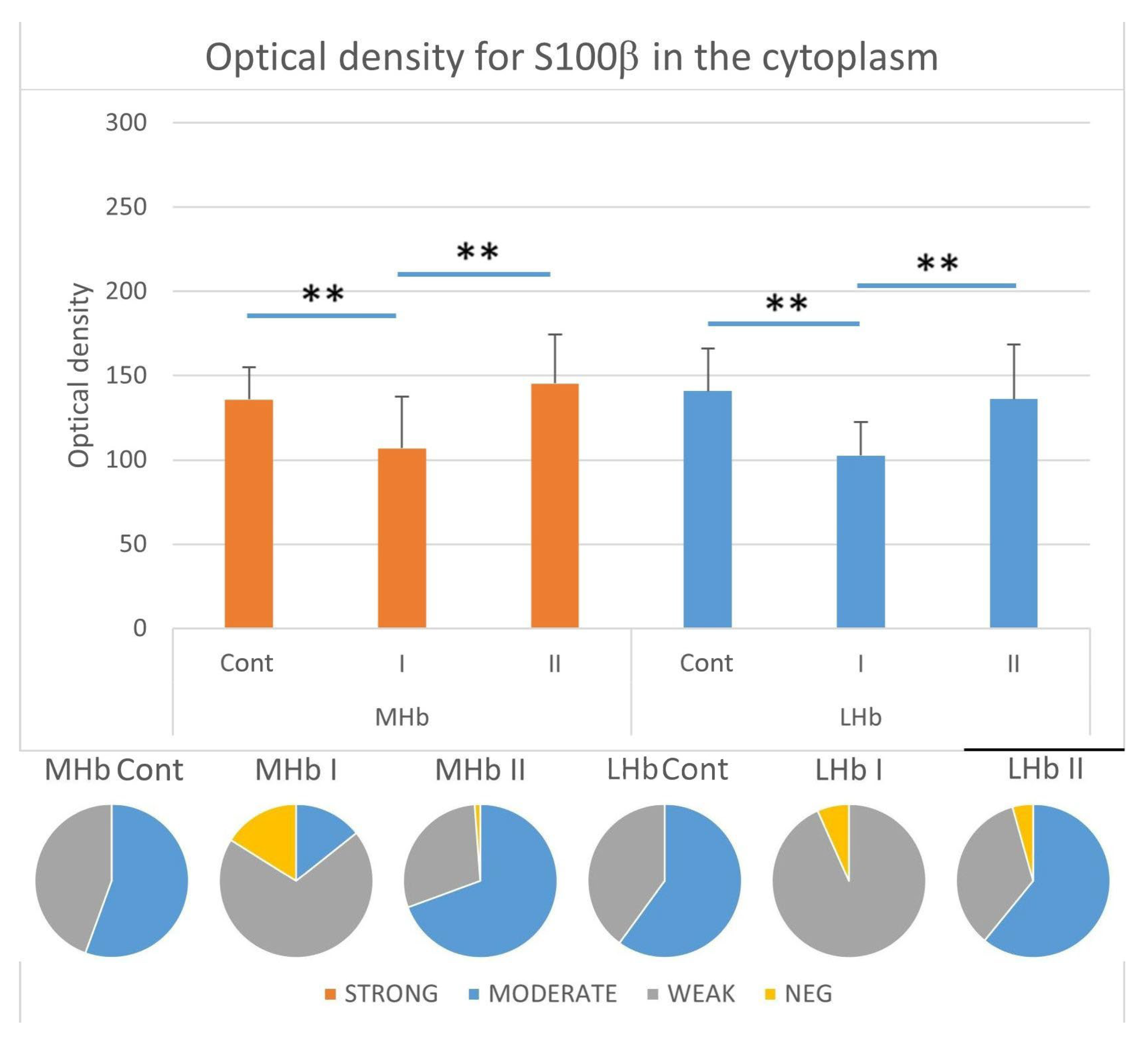
3.3.5. Immunostaining Intensity of the Cytoplasm of S100β-IR Cells
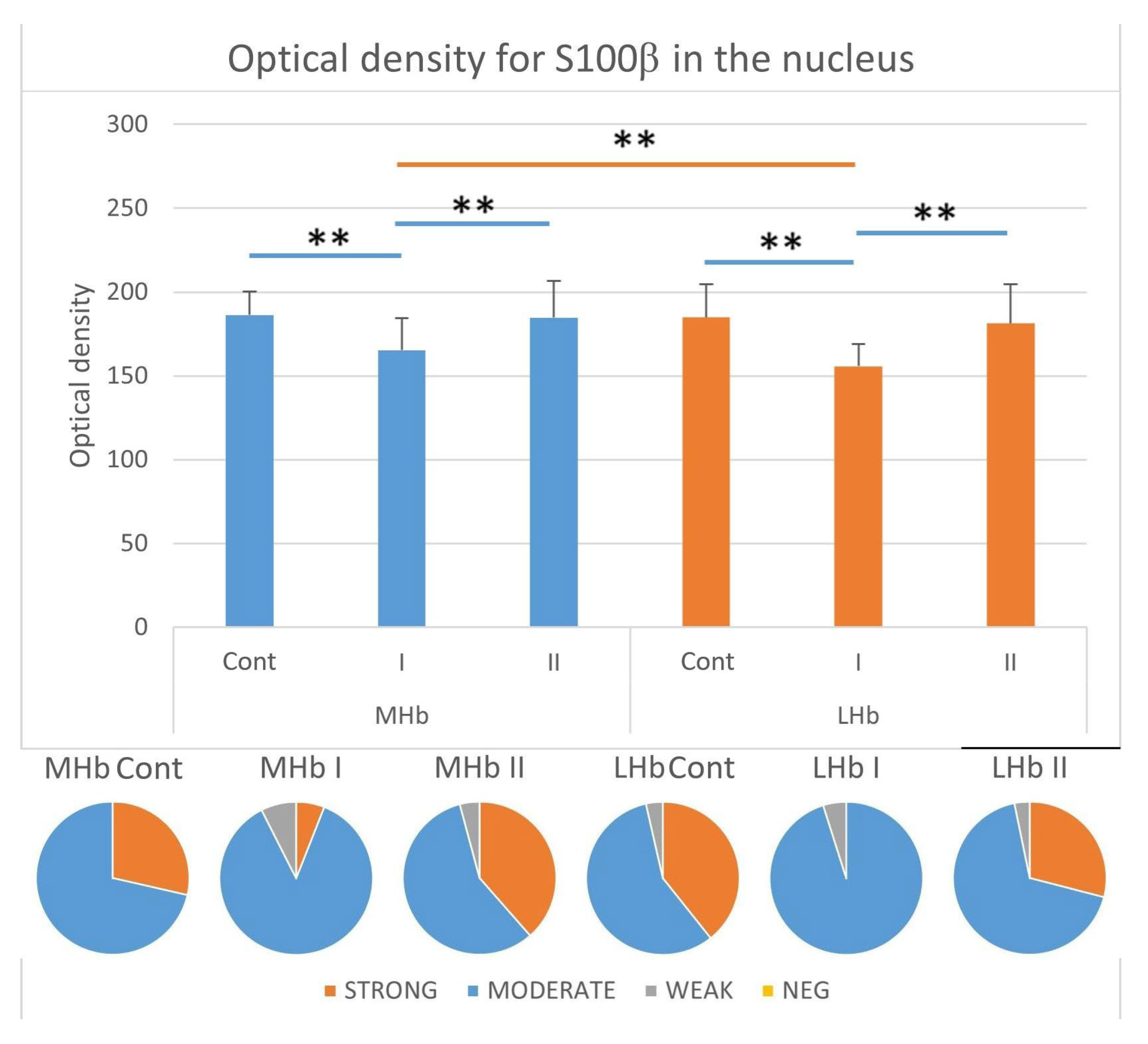
3.3.6. Immunostaining Intensity of the Nucleus of S100β-IR Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Hb | Habenula |
| MHb | Medial part of habenula |
| LHb | Lateral part of habenula |
| CNS | Central nervous system |
| Glu | Glutamate |
| MSG | Monosodium glutamate |
| GFAP | Glial fibrillary acidic protein |
| GFAP-IR | Immunoreactive for glial fibrillary acidic protein |
| S100β-IR | Immunoreactive for S100β protein |
| NMDA-R | N-methyl-D-aspartate receptor |
| RT | Room temperature |
| DAB | 3,3′-diaminobenzidine tetrachloride |
| Si | Shape index |
| OD | Optical density |
| ANOVA | One-way analysis of variance |
| GLAST | Glutamate-aspartate transporter |
| GLT-1 | Glutamate transporter-1 |
| GABA | ℽ-ammobutyric acid |
| TNFα | Tumor necrosis factor |
| IL-1β | Interleukin 1β |
| NF-κB | Nuclear transcription factor kappa B |
References
- Boulos, L.J.; Darcq, E.; Kieffer, B.L. Translating the Habenula-From Rodents to Humans. Biol. Psychiatry 2017, 81, 296–305. [Google Scholar] [CrossRef]
- Groos, D.; Helmchen, F. The Lateral Habenula: A Hub for Value-Guided Behavior. Cell Rep. 2024, 43, 113968. [Google Scholar] [CrossRef]
- Loonen, A.J.M. Role of Neuroglia in the Habenular Connection Hub of the Dorsal Diencephalic Conduction System. Neuroglia 2023, 4, 34–51. [Google Scholar] [CrossRef]
- Piper, J.A.; Musumeci, G.; Castorina, A. The Neuroanatomy of the Habenular Complex and Its Role in the Regulation of Affective Behaviors. J. Funct. Morphol. Kinesiol. 2024, 9, 14. [Google Scholar] [CrossRef]
- Aizawa, H.; Cui, W.; Tanaka, K.; Okamoto, H. Hyperactivation of the Habenula as a Link between Depression and Sleep Disturbance. Front. Hum. Neurosci. 2013, 7, 826. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Zhu, M. Toward an Understanding of the Habenula’s Various Roles in Human Depression. Psychiatry Clin. Neurosci. 2019, 73, 607–612. [Google Scholar] [CrossRef]
- Cui, W.; Mizukami, H.; Yanagisawa, M.; Aida, T.; Nomura, M.; Isomura, Y.; Takayanagi, R.; Ozawa, K.; Tanaka, K.; Aizawa, H. Glial Dysfunction in the Mouse Habenula Causes Depressive-like Behaviors and Sleep Disturbance. J. Neurosci. 2014, 34, 16273–16285. [Google Scholar] [CrossRef]
- Yang, L.M.; Hu, B.; Xia, Y.H.; Zhang, B.L.; Zhao, H. Lateral Habenula Lesions Improve the Behavioral Response in Depressed Rats via Increasing the Serotonin Level in Dorsal Raphe Nucleus. Behav. Brain Res. 2008, 188, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the Lateral Habenula Drives Neuronal Bursts in Depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- Gradisnik, L.; Velnar, T. Astrocytes in the Central Nervous System and Their Functions in Health and Disease: A Review. World J. Clin. Cases 2023, 11, 3385–3394. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in Human Central Nervous System Diseases: A Frontier for New Therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Freude, K. Astrocytes: The Stars in Neurodegeneration? Biomolecules 2024, 14, 289. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.S. Astrogliosis in CNS Pathologies: Is There a Role for Microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef]
- Xu, L.; Sapolsky, R.M.; Giffard, R.G. Differential Sensitivity of Murine Astrocytes and Neurons from Different Brain Regions to Injury. Exp. Neurol. 2001, 169, 416–424. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of Astrocyte Functions and Phenotypes in Neural Circuits. Nat. Neurosci. 2015, 18, 942. [Google Scholar] [CrossRef]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and Therapeutic Value of Astrocytes in Neurological Diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and Stroke: Identifying Novel Targets for Neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular Mechanisms of Ischemia and Glutamate Excitotoxicity. Life Sci. 2023, 328, 121814. [Google Scholar] [CrossRef]
- Plotegher, N.; Filadi, R.; Pizzo, P.; Duchen, M.R. Excitotoxicity Revisited: Mitochondria on the Verge of a Nervous Breakdown. Trends Neurosci. 2021, 44, 342–351. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef] [PubMed]
- Tannenberg, R.K.; Scott, H.L.; Westphalen, R.I.; Dodd, P.R. The Identification and Characterization of Excitotoxic Nerve-Endings in Alzheimer Disease. Curr. Alzheimer Res. 2004, 1, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef]
- Bing, H.; Russell, F. Glutamatergic Dysfunction of Lateral Habenula Promotes Depression. Neuropsychiatry 2018, 08, 1683–1686. [Google Scholar] [CrossRef]
- Noble, L.J.; Hall, J.J.; Chen, S.; Chan, P.H. Morphologic Changes in Cultured Astrocytes after Exposure to Glutamate. J. Neurotrauma 1992, 9, 255–267. [Google Scholar] [CrossRef]
- Yang, S.H.; Sun, Y.; Berry, R.; Choudhury, G.R.; Winters, A.; Chaudhari, K.; Liu, R. Glutamate Provides Cytoprotective Effect for Astrocytes Against Ischemic Insult and Promotes Astrogliosis. Aging Dis. 2024, 15, 2742. [Google Scholar] [CrossRef]
- Monno, A.; Vezzani, A.; Bastone, A.; Salmona, M.; Garattini, S. Extracellular Glutamate Levels in the Hypothalamus and Hippocampus of Rats after Acute or Chronic Oral Intake of Monosodium Glutamate. Neurosci. Lett. 1995, 193, 45–48. [Google Scholar] [CrossRef]
- Bogdanov, M.B.; Tjurmina, O.A.; Wurtman, R.J. Consumption of a High Dietary Dose of Monosodium Glutamate Fails to Affect Extracellular Glutamate Levels in the Hypothalamic Arcuate Nucleus of Adult Rats. Brain Res. 1996, 736, 76–81. [Google Scholar] [CrossRef]
- Torii, K.; Takasaki, Y.; Iwata, S.; Wurtman, R.J. Changes in Blood Osmolarity Electrolytes, and Metabolites among Adult Rats Treated with a Neurotoxic Dose of MSG. Life Sci. 1981, 28, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W. Brain Lesions, Obesity, and Other Disturbances in Mice Treated with Monosodium Glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Farhat, F. Monosodium Glutamate Safety, Neurotoxicity and Some Recent Studies. Al-Azhar J. Pharm. Sci. 2021, 64, 222–243. [Google Scholar] [CrossRef]
- Rivera-Cervantes, M.C.; Torres, J.S.; Feria-Velasco, A.; Armendariz-Borunda, J.; Beas-Zárate, C. NMDA and AMPA Receptor Expression and Cortical Neuronal Death Are Associated with P38 in Glutamate-Induced Excitotoxicity in Vivo. J. Neurosci. Res. 2004, 76, 678–687. [Google Scholar] [CrossRef]
- Hazzaa, S.M.; Abdelaziz, S.A.M.; Abd Eldaim, M.A.; Abdel-Daim, M.M.; Elgarawany, G.E. Neuroprotective Potential of Allium Sativum against Monosodium Glutamate-Induced Excitotoxicity: Impact on Short-Term Memory, Gliosis, and Oxidative Stress. Nutrients 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Aboul Fotouh, G.I.; Sayed, S.S.E.; Altayeb, Z.M.; Zaher, E.A. The Possible Neuroprotective Effect of Astaxanthin on Monosodium Glutamate and Aspartame Induced Hippocampal Changes in Albino Rats: (Histological and Immuno-Histochemical Study). Egypt. J. Histol. 2020, 43, 684–701. [Google Scholar] [CrossRef]
- Hashem, H.E.; El-Din Safwat, M.D.; Algaidi, S. The Effect of Monosodium Glutamate on the Cerebellar Cortex of Male Albino Rats and the Protective Role of Vitamin C (Histological and Immunohistochemical Study). J. Mol. Histol. 2012, 43, 179–186. [Google Scholar] [CrossRef]
- Rosa, S.G.; Chagas, P.M.; Pesarico, A.P.; Nogueira, C.W. Monosodium Glutamate Induced Nociception and Oxidative Stress Dependent on Time of Administration, Age of Rats and Susceptibility of Spinal Cord and Brain Regions. Toxicol. Appl. Pharmacol. 2018, 351, 64–73. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P. Protective Effect of Calendula Officinalis, L. Flowers Against Monosodium Glutamate Induced Oxidative Stress and Excitotoxic Brain Damage in Rats. Indian J. Clin. Biochem. IJCB 2013, 28, 292–298. [Google Scholar] [CrossRef]
- Liao, R.; Wood, T.R.; Nance, E. Superoxide Dismutase Reduces Monosodium Glutamate-Induced Injury in an Organotypic Whole Hemisphere Brain Slice Model of Excitotoxicity. J. Biol. Eng. 2020, 14, 3. [Google Scholar] [CrossRef]
- Jaworska-Adamu, J.; Krawczyk, A.; Rycerz, K. Influence of monosodium glutamate on calretinin immunoreactivity in the dorsal raphe nucleus in adult rats. Med. Weter. 2019, 75, 426–430. [Google Scholar] [CrossRef]
- Krawczyk, A.; Jaworska-Adamu, J. Evaluation of Immunoreactive for Glial Fibrillary Acidic Protein Astrocytes in the Periaqueductal Gray Matter of Rats Treated with Monosodium Glutamate. Med. Weter. 2023, 77, 22–27. [Google Scholar] [CrossRef]
- Krawczyk, A.; Jaworska-Adamu, J. Reactivity of Astrocytes in the Periaqueductal Gray Matter of Rats Treated with Monosodium Glutamate. Folia Histochem. Cytobiol. 2020, 58, 147–155. [Google Scholar] [CrossRef]
- Rycerz, K.; Krawczyk, A.; Jaworska-Adamu, J.; Arciszewski, M.B. Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats. Biomedicines 2024, 12, 2763. [Google Scholar] [CrossRef]
- Suárez, I.; Bodega, G.; Rubio, M.; Fernández, B. Sexual Dimorphism in the Hamster Cerebellum Demonstrated by Glial Fibrillary Acidic Protein (GFAP) and Vimentin Immunoreactivity. Glia 1992, 5, 10–16. [Google Scholar] [CrossRef] [PubMed]
- König, J.F.R.; Klippel, R.A. A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem; Williams and Wilkins: Baltimore, MD, USA, 1963. [Google Scholar]
- de Matos, L.L.; Stabenow, E.; Tavares, M.R.; Ferraz, A.R.; Capelozzi, V.L.; Pinhal, M.A.S. Immunohistochemistry Quantification by a Digital Computer-Assisted Method Compared to Semiquantitative Analysis. Clinics 2006, 61, 417–424. [Google Scholar] [CrossRef][Green Version]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta Histochem. Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Hughes, E.G.; Maguire, J.L.; McMinn, M.T.; Scholz, R.E.; Sutherland, M.L. Loss of Glial Fibrillary Acidic Protein Results in Decreased Glutamate Transport and Inhibition of PKA-Induced EAAT2 Cell Surface Trafficking. Brain Res. Mol. Brain Res. 2004, 124, 114–123. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.R.; Binder, D.K. Regulation of Synaptosomal GLT-1 and GLAST during Epileptogenesis. Neuroscience 2019, 411, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Bravo, D.; Rojas, X.; Concha, M.L. Morphologic and Immunohistochemical Organization of the Human Habenular Complex. J. Comp. Neurol. 2011, 519, 3727–3747. [Google Scholar] [CrossRef]
- Zhou, J.; Sutherland, M.l. Glutamate Transporter Cluster Formation in Astrocytic Processes Regulates Glutamate Uptake Activity. J. Neurosci. 2004, 24, 6301–6306. [Google Scholar] [CrossRef]
- Sullivan, S.M.; Björkman, S.T.; Miller, S.M.; Colditz, P.B.; Pow, D.V. Structural Remodeling of Gray Matter Astrocytes in the Neonatal Pig Brain after Hypoxia/Ischemia. Glia 2010, 58, 181–194. [Google Scholar] [CrossRef]
- Gegelashvili, G.; Civenni, G.; Racagni, G.; Danbolt, N.C.; Schousboe, I.; Schousboe, A. Glutamate Receptor Agonists Up-Regulate Glutamate Transporter GLAST in Astrocytes. Neuroreport 1996, 8, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.M.; Lee, A.; Björkman, S.T.; Miller, S.M.; Sullivan, R.K.; Poronnik, P.; Colditz, P.B.; Pow, D.V. Cytoskeletal Anchoring of GLAST Determines Susceptibility to Brain Damage: An Identified Role for GFAP. J. Biol. Chem. 2007, 282, 29414–29423. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. The Problem of Astrocyte Identity. Neurochem. Int. 2004, 45, 191–202. [Google Scholar] [CrossRef]
- Björklund, H.; Olson, L.; Dahl, D.; Schwarcz, R. Short- and Long-Term Consequences of Intracranial Injections of the Excitotoxin, Quinolinic Acid, as Evidenced by GFA Immunohistochemistry of Astrocytes. Brain Res. 1986, 371, 267–277. [Google Scholar] [CrossRef]
- Dihné, M.; Block, F.; Korr, H.; Töpper, R. Time Course of Glial Proliferation and Glial Apoptosis Following Excitotoxic CNS Injury. Brain Res. 2001, 902, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Giulian, D.; Woodward, J.; Young, D.G.; Krebs, J.F.; Lachman, L.B. Interleukin-1 Injected into Mammalian Brain Stimulates Astrogliosis and Neovascularization. J. Neurosci. 1988, 8, 2485–2490. [Google Scholar] [CrossRef]
- Rostworowski, M.; Balasingam, V.; Chabot, S.; Owens, T.; Yong, V.W. Astrogliosis in the Neonatal and Adult Murine Brain Post-Trauma: Elevation of Inflammatory Cytokines and the Lack of Requirement for Endogenous Interferon-Gamma. J. Neurosci. 1997, 17, 3664–3674. [Google Scholar] [CrossRef]
- Chen, R.; Xue, G.; Hölscher, C. The Role of the TNFα-Mediated Astrocyte Signaling Pathway in Epilepsy. Acta Epileptol. 2021, 3, 24. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s Double Life: Intracellular Regulator and Extracellular Signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B Story: From Biomarker to Active Factor in Neural Injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2018, 8, 1908. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, R.; Di Iorio, P.; Bruno, V.; Battaglia, G.; D’Alimonte, I.; D’Onofrio, M.; Nicoletti, F.; Caciagli, F. Activation of A(1) Adenosine or mGlu3 Metabotropic Glutamate Receptors Enhances the Release of Nerve Growth Factor and S-100beta Protein from Cultured Astrocytes. Glia 1999, 27, 275–281. [Google Scholar] [CrossRef]
- Sakatani, S.; Seto-Ohshima, A.; Shinohara, Y.; Yamamoto, Y.; Yamamoto, H.; Itohara, S.; Hirase, H. Neural-Activity-Dependent Release of S100B from Astrocytes Enhances Kainate-Induced Gamma Oscillations in Vivo. J. Neurosci. 2008, 28, 10928–10936. [Google Scholar] [CrossRef]
- Bianchi, R.; Giambanco, I.; Donato, R. S100B/RAGE-Dependent Activation of Microglia via NF-kappaB and AP-1 Co-Regulation of COX-2 Expression by S100B, IL-1beta and TNF-Alpha. Neurobiol. Aging 2010, 31, 665–677. [Google Scholar] [CrossRef]
- Farombi, E.O.; Onyema, O.O. Monosodium Glutamate-Induced Oxidative Damage and Genotoxicity in the Rat: Modulatory Role of Vitamin C, Vitamin E and Quercetin. Hum. Exp. Toxicol. 2006, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, P.C. Neuroglial Responses to Elevated Glutamate in the Medial Basal Hypothalamus of the Infant Mouse. J. Nutr. 2000, 130, 1032S–1038S. [Google Scholar] [CrossRef]
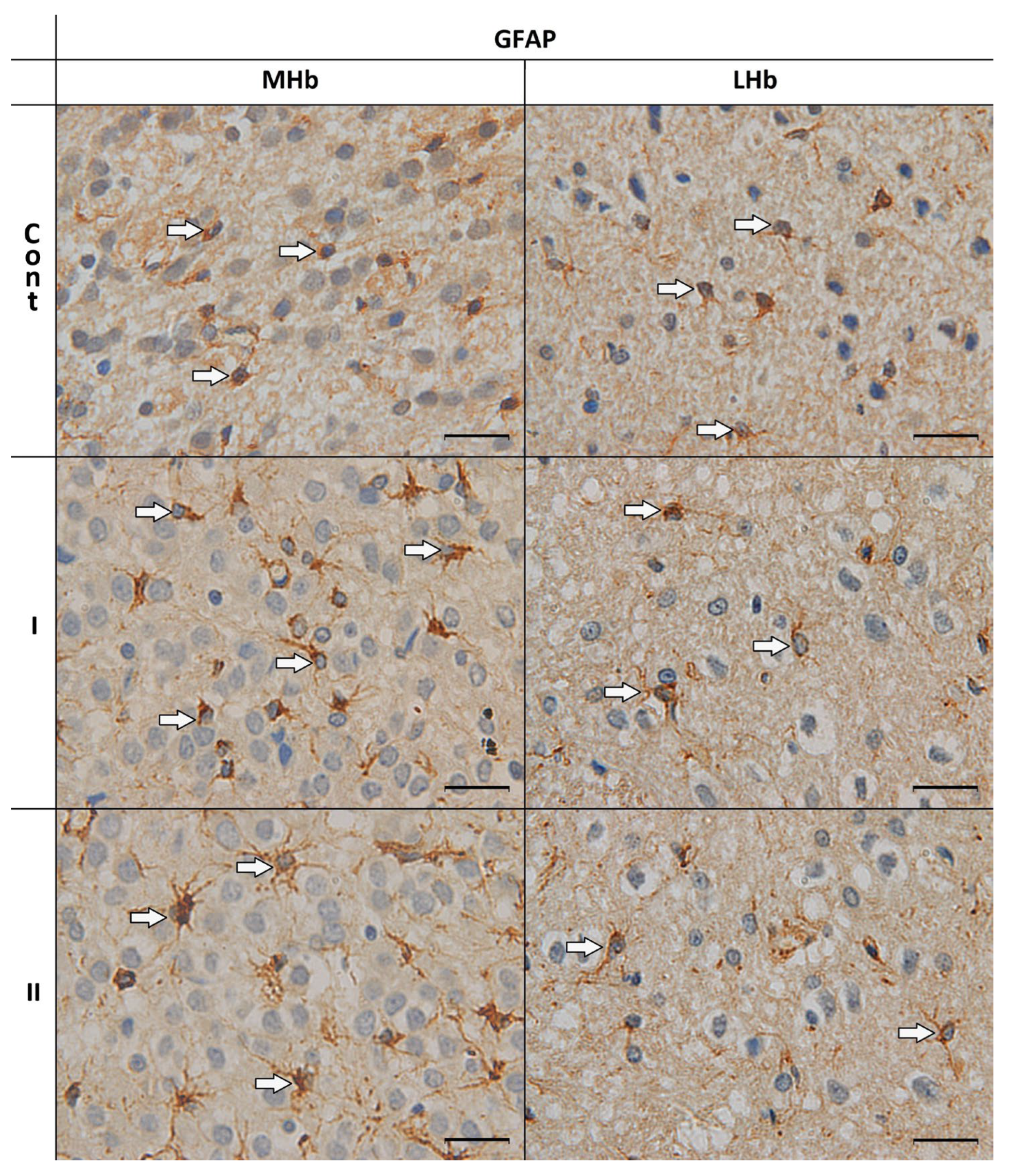
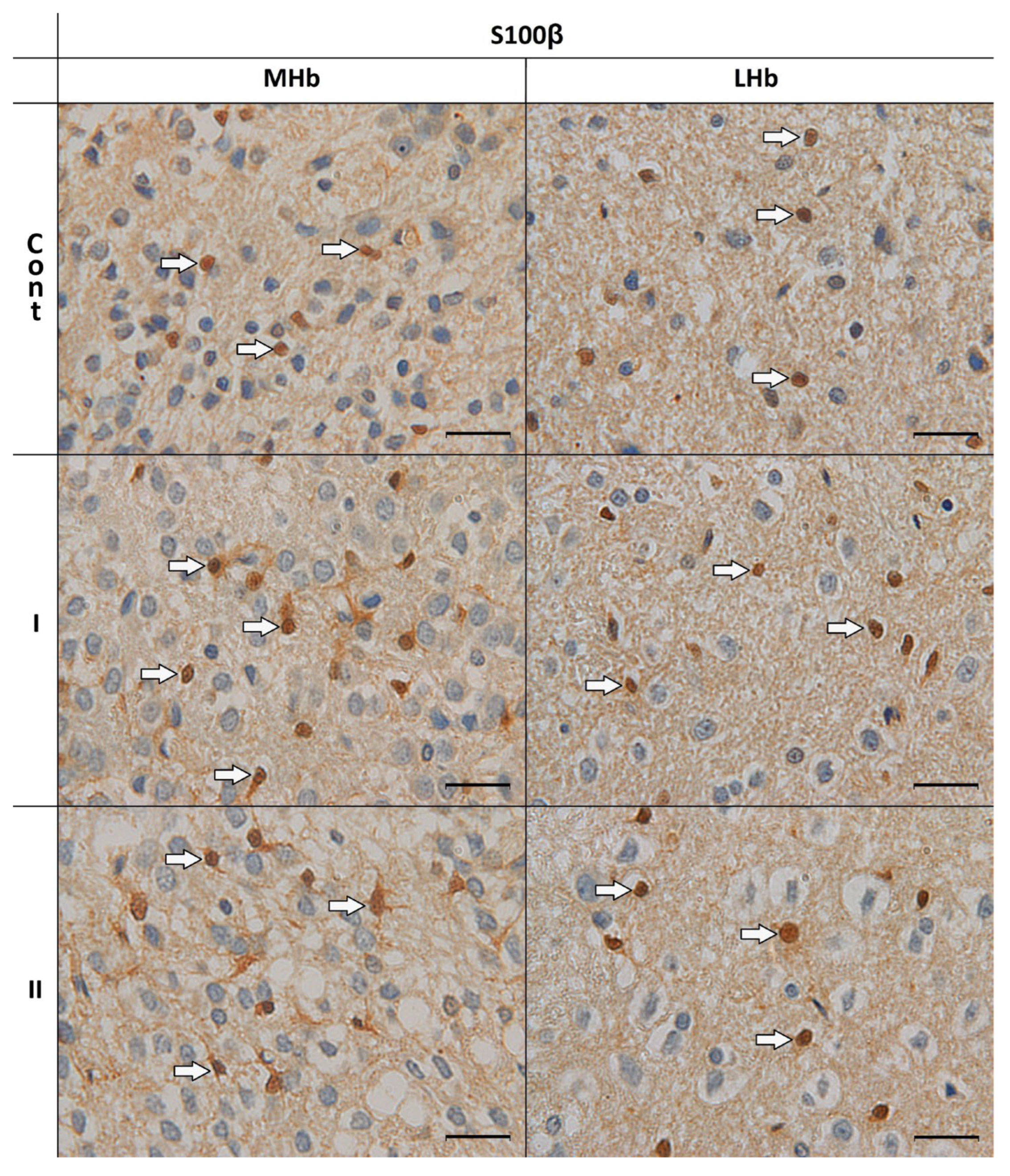
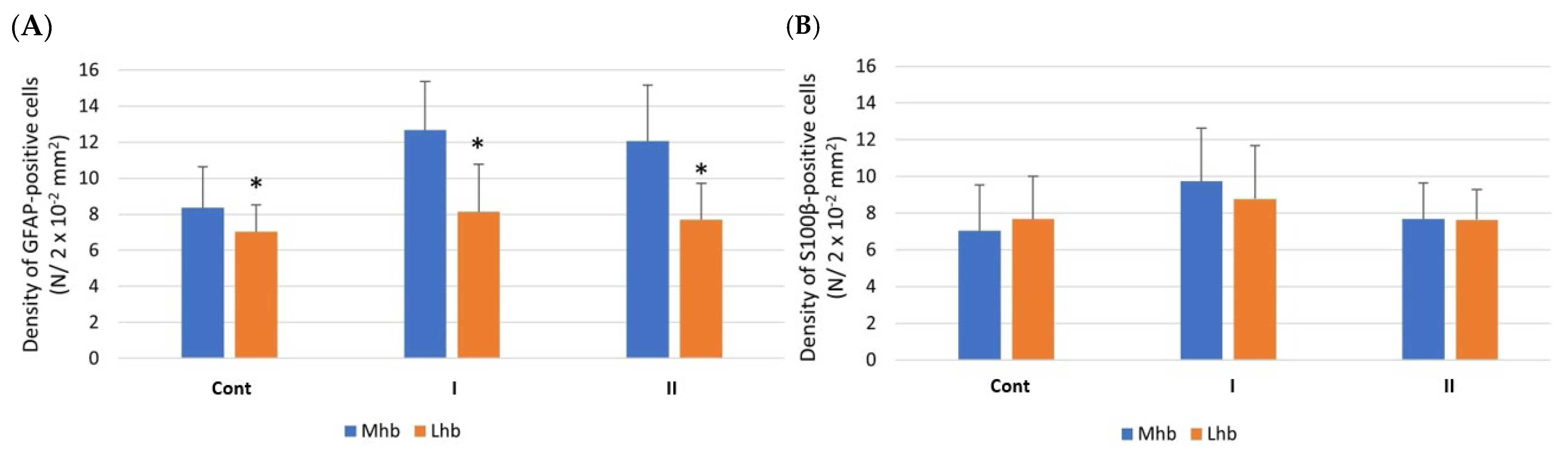
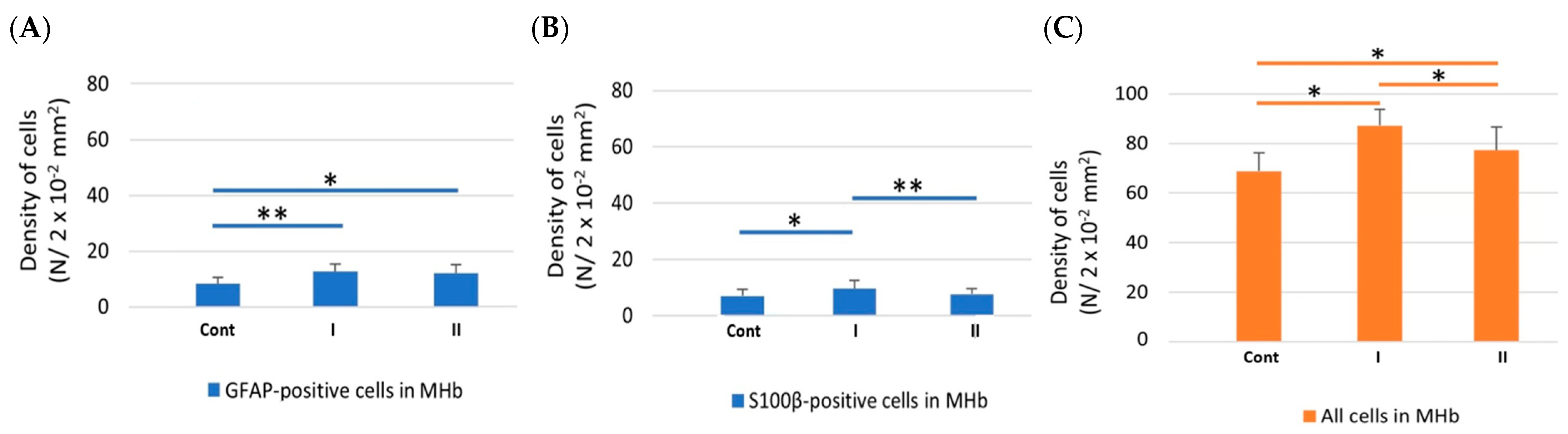
| GFAP-IR Cells | ||||||
|---|---|---|---|---|---|---|
| MHb | LHb | |||||
| Cont | I | II | Cont | I | II | |
| Processes number | 2.24 ± 0.98 a | 3.15 ± 1.09 b | 4.25 ± 1.3 c | 2.08 ± 1.04 | 2.41 ± 1.1 * | 2.07 ± 0.98 * |
| Processes length | 5.66 ± 2.09 d | 8.48 ± 3.44 e | 11.68 ± 4.47 f | 5.82 ± 2.36 | 7.27 ± 3.53 (**) | 6.99 ± 3.02 ** |
| Area | 25.16 ± 5.53 d | 37.63 ± 8.37 e | 44.66± 11.86 f | 26.32 ± 6.9 | 27.96 ± 8.14 ** | 28.64 ± 7.4 ** |
| Perimeter | 19.48 ± 2.63 d | 23.83 ± 2.89 e | 25.95 ± 3.48 f | 20.14 ± 2.58 | 21.41 ± 3.44 ** | 21.09 ± 2.5 ** |
| Shape index | 0.83 ± 0.1 | 0.83 ± 0.09 | 0.82 ± 0.09 | 0.8 ± 0.06 (ab) | 0.76 ± 0.08 (a)* | 0.8 ± 0.09 (b) |
| S100β-IR Cells | ||||||
|---|---|---|---|---|---|---|
| MHb | LHb | |||||
| Cont | I | II | Cont | I | II | |
| Processes number | 0.8 ± 1.02 a | 1.42 ± 1.15 b | 2.32 ± 1.56 c | 0.33 ± 0.56 a* | 0.69 ± 0.66 b* | 0.81 ± 0.68 b* |
| Processes length | 1.01 ± 1.42 d | 5.13 ± 2.6 e | 5.19 ± 3.32 e | 0.64 ± 1.43 d | 1.3 ± 1.43 (e)** | 1.97 ± 2.03 e** |
| Area | 19.21 ± 4.21 d | 27.01 ± 6.9 e | 27.57 ± 7.87 e | 21.46± 4.97 (**) | 21.1 ± 4.49 ** | 21.84 ± 5.93 ** |
| Perimeter | 17.21 ± 1.89 d | 20.64 ± 2.72 e | 20.78 ± 2.98 e | 18.46± 2.36 (**) | 18.21 ± 2.28 ** | 18.86 ± 2.76 ** |
| Shape index | 0.81 ± 0.08 | 0.79 ± 0.08 | 0.79 ± 0.09 | 0.79 ± 0.08 | 0.8 ± 0.09 | 0.76 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, A.; Rycerz, K.; Jaworska-Adamu, J.; Arciszewski, M.B. Influence of Monosodium Glutamate on Astroglia of Rat Habenula. Biomolecules 2025, 15, 1111. https://doi.org/10.3390/biom15081111
Krawczyk A, Rycerz K, Jaworska-Adamu J, Arciszewski MB. Influence of Monosodium Glutamate on Astroglia of Rat Habenula. Biomolecules. 2025; 15(8):1111. https://doi.org/10.3390/biom15081111
Chicago/Turabian StyleKrawczyk, Aleksandra, Karol Rycerz, Jadwiga Jaworska-Adamu, and Marcin B. Arciszewski. 2025. "Influence of Monosodium Glutamate on Astroglia of Rat Habenula" Biomolecules 15, no. 8: 1111. https://doi.org/10.3390/biom15081111
APA StyleKrawczyk, A., Rycerz, K., Jaworska-Adamu, J., & Arciszewski, M. B. (2025). Influence of Monosodium Glutamate on Astroglia of Rat Habenula. Biomolecules, 15(8), 1111. https://doi.org/10.3390/biom15081111







