A Novel Synthetic Tag Induces Palmitoylation and Directs the Subcellular Localization of Target Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Constructs and Transfection
2.3. EV Production and Isolation
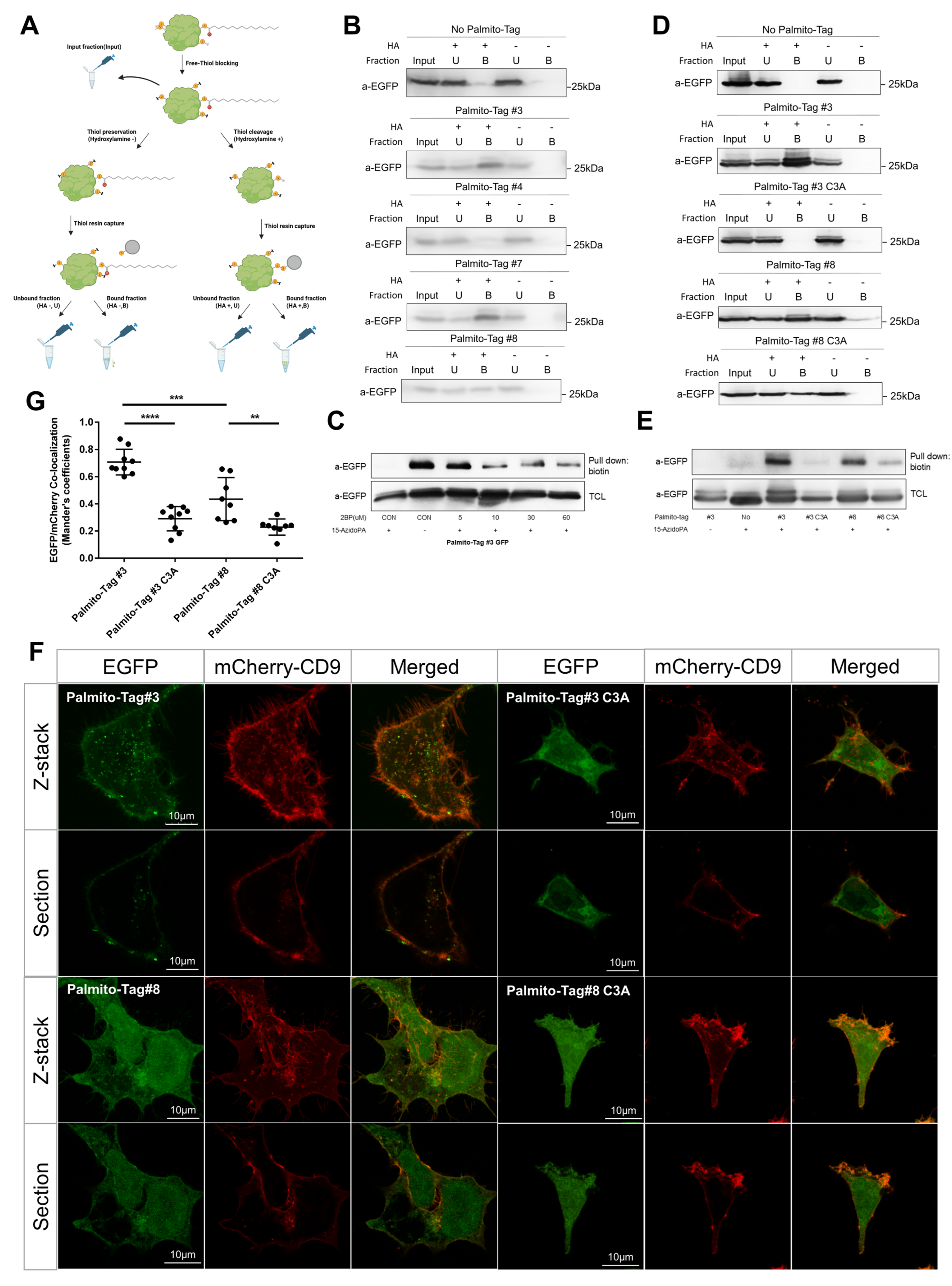
2.4. Detection of Palmitoylated Proteins
2.5. Immunoblotting
2.6. Confocal Microscopy for Cell Imaging
2.7. EV Uptake Analysis
2.8. Luciferase Activity Assay
2.9. Statistical Analyses
3. Results
3.1. Design and Optimization of the Palmito-Tag
3.2. Subcellular Localization of EGFP Was Altered by the Palmito-Tag
3.3. Palmito-Tag Alters Subcellular Localization Without Affecting the Biological Function of a Target Protein
3.4. The Presence of Palmito-Tag Is Sufficient to Induce Ectopic Palmitoylation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD9 | Clusters of differentiation 9 |
| EGFP | Enhanced green fluorescent protein |
| HEK293T | Human embryonic kidney 293T |
| HUVEC | Human umbilical vein endothelial cell |
| GAP43 | Growth associated protein 43 |
| PAT/DHHC | Palmitoyl-acyltransferases |
| PPT | Palmitoyl protein thioesterases |
References
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res 2017, 6, 2050. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, H.; Xi, Y.; Hu, Q.; Liu, H.; Fan, J.; Xiang, Y.; Zhang, X.; Shui, W.; Lai, Y. Vesicle trafficking and vesicle fusion: Mechanisms, biological functions, and their implications for potential disease therapy. Mol. Biomed. 2022, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.C.; Doetsch, P.W.; Corbett, A.H. Mechanisms Regulating Protein Localization. Traffic 2015, 16, 1039–1061. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Nevo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; An, Z.; et al. Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications. MedComm 2023, 4, e261. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, P.; Resh, M.D. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol. Cell Biol. 2010, 30, 4094–4107. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.F.; Liras, P. Targeting of Specialized Metabolites Biosynthetic Enzymes to Membranes and Vesicles by Posttranslational Palmitoylation: A Mechanism of Non-Conventional Traffic and Secretion of Fungal Metabolites. Int. J. Mol. Sci. 2024, 25, 1224. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, Y.; Niu, J.; Jarugumilli, G.K.; Wu, X. Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell Chem. Biol. 2018, 25, 817–831. [Google Scholar] [CrossRef] [PubMed]
- De, I.; Sadhukhan, S. Emerging Roles of DHHC-mediated Protein S-palmitoylation in Physiological and Pathophysiological Context. Eur. J. Cell Biol. 2018, 97, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Q.; Sun, C.; Song, J.; Gao, L.; Zhang, S.; Munoz, A.; Read, N.D.; Lu, L. Palmitoylation of the Cysteine Residue in the DHHC Motif of a Palmitoyl Transferase Mediates Ca2+ Homeostasis in Aspergillus. PLoS Genet. 2016, 12, e1005977. [Google Scholar] [CrossRef] [PubMed]
- Stix, R.; Lee, C.J.; Faraldo-Gomez, J.D.; Banerjee, A. Structure and Mechanism of DHHC Protein Acyltransferases. J. Mol. Biol. 2020, 432, 4983–4998. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hancock, J.F. Ras trafficking, localization and compartmentalized signalling. Semin. Cell Dev. Biol. 2012, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, L.; Xu, Y.; Yang, M.; Xu, Y.; Komaniecki, G.P.; Kosciuk, T.; Chen, X.; Lu, X.; Zou, X.; et al. A STAT3 palmitoylation cycle promotes T(H)17 differentiation and colitis. Nature 2020, 586, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, J.; Vagner, T.; Kim, M.; Zhou, B.; Chin, A.; Zandian, M.; Freeman, M.R.; You, S.; Zijlstra, A.; Yang, W.; et al. Comprehensive palmitoyl-proteomic analysis identifies distinct protein signatures for large and small cancer-derived extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1764192. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Hwang, H.; Choi, Y.; Jung, S.; Hong, J.H.; Yoon, B.J.; Rhim, H.; Park, M. Myristoylation-dependent palmitoylation of cyclin Y modulates long-term potentiation and spatial learning. Prog. Neurobiol. 2022, 218, 102349. [Google Scholar] [CrossRef] [PubMed]
- Udenwobele, D.I.; Su, R.C.; Good, S.V.; Ball, T.B.; Varma Shrivastav, S.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Nazarian, A.; Erdjument-Bromage, H.; Bornmann, W.; Tempst, P.; Resh, M.D. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 2001, 276, 30987–30994. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.T.; Dillard, M.E.; Stewart, D.P.; Zhang, Y.; Wagner, B.; Levine, R.M.; Pruett-Miller, S.M.; Sykes, A.; Temirov, J.; Cheney, R.E.; et al. Cytoneme delivery of Sonic Hedgehog from ligand-producing cells requires Myosin 10 and a Dispatched-BOC/CDON co-receptor complex. Elife 2021, 10, e61432. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hannoush, R.N. A Decade of Click Chemistry in Protein Palmitoylation: Impact on Discovery and New Biology. Cell Chem. Biol. 2018, 25, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Leymarie, N.; Haeussler, D.J.; Bachschmid, M.M.; Costello, C.E.; Lin, C. Direct detection of S-palmitoylation by mass spectrometry. Anal. Chem. 2013, 85, 11952–11959. [Google Scholar] [CrossRef] [PubMed]
- Grangier, A.; Branchu, J.; Volatron, J.; Piffoux, M.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Technological advances towards extracellular vesicles mass production. Adv. Drug Deliv. Rev. 2021, 176, 113843. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Grassi, C. Nutrient-Dependent Changes of Protein Palmitoylation: Impact on Nuclear Enzymes and Regulation of Gene Expression. Int. J. Mol. Sci. 2018, 19, 3820. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Jiang, P.; Guo, Y.; Wang, C.; Tan, X.; Zhang, W.; Peng, D.; Xue, Y. GPS-Palm: A deep learning-based graphic presentation system for the prediction of S-palmitoylation sites in proteins. Brief. Bioinform. 2021, 22, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Kumar, R.; Kumar, M. PalmPred: An SVM based palmitoylation prediction method using sequence profile information. PLoS ONE 2014, 9, e89246. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023, 31, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.F.; Wan, J.; Bailey, A.O.; Sun, B.; Kuchar, J.A.; Green, W.N.; Phinney, B.S.; Yates, J.R., 3rd; Davis, N.G. Global analysis of protein palmitoylation in yeast. Cell 2006, 125, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kashio, A.; Ogata, R.; Ishitomi, A.; Yamazaki, Y.; Kihara, A. Analysis of substrate specificity of human DHHC protein acyltransferases using a yeast expression system. Mol. Biol. Cell 2012, 23, 4543–4551. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ka, J.; Lee, G.; Han, S.; Jeong, H.; Jin, S.-W. A Novel Synthetic Tag Induces Palmitoylation and Directs the Subcellular Localization of Target Proteins. Biomolecules 2025, 15, 1076. https://doi.org/10.3390/biom15081076
Ka J, Lee G, Han S, Jeong H, Jin S-W. A Novel Synthetic Tag Induces Palmitoylation and Directs the Subcellular Localization of Target Proteins. Biomolecules. 2025; 15(8):1076. https://doi.org/10.3390/biom15081076
Chicago/Turabian StyleKa, Jun, Gwanyeob Lee, Seunghyun Han, Haekwan Jeong, and Suk-Won Jin. 2025. "A Novel Synthetic Tag Induces Palmitoylation and Directs the Subcellular Localization of Target Proteins" Biomolecules 15, no. 8: 1076. https://doi.org/10.3390/biom15081076
APA StyleKa, J., Lee, G., Han, S., Jeong, H., & Jin, S.-W. (2025). A Novel Synthetic Tag Induces Palmitoylation and Directs the Subcellular Localization of Target Proteins. Biomolecules, 15(8), 1076. https://doi.org/10.3390/biom15081076





