The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation
Abstract
1. Introduction
2. Lf Release from Neutrophils
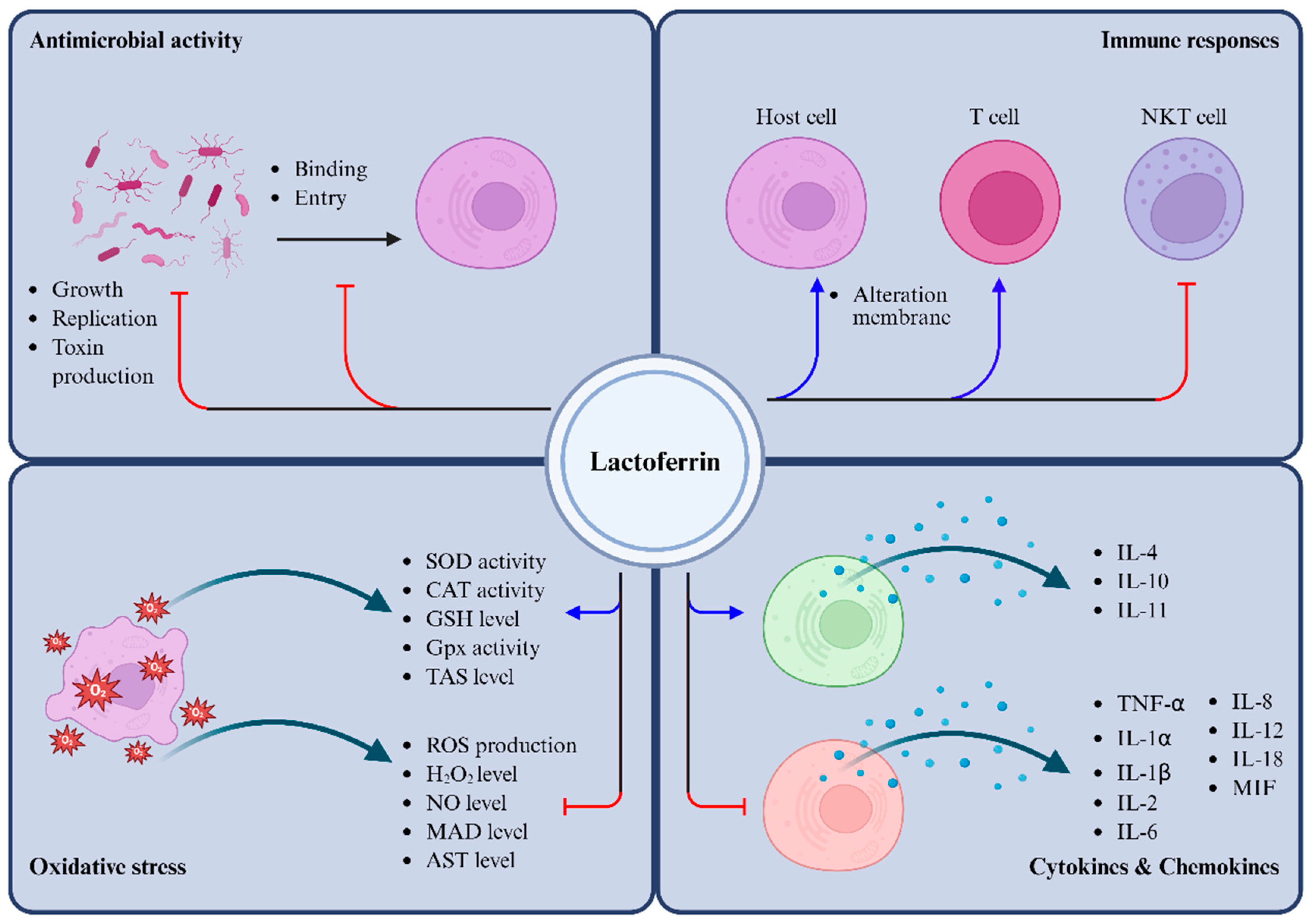
3. Antimicrobial Activity
3.1. Antibacterial Activity
3.2. Antiviral Activity
3.3. Antifungal Activity and Antiparasitic Activity
4. Anti-Inflammatory Activity
4.1. Regulation of Cytokines
4.2. Mitigation of Oxidative Stress
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Montoya, I.A.; Cendon, T.S.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H. The physiology of lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Lonnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.F.; Zhang, Y.H.; Wang, S.; Pang, Z.Q.; Fan, Y.G.; Li, J.Y.; Wang, Z.Y.; Guo, C. Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox Biol. 2019, 21, 101090. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rumball, S.V.; Baker, E.N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature 1990, 344, 784–787. [Google Scholar] [CrossRef]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquie, C.; Le Parc, A.; Barile, D. Studying lactoferrin N-glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Karav, S. Selective deglycosylation of lactoferrin to understand glycans’ contribution to antimicrobial activity of lactoferrin. Cell. Mol. Biol. 2018, 64, 52–57. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.; Das, M.R. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grondahl, L.; Bhandari, B. Physico-chemical properties of different forms of bovine lactoferrin. Food Chem. 2013, 141, 3007–3013. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2022, 9, 1018336. [Google Scholar] [CrossRef]
- Ostertag, F.; Grimm, V.J.; Hinrichs, J. Iron saturation and binding capacity of lactoferrin—Development and validation of a colorimetric protocol for quality control. Food Chem. 2025, 463, 141365. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Shoji, H.; Oguchi, S.; Shinohara, K.; Shimizu, T.; Yamashiro, Y. Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr. Res. 2007, 61, 89–92. [Google Scholar] [CrossRef]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron absorption is greater from apo-lactoferrin and is similar between holo-lactoferrin and ferrous sulfate: Stable iron isotope studies in Kenyan infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Nonnecke, B.J.; Smith, K.L. Inhibition of mastitic bacteria by bovine milk apo-lactoferrin evaluated by in vitro microassay of bacterial growth. J. Dairy Sci. 1984, 67, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.G.; Schanbacher, F.L.; Ferguson, L.C.; Smith, K.L. In vitro growth inhibition of mastitis-causing coliform bacteria by bovine apo-lactoferrin and reversal of inhibition by citrate and high concentrations of apo-lactoferin. Infect. Immun. 1976, 14, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Gómez, C.; Reyes-López, M.; Ramírez-Rico, G.; Díaz-Aparicio, E.; Zenteno, E.; González-Ruiz, C.; de la Garza, M. Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2. Vet. Res. 2020, 51, 36. [Google Scholar] [CrossRef]
- Majka, G.; Śpiewak, K.; Kurpiewska, K.; Heczko, P.; Stochel, G.; Strus, M.; Brindell, M. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013, 405, 5191–5200. [Google Scholar] [CrossRef]
- Gerstein, M.; Anderson, B.F.; Norris, G.E.; Baker, E.N.; Lesk, A.M.; Chothia, C. Domain closure in lactoferrin: Two hinges produce a see-saw motion between alternative close-packed interfaces. J. Mol. Biol. 1993, 234, 357–372. [Google Scholar] [CrossRef]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Visca, P.; Berlutti, F.; Vittorioso, P.; Dalmastri, C.; Thaller, M.C.; Valenti, P. Growth and adsorption of Streptococcus mutans 6715-13 to hydroxyapatite in the presence of lactoferrin. Med. Microbiol. Immunol. 1989, 178, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Kumar, P.; Paramasivam, M.; Yadav, R.S.; Sahani, M.S.; Sharma, S.; Srinivasan, A.; Singh, T.P. Camel lactoferrin, a transferrin-cum-lactoferrin: Crystal structure of camel apolactoferrin at 2.6Å resolution and structural basis of its dual role. J. Mol. Biol. 2001, 309, 751–761. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef]
- Dionysius, D.A.; Grieve, P.A.; Milne, J.M. Forms of lactoferrin: Their antibacterial effect on enterotoxigenic Escherichia coli. J. Dairy Sci. 1993, 76, 2597–2606. [Google Scholar] [CrossRef]
- Longhi, C.; Conte, M.P.; Seganti, L.; Polidoro, M.; Alfsen, A.; Valenti, P. Influence of lactoferrin on the entry process of Escherichia coli HB101(pRI203) in HeLa cells. Med. Microbiol. Immunol. 1993, 182, 25–35. [Google Scholar] [CrossRef]
- Liang, Y.; Ikeda, S.-i.; Chen, J.; Zhang, Y.; Negishi, K.; Tsubota, K.; Kurihara, T. Myopia is suppressed by digested lactoferrin or holo-lactoferrin administration. Int. J. Mol. Sci. 2023, 24, 5815. [Google Scholar] [CrossRef]
- McAbee, D.D.; Esbensen, K. Binding and endocytosis of apo- and holo-lactoferrin by isolated rat hepatocytes. J. Biol. Chem. 1991, 266, 23624–23631. [Google Scholar] [CrossRef]
- Sreedhara, A.; Flengsrud, R.; Langsrud, T.; Kaul, P.; Prakash, V.; Vegarud, G.E. Structural characteristic, pH and thermal stabilities of apo and holo forms of caprine and bovine lactoferrins. BioMetals 2010, 23, 1159–1170. [Google Scholar] [CrossRef]
- Grossmann, J.G.; Neu, M.; Pantos, E.; Schwab, F.J.; Evans, R.W.; Townes-Andrews, E.; Lindley, P.F.; Appel, H.; Thies, W.-G.; Hasnain, S.S. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J. Mol. Biol. 1992, 225, 811–819. [Google Scholar] [CrossRef]
- Sharma, A.; Rajashankar, K.; Yadav, M.; Singh, T. Structure of mare apolactoferrin: The N and C lobes are in the closed form. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1152–1157. [Google Scholar] [CrossRef]
- Kurokawa, H.; Dewan, J.C.; Mikami, B.; Sacchettini, J.C.; Hirose, M. Crystal structure of hen apo-ovotransferrin: Both lobes adopt an open conformation upon loss of iron*. J. Biol. Chem. 1999, 274, 28445–28452. [Google Scholar] [CrossRef]
- Teraguchi, S.; Wakabayashi, H.; Kuwata, H.; Yamauchi, K.; Tamura, Y. Protection against infections by oral lactoferrin: Evaluation in animal models. Biometals 2004, 17, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, M.A.; Uva, A.; Gernone, F.; Gusatoaia, O.; Donghia, R.; Zatelli, A. Efficacy of a combination of nucleotides and lactoferrin in maintaining stable or improving the clinical picture and laboratory findings of leishmaniotic dogs: A randomized controlled study. Vet. Parasitol. 2024, 332, 110319. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lonnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in aseptic and septic inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed]
- Actis Dato, V.; Chiabrando, G.A. The role of low-density lipoprotein receptor-related protein 1 in lipid metabolism, glucose homeostasis and inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef]
- Bezwoda, W.R.; Baynes, R.D.; Khan, Q.; Mansoor, N. Enzyme-linked immunosorbent assay for lactoferrin. Plasma and tissue measurements. Clin. Chim. Acta 1985, 151, 61–69. [Google Scholar] [CrossRef]
- Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal interactions between lactoferrin and bacterial endotoxins and their role in the regulation of the immune response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Berrington, J.E.; McGuire, W.; Stewart, C.J.; Cummings, S.P. Lactoferrin: Antimicrobial activity and therapeutic potential. Semin. Fetal Neonatal Med. 2013, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Birgens, H.S. Lactoferrin in plasma measured by an ELISA technique: Evidence that plasma lactoferrin is an indicator of neutrophil turnover and bone marrow activity in acute leukaemia. Scand. J. Haematol. 1985, 34, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Rado, T.A.; Wei, X.P.; Benz, E.J., Jr. Isolation of lactoferrin cDNA from a human myeloid library and expression of mRNA during normal and leukemic myelopoiesis. Blood 1987, 70, 989–993. [Google Scholar] [CrossRef]
- Rascon-Cruz, Q.; Espinoza-Sanchez, E.A.; Siqueiros-Cendon, T.S.; Nakamura-Bencomo, S.I.; Arevalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Ellison, R.T., 3rd; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [CrossRef]
- Leitch, E.C.; Willcox, M.D. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr. Eye Res. 1999, 19, 12–19. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.; Pauwels, E.K.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andres, M.T.; Perez-Gil, J.; Sanchez, M.; Fierro, J.F. Potassium efflux induced by a new lactoferrin-derived peptide mimicking the effect of native human lactoferrin on the bacterial cytoplasmic membrane. Biochem. 2003, 68, 217–227. [Google Scholar] [CrossRef]
- Velliyagounder, K.; Kaplan, J.B.; Furgang, D.; Legarda, D.; Diamond, G.; Parkin, R.E.; Fine, D.H. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect. Immun. 2003, 71, 6141–6147. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Arnold, R.R.; Russell, J.E.; Champion, W.J.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Differentiation from the stasis of iron deprivation. Infect. Immun. 1982, 35, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75, ftx054. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Inhibitory effects of bovine lactoferrin and lactoferricin B on Enterobacter sakazakii. Biocontrol Sci. 2008, 13, 29–32. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Noguera-Obenza, M.; Ebel, F.; Guzman, C.A.; Gomez, H.F.; Cleary, T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003, 71, 5149–5155. [Google Scholar] [CrossRef]
- Nascimento de Araujo, A.; Giugliano, L.G. Human milk fractions inhibit the adherence of diffusely adherent Escherichia coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 2000, 184, 91–94. [Google Scholar] [CrossRef]
- Qiu, J.; Hendrixson, D.R.; Baker, E.N.; Murphy, T.F.; St Geme, J.W., 3rd; Plaut, A.G. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 1998, 95, 12641–12646. [Google Scholar] [CrossRef]
- Dial, E.J.; Romero, J.J.; Headon, D.R.; Lichtenberger, L.M. Recombinant human lactoferrin is effective in the treatment of Helicobacter felis-infected mice. J. Pharm. Pharmacol. 2000, 52, 1541–1546. [Google Scholar] [CrossRef]
- Superti, F.; Ammendolia, M.G.; Valenti, P.; Seganti, L. Antirotaviral activity of milk proteins: Lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microbiol. Immunol. 1997, 186, 83–91. [Google Scholar] [CrossRef]
- Morici, P.; Florio, W.; Rizzato, C.; Ghelardi, E.; Tavanti, A.; Rossolini, G.M.; Lupetti, A. Synergistic activity of synthetic N-terminal peptide of human lactoferrin in combination with various antibiotics against carbapenem-resistant Klebsiella pneumoniae strains. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1739–1748. [Google Scholar] [CrossRef]
- Morita, Y.; Ishikawa, K.; Nakano, M.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Ooka, T.; Hironaka, S. Effects of lactoferrin and lactoperoxidase-containing food on the oral hygiene status of older individuals: A randomized, double-blinded, placebo-controlled clinical trial. Geriatr. Gerontol. Int. 2017, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, Y.; Du, C.; Piao, J.; Yang, L.; Yang, X. The effect of recombinant human lactoferrin from the milk of transgenic cows on Salmonella enterica serovar typhimurium infection in mice. Food Funct. 2016, 7, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sijbrandij, T.; Ligtenberg, A.J.; Nazmi, K.; van den Keijbus, P.A.M.; Veerman, E.C.I.; Bolscher, J.G.M.; Bikker, F.J. Lfchimera protects HeLa cells from invasion by Yersinia spp. in vitro. Biometals 2018, 31, 941–950. [Google Scholar] [CrossRef]
- Garcia-Borjas, K.A.; Ceballos-Olvera, I.; Luna-Castro, S.; Pena-Avelino, Y. Bovine lactoferrin can decrease the in vitro biofilm production and show synergy with antibiotics against Listeria and Escherichia coli isolates. Protein Pept. Lett. 2021, 28, 101–107. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.; Valdiviezo-Godina, N.; Siqueiros-Cendon, T.; Sinagawa-Garcia, S.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. High-level expression of recombinant bovine lactoferrin in Pichia pastoris with antimicrobial Activity. Int. J. Mol. Sci. 2016, 17, 902. [Google Scholar] [CrossRef]
- van Berkel, P.H.; Geerts, M.E.; van Veen, H.A.; Mericskay, M.; de Boer, H.A.; Nuijens, J.H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997, 328, 145–151. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Y.; Wang, Y.; Ding, L.; Huan, W.; Yang, Y.; Fang, F.; Cui, W. Global bibliometric and visualized analysis of research on lactoferrin from 1978 to 2024. Mol. Nutr. Food Res. 2024, 68, e2400379. [Google Scholar] [CrossRef]
- Beeckman, D.S.; Van Droogenbroeck, C.M.; De Cock, B.J.; Van Oostveldt, P.; Vanrompay, D.C. Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res. 2007, 38, 729–739. [Google Scholar] [CrossRef]
- Wang, X.; Hirmo, S.; Willen, R.; Wadstrom, T. Inhibition of Helicobacter pylori infection by bovine milk glycoconjugates in a BAlb/cA mouse model. J. Med. Microbiol. 2001, 50, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin adsorbed onto biomimetic hydroxyapatite nanocrystals controlling—In vivo—The Helicobacter pylori infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [PubMed]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized bovine lactoferrin counteracts infection, inflammation and iron dysbalance in a cystic fibrosis mouse model of Pseudomonas aeruginosa chronic lung infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef] [PubMed]
- Mosquito, S.; Ochoa, T.J.; Cok, J.; Cleary, T.G. Effect of bovine lactoferrin in Salmonella ser. Typhimurium infection in mice. Biometals 2010, 23, 515–521. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Rivera-Aguilar, V.; Resendiz-Albor, A.A.; Campos-Rodriguez, R. Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immunol. Lett. 2010, 134, 35–46. [Google Scholar] [CrossRef]
- Sato, N.; Kurotaki, H.; Ikeda, S.; Daio, R.; Nishinome, N.; Mikami, T.; Matsumoto, T. Lactoferrin inhibits Bacillus cereus growth, and heme analogs recover its growth. Biol. Pharm. Bull. 1999, 22, 197–199. [Google Scholar] [CrossRef]
- Jugert, C.S.; Didier, A.; Plotz, M.; Jessberger, N. Strain-specific antimicrobial activity of lactoferrin-based food supplements. J. Food Prot. 2023, 86, 100153. [Google Scholar] [CrossRef]
- Chilton, C.H.; Crowther, G.S.; Spiewak, K.; Brindell, M.; Singh, G.; Wilcox, M.H.; Monaghan, T.M. Potential of lactoferrin to prevent antibiotic-induced Clostridium difficile infection. J. Antimicrob. Chemother. 2016, 71, 975–985. [Google Scholar] [CrossRef]
- Hwang, S.A.; Kruzel, M.L.; Actor, J.K. Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int. Immunopharmacol. 2014, 20, 157–163. [Google Scholar] [CrossRef]
- Bhimani, R.S.; Vendrov, Y.; Furmanski, P. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 1999, 86, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Guillen, C.; McInnes, I.B.; Vaughan, D.M.; Kommajosyula, S.; Van Berkel, P.H.; Leung, B.P.; Aguila, A.; Brock, J.H. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J. Immunol. 2002, 168, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-A.; Chiu, C.-C.; Huang, C.-Y.; Chen, L.-J.; Tsai, C.-C.; Hsu, T.-C.; Tzang, B.-S. Lactoferrin increases antioxidant activities and ameliorates hepatic fibrosis in lupus-prone mice fed with a high-cholesterol diet. J. Med. Food 2016, 19, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Ajello, M.; Bosso, P.; Morea, C.; Petrucca, A.; Antonini, G.; Valenti, P. Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals 2004, 17, 271–278. [Google Scholar]
- Velusamy, S.K.; Markowitz, K.; Fine, D.H.; Velliyagounder, K. Human lactoferrin protects against Streptococcus mutans-induced caries in mice. Oral Dis. 2016, 22, 148–154. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Erturk, M.; Karav, S. The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases. Front. Immunol. 2024, 15, 1402135. [Google Scholar] [CrossRef]
- Cheneau, C.; Eichholz, K.; Tran, T.H.; Tran, T.T.P.; Paris, O.; Henriquet, C.; Bajramovic, J.J.; Pugniere, M.; Kremer, E.J. Lactoferrin retargets human adenoviruses to TLR4 to induce an abortive NLRP3-associated pyroptotic response in human phagocytes. Front. Immunol. 2021, 12, 685218. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Pietrantoni, A.; Tinari, A.; Valenti, P.; Superti, F. Bovine lactoferrin inhibits echovirus endocytic pathway by interacting with viral structural polypeptides. Antiviral Res. 2007, 73, 151–160. [Google Scholar] [CrossRef]
- Chien, Y.J.; Chen, W.J.; Hsu, W.L.; Chiou, S.S. Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein. Virology 2008, 379, 143–151. [Google Scholar] [CrossRef]
- Kaito, M.; Iwasa, M.; Fujita, N.; Kobayashi, Y.; Kojima, Y.; Ikoma, J.; Imoto, I.; Adachi, Y.; Hamano, H.; Yamauchi, K. Effect of lactoferrin in patients with chronic Hepatitis C: Combination therapy with interferon and ribavirin. J. Gastroenterol. Hepatol. 2007, 22, 1894–1897. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Marchetti, M.; Superti, F. Bovine lactoferrin prevents the entry and intercellular spread of Herpes simplex virus type 1 in green monkey kidney cells. Antiviral Res. 2007, 76, 252–262. [Google Scholar] [CrossRef]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001, 51, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Beljaars, L.; van der Strate, B.W.; Bakker, H.I.; Reker-Smit, C.; van Loenen-Weemaes, A.M.; Wiegmans, F.C.; Harmsen, M.C.; Molema, G.; Meijer, D.K. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antiviral Res. 2004, 63, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Baktiroglu, M.; Kalkan, A.E.; Canbolat, A.A.; Lombardo, M.; Raposo, A.; de Brito Alves, J.L.; Witkowska, A.M.; Karav, S. Lactoferrin: A promising therapeutic molecule against human papillomavirus. Nutrients 2024, 16, 3073. [Google Scholar] [CrossRef]
- Carvalho, C.A.M.; Casseb, S.M.M.; Goncalves, R.B.; Silva, E.V.P.; Gomes, A.M.O.; Vasconcelos, P.F.C. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2017, 98, 1749–1754. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Karav, S. Bovine colostrum and its potential contributions for treatment and prevention of COVID-19. Front. Immunol. 2023, 14, 121451433. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Isakova-Sivak, I.N.; Mezhenskaya, D.A.; Kostevich, V.A.; Gorbunov, N.P.; Elizarova, A.Y.; Matyushenko, V.A.; Berson, Y.M.; Grudinina, N.A.; Kolmakov, N.N.; et al. Molecular mimicry of the receptor-binding domain of the SARS-CoV-2 spike protein: From the interaction of spike-specific antibodies with transferrin and lactoferrin to the antiviral effects of human recombinant lactoferrin. Biometals 2023, 36, 437–462. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; El-Gendi, H.; El-Maradny, Y.A.; Abu-Serie, M.M.; Abdel-Wahhab, K.G.; Shabana, M.E.; Ashry, M. Inhibitory effect of lactoferrin-coated zinc nanoparticles on SARS-CoV-2 replication and entry along with improvement of lung fibrosis induced in adult male albino rats. Int. J. Biol. Macromol. 2023, 245, 125552. [Google Scholar] [CrossRef]
- He, S.T.; Qin, H.; Guan, L.; Liu, K.; Hong, B.; Zhang, X.; Lou, F.; Li, M.; Lin, W.; Chen, Y.; et al. Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model. J. Med. Virol. 2023, 95, e28281. [Google Scholar] [CrossRef]
- Alves, N.S.; Azevedo, A.S.; Dias, B.M.; Horbach, I.S.; Setatino, B.P.; Denani, C.B.; Schwarcz, W.D.; Lima, S.M.B.; Missailidis, S.; Ano Bom, A.P.D.; et al. Inhibition of SARS-CoV-2 infection in Vero cells by bovine lactoferrin under different iron-saturation states. Pharmaceuticals 2023, 16, 1352. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Valenti, P.; Seganti, L.; Superti, F. Antiadenovirus activity of milk proteins: Lactoferrin prevents viral infection. Antiviral Res. 2002, 53, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; Lenman, A.; Frangsmyr, L.; Schmid, M.; Ahlm, C.; Pluckthun, A.; Jenssen, H.; Arnberg, N. Lactoferrin-hexon interactions mediate CAR-independent adenovirus infection of human respiratory cells. J. Virol. 2020, 94, e00542-20. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Jonsson, M.; Marttila, M.; Persson, D.; Fan, X.L.; Skog, J.; Frangsmyr, L.; Wadell, G.; Arnberg, N. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 2007, 81, 954–963. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, P.; Han, S.; He, H. Lactoferrin alleviates inflammation and regulates gut microbiota composition in H5N1-infected mice. Nutrients 2023, 15, 3362. [Google Scholar] [CrossRef]
- Wrobel, M.; Malaczewska, J.; Kaczorek-Lukowska, E. Antiviral effect of bovine lactoferrin against Enterovirus E. Molecules 2022, 27, 5569. [Google Scholar] [CrossRef]
- Weng, T.Y.; Chen, L.C.; Shyu, H.W.; Chen, S.H.; Wang, J.R.; Yu, C.K.; Lei, H.Y.; Yeh, T.M. Lactoferrin inhibits Enterovirus 71 infection by binding to VP1 protein and host cells. Antiviral Res. 2005, 67, 31–37. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chu, C.; Chiu, C.H. Lactoferrin inhibits Enterovirus 71 infection of human embryonal rhabdomyosarcoma cells in vitro. J. Infect. Dis. 2002, 186, 1161–1164. [Google Scholar] [CrossRef]
- Luo, Y.; Xiang, K.; Liu, J.; Song, J.; Feng, J.; Chen, J.; Dai, Y.; Hu, Y.; Zhuang, H.; Zhou, Y. Inhibition of in vitro infection of Hepatitis B virus by human breastmilk. Nutrients 2022, 14, 1561. [Google Scholar] [CrossRef]
- Florian, P.E.; Macovei, A.; Lazar, C.; Milac, A.L.; Sokolowska, I.; Darie, C.C.; Evans, R.W.; Roseanu, A.; Branza-Nichita, N. Characterization of the anti-HBV activity of HLP1-23, a human lactoferrin-derived peptide. J. Med. Virol. 2013, 85, 780–788. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, Y.; Liu, N.; Zhang, W.; Han, J. Effect of iron saturation of bovine lactoferrin on the inhibition of Hepatitis B virus in vitro. PeerJ 2024, 12, e17302. [Google Scholar] [CrossRef]
- Redwan, E.M.; El-Fakharany, E.M.; Uversky, V.N.; Linjawi, M.H. Screening the anti-infectivity potentials of native N- and C-lobes derived from the camel lactoferrin against Hepatitis C virus. BMC Complement. Altern. Med. 2014, 14, 219. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Ikeda, M.; Nozaki, A.; Sugiyama, K.; Tanaka, T.; Naganuma, A.; Tanaka, K.; Sekihara, H.; Shimotohno, K.; Saito, M.; Kato, N. Characterization of antiviral activity of lactoferrin against Hepatitis C virus infection in human cultured cells. Virus Res. 2000, 66, 51–63. [Google Scholar] [CrossRef]
- Picard-Jean, F.; Bouchard, S.; Larivee, G.; Bisaillon, M. The intracellular inhibition of HCV replication represents a novel mechanism of action by the innate immune lactoferrin protein. Antiviral Res. 2014, 111, 13–22. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, E.M.; Sanchez, L.; Al-Mehdar, H.A.; Redwan, E.M. Effectiveness of human, camel, bovine and sheep lactoferrin on the Hepatitis C virus cellular infectivity: Comparison study. Virol. J. 2013, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ikeda, M.; Nozaki, A.; Kato, N.; Tsuda, H.; Saito, S.; Sekihara, H. Lactoferrin inhibits Hepatitis C virus viremia in patients with chronic Hepatitis C: A pilot study. Jpn. J. Cancer Res. 1999, 90, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergstrom, T. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef]
- Wang, X.; Yue, L.; Dang, L.; Yang, J.; Chen, Z.; Wang, X.; Shu, J.; Li, Z. Role of sialylated glycans on bovine lactoferrin against Influenza virus. Glycoconj. J. 2021, 38, 689–696. [Google Scholar] [CrossRef]
- Superti, F.; Agamennone, M.; Pietrantoni, A.; Ammendolia, M.G. Bovine lactoferrin prevents Influenza A virus infection by interfering with the fusogenic function of viral hemagglutinin. Viruses 2019, 11, 51. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Dofrelli, E.; Tinari, A.; Ammendolia, M.G.; Puzelli, S.; Fabiani, C.; Donatelli, I.; Superti, F. Bovine lactoferrin inhibits Influenza A virus induced programmed cell death in vitro. Biometals 2010, 23, 465–475. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of Influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Ammendolia, M.G.; Superti, F. Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of Influenza virus infection. Biochem. Cell Biol. 2012, 90, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.A.; Sousa, I.P., Jr.; Silva, J.L.; Oliveira, A.C.; Goncalves, R.B.; Gomes, A.M. Inhibition of mayaro virus infection by bovine lactoferrin. Virology 2014, 452–453, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Denani, C.B.; Real-Hohn, A.; de Carvalho, C.A.M.; Gomes, A.M.O.; Goncalves, R.B. Lactoferrin affects rhinovirus B-14 entry into H1-HeLa cells. Arch. Virol. 2021, 166, 1203–1211. [Google Scholar] [CrossRef]
- Superti, F.; Siciliano, R.; Rega, B.; Giansanti, F.; Valenti, P.; Antonini, G. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Biophys. Acta 2001, 1528, 107–115. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective effects of lactoferrin against SARS-CoV-2 infection in vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin against SARS-CoV-2: In vitro and in silico evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- da Silva, A.M.V.; Machado, T.L.; Nascimento, R.S.; Rodrigues, M.; Coelho, F.S.; Tubarao, L.N.; da Rosa, L.C.; Bayma, C.; Rocha, V.P.; Frederico, A.B.T.; et al. Immunomodulatory effect of bovine lactoferrin during SARS-CoV-2 infection. Front. Immunol. 2024, 15, 1456634. [Google Scholar] [CrossRef]
- Pietrantoni, A.; Fortuna, C.; Remoli, M.E.; Ciufolini, M.G.; Superti, F. Bovine lactoferrin inhibits Toscana virus infection by binding to heparan sulphate. Viruses 2015, 7, 480–495. [Google Scholar] [CrossRef]
- Dierick, M.; Van der Weken, H.; Rybarczyk, J.; Vanrompay, D.; Devriendt, B.; Cox, E. Porcine and bovine forms of lactoferrin inhibit growth of porcine enterotoxigenic Escherichia coli and degrade its virulence factors. Appl. Environ. Microbiol. 2020, 86, e00524-20. [Google Scholar] [CrossRef]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of use of antiviral peptides against Influenza virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin—A natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef]
- van der Strate, B.W.; Harmsen, M.C.; Schafer, P.; Swart, P.J.; The, T.H.; Jahn, G.; Speer, C.P.; Meijer, D.K.; Hamprecht, K. Viral load in breast milk correlates with transmission of human cytomegalovirus to preterm neonates, but lactoferrin concentrations do not. Clin. Diagn. Lab. Immunol. 2001, 8, 818–821. [Google Scholar] [CrossRef]
- Marchetti, M.; Superti, F.; Ammendolia, M.G.; Rossi, P.; Valenti, P.; Seganti, L. Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin. Med. Microbiol. Immunol. 1999, 187, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Papadopoulou, A.; Syridou, G.; Marchisio, E.; Kapsabeli, E.; Daskalaki, A.; Papaevangelou, V. Early human milk lactoferrin during SARS-CoV-2 infection. J. Matern. Fetal Neonatal Med. 2022, 35, 6704–6707. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Wakabayashi, H.; Takase, M.; Kawase, K.; Shimamura, S.; Tomita, M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993, 182, 97–105. [Google Scholar] [CrossRef]
- Kuipers, M.E.; de Vries, H.G.; Eikelboom, M.C.; Meijer, D.K.; Swart, P.J. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 1999, 43, 2635–2641. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin is broadly active against yeasts and highly synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, 02284-19. [Google Scholar] [CrossRef]
- Gruden, S.; Poklar Ulrih, N. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Zarember, K.A.; Sugui, J.A.; Chang, Y.C.; Kwon-Chung, K.J.; Gallin, J.I. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 2007, 178, 6367–6373. [Google Scholar] [CrossRef]
- Zarember, K.A.; Cruz, A.R.; Huang, C.Y.; Gallin, J.I. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2009, 53, 2654–2656. [Google Scholar] [CrossRef]
- Pawar, S.; Markowitz, K.; Velliyagounder, K. Effect of human lactoferrin on Candida albicans infection and host response interactions in experimental oral candidiasis in mice. Arch. Oral Biol. 2022, 137, 105399. [Google Scholar] [CrossRef]
- Lupetti, A.; Brouwer, C.P.; Bogaards, S.J.; Welling, M.M.; de Heer, E.; Campa, M.; van Dissel, J.T.; Friesen, R.H.; Nibbering, P.H. Human lactoferrin-derived peptide’s antifungal activities against disseminated Candida albicans infection. J. Infect. Dis. 2007, 196, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Visca, P.; Antonini, G.; Orsi, N. Interaction between lactoferrin and ovotransferrin and Candida cells. FEMS Microbiol. Lett. 1986, 33, 271–275. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 1993, 38, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Samaranayake, Y.; Samaranayake, L.; Nikawa, H. In vitro susceptibility of Candida species to lactoferrin. Sabouraudia 1999, 37, 35–41. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.; Tenovuo, J.; Hamada, T. The effect of antifungal agents on the in vitro susceptibility of Candida albicans to apo-lactoferrin. Arch. Oral Biol. 1994, 39, 921–923. [Google Scholar] [CrossRef]
- Okutomi, T.; Abe, S.; Tansho, S.; Wakabayashi, H.; Kawase, K.; Yamaguchi, H. Augmented inhibition of growth of Candida albicans by neutrophils in the presence of lactoferrin. FEMS Immunol. Med. Microbiol. 1997, 18, 105–112. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.; Hamada, T. Modulation of the anti-Candida activity of apo-lactoferrin by dietary sucrose and tunicamycin in vitro. Arch. Oral Biol. 1995, 40, 581–584. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Uchida, K.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H.; Yamaguchi, H. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 2000, 46, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Samaranayake, L.P.; Wu, P.C.; So, M. The antifungal effect of lactoferrin and lysozyme on Candida krusei and Candida albicans. APMIS 1997, 105, 875–883. [Google Scholar] [CrossRef]
- Lai, Y.W.; Pang, C.N.I.; Campbell, L.T.; Chen, S.C.A.; Wilkins, M.R.; Carter, D.A. Different pathways mediate amphotericin-lactoferrin drug synergy in Cryptococcus and Saccharomyces. Front. Microbiol. 2019, 10, 2195. [Google Scholar] [CrossRef]
- Kang, J.J.; Schaber, M.D.; Srinivasula, S.M.; Alnemri, E.S.; Litwack, G.; Hall, D.J.; Bjornsti, M.-A. Cascades of mammalian caspase activation in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 3189–3198. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takakura, N.; Yamauchi, K.; Teraguchi, S.; Uchida, K.; Yamaguchi, H.; Tamura, Y. Effect of lactoferrin feeding on the host antifungal response in guinea-pigs infected or immunised with Trichophyton mentagrophytes. J. Med. Microbiol. 2002, 51, 844–850. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Viruses: Structure, function, and uses. In Molecular Cell Biology, 4th ed.; WH Freeman: New York, NY, USA, 2000. [Google Scholar]
- Anand, N. Antiparasitic activity of the iron-containing milk protein lactoferrin and its potential derivatives against human intestinal and blood parasites. Front. Parasitol. 2023, 2, 1330398. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gomez, C.; Ramirez-Texcalco, L.S.; Torres-Lopez, E.; Ramirez-Mondragon, R.; Hernandez-Ramirez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Leon-Sicairos, N.; Reyes-Lopez, M.; Ordaz-Pichardo, C.; de la Garza, M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem. Cell Biol. 2006, 84, 327–336. [Google Scholar] [CrossRef]
- Leon-Sicairos, N.; Lopez-Soto, F.; Reyes-Lopez, M.; Godinez-Vargas, D.; Ordaz-Pichardo, C.; de la Garza, M. Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin. Med. Res. 2006, 4, 106–113. [Google Scholar] [CrossRef]
- Ikadai, H.; Tanaka, T.; Shibahara, N.; Tanaka, H.; Matsuu, A.; Kudo, N.; Shimazaki, K.; Igarashi, I.; Oyamada, T. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.L.; Sparks, H.; White, A.C., Jr.; Martinez-Traverso, G.; Ochoa, T.; Castellanos-Gonzalez, A. Killing of Cryptosporidium sporozoites by lactoferrin. Am. J. Trop. Med. Hyg. 2017, 97, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Soto, F.; Leon-Sicairos, N.; Nazmi, K.; Bolscher, J.G.; de la Garza, M. Microbicidal effect of the lactoferrin peptides lactoferricin17-30, lactoferrampin265-284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals 2010, 23, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Godinez, C.; Gonzalez-Galindo, X.; Meza-Menchaca, T.; Bobes, R.J.; de la Garza, M.; Leon-Sicairos, N.; Laclette, J.P.; Carrero, J.C. Synthetic bovine lactoferrin peptide lfampin kills Entamoeba histolytica trophozoites by necrosis and resolves amoebic intracecal infection in mice. Biosci. Rep. 2019, 39, BSR20180850. [Google Scholar] [CrossRef]
- Turchany, J.M.; Aley, S.B.; Gillin, F.D. Giardicidal activity of lactoferrin and N-terminal peptides. Infect. Immun. 1995, 63, 4550–4552. [Google Scholar] [CrossRef]
- Asthana, S.; Gupta, P.K.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Targeted chemotherapy of visceral leishmaniasis by lactoferrin-appended amphotericin B-loaded nanoreservoir: In vitro and in vivo studies. Nanomedicine 2015, 10, 1093–1109. [Google Scholar] [CrossRef]
- Anand, N.; Kanwar, R.K.; Sehgal, R.; Kanwar, J.R. Antiparasitic and immunomodulatory potential of oral nanocapsules encapsulated lactoferrin protein against Plasmodium berghei. Nanomedicine 2016, 11, 47–62. [Google Scholar] [CrossRef]
- Obayashi, M.; Kimura, M.; Haraguchi, A.; Gotanda, M.; Kitagawa, T.; Matsuno, M.; Sakao, K.; Hamanaka, D.; Kusakisako, K.; Kameda, T.; et al. Bovine lactoferrin inhibits Plasmodium berghei growth by binding to heme. Sci. Rep. 2024, 14, 20344. [Google Scholar] [CrossRef]
- Fritsch, G.; Sawatzki, G.; Treumer, J.; Jung, A.; Spira, D.T. Plasmodium falciparum: Inhibition in vitro with lactoferrin, desferriferrithiocin, and desferricrocin. Exp. Parasitol. 1987, 63, 1–9. [Google Scholar] [CrossRef]
- Eda, S.; Eda, K.; Prudhomme, J.G.; Sherman, I.W. Inhibitory activity of human lactoferrin and its peptide on chondroitin sulfate A-, CD36-, and thrombospondin-mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. Blood 1999, 94, 326–332. [Google Scholar] [CrossRef]
- Anand, N.; Sehgal, R.; Kanwar, R.K.; Dubey, M.L.; Vasishta, R.K.; Kanwar, J.R. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int. J. Nanomedicine 2015, 10, 6355–6369. [Google Scholar]
- Lu, J.M.; Jin, G.N.; Xin, Y.; Ma, J.W.; Shen, X.Y.; Quan, Y.Z.; Liu, Y.M.; Zhou, J.Y.; Wang, B.Z.; Li, Y.B.; et al. Lactoferrin-modified nanoemulsions enhance brain-targeting and therapeutic efficacy of arctigenin against Toxoplasma gondii-induced neuronal injury. Int. J. Parasitol. Drugs Drug Resist. 2025, 27, 100575. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hyung, S.; Lee, J.W.; Kim, J.; Shin, M.H.; Ryu, J.S.; Park, S.J. Identification of antigenic proteins in Trichomonas vaginalis. Korean J. Parasitol. 2011, 49, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Chen, R.M.; Ong, S.C.; Yeh, Y.M.; Huang, P.J.; Lee, C.C. Interaction of human neutrophils with Trichomonas vaginalis protozoan highlights lactoferrin secretion. J. Microbiol. Immunol. Infect. 2025, 58, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.F.; Kierszenbaum, F. Lactoferrin effects on the interaction of blood forms of Trypanosoma cruzi with mononuclear phagocytes. Int. J. Parasitol. 1987, 17, 1205–1208. [Google Scholar] [CrossRef]
- Ando, K.; Hasegawa, K.; Shindo, K.; Furusawa, T.; Fujino, T.; Kikugawa, K.; Nakano, H.; Takeuchi, O.; Akira, S.; Akiyama, T.; et al. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010, 277, 2051–2066. [Google Scholar] [CrossRef]
- Crouch, S.P.; Slater, K.J.; Fletcher, J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 1992, 80, 235–240. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- Sorimachi, K.; Akimoto, K.; Hattori, Y.; Ieiri, T.; Niwa, A. Activation of macrophages by lactoferrin: Secretion of TNF-alpha, IL-8, and NO. Biochem. Mol. Biol. Int. 1997, 43, 79–87. [Google Scholar] [CrossRef]
- Hwang, S.A.; Wilk, K.M.; Bangale, Y.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med. Microbiol. Immunol. 2007, 196, 171–180. [Google Scholar] [CrossRef]
- Wang, W.P.; Iigo, M.; Sato, J.; Sekine, K.; Adachi, I.; Tsuda, H. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn. J. Cancer Res. 2000, 91, 1022–1027. [Google Scholar] [CrossRef]
- Martínez-García, J.J.; Canizalez-Roman, A.; Angulo-Zamudio, U.A.; Velazquez-Roman, J.; Flores-Villaseñor, H.; Valdez-Flores, M.A.; Rios-Burgueño, E.; Moran-Portela, D.; León-Sicairos, N. Lactoferrin and metoprolol supplementation increase mouse survival in an experimental LPS-induced sepsis model. Int. J. Pept. Res. Ther. 2022, 28, 141. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tomé, D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem. Cell Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Pattamatta, U.; Mark, W.; Fiona, S.; Garrett, Q. Bovine lactoferrin promotes corneal wound healing and suppresses IL-1 expression in alkali wounded mouse cornea. Curr. Eye Res. 2013, 38, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic efficacy of lactoferrin in type 2 diabetic pediatrics; controlling impact on PPAR-gamma, SIRT-1, and TLR4 downstream signaling pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, F.; Yun, Z.; An, Y.; Wang, C.; Yan, X. Determination of the effects of lactoferrin in a preclinical mouse model of experimental colitis. Mol. Med. Rep. 2013, 8, 1125–1129. [Google Scholar] [CrossRef]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Nakajima, A.; Ueno, N.; Saito, T.; Sekihara, H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 2002, 17, 1291–1298. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin attenuates intestinal barrier dysfunction and inflammation by modulating the MAPK pathway and gut microbes in mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef]
- Na, Y.J.; Han, S.B.; Kang, J.S.; Yoon, Y.D.; Park, S.K.; Kim, H.M.; Yang, K.H.; Joe, C.O. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int. Immunopharmacol. 2004, 4, 1187–1199. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, H.G.; Wu, H.M.; Yao, Q.Q.; Zhang, Z.Y.; Meng, Q.S.; Fan, L.L.; Wang, J.Q.; Zheng, N. Inhibitory effects of lactoferrin on pulmonary inflammatory processes induced by lipopolysaccharide by modulating the TLR4-related pathway. J. Dairy Sci. 2021, 104, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.A.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Pu, T.-Y.; Chuang, K.-C.; Tung, M.-C.; Yen, C.-C.; Chen, Y.-H.; Cidem, A.; Ko, C.-H.; Chen, W.; Chen, C.-M. Lactoferrin as a therapeutic agent for attenuating hepatic stellate cell activation in thioacetamide-induced liver fibrosis. Biomed. Pharmacother. 2024, 174, 116490. [Google Scholar] [CrossRef]
- Welsh, K.J.; Hwang, S.A.; Hunter, R.L.; Kruzel, M.L.; Actor, J.K. Lactoferrin modulation of mycobacterial cord factor trehalose 6-6′-dimycolate induced granulomatous response. Transl. Res. 2010, 156, 207–215. [Google Scholar] [CrossRef]
- Actor, J.K. Lactoferrin: A modulator for immunity against tuberculosis related granulomatous pathology. Mediators Inflamm. 2015, 2015, 409596. [Google Scholar] [CrossRef]
- Nakamura, A.; Kimura, F.; Tsuji, S.; Hanada, T.; Takebayashi, A.; Takahashi, A.; Kitazawa, J.; Morimune, A.; Amano, T.; Kushima, R.; et al. Bovine lactoferrin suppresses inflammatory cytokine expression in endometrial stromal cells in chronic endometritis. J. Reprod. Immunol. 2022, 154, 103761. [Google Scholar] [CrossRef]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Umezawa, T.; Nakajima, A.; Naito, M.; Sato, S.; Saito, T.; Sekihara, H. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G187–G195. [Google Scholar] [CrossRef]
- Valenti, P.; Catizone, A.; Pantanella, F.; Frioni, A.; Natalizi, T.; Tendini, M.; Berlutti, F. Lactoferrin decreases inflammatory response by cystic fibrosis bronchial cells invaded with Burkholderia cenocepacia iron-modulated biofilm. Int. J. Immunopathol. Pharmacol. 2011, 24, 1057–1068. [Google Scholar] [CrossRef]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar]
- Maritati, M.; Comar, M.; Zanotta, N.; Seraceni, S.; Trentini, A.; Corazza, F.; Vesce, F.; Contini, C. Influence of vaginal lactoferrin administration on amniotic fluid cytokines and its role against inflammatory complications of pregnancy. J. Inflamm. 2017, 14, 5. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Lee, C.; Zhu, H.; Zheng, S.; Pierro, A. Protective effects of lactoferrin on injured intestinal epithelial cells. J. Pediatr. Surg. 2019, 54, 2509–2513. [Google Scholar] [CrossRef]
- Mattsby-Baltzer, I.; Roseanu, A.; Motas, C.; Elverfors, J.; Engberg, I.; Hanson, L.Å. Lactoferrin or a fragment thereof inhibits the endotoxin-induced Interleukin-6 response in human monocytic cells. Pediatr. Res. 1996, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin downregulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Li, H.; Li, G.; Shen, Y.; Fei, M.; Nan, Y. Effect of bovine lactoferrin as a novel therapeutic agent in a rat model of sepsis-induced acute lung injury. AMB Express 2019, 9, 177. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.-A.; Olsen, M.; Zimecki, M.; Hunter, R.L.; Kruzel, M.L. Lactoferrin immunomodulation of DTH response in mice. Int. Immunopharmacol. 2002, 2, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Tanaka, A.; Yamauchi, K.; Iwatsuki, K. Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis. Br. J. Nutr. 2014, 111, 1801–1810. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Mailman, D.; Actor, J.K.; Zimecki, M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 2002, 130, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, A.J.; Kim, G.Y.; Jo, A.; Lee, J.E.; Leem, S.H.; Yoon, J.H.; Ye, S.K.; Chung, J.W. Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J. Microbiol. Biotechnol. 2017, 27, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Imase, M.; Oda, H.; Wakabayashi, H.; Ishii, K. Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: A possible role in protection against oxidative DNA damage. Int. J. Mol. Sci. 2014, 15, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Al Zharani, M.M.; Almuqri, E.A.; Ahmed, M.M.; Aljarba, N.H.; Rudayni, H.A.; Yaseen, K.N.; Alkahtani, S.H.; Nasr, F.A.; Al Doaiss, A.A. Use of lactoferrin supplement as an efficient antioxidant to ameliorate the effects of mercury-induced oxidative stress in male Wistar rats. Biomed. Biotechnol. Res. J. 2024, 8, 45–52. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, J.K.; Lee, J.H.; Lee, Y.J.; Seol, J.W.; Kim, S.J.; Hur, T.Y.; Jung, Y.H.; Kang, S.J.; Park, S.Y. Lactoferrin protects against prion protein-induced cell death in neuronal cells by preventing mitochondrial dysfunction. Int. J. Mol. Med. 2013, 31, 325–330. [Google Scholar] [CrossRef]
- Safaeian, L.; Zabolian, H. Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. ISRN Pharmacol. 2014, 2014, 943523. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, S.; Liu, M.; Guo, J.; Chen, Y.; Shen, X.; Deng, X.; Lu, J. Preventive effects of lactoferrin on acute alcohol-induced liver injury via iron chelation and regulation of iron metabolism. J. Dairy Sci. 2024, 107, 5316–5329. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Mazurier, J.; Legrand, D. Lactoferrin inhibits the binding of lipopolysaccharides to L-selectin and subsequent production of reactive oxygen species by neutrophils. FEBS Lett. 2000, 469, 5–8. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, S.; Hong, J.Y.; Shim, K.S.; Kim, T.H.; Park, K.; Lee, S.H. Accelerated osteogenic differentiation of MC3T3-E1 cells by lactoferrin-conjugated nanodiamonds through enhanced anti-oxidant and anti-inflammatory effects. Nanomaterials 2019, 10, 50. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef]
- Anand, N.; Kanwar, R.K.; Dubey, M.L.; Vahishta, R.K.; Sehgal, R.; Verma, A.K.; Kanwar, J.R. Effect of lactoferrin protein on red blood cells and macrophages: Mechanism of parasite-host interaction. Drug Des. Devel. Ther. 2015, 9, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.T.; Viejo-Diaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Bodur, M.; Aydogdu, G.; Ozcelik, A.O.; Yilmaz, E. An in vitro approach to protective effect of lactoferrin on Acrylamide-induced oxidative damage. An. Acad. Bras. Cienc. 2022, 94, e20201882. [Google Scholar] [CrossRef]
- Fan, Y.G.; Ge, R.L.; Ren, H.; Jia, R.J.; Wu, T.Y.; Lei, X.F.; Wu, Z.; Zhou, X.B.; Wang, Z.Y. Astrocyte-derived lactoferrin inhibits neuronal ferroptosis by reducing iron content and GPX4 degradation in APP/PS1 transgenic mice. Pharmacol. Res. 2024, 209, 107404. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Zong, Q.; Hu, P.; Bao, W.; Liu, H.Y.; Cai, D. Lactoferrin restores the deoxynivalenol-impaired spermatogenesis and blood-testis barrier integrity via improving the antioxidant capacity and modifying the cell adhesion and inflammatory response. Antioxidants 2023, 12, 152. [Google Scholar] [CrossRef]
- Abd El-Rahman, S.S.; Ashwish, N.M.; Ali, M.E. Appraisal of the pre-emptive effect of lactoferrin against chromium-induced testicular toxicity in male rats. Biol. Trace Elem. Res. 2023, 201, 5321–5334. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Kruzel, M.; Hwang, S.-A.; Ba, X.; Boldogh, I. Novel recombinant human lactoferrin: Differential activation of oxidative stress-related gene expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Li, F.; Zhou, Y.; Qi, L.; Liu, L.; Chen, Z. Lactoferrin improves cognitive function and attenuates brain senescence in aged mice. J. Funct. Foods 2020, 65, 103736. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Wang, Y.; Zhang, L.; Chua, N.; Dai, L.; Chen, J.; Ho, C.L. Evaluation of the anti-inflammatory and anti-oxidative effects of therapeutic human lactoferrin fragments. Front. Bioeng. Biotechnol. 2021, 9, 779018. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Radak, Z.; Bacsi, A.; Saavedra-Molina, A.; Boldogh, I. Lactoferrin decreases LPS-induced mitochondrial dysfunction in cultured cells and in animal endotoxemia model. Innate Immun. 2010, 16, 67–79. [Google Scholar] [CrossRef]
- Safaeian, L.; Javanmard, S.H.; Mollanoori, Y.; Dana, N. Cytoprotective and antioxidant effects of human lactoferrin against H2O2-induced oxidative stress in human umbilical vein endothelial cells. Adv. Biomed. Res. 2015, 4, 188. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, K.G.; Ashry, M.; Hassan, L.K.; El-Azma, M.H.; Elqattan, G.M.; Gadelmawla, M.H.; Mannaa, F.A. Hepatic and immune modulatory effectiveness of lactoferrin loaded selenium nanoparticles on bleomycin induced hepatic injury. Sci. Rep. 2024, 14, 21066. [Google Scholar] [CrossRef] [PubMed]
- Gulmez, C.; Dalginli, K.Y.; Atakisi, E.; Atakisi, O. The protective effect of lactoferrin on adenosine deaminase, nitric oxide and liver enzymes in lipopolysaccharide-induced experimental endotoxemia model in rats. Kafkas Univ. Vet. Fak. Derg. 2020, 26, 801–806. [Google Scholar]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A pilot study on the effect of lactoferrin on Alzheimer’s disease pathological sequelae: Impact of the p-Akt/PTEN pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef]
- Maneva, A.; Taleva, B.; Maneva, L. Lactoferrin-protector against oxidative stress and regulator of glycolysis in human erythrocytes. Z. Naturforsch. C J. Biosci. 2003, 58, 256–262. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal lactoferrin administration decreases oxidative stress in the amniotic fluid of pregnant women: An open-label randomized pilot study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef]
- El-Hameed, S.A.; Ibrahim, I.; Awadin, W.; El-Shaieb, A. Assessment of single and combined administration of ubiquinone and lactoferrin on histopathology, ultrastructure, oxidative stress, and WNT4 expression gene induced by thioacetamide on hepatorenal system of adult male rats. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 41. [Google Scholar] [CrossRef]
- Farid, A.S.; El Shemy, M.A.; Nafie, E.; Hegazy, A.M.; Abdelhiee, E.Y. Anti-inflammatory, anti-oxidant and hepatoprotective effects of lactoferrin in rats. Drug Chem. Toxicol. 2021, 44, 286–293. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; An, Z.; Liu, J.; Feng, J. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008, 140, 326–336. [Google Scholar] [CrossRef]
- Fan, L.; Wang, F.; Yao, Q.; Wu, H.; Wen, F.; Wang, J.; Li, H.; Zheng, N. Lactoferrin could alleviate liver injury caused by Maillard reaction products with furan ring through regulating necroptosis pathway. Food Sci. Nutr. 2021, 9, 3449–3459. [Google Scholar] [CrossRef]
- Li, Y.-C.; Hsieh, C.-C. Lactoferrin dampens high-fructose corn syrup-induced hepatic manifestations of the metabolic syndrome in a murine model. PLoS ONE 2014, 9, e97341. [Google Scholar] [CrossRef]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstet. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of oral administration mode on the efficacy of commercial bovine Lactoferrin against iron and inflammatory homeostasis disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Tsuda, H.; Sekine, K.; Fujita, K.-i.; Iigo, M. Cancer prevention by bovine lactoferrin and underlying mechanisms a review of experimental and clinical studies. Biochem. Cell Biol. 2002, 80, 131–136. [Google Scholar] [CrossRef]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Vishwanath-Deutsch, R.; Dallas, D.C.; Besada-Lombana, P.; Katz, L.; Conze, D.; Kruger, C.; Clark, A.J.; Peterson, R.; Malinczak, C.A. A review of the safety evidence on recombinant human lactoferrin for use as a food ingredient. Food Chem. Toxicol. 2024, 189, 114727. [Google Scholar] [CrossRef]

| Function | Apo-Lf (Iron-Free) | Holo-Lf (Iron-Bound) | Ref. |
|---|---|---|---|
| Iron scavenging | High capacity to bind iron (good at chelating iron from the environment) | Cannot bind more iron (saturated) | [15,16,17,18,19,20,21,22,23,24,25] |
| Interaction with bacteria | Disrupt bacterial membranes and inhibit growth | Less effective | [18,26,27] |
| Antimicrobial activity | Stronger: depriving pathogens of the iron they need to grow | Weaker: no longer chelates iron | [16,17,18,21,22,26] |
| Immunomodulation | More potent in anti-inflammatory effects | Moderate to weak | [25,28] |
| Stability | Less stable (more prone to degrade in acidic environments) | More stable due to an iron-induced conformational change | [20,29,30,31,32,33] |
| Bacteria | Host | Function and Mechanism | Ref. |
|---|---|---|---|
| Chlamydophila psittaci | in vitro | Inhibit attachment and entry | [70] |
| Chlamydia trachomatis | in vitro | Inhibit entry Reduce IL-6 and IL-8 | [53,54] |
| Enterobacter sakazakii | in vitro | Inhibit growth | [55] |
| Escherichia coli | in vitro | Inhibit adherence | [57] |
| in vitro | Impair type III secretory system | [56] | |
| in vitro | Inhibit growth | [67] | |
| in vitro | Inhibit biofilm formation | [66] | |
| Haemophilus influenzae | in vitro | Inactivate colonization factors | [58] |
| Helicobacter felis | Mouse | Reverse gastritis, infection rate, and gastric surface hydrophobicity changes | [59] |
| Helicobacter pylori | Mouse | Inhibit gastric colonization and inflammation | [71] |
| Mouse | Reduce bacterial load Inhibit TNF-α, IFN-γ, IL-17, COX-2 Increase IL-4, IL-10, IL-12 Regulate blood parameters Alleviate histopathological changes | [72] | |
| in vitro | Inhibit growth | [73] | |
| Klebsiella pneumoniae | in vitro | Enhance sensitivity to antibiotics | [61] |
| Porphyromonas gingivalis | Human | Inhibit growth | [62] |
| Pseudomonas aeruginosa | in vitro | Inhibit biofilm formation | [63] |
| Mouse | Decrease weight loss Inhibit growth Decrease cell infiltration Decrease MCP-1 and MIP-1 | [74] | |
| Salmonella enterica s erovar Typhimurium | Mouse | Decrease bacterial load in the liver and spleen Reduce hepatomegaly and splenomegaly | [64] |
| Mouse | Increase survival Decrease weight loss Inhibit infection | [75] | |
| Mouse | Increase survival Inhibit infection Increase IgA and IgG | [76] | |
| Yersinia | in vitro | Inhibit entry | [65] |
| Bacteria | Host | Function and Mechanism | Ref. |
|---|---|---|---|
| Bacillus cereus | in vitro | Inhibit growth | [77,78] |
| Clostridium difficile | in vitro | Delay growth Prevent toxin production | [79] |
| Listeria monocytogenes | in vitro | Inhibit biofilm formation | [66] |
| Staphylococcus aureus | Mouse | Decrease IL-17 and IL-1β | [80] |
| in vitro | Increase IL-1β, IFN-γ, IL-2, Reduce IL-6, IL-1β, IL-12p40 | [80] | |
| Mouse | Inhibit kidney infection | [81] | |
| Mouse | Increase the spleen cell number Increase IFN-γ and TNF-α Reduce IL-5 and IL-10 | [82] | |
| in vitro | Decrease cell viability | [83] | |
| in vitro | Inhibit growth | [67] | |
| Streptococcus mutans | in vitro | Inhibit aggregation and biofilm | [84] |
| Mouse | Reduce cavity development | [85] | |
| in vitro | Inhibit infection | [85] |
| Viruses | Host | Function and Mechanism | Ref. |
|---|---|---|---|
| Adenovirus | in vitro | Inhibit viral antigen synthesis | [102] |
| in vitro | Promote binding and infection | [103,104] | |
| Avian influenza | Mouse | Decrease weight loss Decrease IL-17, IL-22, TNF-α | [105] |
| Cytomegalovirus | Mouse | Reduce infection | [93] |
| Enterovirus E | in vitro | Inhibit virus replication | [106] |
| Enterovirus 71 | Mouse | Increase survival | [107] |
| in vitro | Inhibit infection Decrease IL-6 Increase IFN-α | [107] | |
| in vitro | Inhibit infection | [108] | |
| Hepatitis B virus (HBV) | in vitro | Inhibit virus binding Inhibit infection | [109,110] |
| in vitro | Inhibit growth | [111] | |
| Hepatitis C virus (HCV) | in vitro | Inhibit entry | [112,113,114] |
| Inhibit virus replication | [114] | ||
| in vitro | Inhibit virus replication Inhibit viral ATPase/helicase | [115] | |
| in vitro | Inhibit entry Inhibit virus replication | [112,116] | |
| Human | Decrease serum ALT Decrease HCV RNA level | [117] | |
| Herpes simplex virus | in vitro | Inhibit infection | [118] |
| Influenza | in vitro | Reduce infection | [119] |
| in vitro | Suppress viral antigen synthesis Reduce infection | [120] | |
| in vitro | Inhibit cell apoptosis Inhibit DNA fragmentation Reduce caspase-3 activity | [121] | |
| in vitro | Inhibit virus replication | [122] | |
| in vitro | Inhibit infection | [123] | |
| Mayaro virus | in vitro | Inhibit infection Inhibit entry | [124] |
| Rhinovirus B-14 | in vitro | Reduce virus binding | [125] |
| Rotavirus | in vitro | Inhibit viral cytopathic effect | [60,126] |
| SARS-CoV-2 | in vitro | Inhibit infection and replication Reduce thymic stromal lymphopoietin Upregulate TGF-β1 | [127] |
| in vitro | Reduce virus binding Obscure host cell receptors | [128] | |
| in vitro | Reduce virus binding | [98] | |
| Rat | Decrease TNF-α, IL-4, IL-1β, IL-6, IL-10 Increase CD4 cells Alleviate pulmonary fibrosis | [99] | |
| in vitro | Increase virus neutralization Inhibit virus propagation | [99] | |
| in vitro | Decrease virus infection | [100] | |
| Hamster | Decrease virus infection Alleviate pulmonary histopathological changes | [100] | |
| in vitro | Decrease virus infection Inhibit entry | [101] | |
| Mouse | Decrease IFN-γ Increase IL-1β, IL-2, IL-6, GM-CSF Increase TLR-4 and TLR-9 | [129] | |
| in vitro | Decrease NK and NKT cells Activate CD4 cells Decrease programmed death of CD4 and CD8 cells Increase CCL5 | [129] | |
| Toscana virus | in vitro | Inhibit viral cytopathic effect | [130] |
| Fungi | Host | Function and Mechanism | Ref. |
|---|---|---|---|
| Aspergillus fumigatus | Human | Inhibit growth Iron deprivation | [142] |
| in vitro | Iron deprivation | [142,143] | |
| in vitro | Prevent biofilm | [140] | |
| Candida albicans | Mouse | Inhibit growth Downregulate EGF1 | [144] |
| Mouse | Inhibit growth Increase IL-10, TNF-α, IFN-γ, MCP-1 | [145] | |
| Galleria mellonella | Decrease fungal burden | [140] | |
| in vitro | Iron deprivation Interact with cell surface Alter cell membrane Alter cell membrane H+ ATPase | [137,139,146,147,148,149,150] | |
| Candida glabrata | in vitro | Interact with cell surface Alter cell membrane | [139,148] |
| Candida guilliermondii | in vitro | [148] | |
| Candida krusei | in vitro | [147,151,152] | |
| Candida parapsilosis | in vitro | [148] | |
| Candida tropicalis | in vitro | [148] | |
| Cryptococcus gattii | in vitro | Iron deprivation | [153] |
| Cryptococcus neoformans | in vitro | Iron deprivation Alter responses to stress | [153,154] |
| in vitro | Disrupt iron transport Inhibit growth | [155] | |
| Galleria mellonella | Inhibit growth Interact with cell surface Reduce cell and capsule size | [140] | |
| Saccharomyces cerevisiae | in vitro | Regulate cell death | [156] |
| in vitro | Iron deprivation | [153] | |
| Trichophyton mentagrophytes | in vitro | Inhibit growth | [152] |
| Guinea pig | Inhibit growth | [152] | |
| Guinea pig | Modulate mononuclear cell function | [157] | |
| Trichophyton spp. | in vitro | Interact with cell surface Alter cell membrane | [158] |
| Cytokines | Stimuli | Lf Effects | Ref. |
|---|---|---|---|
| IFN-γ | Co26Lu tumor | I | [184] |
| LPS | D | [185] | |
| Toxoplasma gondii cysts | D | [186] | |
| IL-1α | NaOH | D | [187] |
| IL-1β | No stimulus | D | [188] |
| Dextran sulfate sodium (DSS) | D | [189,190] | |
| Deoxynivalenol | D | [191] | |
| LPS | I | [192] | |
| LPS | D | [35,179,193,194] | |
| LPS + IFN-γ | D | [35] | |
| NaOH | D | [187] | |
| Thioacetamide (TAA) | D | [195] | |
| Trehalose 6,6′-dimycolate (TDM) | D | [196,197] | |
| TNF-α | D | [198] | |
| 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) | D | [199] | |
| Burkholderia cenocepacia | D | [200] | |
| Prevotella intermedia | D | [201] | |
| IL-2 | LPS | D | [179] |
| IL-4 | No stimulus | D | [202] |
| DSS | I | [190] | |
| TNBS | I | [199] | |
| IL-6 | No stimulus | I | [203,204] |
| No stimulus | D | [188] | |
| CCl4 | D | [187] | |
| DSS | D | [190] | |
| H2O2 | D | [205] | |
| LPS | I | [181,192] | |
| LPS | D | [35,185,206] | |
| LPS + IFN-γ | D | [35] | |
| TAA | D | [195] | |
| TDM | D | [197] | |
| TNF-α | D | [198] | |
| Chlamydia trachomatis | D | [54] | |
| Escherichia coli HB101(pRI203) | D | [207] | |
| Mycobacterium tuberculosis | D | [196] | |
| P. intermedia | D | [201] | |
| IL-8 (CXCL8) | No stimulus | I | [182] |
| Deoxynivalenol | D | [191] | |
| H2O2 | D | [205] | |
| LPS | D | [35] | |
| Sepsis-induced acute lung injury | D | [208] | |
| C. trachomatis | D | [54] | |
| E. coli HB101(pRI203) | D | [207] | |
| P. intermedia | D | [201] | |
| IL-10 | No stimulus | D | [209] |
| Deoxynivalenol | I | [191] | |
| DSS | I | [190] | |
| LPS | D | [35,183,185] | |
| LPS + IFN-γ | I | [35] | |
| TAA | I | [195] | |
| TDM | I | [196] | |
| TNBS | I | [199] | |
| T. gondii cysts | I | [186] | |
| IL-11 | Zymosan | I | [210] |
| B. cenocepacia | I | [200] | |
| IL-12 | LPS | I | [183] |
| IL-18 | No stimulus | D | [188] |
| Co26Lu tumor | I | [184] | |
| T. gondii cysts | D | [186] | |
| MIF | Sepsis-induced acute lung injury | D | [208] |
| Pseudomonas aeruginosa | D | [74] | |
| TNF-α | No stimulus | I | [182,183] |
| No stimulus | D | [198,202,211] | |
| Deoxynivalenol | D | [191] | |
| DSS | D | [189,190] | |
| LPS | D | [35,179,181,192,194,212] | |
| Sepsis-induced acute lung injury | D | [208] | |
| TDM | D | [197] | |
| TNBS | D | [199] | |
| E. coli HB101(pRI203) | D | [207] | |
| M. tuberculosis | I | [196] | |
| P. intermedia | D | [201] |
| Oxidative-Stress | ||||
| Oxidative Stress | Study Model | Stimuli | LF Effects | Ref. |
| Intracellular ROS | in vitro (A549) | Ragweed pollen extract (RWE), Glucose oxidase (Gox) | D | [25] |
| in vitro (NHBE) | RWE | |||
| in vitro (U937) | Gox | D | [228] | |
| in vitro (SH-SY5Y) | PrP (106–126) | D | [216] | |
| in vitro (RBC) | D | [222] | ||
| in vitro (hMSC) | H2O2 | D | [213] | |
| in vitro (MC3T3-E1) | H2O2 | D | [220] | |
| in vivo (hippocampus) | Age | D | [229] | |
| in vitro (N2a) | Ferric ammonium citrate (FAC) | D | [225] | |
| in vitro (AML-12) | Ethanol | D | [218] | |
| in vitro (FL83B) | Thioacetamide | D | [195] | |
| in vitro (CCD-841-CON, CCD-18co, HT29) | Lipopolysaccharide (LPS) | D | [230] | |
| in vitro (U937, AML-12) | LPS, H2O2, Gox | D | [231] | |
| H2O2 | in vitro (neutrophil) | LPS | D | [219] |
| in vitro (A549) | RWE | D | [25] | |
| in vivo (BAL fluid) | ||||
| in vivo (plasma) | Dexamethasone | D | [217] | |
| in vitro (HUVEC) | H2O2 | D | [232] | |
| in vivo (serum, liver, kidney) | HgCl2 | D | [215] | |
| in vitro (U937, AML-12) | LPS, H2O2 | D | [231] | |
| in vivo (liver, heart, muscle, brain) | LPS, H2O2 | D | [231] | |
| Nitric oxide (NO) | in vivo (liver) | Bleomycin | D | [233] |
| in vivo (liver) | LPS | D | [234] | |
| in vitro (peripheral blood, lymphocytes) | Alzheimer’s disease | D | [235] | |
| Malondialdehyde (MDA) | in vitro (erythrocytes) | D | [236] | |
| in vivo (BAL fluid) | RWE | D | [25] | |
| in vivo (serum, liver) | Cholesterol | D | [83] | |
| in vivo (lung) | Acute lung injury (ALI) | D | [208] | |
| in vitro (U937) | H2O2 | D | [237] | |
| in vivo (amniotic fluid) | ||||
| in vivo (hippocampus) | Age | D | [229] | |
| in vitro (HepG2) | Acrylamide | D | [224] | |
| in vivo (testis) | Deoxynivalenol | D | [226] | |
| in vivo (serum, liver, kidney) | HgCl2 | D | [215] | |
| in vitro (N2a) | FAC | D | [225] | |
| in vitro (AML-12), in vivo (liver) | Ethanol | D | [218] | |
| in vivo (liver, kidney) | Thioacetamide | D | [238] | |
| in vivo (liver) | Bleomycin | D | [233] | |
| in vitro (peripheral blood, lymphocytes) | Alzheimer’s disease | D | [235] | |
| in vivo (liver) | CCl4 | D | [239] | |
| in vivo (serum, longissimus muscle) | D | [240] | ||
| Aspartate Aminotransferase (AST) | in vivo (blood) | D-galactosamine, CCl4, LPS | D | [210] |
| in vivo (serum) | Cholesterol | D | [83] | |
| in vivo (serum, liver) | Furosine, Pyralline, 5-Hydroxymethylfurfural | D | [241] | |
| in vivo (serum, liver, kidney) | HgCl2 | D | [215] | |
| in vivo (serum, liver) | Ethanol | D | [218] | |
| in vivo (serum) | Thioacetamide | D | [238] | |
| in vivo (blood) | Thioacetamide | D | [195] | |
| in vivo (serum) | HgCl2 | D | [215] | |
| in vivo (serum) | Bleomycin | D | [233] | |
| in vivo (serum) | LPS | D | [234] | |
| Alanine Aminotransferase (ALT) | in vivo (serum) | HgCl2 | D | [215] |
| in vivo (blood) | Thioacetamide | D | [195] | |
| in vivo (serum) | Bleomycin | D | [233] | |
| in vivo (liver) | High-fructose corn syrup | D | [242] | |
| NADPH oxidase (NOX2) | in vivo (hippocampus) | Age | D | [229] |
| Antioxidants | ||||
| Antioxidants | Study model | Inhibitor | LF effects | Ref. |
| Superoxide dismutase (SOD) | in vitro (WBC) | I | [228] | |
| in vivo (lung) | ALI | I | [208] | |
| in vivo (hippocampus) | Age | I | [229] | |
| in vitro (HepG2) | Acrylamide | I | [224] | |
| in vivo (testis) | Potassium dichromate (PDC) | I | [227] | |
| in vivo (cortex, hippocampus) | FAC | I | [225] | |
| in vivo (liver, kidney) | Thioacetamide | I | [238] | |
| in vivo (liver) | CCl4 | I | [239] | |
| Catalase (CAT) | in vivo (lung) | ALI | I | [208] |
| in vitro (HepG2) | Acrylamide | I | [224] | |
| in vivo (testis) | PDC | I | [227] | |
| in vivo (serum, liver, kidney) | HgCl2 | I | [215] | |
| in vivo (liver, kidney) | Thioacetamide | I | [238] | |
| in vivo (liver) | Thioacetamide | I | [195] | |
| in vivo (serum, longissimus muscle) | I | [240] | ||
| Glutathione reductase (GSH) | in vitro (erythrocytes) | I | [236] | |
| in vivo (serum, liver) | Cholesterol | I | [83] | |
| in vivo (lung) | ALI | I | [208] | |
| in vitro (HepG2) | Acrylamide | I | [224] | |
| in vivo (testis) | PDC | I | [227] | |
| in vivo (serum, liver, kidney) | HgCl2 | I | [215] | |
| in vitro (N2a) | FAC | I | [225] | |
| in vitro (AML-12) in vivo (liver) | Ethanol | I | [218] | |
| in vivo (liver) | Bleomycin | I | [233] | |
| in vitro (peripheral blood, lymphocytes) | Alzheimer’s disease | I | [235] | |
| Glutathione peroxidase (GPX) | in vitro (WBC) | I | [228] | |
| in vivo (lung) | ALI | I | [208] | |
| in vivo (testis) | Deoxynivalenol | I | [226] | |
| in vitro (N2a, SH-SY5Y) in vivo (cortex, hippocampus) | FAC | I | [225] | |
| in vitro (AML-12) in vivo (liver) | Ethanol | I | [218] | |
| in vivo (liver) | Bleomycin | I | [233] | |
| in vivo (liver) | CCl4 | I | [239] | |
| in vivo (serum, longissimus muscle) | I | [240] | ||
| Total antioxidant status (TAS) | in vivo (amniotic fluid) | I | [237] | |
| Total antioxidant capacity (TAC) | in vivo (serum, liver, kidney) | HgCl2 | I | [215] |
| in vitro (peripheral blood, lymphocytes) | Alzheimer’s disease | I | [235] | |
| Actions | Mode of Action | Target Pathogens/Molzecules | LF Effects | Ref. | |
|---|---|---|---|---|---|
| Antimicrobial action | Iron chelation | Bacteria (G+/C−), Viruses, Fungi, Parasites | − | Inhibit growth, Prevent infection by blocking binding to host cells | [55,56,57,58,59,63,70,71,73,75,76,77,79,80,81,82,84,85,93,102,104,105,106,107,108,110,114,115,116,118,121,126,129,130,137,139,142,143,145,146,147,148,149,150,151,152,153,154,155,156,157,158] |
| Membrane disruption | |||||
| Interaction impairment | |||||
| Immune system reaction | Signaling molecules | MAPK, NF-κB | D I | Reduce the expression of pro-inflammatory genes | [178] |
| Cytokines | IL-4, IL-10, IL-11, IL-12, | [35,183,185,186,190,191,195,196,199,200,202,209,210] | |||
| IFN-γ, IL-1α, IL-1β, IL-2, IL-6, IL-8, TNF-α, MIF | D | [35,54,74,179,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,200,201,202,203,204,205,206,207,208,211,212] | |||
| Redox regulation | Oxidative stress | ROS, H2O2, NO, MDA, AST, ALT, NOX2 | D I | Protect host cells from damage caused by excessive oxidative stress | [24,25,83,195,197,208,210,213,215,217,218,219,220,222,224,226,228,229,231,232,233,234,235,236,237,238,239,240,241,242] |
| Antioxidant activity | SOD, CAT, GSH, GPX, TAS, TAC | [83,195,208,215,218,224,225,227,228,229,233,235,236,237,238,239,240] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Lee, J.S.; Choi, Y.J.; Kim, C. The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation. Biomolecules 2025, 15, 1174. https://doi.org/10.3390/biom15081174
Kim JW, Lee JS, Choi YJ, Kim C. The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation. Biomolecules. 2025; 15(8):1174. https://doi.org/10.3390/biom15081174
Chicago/Turabian StyleKim, Jung Won, Ji Seok Lee, Yu Jung Choi, and Chaekyun Kim. 2025. "The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation" Biomolecules 15, no. 8: 1174. https://doi.org/10.3390/biom15081174
APA StyleKim, J. W., Lee, J. S., Choi, Y. J., & Kim, C. (2025). The Multifaceted Functions of Lactoferrin in Antimicrobial Defense and Inflammation. Biomolecules, 15(8), 1174. https://doi.org/10.3390/biom15081174





