Abstract
3-Nitropropionic acid (3-NPA) is a deadly neurotoxic nitroalkane found in numerous fungi and leguminous plants. 3-NPA, known as an antimetabolite of succinate, irreversibly inhibits succinate dehydrogenase and disrupts mitochondrial oxidative phosphorylation. Its utility in modeling Huntington’s disease (HD) and oxidative stress has garnered significant research interest. Derivatives of 3-NPA, formed through esterification, have a wide range of biological activities including neurotoxic, antiviral, insecticidal, antimicrobial and antioxidant properties. This review systematically summarizes the structural characteristics, biological activities, and chemical synthesis of 3-NPA-derived compounds, providing valuable insights for further research and therapeutic applications.
1. Introduction
3-Nitropropionic acid (3-NPA) (1) (Figure 1) is a potent neurotoxic nitroalkane initially isolated from fungi such as Aspergillus flavus, Aspergillus oryzae, and Arthrinium spp. and is occasionally found in some leguminous plants [1,2,3]. Compound 1, known as an antimetabolite of succinate, irreversibly inhibits succinate dehydrogenase and disrupts mitochondrial oxidative phosphorylation. While 1 was first reported in 1920 [4], its structure was not elucidated until 1949 [5]. In 1959, Britten, E. J. identified 3-NPA as the major toxic compound of indigo by comparing sodium nitrite and 3-NPA [6]. In China, nearly 1000 cases of acute encephalopathy and gastrointestinal disorders (e.g., nausea, vomiting, and abdominal pain) were linked to moldy sugarcane consumption, with severe cases resulting in death or delayed dystonia [7]. Researchers isolated Arthrinium spp. from moldy sugarcane and identified 3-NPA as the causative toxin [8]. In 1991, Ludolph et al. [9] investigated the pathological mechanisms underlying 3-NPA-mediated human mortality and delayed-onset dystonia. Their research revealed 3-NPA’s neurotoxic properties in experimental animals, leading to the proposal of a standardized animal model for studying selective neuronal metabolic damage. Subsequently, animal models based on 3-NPA toxicity emerged as a prominent research focus. 3-NPA has been primarily utilized to study therapeutic interventions across various symptomatic stages of Huntington’s disease (HD). Furthermore, pathological investigations of HD and 3-NPA have established animal models characterized by mitochondrial morphological alterations, cerebral hypoxia, caspase-3 activation, bradykinesia, and memory impairment [10,11,12,13,14]. 3-NPA HD models could be a better option for screening novel drugs to manage HD [15]. The derivatives of 3-Nitropropionic acid exhibit toxicity similar to 3-NPA. Carter et al. [16] first reported the lethality of karaka fruit to livestock and isolated 3-NPA and its glycoside derivative karakin (3), confirming its role as a primary toxin. Because the prolonged consumption of the seeds of some species of the genus Lathyrus (Leguminosae), e.g., L. sativus, can cause lathyrism, a neuronal disease, many of the secondary metabolites in these plants, such as those derived from isoxazolin-5-one and 3-NPA, have been characterized in terms of their neurotoxicity [17]. Subsequently, toxicological investigations of 3-Nitropropionic acid derivatives emerged as a prominent research focus. Byers et al. [18] further demonstrated that the majority of these derivatives exhibit potent insecticidal and antifeedant activities, along with neuroactive effects beyond classical toxicological mechanisms. The neuroregulatory properties of these compounds were elucidated by Acheson’s group [19]. Notably, α-L-galactopyranose 2,3,4,6-tetrakis (3-nitropropanoate) was found to specifically inhibit the binding of the neurotrophic precursor protein p75 receptor and disrupt the cellular internalization of pro-nerve growth factor (proNGF).
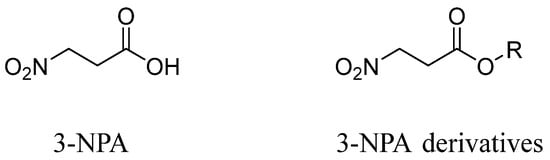
Figure 1.
Chemical structures of 3-Nitropropionic acid and its derivatives.
In 2024, Tang, Y’s group elucidated the biosynthetic pathway of 3-nitropropanoic acid (3-NPA) in the koji mold Aspergillus oryzae [20] (Scheme 1). The identified biosynthetic gene cluster (BGC) contains genes encoding an amine oxidase, NpaA, and a decarboxylase, NpaB. Genetic knockout experiments confirmed that NpaA functions as an (S)-nitrosuccinate synthase, catalyzing the oxidation of L-aspartate to (S)-nitrosuccinic acid. The decarboxylase NpaB potentially accelerates the subsequent decarboxylation step. Notably, a conserved BGC for 3-nitropropanoic acid was identified across various fungi used in food processing, despite the absence of reported 3-NPA production in these organisms [20].
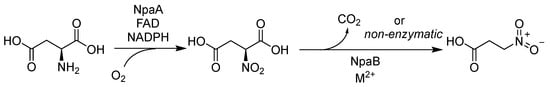
Scheme 1.
Biosynthetic pathways of 3-Nitropropionic acid.
Most 3-Nitropropionic acid derivatives are small-molecule compounds with significant potential in the development of novel therapeutics and biomedical applications. This review categorizes known derivatives based on three aspects: structural features, physiological activities, and biosynthetic pathways, aiming to facilitate future studies.
2. Structural Classification of 3-Nitropropionic Acid Derivatives
3-NPA derivatives are compounds formed through the dehydrogenation of the carboxylic acid group in 3-NPA. Based on the esterified groups, these compounds are classified into three categories: 3-NPA sugar esters, 3-NPA non-sugar esters, and other 3-NPA derivatives.
3. 3-Nitropropionic Acid Sugar Esters
3-NPA sugar esters are a class of compounds synthesized through the dehydration condensation of sugar (or a derivative of sugar) with 3-NPA via esterification. This classification represents the largest group of 3-NPA derivatives with 44 compounds, accounting for approximately 80% of all reported compounds. These 3-Nitropropionic acid sugar esters are predominantly isolated from higher plants, with a minor fraction derived from fungi and insects.
In 1920, Gorter [4] first isolated a 3-Nitropropionic acid glycoside, Hiptagin (2) (Figure 2), from Hiptage benghalensis Kurz. Gnanasunderam et al. [21] also isolated compound 2 from Lotus pedunculatus (Fabaceae), a Fabaceae species native to Europe. Utilizing feeding bioassays, they demonstrated that compound 2, the bioactive compound derived from this plant, exhibits potent antifeedant activity against the third-instar larvae of Costelytra zealandica, a key subterranean pest in New Zealand. This discovery facilitated the development of botanical insecticides targeting C. zealandica and related soil-dwelling pests. Mechanistic studies of Hiptagin’s activity have provided critical insights for designing eco-friendly pest control strategies. A research group at the University of Sheffield [22] elucidated 75 compounds that exhibit therapeutic potential for neurodegenerative disorders. Notably, compound 2 was characterized as a potent activator of the Nrf2-ARE pathway, demonstrating applicability in managing oxidative stress-mediated pathologies, including motor neuron diseases.
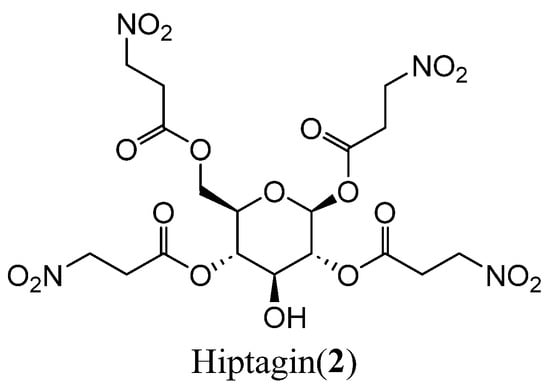
Figure 2.
Structure of compound 2.
Pfeffer et al. [23] characterized seven 3-Nitropropionic acid sugar esters, including 4,6-di-O-(3-nitropropanoyl)-β-D-glucopyranose (4), 4,6-di-O-(3-nitropropanoyl)-α-D-glucopyranose (5), 2,6-di-O-(3-nitropropanoyl)-β-D-glucopyranose (6), 1,6-di-O-(3-nitropropanoyl)-α-D-glucopyranose (7), 1,3,6-tri-O-(3-nitropropanoyl)-β-D-glucopyranose (8), and 1,4,6-tri-O-(3-nitropropanoyl)-α-D-glucopyranose (9) (Figure 3). Furthermore, Moyer et al. [24] isolated five additional 3-Nitropropionic acid sugar esters, including karakin (3), corollin (10), coronillin (11), corynocarpin (12), cibarian (13), and coronarian (14) (Figure 3), from Coronilla varia L. (Fabaceae). Through 13C-NMR spectroscopy and linear regression analysis of 13C substitution parameters at varying esterification sites on the α/β-glucopyranose scaffold, two distinct sets of chemical shift constants were derived. The theoretical chemical shifts calculated using these parameters exhibited high congruence with experimental data. Furthermore, the inductive and electron affinity effects of the substituents were correlated with their esterification positions and spatial orientations. These parameters were subsequently utilized to elucidate the structures of additional derivatives.
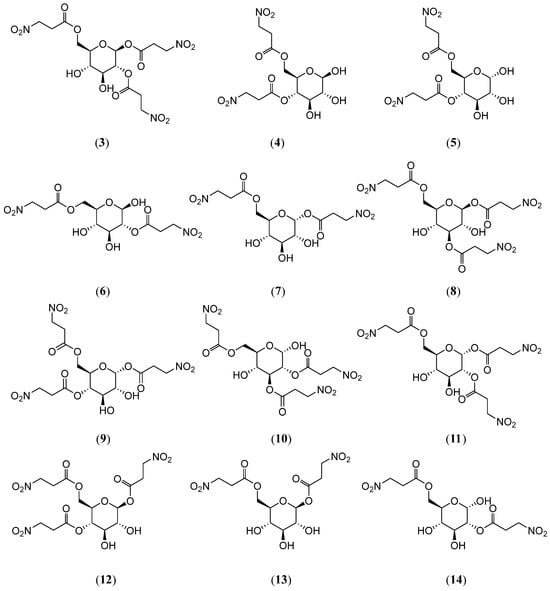
Figure 3.
Structures of compounds 3–14.
Byers et al. [18] also isolated 3-NPA, karakin (3) (Figure 3), compound 11, and compound 13 from Coronilla varia L. (Fabaceae). They employed Trichoplusia ni, a polyphagous species with an extensive host range, as the experimental model organism, observing that 3-NPA and its glycoside derivatives significantly increased mortality in first-instar larvae, with toxicity ranked in the following order: 3-NPA > trisubstituted derivatives > disubstituted derivatives. These compounds also reduced pupal weight and impaired reproductive capacity in Trichoplusia ni although egg hatchability remained unaffected. Byers et al. [25] further demonstrated that 3-NPA, compound 3, and compound 13 exhibited toxicity not only in non-ruminants (e.g., chickens and pigs) but also significantly increased mortality and reduced pupal weight in Spa-rganothis fruitworm larvae. Kigel [26,27] reported that the intravenous administration of compound 11 in cats at 0.03 mg/kg induced negative chronotropic effects (heart rate reduction) and positive inotropic effects (myocardial contractility enhancement), with a duration of action lasting 6 h, comparable to cardiac glycosides like Gophruzid and Periplocin. These findings confirm that compound 11 shares the hallmark cardiotonic properties of cardiac glycosides.
Benn et al. [28] isolated three 3-Nitropropionic acid sugar esters, karakin (3), 2,3,4,6-tetra-O-(3-nitropropanoyl)-α-D-glucopyranose (15), and 3,4,6-tri-O-(3-nitropropanoyl)-α-D-glucopyranose (16) (Figure 4), from Indigofera linnaei (Fabaceae). A research group at Cornell University [29] identified that the 2,3,4,6-tetra-O-(3-nitropropanoyl)-α-L-galactopyranose (17) (Figure 4) inhibits proNGF signaling by suppressing its cellular internalization. The experimental data revealed a 70.96% reduction in proNGF uptake. This compound’s ability to disrupt the cellular trafficking of neurotrophic precursor proteins positions it as a candidate for treating neurological diseases characterized by apoptotic pathways and aberrant neurotrophic factor signaling.
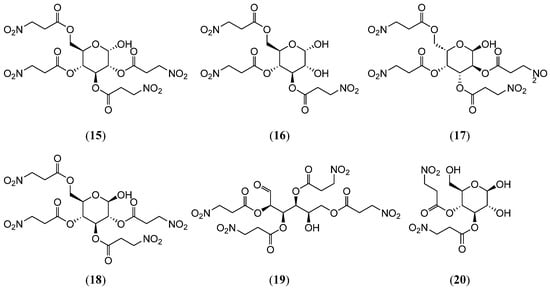
Figure 4.
Structures of compounds 15–20.
Roman et al. [30] isolated 2,3,4,6-tetra-O-(3-nitropropanoyl)-β-D-glucopyranose (18) (Figure 4), a 3-nitropropanoate acid derivative, from the root extracts of the endemic Brazilian species Heteropteris aphrodisiaca. Antibacterial susceptibility testing revealed the broad-spectrum activity of this compound against Staphylococcus aureus, Bacillus subtilis, C. albicans, C. tropicalis, C. parapsilosis, and C. krusei. Moreover, researchers at the Federal University of São Paulo [31] identified D-Glucose, 2,3,4,6-tetrakis (3-nitropropanoate) (19) (Figure 4), a structurally analogous 3-Nitropropionic acid glycoside, from the same botanical source. This compound displayed a minimum inhibitory concentration (MIC) of 250 μg/mL and a minimum bactericidal concentration (MBC) of 500 μg/mL against S. aureus, alongside an MIC/MBC profile of 125/250 μg/mL for B. subtilis. Furthermore, this compound exhibited marked antifungal efficacy against C. albicans, C. tropicalis, and C. parapsilosis and demonstrated dose-dependent antiviral activity against the herpes simplex virus (HSV) and poliovirus, achieving inhibition rates of 69–94% within a concentration range of 12.5–50 μg/mL.
Indigofera kirilowii, a plant native to the Shaanxi, Henan, Hubei, and Shanxi provinces, is primarily used as a source of the traditional Chinese medicine Shandougen (Euchresta japonica Benth. ex Oliv.) and is known for its heat-clearing and detoxifying properties. However, the substitution of Shandougen with I. kirilowii roots has led to neurotoxicity in some patients, with severe cases resulting in death. Thus, investigating the chemical composition of I. kirilowii root is critical for identifying its toxic components. Yang, et al. [32] isolated four 3-Nitropropionic acid sugar esters from the roots of I. kirilowii (Fabaceae): 3,4-di-O-(3-nitropropanoyl)-β-D-glucopyranose (20) (Figure 4), 3,4-di-O-(3-nitropropanoyl)-α-D-glucopyranose (21), 6-O-(3-nitropropanoyl)-β-D-glucopyranose (22), and 6-O-(3-nitropropanoyl)-α-D-glucopyranose (23) (Figure 5). Subsequent studies [33,34] isolated 10 additional 3-Nitropropionic acid sugar esters (kirilowin A-H (24–31)) (Figure 5) from the same plant. Notably, β-D-Glucopyranose (22), originally isolated from Securigera varia (Fabaceae) by Sientzoff et al. [35], demonstrated DPPH radical scavenging activity with an EC50 value of 133.5 ± 2.8 μg/mL, indicating significant antioxidant potential. Finnegan et al. [36] synthesized the tetra-acylated derivative Hiptagin methyl ether (32) (Figure 5) via the acylation of 3-O-methyl-α-D-glucopyranose with 3-nitropropanoyl chloride in N-methylpyrrolidone solution.
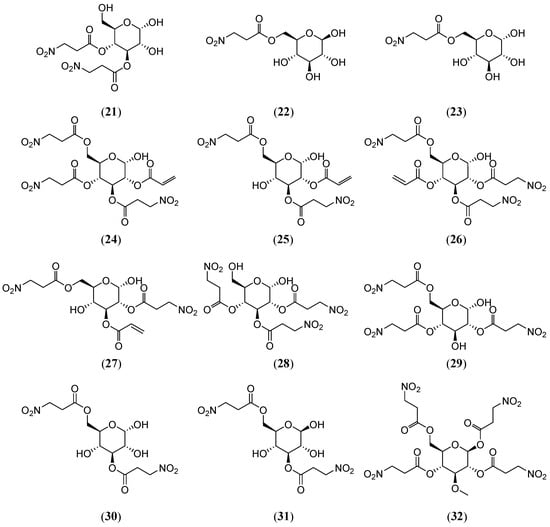
Figure 5.
Structures of compounds 21–32.
Majak et al. [37] isolated seven compounds from the New Zealand plant Corynocarpus laevigatus: compounds 2, 8, 16, and 22–23, 1,3,4,6-Tetrakis-O-(3-nitropropanoyl)-β-D-glucopyranose (33), and 1,2,3,6-Tetrakis-O-(3-nitropropanoyl)-β-D-glucopyranose (34) (Figure 6). Sugeno et al. [38] identified five 3-Nitropropionic acid derivatives in the secretions of four Japanese Chrysomelidae beetles: compounds 3 and 13, 2-[3′,6′-di-(3″-nitropropanoyl)-β-D-glucopyranosyl]-3-isoxazolin-5-one (35), 2-[2′,6′-di-(3″-nitropropanoyl)-β-D-glucopyranosyl]-3-isoxazolin-5-one (36), and 2-[6′-(3″-nitropropanoyl)-β-D-glucopyranosyl]-3-isoxazolin-5-one (37) (Figure 6). Among these, compounds 3, 13, 35, and 36 exhibited potent repellent effects against Tetramorium caespitum (p < 0.01). Compounds 3 and 36 showed significant repellency at the tested concentrations (p < 0.05), highlighting their roles in the defense mechanisms of Chrysomelidae beetles and their potential as natural repellents. Pasteels et al. [39,40] isolated compounds 37 and 2-[2,3,4-tri-O-acetyl-6-O-(3-nitro-1-oxopropyl)-β-D-glucopyranosyl]-5(2H)-isoxazolone (38) (Figure 6) from the eggs, neonate larvae, and defensive secretions of beetles within the Chrysomelina and Phyllo-dectina subfamilies. Bioassays demonstrated that compound 37 had a significant repellent effect on red ants (Myrmica rubra) at the concentration found in the eggs (about 10−2 M); this repellent effect persisted at lower concentrations (10−3 M) and higher concentrations. The above results suggest that, in Chrysomelina subfamilies, isoxazolone (37) protects eggs from predators through its strong repellent effects. Concurrently, compound 38, identified in adult secretions, functions as a defensive compound, mediating predator avoidance and thereby facilitating ecological fitness in chrysomelid beetles.
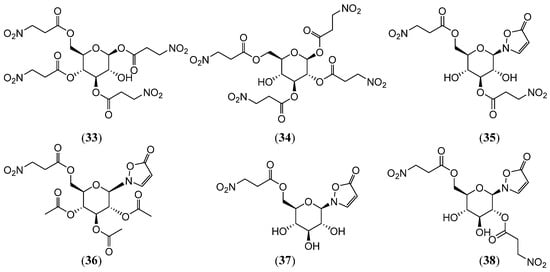
Figure 6.
Structures of compounds 33–38.
Carter et al. [41] developed a protocol for synthesizing and purifying compound 3 and its derivative, compound 12, involving crystallization, acetylation, hydrolysis, and chromatographic separation. Notably, the structural elucidation of compound 12 was further confirmed by synthesizing its acetylated derivative, diacetylkarakin (39) (Figure 7).
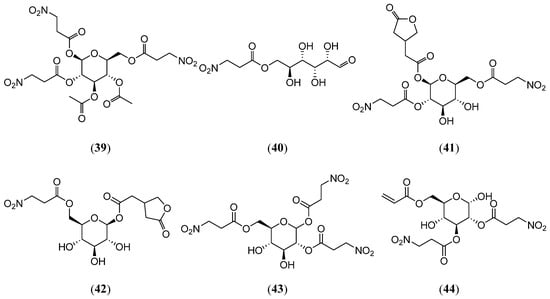
Figure 7.
Structures of compounds 39–44.
Astragalus canadensis, the most widespread Astragalus species in North America, exhibits toxicity to sheep and cattle [42]. Benn et al. [43] identified seven 3-Nitropropionic acid sugar esters in this plant: compounds 3, 8, 12, and 13 and D-Glucose, 6-(3-nitropropanoate) (40), 1-O-[5-oxotetrahydrofuran-3-yl]acetyl-2,6-di-O-(3-nitropropanoyl)-β-D-glucopyranose (41), and 1-O-[5-oxotetrahydrofuran-3-yl]acetyl-6-O-(3-nitropropanoyl)-β-D-glucopyranose (42) (Figure 7). The hydrolysis of these glycosides releases 3-NPA, amplifying the plant’s toxicity.
Three 3-Nitropropionic acid sugar esters, compounds 13–14, and 1,2,6-tri-(3-nitropropanoyl)-α-D-glucopyranose (43) were isolated from Coronilla varia (Fabaceae) by Gustine et al. (Figure 7) [44]. In vitro and in vivo experiments confirmed that rumen microbiota metabolize these glycosides and that compound 43 was rapidly hydrolyzed to 3-NPA and glucose within 24 h, with 3-NPA further degrading to unknown metabolites. When sheep ate C. varia, the 3-NPA in their rumen completely disappeared within 6 h without any signs of toxicity. Voles did not show symptoms of toxicity after consuming the rumen-fluid-treated C. varia, indicating that the 3-Nitropropionic acid sugar esters were successfully detoxified via rumen microorganisms. Zhang et al. [45] isolated and characterized two novel aliphatic nitro compounds, compound 25 and 6-O-acryl-2,3-di-(3 nitropropanoyl)-α-D-glucopyranose (44) (Figure 7), from Indigofera carlesii (Fabaceae), providing new chemical insights for exploring its medicinal potential.
4. 3-Nitropropionic Acid Non-Sugar Esters
3-Nitropropionic acid non-sugar esters are organic compounds formed through the esterification of 3-NPA carboxylic acid, linked via single, double, or triple bonds to form linear or branched structures. Linear derivatives are primarily used in chemical synthesis. Despite their simple structures, they exhibit diverse functionalities and are applied in materials science, pharmaceuticals, and agriculture. Investigating their structural and physicochemical properties can facilitate the development of compounds with enhanced practical applications.
Zhang et al. [46] synthesized tert-butyl 3-nitropropionate (45) (Scheme 2) via a three-step process, starting with the nitration of acrolein to yield 3-nitropropanal, followed by the oxidation of 3-nitropropanal to 3-NPA and finally the esterification of 3-NPA with isobutylene catalyzed via concentrated sulphuric acid. The overall isolated yield was 30%.
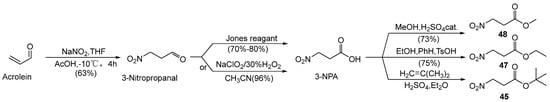
Scheme 2.
Preparation of compounds 45, 47, and 48.
A research group from the China Pharmaceutical University [47] identified tert-butyl 3-nitropropionate (45) (Scheme 3) as a key starting material for synthesizing the emricasan intermediate 1,1-dimethylethyl 3-amino-5-bromo-4-oxopentanoate, an inhibitor of the Caspase family of cysteine proteases. The nitro and tert-butoxy ester groups in its structure provide critical functional groups for subsequent reactions. This synthetic route offers advantages such as readily available raw materials, mild reaction conditions, and high yields, making it suitable for industrial-scale production. The overall yield was 52.1%.
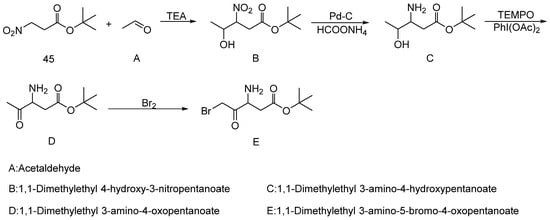
Scheme 3.
Preparation of 1,1-dimethylethyl 3-amino-5-bromo-4-oxopentanoate.
Compound 45 serves as a key synthetic intermediate for introducing nitro and carboxyl groups, constructing molecular scaffolds, and serving as a protecting group in the synthesis of bioactive compounds. Through sequential reactions, it is converted into ICE/CED-3 family inhibitors that suppress apoptosis. These inhibitors have significant applications in expanding hematopoietic cell populations, prolonging organ transplant survival and enhancing bioproduction efficiency [48,49,50,51,52,53]. Roesch et al. [54] demonstrated that compound 45 acts as a key intermediate in synthesizing bifunctional chelating agents (BFCs) to construct 1,4-diazepane (DAZA) scaffolds. These BFCs are used to chelate radiometals, primarily in non-invasive molecular imaging applications (Scheme 4).
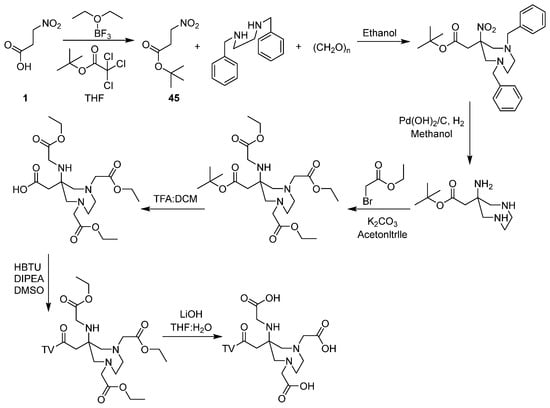
Scheme 4.
Preparation of BFCs from compound 45.
Xu [55] presented an enzymatic method for synthesizing the key intermediate of aliskiren (2,2-dimethyl-3-aminopropionamide). The esterification reaction employs isopropyl 3-nitropropanoate (46), ethyl 3-nitropropionate (47), or methyl 3-nitropropionate (48) (Scheme 5) as the critical intermediates, synthesized via the esterification of 3-nitropropanoic acid with aliphatic alcohols (e.g., isopropanol, ethanol, or methanol, with methanol being the preferred substrate). These intermediates serve as essential precursors for subsequent methylation reactions. This enzymatic approach reduces reliance on the high-temperature and high-pressure conditions in traditional synthesis, minimizes equipment and operational demands, and ensures the high purity and yield of the antihypertensive drug intermediate. The yield of the final enzymatic synthesis step is 92%.
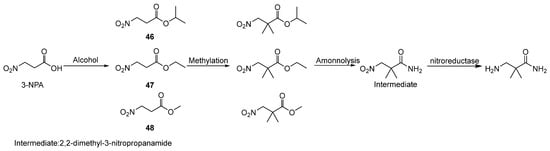
Scheme 5.
Preparation of key intermediate 2,2-dimethyl-3-aminopropionamide.
Seebach et al. [56] introduced a titanate-catalyzed transesterification method for synthesizing diverse functionalized esters. For example, compound 48 can be converted to compound 46. This method features mild reaction conditions, high-functional group tolerance, strong selectivity, and high product purity, highlighting its potential utility in organic synthesis (Scheme 6). The yield was 50%.
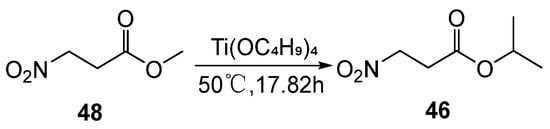
Scheme 6.
Titanate-catalyzed transesterification reaction.
Silva et al. [57] developed a simple, rapid, and high-yield synthetic route to produce 3-NPA and compounds 47 and 48 from commercially available acrolein in two steps, addressing the demand for large-scale preparation (Scheme 2).
Su et al. [58] isolated compound 47 and five 3-Nitropropionic acid sugar esters from the roots of Indigofera kirilowii (Fabaceae). Finnegan et al. [59] isolated toxic compound 3-NPA and compounds 2 and 47 from Indigofera endecaphylla (Fabaceae). Herdwiani et al. [60] isolated compound 47 from Cinnamomum burmanni (Nees and T. Nees) Blume (Lauraceae). Sarkar et al. screened 10 fungal metabolites including compound 47 from Aspergillus flavus and Aspergillus oryzae as inhibitors of the SARS-CoV-2 main protease. Molecular docking simulations revealed that compound 47 exhibited a docking score of −5.1 kcal/mol with main protease 6LU7, which is comparable to chloroquine (−6.29 kcal/mol), suggesting its potential as an effective antiviral agent. Ma et al. [61] demonstrated that the addition of an isocitrate lyase-specific inhibitor effectively reduced succinic acid accumulation while improving lactic acid purity and productivity. Dose–response experiments revealed that ethyl 3-nitropropanoate (0–0.1 mM) at 0.1 mM decreased succinic acid levels from 2000 ppm to 215 ppm, corresponding to an 89.25% inhibition efficiency. These findings highlight ethyl 3-nitropropanoate’s capacity to selectively suppress succinic acid biosynthesis at sub-millimolar concentrations without compromising lactic acid fermentation kinetics.
Somanthan et al. [62] isolated 3-NPA and compound 48 from Astragalus spp. (Fabaceae). Swargiary et al. [63] isolated compound 48 from Hydrocotyle sibthorpioides Lam. (Araliaceae) using GC-MS. SwissADME and ADMETlab predictions indicated compound 48’s favorable pharmacokinetics, low toxicity, and drug-likeness. Its binding affinity to α-amylase and α-glucosidase suggests potential hypoglycemic activity through enzyme inhibition. Safdar, Sahar et al. identified compound 48 in the ethanol extract of Selenicereus undatus (Cactaceae). Antibacterial assays confirmed its antibacterial activity, suggesting its role in the plant’s observed antibacterial and anticancer properties.
Marom et al. [64] employed 3-Nitropropionic anhydride (49) (Scheme 7) as an acylating agent to synthesize fingolimod (FTY720) and its pharmaceutical salts. Compared with traditional acylation methods (e.g., using carboxylic acids and dehydrating agents), this approach achieved higher reaction efficiency and selectivity. Compound 49, serving as a pivotal starting reagent, facilitates a streamlined synthesis of FTY720 via its high-yielding and regioselective acylation protocols. This methodologically advanced approach enhances reaction kinetics, reduces procedural complexity, and elevates product purity, underscoring its substantial industrial utility in large-scale pharmaceutical manufacturing.
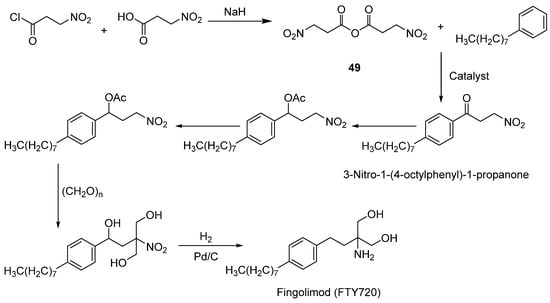
Scheme 7.
Preparation of fingolimod.
Jin et al. [65] synthesized a novel aliphatic energetic plasticizer, NDAE (50) (Scheme 8), by introducing nitro, azido, and ester groups into an aliphatic framework. This compound exhibits low viscosity, low glass-transition temperature, reduced sensitivity, and excellent safety properties, making it suitable for applications in explosives and propellant formulations.
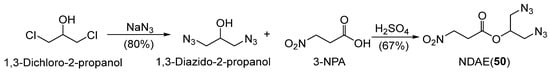
Scheme 8.
Preparation of compound 50.
Salem et al. [66] surveyed 148 angiosperm species (primarily European) and found a limited distribution of 3-Nitropropionic acid derivatives in European Fabaceae, mainly within Trib. Loteae DC. and Trib. Coronilleae. In extracts of Hippocrepis comosa, 4-(3-nitropropanoyloxy) butanoic acid (51) (Figure 8) was identified, which may pose toxicity to livestock.
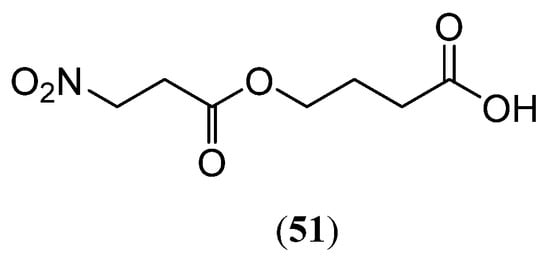
Figure 8.
Structure of compound 51.
Becker et al. [67] elucidated the biosynthetic pathway from β-alanine to compound 37 and 3-Nitropropionic acid derivatives using stable isotope-labeled precursors. They identified 2,2,2-trichloroethyl-3-nitropropanoate (52) (Scheme 9) as a key intermediate in synthesizing compound 37 and confirmed the universality of this pathway across multiple Chrysomelidae beetle larvae species.
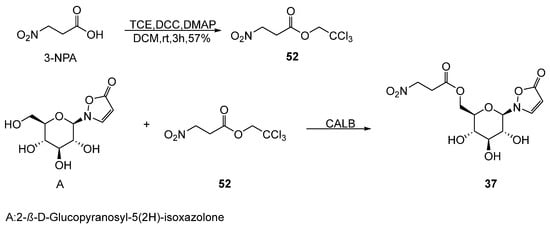
Scheme 9.
Biosynthetic pathway of compound 37.
Compound 52 can also be chemically converted to compound 37. Tobias Becker et al. [68] developed a novel synthetic route utilizing cascade reactions to construct compound 37. A critical step involved the enzymatic esterification of 3-Nitropropionic acid with 2-β-D-Glucopyranosyl-5(2H)-isoxazolone and 2-α-D-Glucopyranosyl-5(2H)-isoxazolone, yielding bioactive compound 37 (Scheme 10).
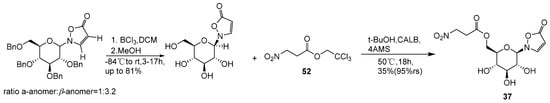
Scheme 10.
Chemical synthesis pathway of compound 37.
5. Other 3-Nitropropionic Acid Derivatives
Phenyl 3-nitropropanoate (53) (Figure 9) is a 3-NPA derivative synthesized via the esterification of 3-NPA with phenol, resulting in a phenyl-substituted 3-nitropropanoate ester. The Bridgestone Corporation research group [69] employed compound 53 as a specialized coupling agent to synthesize poly(diene) and poly(diene) copolymers with reduced cold-flow properties.
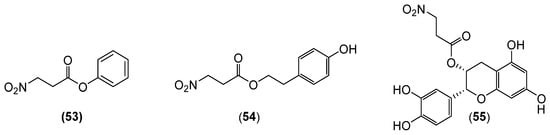
Figure 9.
Structures of compounds 53–55.
Pelletier et al. [70] (Scheme 11) employed cyclic imine as a model substrate to investigate the rate-determining step in the nitro-Mannich/lactamization reaction cascade by comparing the reaction kinetics of nitroesters with different ester groups (compounds 47, 48, and 53) with 3,4-dihydroisoquinoline. Real-time monitoring via 1H NMR spectroscopy revealed that the reaction rate increased significantly with decreasing steric bulk of the ester moiety (phenyl > methyl > ethyl), clearly indicating that the lactamization step is rate-determining. These results support the hypothesis that the observed diastereocontrol originates from the favored pseudoequatorial orientation of the substituents during the lactamization process rather than during the reversible nitro-Mannich reaction. This study successfully developed a highly diastereoselective cascade reaction between methyl 2-alkyl-3-nitropropanoates and in situ generated imines, providing a novel synthetic approach to 1,3,5-trisubstituted-4-nitropyrrolidin-2-one derivatives.
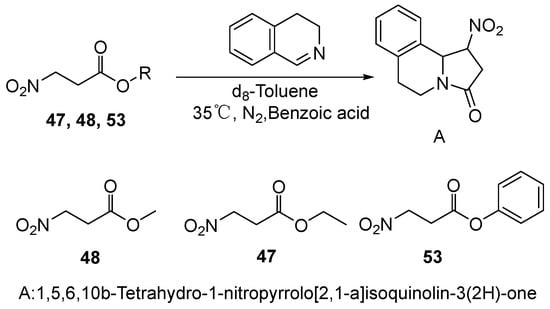
Scheme 11.
Rate-determining step involving compounds 47, 48, and 53.
Phomonitroester (54) (Figure 9) is derived from the endophytic fungi Phomopsis sp. PSU-D15 [2], marine fungi Pestalotiopsis diploclisia (BCC 35283) [71], and Arthrinium spp. [72]. Klaiklay et al. [73] isolated compound 54 from the mangrove-derived endophytic fungus Phomopsis sp. PSU-MA214. This compound exhibited selective cytotoxicity against human oral cancer cells (KB) with an IC50 value of 43 µg/mL. Ignjatović et al. [74] performed molecular docking simulations using Auto-dock v4.2, revealing compound 54’s strong binding affinities to multiple receptors, particularly 3G7B (Staphylococcus aureus gyrase B) and 1F0K (Escherichia coli transferase).
Qiu et al. [75] isolated compound HQM-3 (55) (Figure 9) from Indigofera stachyodes (Fabaceae). Treatment with compound 55 resulted in 122.0% cell viability, surpassing the protective effects of the positive controls (e.g., bifendate and bicyclol tablets) against CCl4-induced hepatocyte damage in vitro. Hao et al. [76] developed methods to formulate compound 55 into injectables, lyophilized powders, oral liquids, tablets, and capsules, providing a scientific and technical foundation for novel hepatoprotective drugs. Compound 55, a unique 3-nitropropanoate-linked flavonol derivative, derives its hepatoprotective activity from the 3-nitropropanoate substituent.
6. Conclusions
Since the first isolation of the 3-Nitropropionic acid derivative Hiptagin (2) in 1920 a total of 55 such compounds have been identified. Based on their structural characteristics, we have systematically classified these compounds into distinct categories. Among them, 3-Nitropropionic acid sugar esters, predominantly derived from leguminous plants, exhibit diverse bioactivities including antiviral, insecticidal, antibacterial, and antioxidant properties. However, among the 44 identified 3-Nitropropionic acid sugar esters, only approximately 25% have been pharmacologically characterized, with limited studies on their chemical syntheses, structural modifications, and mechanisms of action. 3-Nitropropionic acid non-sugar esters and other structural types show broad application potential in medicine, materials science, and agriculture. Linear derivatives, mostly small molecules, are widely used in chemical syntheses due to their reactivity and synthetic accessibility. However, studies on their pharmacological activities are sparse, and naturally occurring linear derivatives remain rare. Furthermore, significant gaps persist in the structural elucidation of linear and other 3-Nitropropionic acid derivatives, requiring further systematic exploration.
As a distinct class of nitro compounds, 3-Nitropropionic acid derivatives hold a unique position in medicinal chemistry. The nitro group, with its strong electron-withdrawing nature, significantly alters molecular electronic distribution, modulating reactivity and bioactivity. This electron-withdrawing effect can create localized electrophilic regions that facilitate interactions with biomacromolecules such as proteins and nucleic acids. Additionally, the selective toxicity of nitro compounds enables targeted action against specific pathogens or cancer cells [77]. Despite their remarkable pharmacological potential, nitro compounds are often associated with adverse effects and toxicity, such as carcinogenicity, hepatotoxicity, mutagenicity, and myelosuppression, which require careful consideration. During metabolic reduction in vivo, nitro groups may generate free radicals or reactive intermediates capable of reacting with DNA, proteins, and other biomolecules, leading to cellular damage or mutagenesis [78,79].
Similarly, while 3-NPA, as a privileged pharmacophore, demonstrates broad therapeutic potential in medicinal chemistry, its inherent toxicity requires cautious management. Through rational structural design and optimization, 3-Nitropropionic acid derivatives may be developed into efficacious therapeutic agents, particularly for oncology and antimicrobial/antiparasitic applications. Future research should prioritize balancing efficacy and toxicity to develop safer and more potent drugs, ultimately unlocking their full therapeutic potentials.
Author Contributions
Conceptualization, B.-B.S.; writing—original draft preparation, M.-L.F. and Z.-H.L.; writing—review and editing, B.-B.S.; supervision, B.-B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Funds for the South-Central MinZu University (YZY24019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3-NPA | 3-Nitropropionic acid |
| HD | Huntington’s disease |
| ProNGF | Pro-nerve growth factor |
| Nrf2-ARE | Nuclear factor erythroid 2-related factor 2-Antioxidant Response Element |
| 13C-NMR | Carbon-13 Nuclear Magnetic Resonance |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| HSV | Herpes simplex virus |
| BFCs | Bifunctional chelating agents |
| DAZA | 1,4-Diazepane |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| ICE | Interleukin-1β-Converting Enzyme |
| CED-3 | Cell Death Abnormal-3 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| FTY720 | Fingolimod |
| PSU | Prince of Songkla University |
| BCC 35283 | BIOTEC Culture Collection No. 35283 |
| IC50 | Half Maximal Inhibitory Concentration |
| DNA | Deoxyribonucleic Acid |
| THF | Tetrahydrofuran |
| TEA | Triethylamine |
| TEMPO | 2,2,6,6-Tetramethylpiperidin-1-oxyl |
| TCE | Trichloroethylene |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| DCM | Dichloromethane |
| CALB | Candida antarctica Lipase B |
| BGC | Biosynthetic gene cluster |
References
- Alston, T.A.; Mela, L.; Bright, H.J. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc. Natl. Acad. Sci. USA 1977, 74, 3767–3771. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Sommart, U.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Metabolites from the endophytic fungus Phomopsis sp. PSU-D15. Phytochemistry 2008, 69, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.T.; Touster, O.; Early, J. The production of beta-nitropropionic acid by a strain of Aspergillus flavus. J. Biol. Chem. 1951, 188, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gorter, K. Hiptagin, a New Glucoside from Hiptage medablota, gaertu. Bull. Jard. Bot. Buitenzorg. 1920, 2, 187–202. [Google Scholar]
- Morris, M.P.; Pagán, C.; Warmke, H.E. Hiptagenic Acid, a Toxic Component of Indigofera endecaphylla. Science 1954, 119, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Britten, E.J.; Matsumoto, H.; Palafox, A.L. Comparative Toxic Effects of 3-Nitropropionic Acid, Sodium Nitrite and Indigofera endecaphylla on Chicks. Agron. J. 1959, 51, 462–464. [Google Scholar] [CrossRef]
- Hamilton, B.F.; Gould, D.H.; Gustine, D.L. History of 3-Nitropropionic Acid. In Mitochondrial Inhibitors and Neurodegenerative Disorders; Sanberg, P.R., Nishino, H., Borlongan, C.V., Eds.; Contemporary Neuroscience; Humana Press: Totowa, NJ, USA, 2000; pp. 21–33. [Google Scholar] [CrossRef]
- Li, M. Moldy Sugarcane Poisoning—A Case Report with a Brief Review. J. Toxicol. Clin. Toxicol. 2008, 33, 363–367. [Google Scholar] [CrossRef]
- Ludolph, A.C.; He, F.; Spencer, P.S.; Hammerstad, J.; Sabri, M. 3-Nitropropionic Acid—Exogenous Animal Neurotoxin and Possible Human Striatal Toxin. Can. J. Neurol. Sci. 1991, 18, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Neurochemistry and Toxin Models in Huntington’s Disease. Curr. Opin. Neurol. 1994, 7, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Saez-Atienzar, S.; Jordan, J.; Galindo, M.F. 3-Nitropropionic acid induces autophagy by forming mitochondrial permeability transition pores rather than activating the mitochondrial fission pathway. Br. J. Pharmacol. 2013, 168, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.A.; Akopian, G.; Ring, J.; Jakowec, M.W.; Petzinger, G.M.; Andersen, J.K.; Vittozzi-Wong, P.; Wang, K.; Farley, C.M.; Charntikov, S.; et al. Acute and long-term response of dopamine nigrostriatal synapses to a single, low-dose episode of 3-nitropropionic acid-mediated chemical hypoxia. Synapse 2011, 65, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.C.; Gao, X.Q.; Xing, Y.; Sun, S.G.; Li, H.G.; Wang, Y.F. Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats. J. Mol. Neurosci. 2004, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wiprich, M.T.; Altenhofen, S.; Gusso, D.; Vasques, R.R.; Zanandrea, R.; Kist, L.W.; Bogo, M.R.; Bonan, C.D. Modulation of Adenosine Signaling Reverses 3-nitropropionic Acid-Induced Bradykinesia and Memory Impairment in Adult Zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110602. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, S.; Yedke, N.G.; Rahi, V.; Singh, S.; Kumar, S.; Arora, A.; Chandolia, P.; Kaur, P.; Kumar, M.; Koshal, P.; et al. An Overview of the Pathophysiological Mechanisms of 3-Nitropropionic Acid (3-NPA) as a Neurotoxin in a Huntington’s Disease Model and Its Relevance to Drug Discovery and Development. Neurochem. Res. 2023, 48, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.L.; Wyatt, G.H. Karakin, the Glucoside of Corynocarpus levigata, and Hiptagenic Acid. J. Chem. Technol. Biotechnol. 2010, 62, 238–240. [Google Scholar] [CrossRef]
- Becker, T.; Pasteels, J.; Weigel, C.; Dahse, H.-M.; Voigt, K.; Boland, W. A tale of four kingdoms—Isoxazolin-5-one- and 3-nitropropanoic acid-derived natural products. Nat. Prod. Rep. 2017, 34, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.A.; Gustine, D.L.; Moyer, B.G. Toxicity of β-Nitropropionic Acid to Trichoplusia ni12. Environ. Entomol. 1977, 6, 229–232. [Google Scholar] [CrossRef]
- Acheson, A.L.; Naujoks, K.; Thoenen, H. Nerve Growth Factor-Mediated Enzyme Induction in Primary Cultures of Bovine Adrenal Chromaffin Cells: Specificity and Level of Regulation. J. Neurosci. 1984, 4, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.W.; Ohashi, M.; Tang, Y. How Fungi Biosynthesize 3-Nitropropanoic Acid: The Simplest Yet Lethal Mycotoxin. Org. Lett. 2024, 26, 3158–3163. [Google Scholar] [CrossRef] [PubMed]
- Gnanasunderam, C.; Sutherland, O.R.W. Hiptagin and Other Aliphatic Nitro Esters in Lotus pedunculatus. Phytochemistry 1986, 25, 409. [Google Scholar] [CrossRef]
- Shaw, P.; Mead, R.; Higginbottom, A.; Barber, S. Therapeutics for Neurological Disorders. U.S. Patent US20110251230A1, 13 October 2011. [Google Scholar]
- Pfeffer, P.E.; Valentine, K.M.; Moyer, B.G. Assessment of Carbon-13 Shift Parameters in Di- and Tri-(3-nitropropanoyl)-D-glucopyranoses. Carbohydr. Res. 1979, 73, 1–8. [Google Scholar] [CrossRef]
- Moyer, B.G.; Pfeffer, P.E.; Moniot, J.L.; Shamma, M.; Gustine, D.L. Corollin, Coronillin and Coronarian: Three New 3-Nitropropanoyl-D-glucopyranoses from Coronilla varia. Phytochemistry 1977, 16, 375–377. [Google Scholar] [CrossRef]
- Byers, R.A.; Gustine, D.L.; Moyer, B.G.; Bierlein, D.L. 3-Nitropropionate in Crownvetch: A Natural Deterrent to Insects? ACS Symp. Ser. 1986, 296, 95–105. [Google Scholar]
- Kigel, T.B. Pharmacological Evaluation of the Cardiac Glycoside Coronillin. Serd. Sosud. Patol. 1964, 5, 141–145. [Google Scholar]
- Kigel, T.B. Pharmacodynamics of the Cardiac Glycoside Coronillin. Farmakol. Toksikol. 1964, 27, 16–19. [Google Scholar]
- Benn, M.; McEwan, D.; Pass, M.A.; Majak, W. Three Nitropropanoyl Esters of Glucose from Indigofera linnaei. Phytochemistry 1992, 31, 2393–2395. [Google Scholar] [CrossRef]
- Hempstead, B.L. Small Molecule Modulators of proNGF Uptake. World Patent WO2010042728A1, 15 April 2010. [Google Scholar]
- Roman-Junior, W.A.; Vilegas, W.; Mello, J. 2,3,4,6-Tetra-O-(3-nitropropanoyl)-O-β-D-glucopyranoside, a New Antibacterial from the Roots of Heteropteris aphrodisiaca. Lat. Am. J. Pharm. 2005, 24, 543–545. [Google Scholar]
- De Mello, J.C.P.; Cardoso, M.L.C.; Nakamura, C.V. Process to Obtain and Isolate a New Compound with Anti-Viral, Fungicide and Antibacterial Properties. Brazil Patent BR PI0302921-6, 15 March 2005. [Google Scholar]
- Yang, F.-Y.; Lü, M.; Su, Y.-F.; Li, C.-Z. New 3-Nitropropionyl-D-glucopyranoses in Root of Indigofera kirilowii. Zhong Cao Yao 2007, 38, 1448–1450. [Google Scholar]
- Su, Y.; Lü, M.; Yang, F.; Li, C.; Di, L.; Wu, D.; Guo, Z.; Lü, J.; Guo, D. Six New Glucose Esters of 3-Nitropropanoic Acid from Indigofera kirilowii. Fitoterapia 2008, 79, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, C.; Gao, Y.; Di, L.; Zhang, X.; Guo, D. Acryloylated Glucose 3-Nitropropanoates from Indigofera kirilowii. J. Nat. Prod. 2005, 68, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Sientzoff, P.; Hubert, J.; Janin, C.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Harakat, D.; Alabdul Magid, A. Fast Identification of Radical Scavengers from Securigera varia by Combining 13C-NMR-Based Dereplication to Bioactivity-Guided Fractionation. Molecules 2015, 20, 14970–14984. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, R.A.; Stephani, R.A. Structure of Hiptagin as 1,2,4,6-Tetra-O-(3-nitropropanoyl)-β-D-glucopyranoside, Its Identity with Endecaphyllin X, and the Synthesis of Its Methyl Ether. J. Pharm. Sci. 1968, 57, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Majak, W.; Benn, M. Additional Esters of 3-Nitropropanoic Acid and Glucose from Fruit of the New Zealand Karaka Tree, Corynocarpus laevigatus. Phytochemistry 1994, 35, 901–903. [Google Scholar] [CrossRef]
- Sugeno, W.; Matsuda, K. Adult Secretions of Four Japanese Chrysomelinae (Coleoptera: Chrysomelidae). Appl. Entomol. Zool. 2002, 37, 191–197. [Google Scholar] [CrossRef]
- Pasteels, J.M.; Daloze, D.; Rowell-Rahier, M. Chemical defense in chrysomelid eggs and neonate larvae. Physiol. Entomol. 1986, 11, 29–37. [Google Scholar] [CrossRef]
- Pasteels, J.M.; Braekman, J.C.; Daloze, D.; Ottinger, R. Chemical defence in chrysomelid larvae and adults. Tetrahedron 1982, 38, 1891–1897. [Google Scholar] [CrossRef]
- Carter, C.L. The constitution of karakin. J. Sci. Food Agric. 1951, 2, 54–55. [Google Scholar] [CrossRef]
- James, L.F.; Hartley, W.J.; Van Kampen, K.R. Syndromes of Astragalus poisoning in livestock. J. Am. Vet. Med. Assoc. 1981, 178, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Bai, Y.; Majak, W. Aliphatic nitro-compounds in Astragalus canadensis. Phytochemistry 1995, 40, 1629–1631. [Google Scholar] [CrossRef]
- Gustine, D.L.; Moyer, B.G.; Wangsness, P.J.; Shenk, J.S. Ruminal metabolism of 3-nitropropanoyl-D-glucopyranoses from crownvetch. J. Anim. Sci. 1977, 44, 1107–1111. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Zhang, Z.-X.; Chen, L.; Su, Y.-F. New aliphatic nitro-compounds from Indigofera carlesii. Fitoterapia 2006, 77, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Li, L.; Yuan, S.J.; Li, Z.Q. Preparation of tert-butyl 3-nitropropionate. Chin. J. Pharm. 2005, 36, 330–331. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, L.; Chang, Y.; Shang, M.; Meng, H.; Chen, G. Process for preparation of tert-butyl 3-amino-5-bromo-4-oxo-pentanoate. Chinese Patent CN106831463A, 13 June 2017. [Google Scholar]
- Aja, T.; Ching, B.W.; Gladstone, P.L. Anti-Apoptotic Agents or Interleukin 1β Converting Enzyme (ICE/CED-3) Inhibitors for Preserving Antigenicity of Markers Associated with Diseases. World Patent WO2002070544A2, 12 September 2002. [Google Scholar]
- Fritz, L.C.; Tomaselli, K.J. Inhibition of Apoptosis Using Interleukin-1β-Converting Enzyme (ICE)/CED-3 Family Inhibitors. World Patent WO9810778A1, 19 March 1998. [Google Scholar]
- Lawrence, F.C.; Tomaselli, K.J.; Karanewsky, D.S.; Linton, S.D.; Bai, X. Treatment of Infectious Disease Using Interleukin-1β-Converting Enzyme (ICE)/CED-3 Family Inhibitors. U.S. Patent US20020128306A1, 12 September 2002. [Google Scholar]
- Fritz, L.C.; Tomaselli, K.J.; Karanewsky, D.S. Preparation of 2-Indolecarbonyl Amino Acid Amides for Inhibition of Inflammation Using Interleukin-1β-Converting Enzyme (ICE)/CED-3 Family Inhibitors. U.S. Patent 6,531,467, 11 March 2003. [Google Scholar]
- Karanewsky, D.S.; Bai, X. Preparation of N-Substituted-2-Indolyl Dipeptides as Inhibitors of the ICE/Ced-3 Family of Cysteine Proteases. World Patent WO1998011129A1, 19 March 1998. [Google Scholar]
- Karanewsky, D.S.; Bai, X. Preparation of C-Terminal Modified N-Substituted 2-Indolylcarbonyl Dipeptides as Inhibitors of the ICE/Ced-3 Family of Cysteine Proteases. World Patent WO2001000658A1, 4 January 2001. [Google Scholar]
- Roesch, F.; Waldron, B.P.; Parker, D. Bifunctional Chelating Agents Based on the 1,4-Diazepine Scaffold (DAZA) for Non-Invasive Molecular Imaging. World Patent WO2014198478A1, 18 December 2014. [Google Scholar]
- Xu, X.L. A Kind of Method that Enzyme Process Prepares Aliskiren Key Intermediate. Chinese Patent CN108192933A, 22 June 2018. [Google Scholar]
- Seebach, D.; Hungerbühler, E.; Naef, R.; Schnurrenberger, P.; Weidmann, B.; Züger, M.F. Titanate-mediated transesterifications with functionalized substrates. Synthesis 1982, 1982, 138–141. [Google Scholar] [CrossRef]
- Silva, P.C.; Costa, J.S.; Pereira, V.L.P. An Expeditious Synthesis of 3-Nitropropionic Acid and Its Ethyl and Methyl Esters. Synth. Commun. 2001, 31, 595–600. [Google Scholar] [CrossRef]
- Su, Y.F.; Di, L.Z.; Lv, M.; Li, C.Z.; Guo, L.P.; Wu, D. Chemical Constituents in Roots of Indigofera kirilowii. Zhongcaoyao 2008, 39, 1626–1628. [Google Scholar]
- Finnegan, R.A.; Mueller, W.H. Chemical examination of a toxic extract of Indigofera endecaphylla: The Endecaphyllins. J. Pharm. Sci. 1965, 54, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Herdwiani, W.; Aa, S.; Mi, T.A.N. Gas Chromatograph-Mass Spectrometer Analysis and Acute Oral Toxicity of Cinnamomum burmannii Ness ex Bl. Essential Oil. Asian J. Pharm. Clin. Res. 2016, 9, 240–245. [Google Scholar]
- Ma, G.; Wang, J.; Yang, F. Method for Reducing Byproduct Succinic Acid During Lactic Acid Fermentation by Adding Isocitrate Lyase Inhibitor. Chinese Patent CN116622785A, 24 August 2023. [Google Scholar]
- Somanthan, R.; Rivero, I.A.; Beltran, R.G. Nitropropanoic acid from five Astragalus species. Rev. Latinoam. Quim. 1990, 21, 101–103. [Google Scholar]
- Swargiary, A.; Daimari, M. GC–MS Analysis of Phytocompounds and Antihyperglycemic Property of Hydrocotyle sibthorpioides Lam. SN Appl. Sci. 2021, 3, 36. [Google Scholar] [CrossRef]
- Marom, E.; Mizhiritskii, M.; Rubnov, S. Intermediate Compounds and Process for the Preparation of Fingolimod. World Patent WO2012056458A2, 3 May 2012. [Google Scholar]
- Jin, Y.-H.; Zhang, Q.-H.; Liu, T.-L.; Zhang, W.-Q.; Huang, S.; Wang, K.-C. Preparation of 1,3-Diazidopropan-2-yl 3-Nitropropanoate Use as Energetic Plasticizer. Chinese Patent CN117567316A, 23 February 2024. [Google Scholar]
- Salem, M.A.; Williams, J.M.; Wainwright, S.J.; Hipkin, C.R. Nitroaliphatic compounds in Hippocrepis comosa and other legumes in the European flora. Phytochemistry 1995, 40, 89–91. [Google Scholar] [CrossRef]
- Becker, T.; Ploss, K.; Boland, W. Biosynthesis of isoxazolin-5-one and 3-nitropropanoic acid containing glucosides in juvenile Chrysomelina. Org. Biomol. Chem. 2016, 14, 6274–6280. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Goerls, H.; Pauls, G.; Wedekind, R.; Kai, M.; von Reuss, S.H.; Boland, W. Synthesis of Isoxazolin-5-one Glucosides by a Cascade Reaction. J. Org. Chem. 2013, 78, 12779–12783. [Google Scholar] [CrossRef] [PubMed]
- Luo, S. Method for Producing Polydienes and Polydiene Copolymers with Reduced Cold Flow. World Patent WO2016057388A1, 14 April 2016. [Google Scholar]
- Pelletier, S.M.C.; Ray, P.C.; Dixon, D.J. Diastereoselective Synthesis of 1,3,5-Trisubstituted 4-Nitropyrrolidin-2-ones via a Nitro-Mannich/Lactamization Cascade. Org. Lett. 2011, 13, 6406–6409. [Google Scholar] [CrossRef] [PubMed]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Nithithanasilp, S.; Choowong, W.; Preedanon, S.; Suetrong, S. Two New Farnesyl Hydroquinones from Pestalotiopsis diploclisia (BCC 35283), the Fungus Associated with Algae. Nat. Prod. Res. 2023, 37, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; Ebada, S.S.; Ashour, M.L.; Özkaya, F.C.; Ebrahim, W.; Singab, A.B.; Proksch, P. Spiroarthrinols A and B, Two Novel Meroterpenoids Isolated from the Sponge-Derived Fungus Arthrinium sp. Phytochem. Lett. 2017, 20, 246–251. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Phongpaichit, S.; Pakawatchai, C.; Saithong, S.; Buatong, J.; Preedanon, S.; Sakayaroj, J. Anthraquinone Derivatives from the Mangrove-Derived Fungus Phomopsis sp. PSU-MA214. Phytochem. Lett. 2012, 5, 738–742. [Google Scholar] [CrossRef]
- Ignjatović, J.; Dajić, N.; Krmar, J.; Protić, A.; Strukelj, B.; Otasević, B. Molecular Docking Study on Biomolecules Isolated from Endophytic Fungi. J. Serb. Chem. Soc. 2021, 86, 125–137. [Google Scholar] [CrossRef]
- Qiu, L.; Liang, Y.; Tang, G.-H.; Yuan, C.-M.; Zhang, Y.; Hao, X.-Y.; Hao, X.-J.; He, H.-P. Two New Flavonols, Including One Flavan Dimer, from the Roots of Indigofera stachyodes. Phytochem. Lett. 2013, 6, 368–371. [Google Scholar] [CrossRef]
- Hao, X.-Y.; He, H.-P.; Qiu, L.; Hao, X.-J.; Liang, Y.; Liu, L.; Yao, C.-F. 3-Nitropropionyl Containing Flavanol, Its Medicinal Composition and Application in Pharmaceuticals Industry. Chinese Patent CN102942551, 27 February 2013. [Google Scholar]
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Sun, G.; Zhao, L.; Cui, X.; Zhong, R. QSAR and Classification Study on Prediction of Acute Oral Toxicity of N-Nitroso Compounds. Int. J. Mol. Sci. 2018, 19, 3015. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Liao, L.; Zhou, Y.; Long, X.; Li, J. Toward the Toxicology of Some Nitro-Compounds. Mod. Org. Chem. Res. 2018, 3, 11–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).