Elucidating the Role of Toxoplasma gondii’s Mitochondrial Superoxide Dismutase
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Cell and Parasite Cultures
2.2. Transcription Quantification of ROS Scavenger Genes in T. gondii Parasites
2.3. Generation of Mutant Parasites
2.4. Parasite Transfection
2.5. Western Blotting
2.6. Parasite Plaque Assay
2.7. Parasite Replication Assay
2.8. Mitochondrial Membrane Potential
2.9. Mitochondrial Morphology Microscopy
3. Results
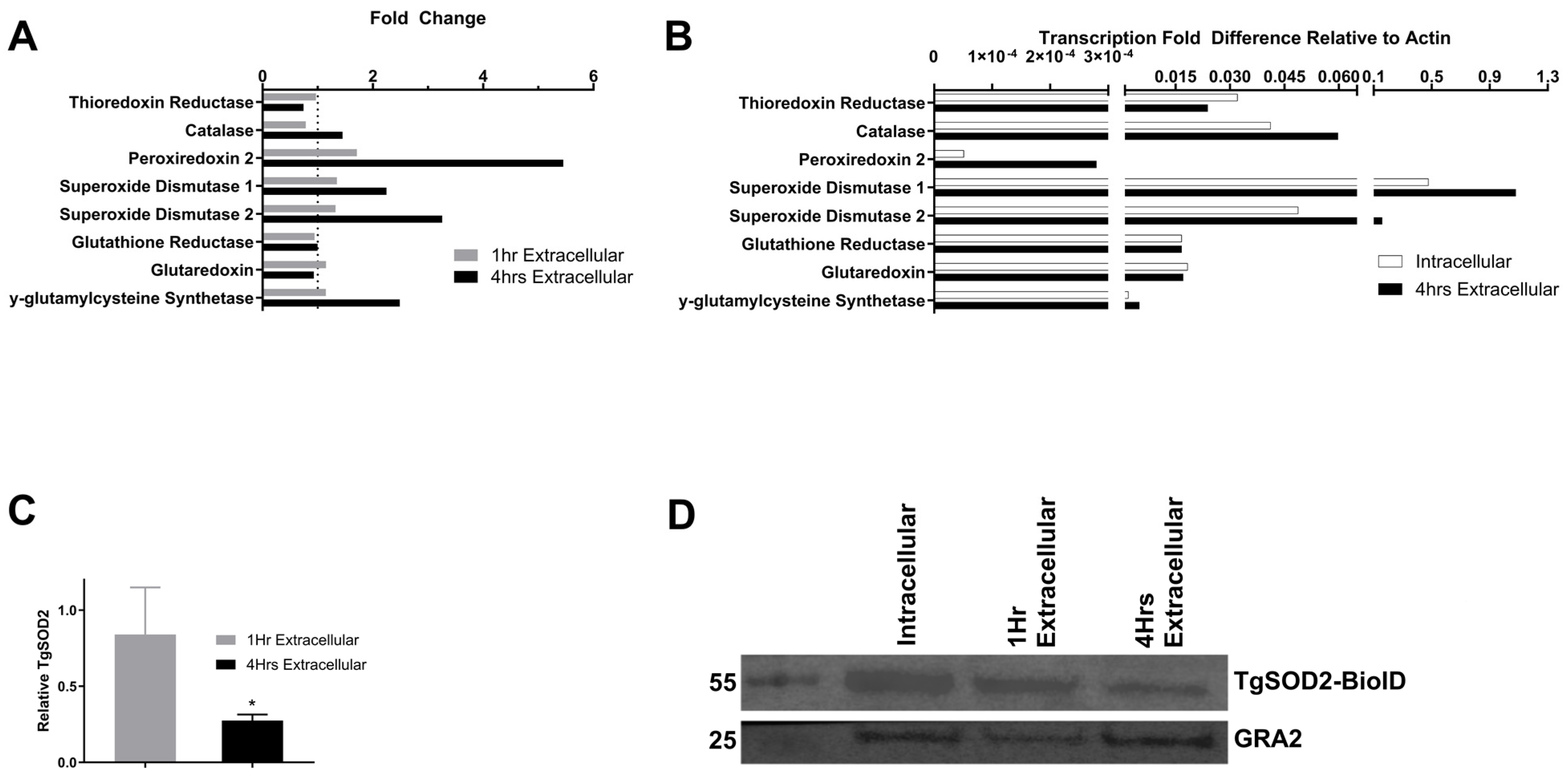
3.1. Expression of T. gondii Superoxide Dismutase 1 and 2 Increases in Extracellular Parasites
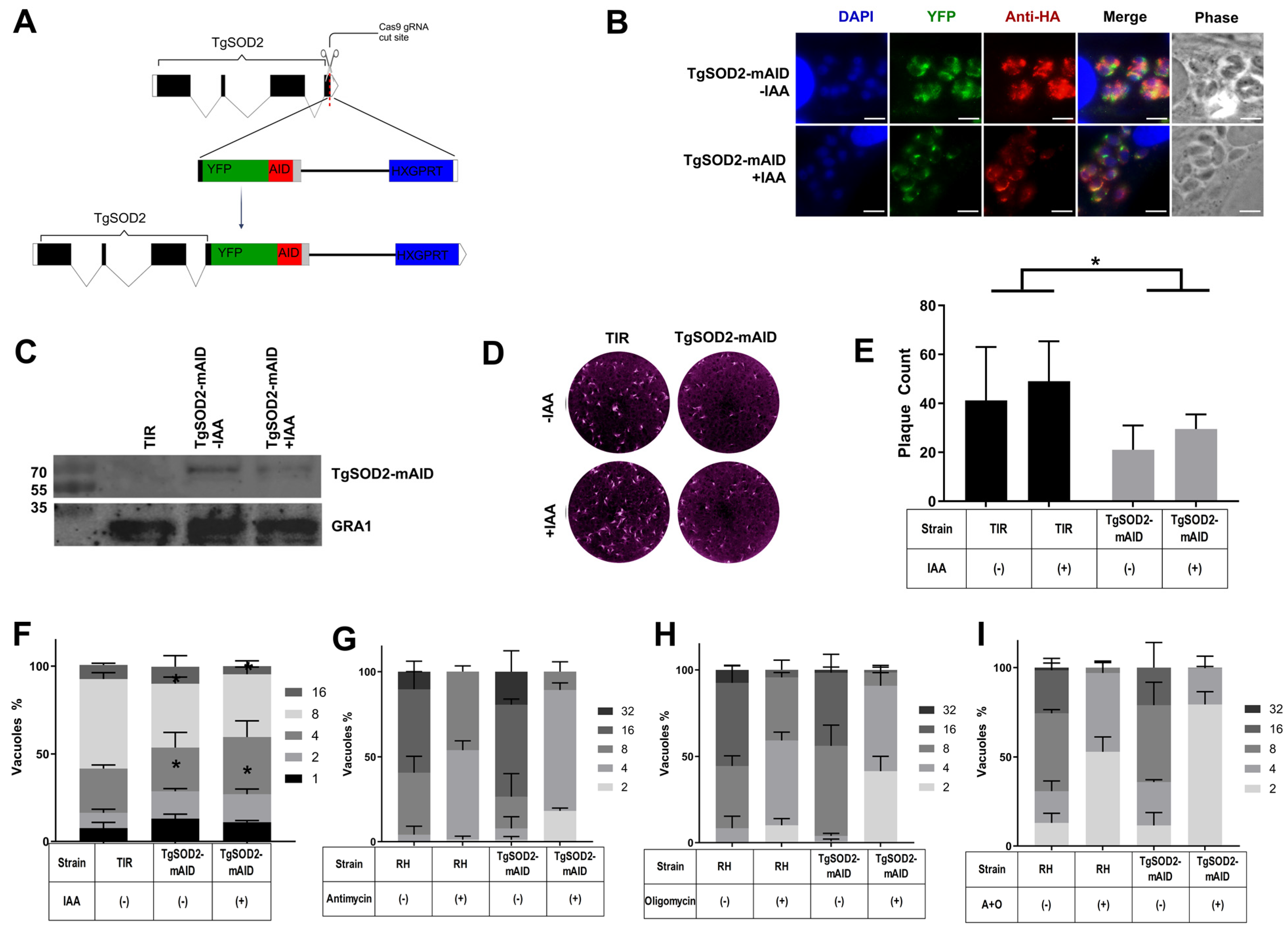
3.2. Generation of an Inducible TgSOD2 Knockdown Clone
3.3. TgSOD2 Depletion Impairs Parasite Growth
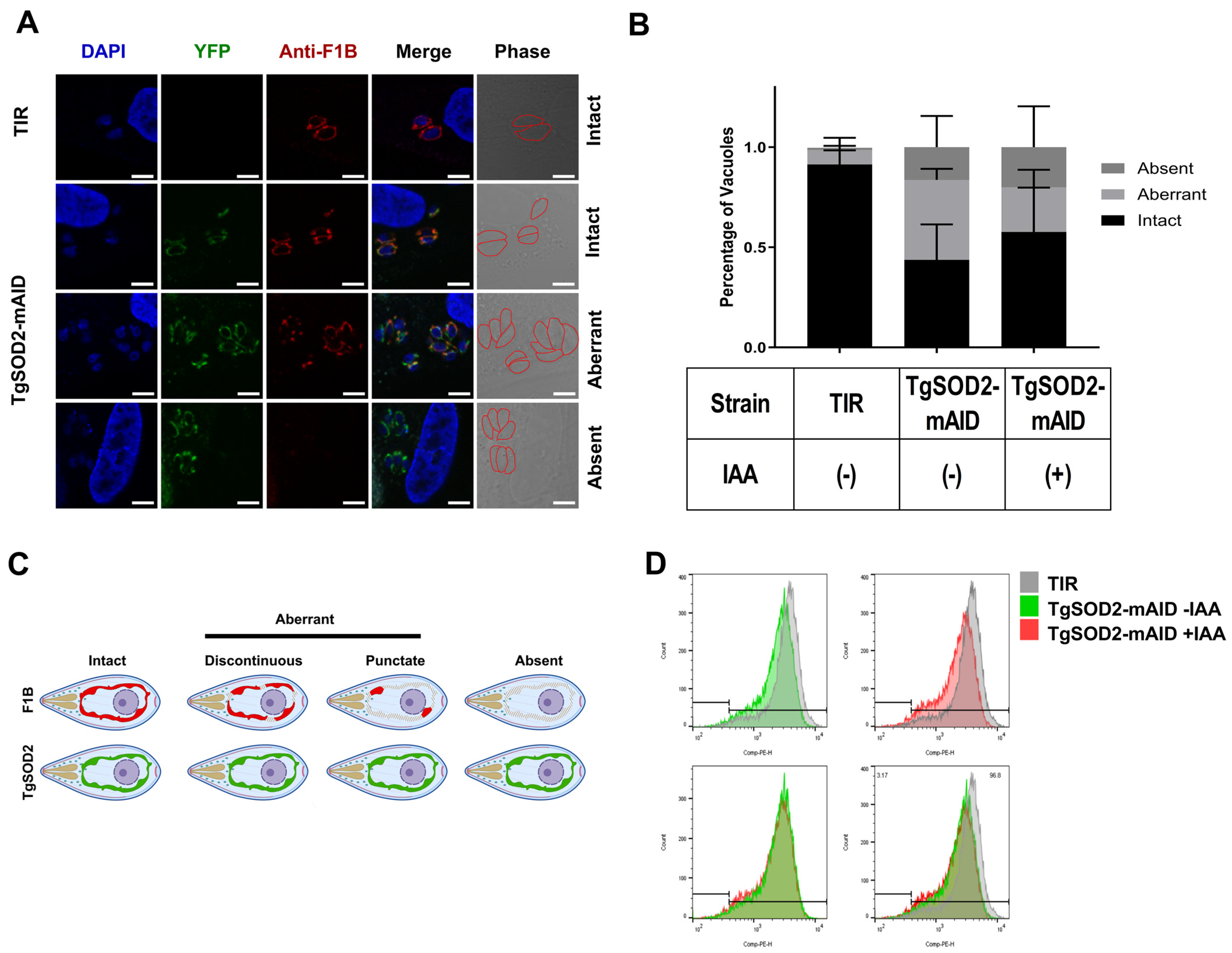
3.4. TgSOD2 Depletion Alters the Parasite Mitochondrial Homeostasis
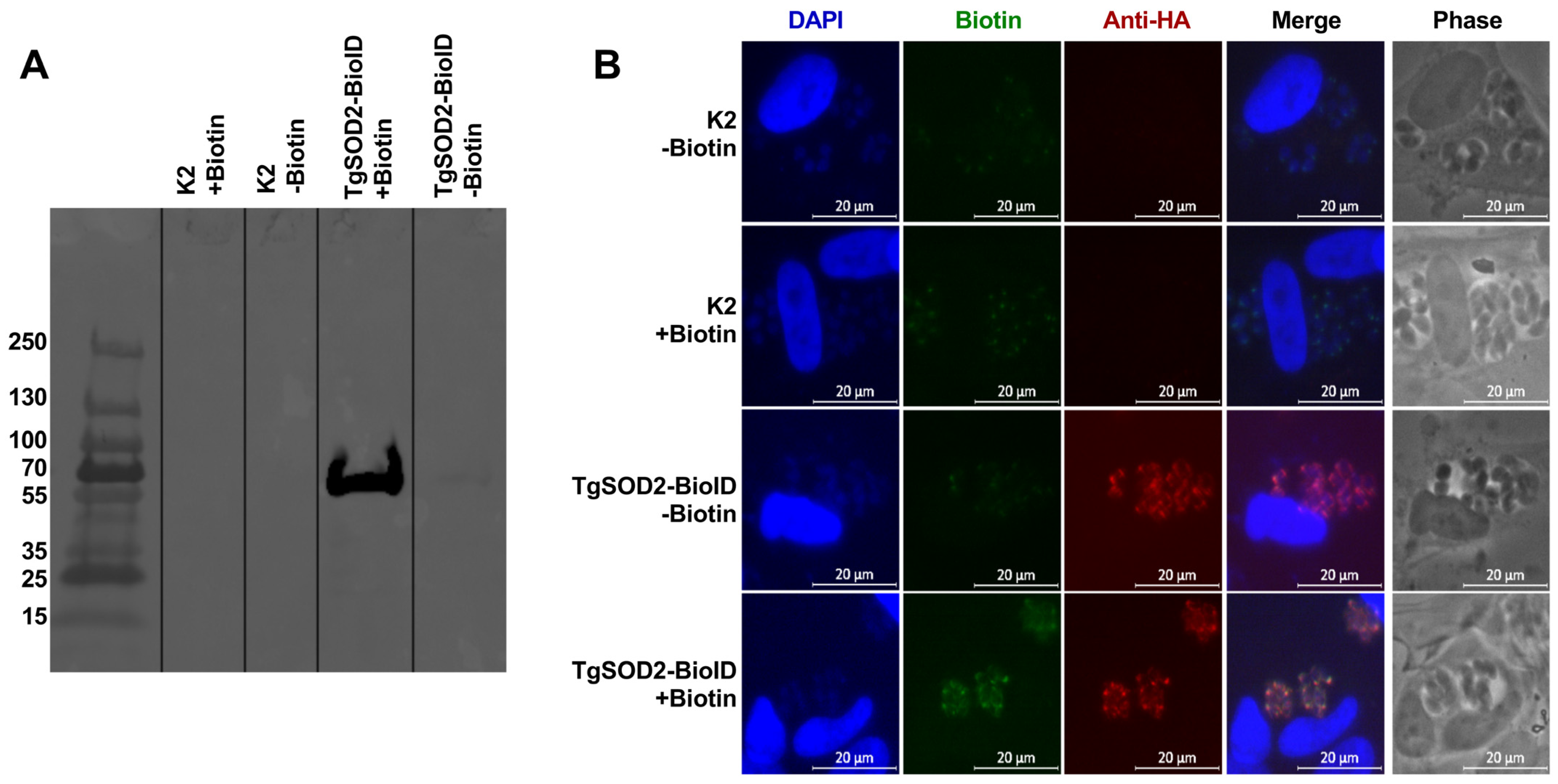
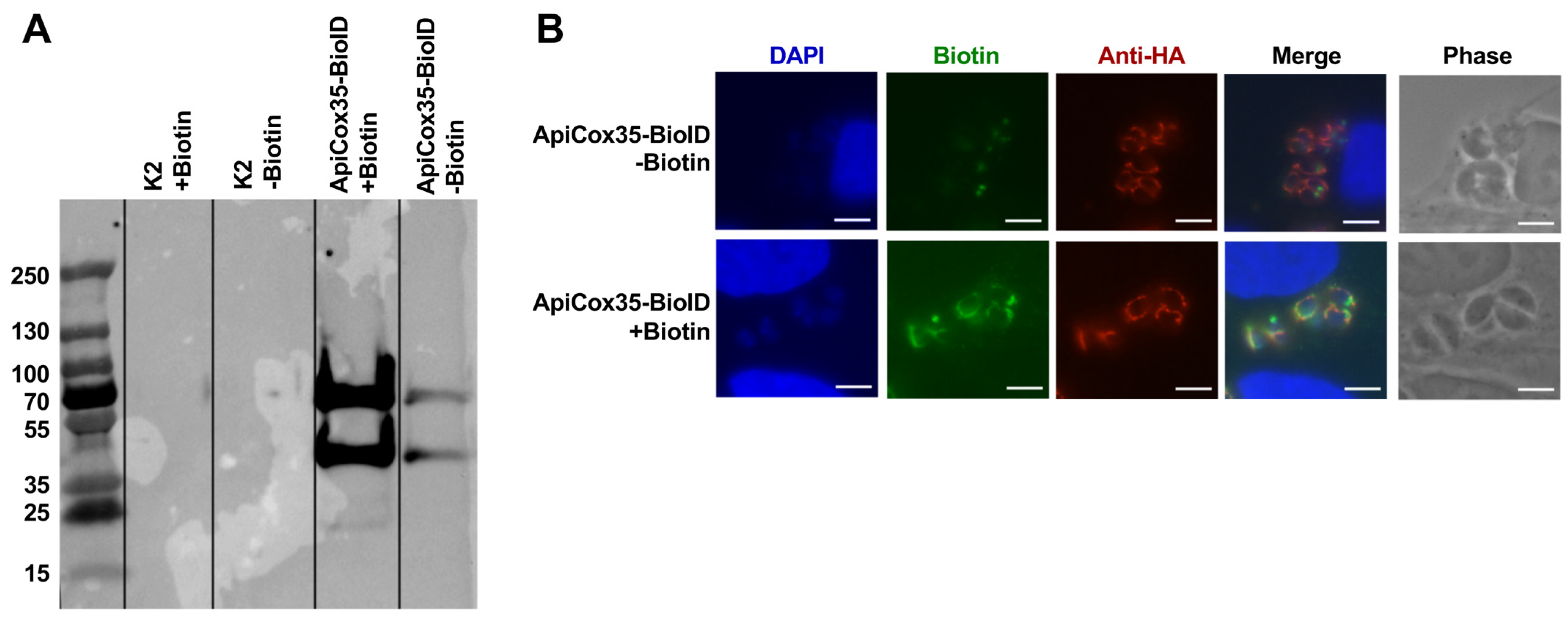
3.5. Generation of TgSOD2-BioID to Evaluate Protein–Protein Interactions
3.6. TgSOD2 Is in Close Proximity to Proteins of Its Mitochondrial Electron Transport Chain
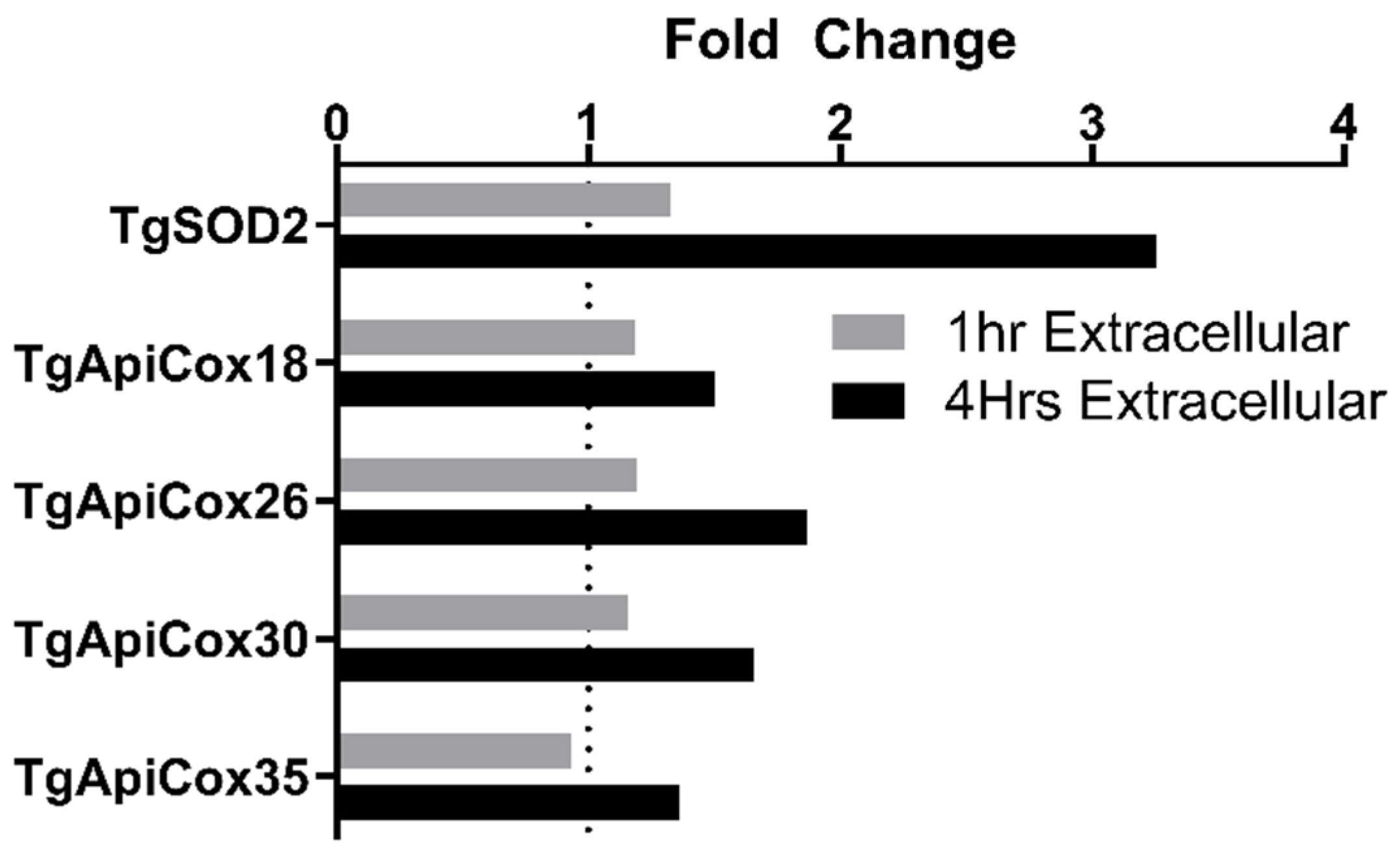
3.7. TgApiCox Transcription Patterns Mirror TgSOD2 Transcription
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| TgSOD2 | T. gondii mitochondrial superoxide dismutase |
| mETC | Mitochondrial electron transport chain |
| HFFs | Human foreskin fibroblasts |
| mAID | Mini auxin inducible degron |
| TIR-1 | Transport inhibitor response 1 |
| BSO | Buthionine sulfoximine |
| PV | Parasitophorous vacuole |
| MMP | Mitochondrial membrane potential |
| SCF | Skp1-Cul1-F-box |
| IAA | Indoleacetic acid |
| TgSODs | T. gondii superoxide dismutases (1,2,3) |
References
- Rahmanian, V.; Rahmanian, K.; Jahromi, A.S.; Bokaie, S. Seroprevalence of Toxoplasma gondii infection: An umbrella review of updated systematic reviews and meta-analyses. J. Family Med. Prim. Care 2020, 9, 3848–3855. [Google Scholar] [CrossRef]
- Pino, P.; Foth, B.J.; Kwok, L.Y.; Sheiner, L.; Schepers, R.; Soldati, T.; Soldati-Favre, D. Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii. PLoS Pathog. 2007, 3, e115. [Google Scholar] [CrossRef]
- Odberg-Ferragut, C.; Renault, J.P.; Viscogliosi, E.; Toursel, C.; Briche, I.; Engels, A.; Lepage, G.; Morgenstern-Badarau, I.; Camus, D.; Tomavo, S.; et al. Molecular cloning, expression analysis and iron metal cofactor characterisation of a superoxide dismutase from Toxoplasma gondii. Mol. Biochem. Parasitol. 2000, 106, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Clayton, C.; Soldati, D. Toxoplasma gondii catalase: Are there peroxisomes in toxoplasma? J. Cell Sci. 2000, 113 Pt 13, 2409–2419. [Google Scholar] [CrossRef]
- Ding, M.; Kwok, L.Y.; Schluter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423–1435.e1412. [Google Scholar] [CrossRef]
- Charvat, R.A.; Arrizabalaga, G. Oxidative stress generated during monensin treatment contributes to altered Toxoplasma gondii mitochondrial function. Sci. Rep. 2016, 6, 22997. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Walton, J.L.; Roepe, P.D.; Sinai, A.P. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012, 14, 589–607. [Google Scholar] [CrossRef]
- Peng, D.; Tarleton, R. EuPaGDT: A web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb. Genom. 2015, 1, e000033. [Google Scholar] [CrossRef]
- Yang, C.; Broncel, M.; Dominicus, C.; Sampson, E.; Blakely, W.J.; Treeck, M.; Arrizabalaga, G. A plasma membrane localized protein phosphatase in Toxoplasma gondii, PPM5C, regulates attachment to host cells. Sci. Rep. 2019, 9, 5924. [Google Scholar] [CrossRef]
- Tagoe, D.N.A.; Ribeiro, E.S.A.; Drozda, A.A.; Coppens, I.; Coleman, B.I.; Gubbels, M.J. Toxoplasma FER1 is a versatile and dynamic mediator of differential microneme trafficking and microneme exocytosis. Sci. Rep. 2024, 14, 21819. [Google Scholar] [CrossRef]
- Back, P.S.; O’Shaughnessy, W.J.; Moon, A.S.; Dewangan, P.S.; Hu, X.; Sha, J.; Wohlschlegel, J.A.; Bradley, P.J.; Reese, M.L. Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 12164–12173. [Google Scholar] [CrossRef]
- Achbarou, A.; Mercereau-Puijalon, O.; Sadak, A.; Fortier, B.; Leriche, M.A.; Camus, D.; Dubremetz, J.F. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology 1991, 103 Pt 3, 321–329. [Google Scholar] [CrossRef]
- Rommereim, L.M.; Bellini, V.; Fox, B.A.; Petre, G.; Rak, C.; Touquet, B.; Aldebert, D.; Dubremetz, J.F.; Cesbron-Delauw, M.F.; Mercier, C.; et al. Phenotypes Associated with Knockouts of Eight Dense Granule Gene Loci (GRA2-9) in Virulent Toxoplasma gondii. PLoS ONE 2016, 11, e0159306. [Google Scholar] [CrossRef]
- Roos, D.S.; Donald, R.G.; Morrissette, N.S.; Moulton, A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994, 45, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Boskovic, Z.V.; Theriault, J.R.; Wang, A.J.; Stern, A.M.; Wagner, B.K.; Shamji, A.F.; Schreiber, S.L. Discovery of small-molecule enhancers of reactive oxygen species that are nontoxic or cause genotype-selective cell death. ACS Chem. Biol. 2013, 8, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.I.; Tirtorahardjo, J.A.; Jan, S.; Schweizer, S.S.; Rosario, S.A.C.; Du, Y.; Zhang, J.J.; Morrissette, N.S.; Andrade, R.M. Auranofin Resistance in Toxoplasma gondii Decreases the Accumulation of Reactive Oxygen Species but Does Not Target Parasite Thioredoxin Reductase. Front Cell Infect Microbiol. 2021, 11, 618994. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Long, S.; Sibley, L.D. Plasma Membrane Association by N-Acylation Governs PKG Function in Toxoplasma gondii. mBio 2017, 8, e00375-17. [Google Scholar] [CrossRef]
- Moss, W.J.; Patterson, C.E.; Jochmans, A.K.; Brown, K.M. Functional Analysis of the Expanded Phosphodiesterase Gene Family in Toxoplasma gondii Tachyzoites. mSphere 2022, 7, e0079321. [Google Scholar] [CrossRef]
- Trauth, J.; Scheffer, J.; Hasenjäger, S.; Taxis, C. Synthetic Control of Protein Degradation during Cell Proliferation and Developmental Processes. Acs Omega 2019, 4, 2766–2778. [Google Scholar] [CrossRef]
- Andrade, R. TgSOD2 Depletion in TgSOD2-mAID Parasites Reduced Growth and Replication. (A) Insertion of the YFP-mAID-HXGPRT at 3’ End of TgSOD2 via CRISPR/CAS9. BioRender. 2025. Available online: https://BioRender.com/dgphjp3 (accessed on 21 June 2025).
- Andrade, R. (C) Schematic Illustration of Staining Patterns Observed for ATPase Synthase. The Aberrant Group Includes Both Discontinuous and Punctate Staining Signals. BioRender. 2025. Available online: https://BioRender.com/90v4ajz (accessed on 21 June 2025).
- Harding, C.R.; Gow, M.; Kang, J.H.; Shortt, E.; Manalis, S.R.; Meissner, M.; Lourido, S. Alveolar proteins stabilize cortical microtubules in Toxoplasma gondii. Nat. Commun. 2019, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.L.; Kim, E.W.; Toh, J.Y.; Vashisht, A.A.; Rashoff, A.Q.; Van, C.; Huang, A.S.; Moon, A.S.; Bell, H.N.; Bentolila, L.A.; et al. Novel components of the Toxoplasma inner membrane complex revealed by BioID. mBio 2015, 6, e02357-02314. [Google Scholar] [CrossRef]
- Gaji, R.Y.; Behnke, M.S.; Lehmann, M.M.; White, M.W.; Carruthers, V.B. Cell cycle-dependent, intercellular transmission of Toxoplasma gondii is accompanied by marked changes in parasite gene expression. Mol. Microbiol. 2011, 79, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Brecht, S.; Bujard, H.; Soldati, D. Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res. 2001, 29, E115. [Google Scholar] [CrossRef]
- Daniel, K.; Icha, J.; Horenburg, C.; Muller, D.; Norden, C.; Mansfeld, J. Conditional control of fluorescent protein degradation by an auxin-dependent nanobody. Nat. Commun. 2018, 9, 3297. [Google Scholar] [CrossRef]
- Natsume, T.; Kanemaki, M.T. Conditional Degrons for Controlling Protein Expression at the Protein Level. Annu. Rev. Genet. 2017, 51, 83–102. [Google Scholar] [CrossRef]
- Ma, X.; Jin, M.; Cai, Y.; Xia, H.; Long, K.; Liu, J.; Yu, Q.; Yuan, J. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem. Biol. 2011, 18, 1474–1481. [Google Scholar] [CrossRef]
- Shchepina, L.A.; Pletjushkina, O.Y.; Avetisyan, A.V.; Bakeeva, L.E.; Fetisova, E.K.; Izyumov, D.S.; Saprunova, V.B.; Vyssokikh, M.Y.; Chernyak, B.V.; Skulachev, V.P. Oligomycin, inhibitor of the F0 part of H+-ATP-synthase, suppresses the TNF-induced apoptosis. Oncogene 2002, 21, 8149–8157. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef]
- Seidi, A.; Muellner-Wong, L.S.; Rajendran, E.; Tjhin, E.T.; Dagley, L.F.; Aw, V.Y.; Faou, P.; Webb, A.I.; Tonkin, C.J.; van Dooren, G.G. Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. Elife 2018, 7, e38131. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; NaveenKumar, S.K.; Geethika, M.; Mugesh, G. A Cerium Vanadate Nanozyme with Specific Superoxide Dismutase Activity Regulates Mitochondrial Function and ATP Synthesis in Neuronal Cells. Angew. Chem. Int. Ed. Engl. 2021, 60, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.D.; Lawson, R.; Weidner, E. Superoxide dismutase and catalase in Toxoplasma gondii. Mol. Biochem. Parasitol. 1986, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Maclean, A.E.; Hayward, J.A.; Huet, D.; van Dooren, G.G.; Sheiner, L. The mystery of massive mitochondrial complexes: The apicomplexan respiratory chain. Trends Parasitol. 2022, 38, 1041–1052. [Google Scholar] [CrossRef]
| ToxoDB GeneID | Protein Annotation | Capture Method | Predicted Protein Mass (kDa) | Protein Localization | Phenotype Score (Sidik et al., 2016 [6]) | Human Homolog Similarity |
|---|---|---|---|---|---|---|
| TGME49_316330 | superoxide dismutase TgSOD2 (SOD2) | Streptavidin Beads | 31.47 | Mitochondrion | −4.09 | 38.97% |
| TGME49_221510 | Tg ApiCox18 | Streptavidin Beads | 17.92 | Mitochondrion | −3.28 | 0.00% |
| TGME49_306670 | Tg ApiCox26 | Streptavidin Beads | 25.84 | Mitochondrion | −3.68 | 0.00% |
| TGME49_297810 | Tg ApiCox30 | Streptavidin Beads | 29.57 | Mitochondrion | −3.64 | 0.00% |
| TGME49_229920 | Tg ApiCox35 | Streptavidin Beads | 34.96 | Mitochondrion | −3.84 | 0.00% |
| TGME49_261950 | ATP synthase beta subunit ATP-B (ATPB) | Streptavidin Beads | 59.93 | Mitochondrion | −4.84 | 79.45% |
| TGME49_209260 | cytochrome c oxidase subunit 5b | Streptavidin Beads | 34.71 | Mitochondrion | −3.07 | 38.60% |
| TGME49_260170 | elongation factor EF-G, putative | Streptavidin Beads | 96.63 | Mitochondrion | −4.87 | 51.36% |
| TGME49_257730 | methionine aminopeptidase, type I, putative | Streptavidin Beads | 52.24 | Mitochondrion | −3.51 | 50.00% |
| TGME49_202680 | peptidase M16, alpha subunit, putative | Streptavidin Beads | 62.21 | Mitochondrion | −4.3 | 32.96% |
| TGME49_236210 | peptidase M16 family protein, putative | Streptavidin Beads | 56.92 | Mitochondrion | −4.74 | 44.15% |
| TGME49_216530 | ribosome recycling factor protein | Streptavidin Beads | 51.08 | Mitochondrion | −3.81 | 0.00% |
| TGME49_316330 | superoxide dismutase SOD2 (SOD2) | Anti-HA Beads | 31.47 | Mitochondrion | −4.09 | 38.97% |
| TGME49_261950 | ATP synthase beta subunit ATP-B (ATPB) | Anti-HA Beads | 59.93 | Mitochondrion | −4.84 | 79.45% |
| TGME49_247550 | heat shock protein HSP60 | Anti-HA Beads | 60.92 | Mitochondrion | −5.1 | 55.51% |
| TGME49_288500 | FAD Malate-dehydrogenase (MDH-FAD) | Anti-HA Beads | 60.39 | Mitochondrion | −0.79 | 0.00% |
| TGME49_243950 | prohibitin, putative | Anti-HA Beads | 30.23 | Mitochondrion | −5.18 | 51.49% |
| TGME49_219550 | dihydrolipoyllysine-residue succinyltransferase component of oxoglutarate dehydrogenase | Anti-HA Beads | 50.13 | Mitochondrion | −4.25 | 60.26% |
| TGME49_215590 | flavoprotein subunit of succinate dehydrogenase | Anti-HA Beads | 72.76 | Mitochondrion | −3.96 | 65.70% |
| TGME49_319920 | 2-oxo acid dehydrogenases acyltransferase (catalytic domain) domain-containing protein | Anti-HA Beads | 70.3 | Mitochondrion | −1.92 | 40.60% |
| TGME49_204400 | ATPase synthase subunit alpha, putative | Anti-HA Beads | 62.16 | Mitochondrion | −3.84 | 74.11% |
| ToxoDB GeneID | Protein Annotation | Protein Localization | Phenotype Score (Sidik et al., 2016 [6]) | Human Homolog Similarity |
|---|---|---|---|---|
| TGME49_229920 | Tg ApiCox35 | Mitochondrion | −3.84 | 0.00% |
| TGME49_265370 | Tg ApiCox16 | Mitochondrion | 1.56 | 0.00% |
| TGME49_221510 | Tg ApiCox18 | Mitochondrion | −3.28 | 0.00% |
| TGME49_247770 | Tg ApiCox19 | Mitochondrion | −2.61 | 0.00% |
| TGME49_286530 | Tg ApiCox24 | Mitochondrion | −2.82 | 0.00% |
| TGME49_264040 | Tg ApiCox25 | Mitochondrion | −2.54 | 0.00% |
| TGME49_306670 | Tg ApiCox26 | Mitochondrion | −3.68 | 0.00% |
| TGME49_297810 | Tg ApiCox30 | Mitochondrion | −3.64 | 0.00% |
| TGME49_209260 | cytochrome c oxidase subunit 5b | Mitochondrion | −3.07 | 38.60% |
| TGME49_234420 | ATPase, AAA family protein | Mitochondrion | −5.08 | 38.83% |
| TGME49_262640 | Cg8 family protein | Mitochondrion | −3.49 | 0.00% |
| TGME49_210790 | dihydroorotate dehydrogenase | Mitochondrion | −2.84 | 47.51% |
| TGME49_251780 | heat shock protein | Mitochondrion | −5.09 | 66.67% |
| TGME49_257730 | methionine aminopeptidase, type I | Mitochondrion | −3.51 | 42.96% |
| TGME49_236210 | peptidase M16 family protein | Mitochondrion | −4.74 | 44.15% |
| TGME49_255910 | PfMNL-2 CISD1 family iron-sulfur protein | Mitochondrion | 1.64 | 48.72% |
| TGME49_207620 | pyridine nucleotide-disulfide oxidoreductase domain-containing protein | Mitochondrion | −1.15 | 34.94% |
| TGME49_284580 | ribose-phosphate diphosphokinase subfamily protein | Mitochondrion | −0.72 | 29.10% |
| TGME49_216530 | ribosome recycling factor protein | Mitochondrion | −3.81 | 0.00% |
| TGME49_319730 | YOU2 family C2C2 zinc finger protein | Mitochondrion | 0.02 | 0.00% |
| TGME49_312160 | hypothetical protein | Mitochondrion | −1.29 | 0.00% |
| TGME49_229420 | cytochrome c | Cytoplasm | 0.61 | 33.93% |
| TGME49_231350 | glucosamine-fructose-6-phosphate aminotransferase | Plastid | −4.57 | 31.11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirtorahardjo, J.A.; Ma, C.I.-H.; Shaikh, A.; Andrade, R.M. Elucidating the Role of Toxoplasma gondii’s Mitochondrial Superoxide Dismutase. Biomolecules 2025, 15, 972. https://doi.org/10.3390/biom15070972
Tirtorahardjo JA, Ma CI-H, Shaikh A, Andrade RM. Elucidating the Role of Toxoplasma gondii’s Mitochondrial Superoxide Dismutase. Biomolecules. 2025; 15(7):972. https://doi.org/10.3390/biom15070972
Chicago/Turabian StyleTirtorahardjo, James Alexander, Christopher I-H. Ma, Areej Shaikh, and Rosa M. Andrade. 2025. "Elucidating the Role of Toxoplasma gondii’s Mitochondrial Superoxide Dismutase" Biomolecules 15, no. 7: 972. https://doi.org/10.3390/biom15070972
APA StyleTirtorahardjo, J. A., Ma, C. I.-H., Shaikh, A., & Andrade, R. M. (2025). Elucidating the Role of Toxoplasma gondii’s Mitochondrial Superoxide Dismutase. Biomolecules, 15(7), 972. https://doi.org/10.3390/biom15070972







