Intratracheal Aerosolization of Nocardia farcinica in Mice Optimizes Bacterial Distribution and Enhances Pathogenicity Compared to Intranasal Inoculation and Intratracheal Instillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Animals and Ethic Statement
2.3. Inoculum Preparation for Lung Infection
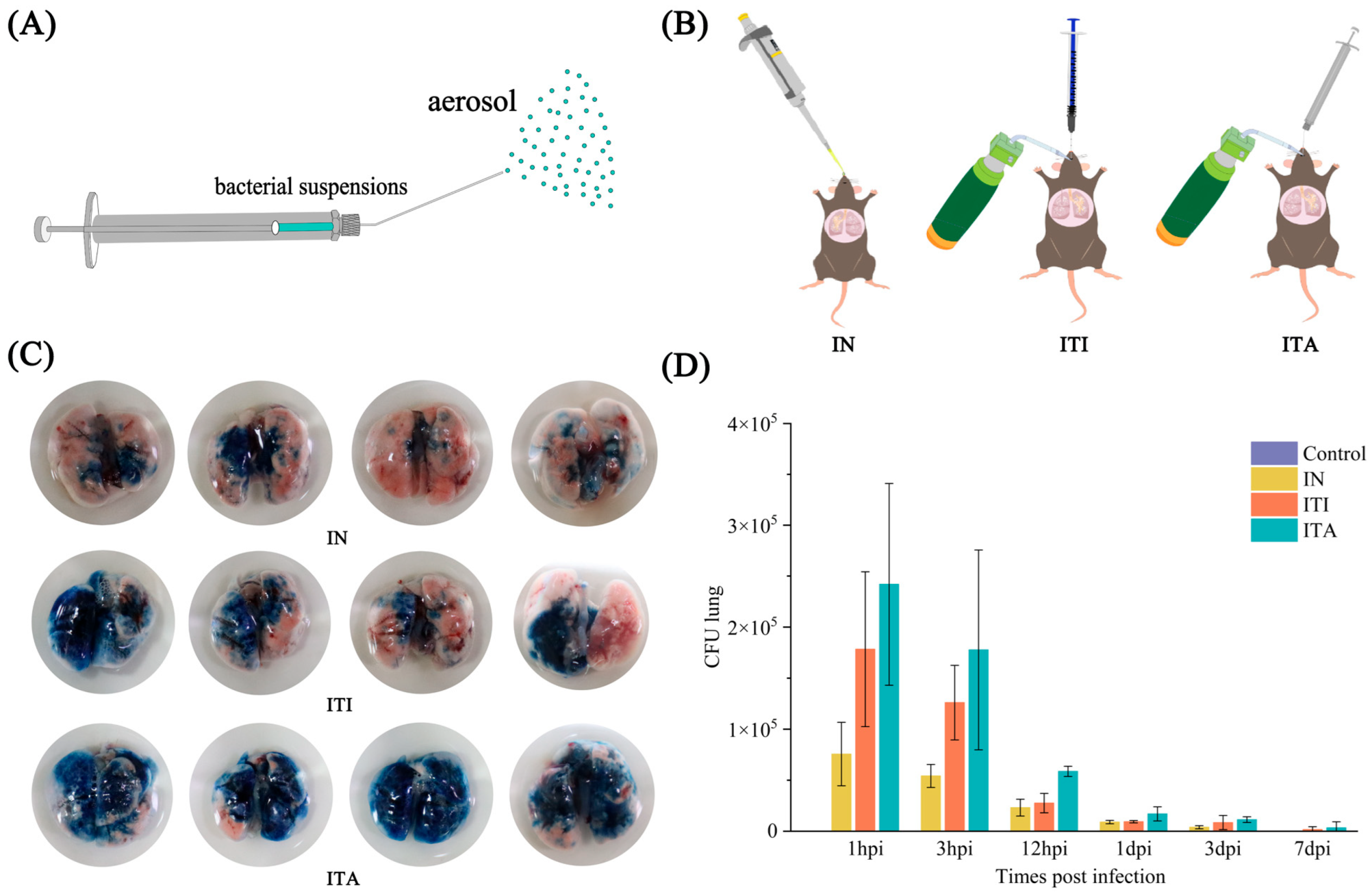
2.4. Mice Models of Pneumonia
2.5. Aerosol Distribution of Trypan Blue
2.6. BALF Collection and Bacterial Load Determination
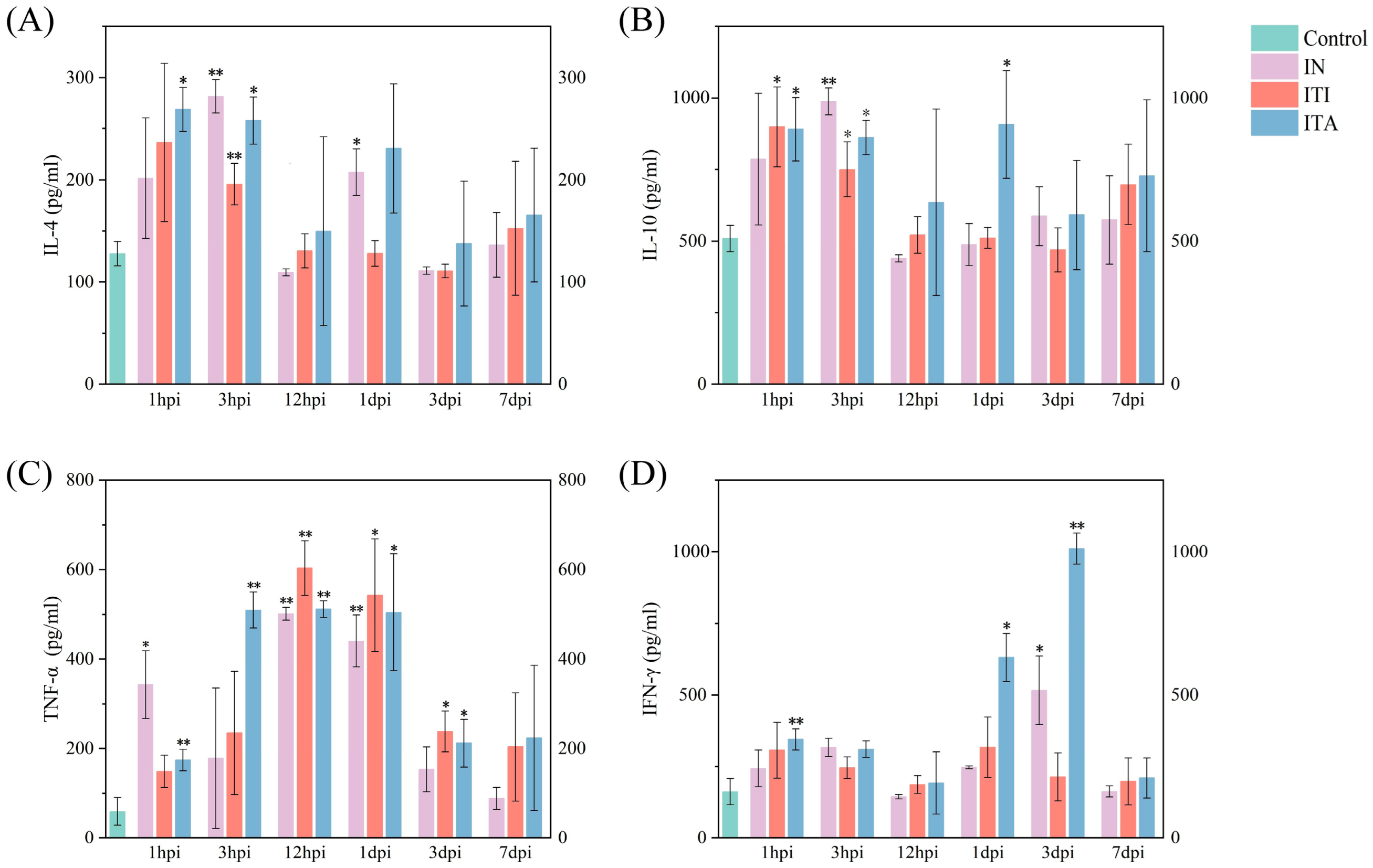
2.7. Detection of Inflammatory Cytokines in Mice BALF
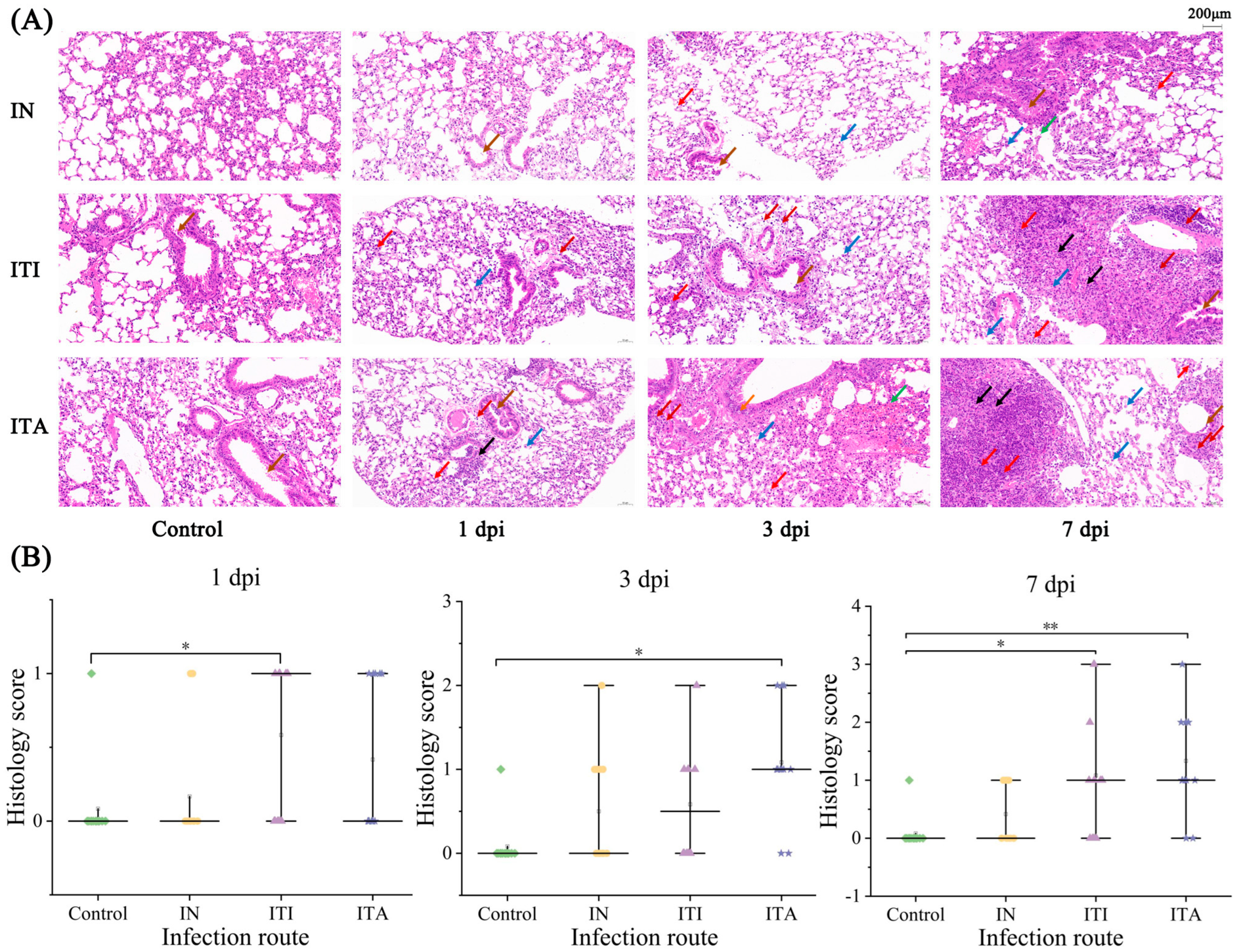
2.8. Histopathological
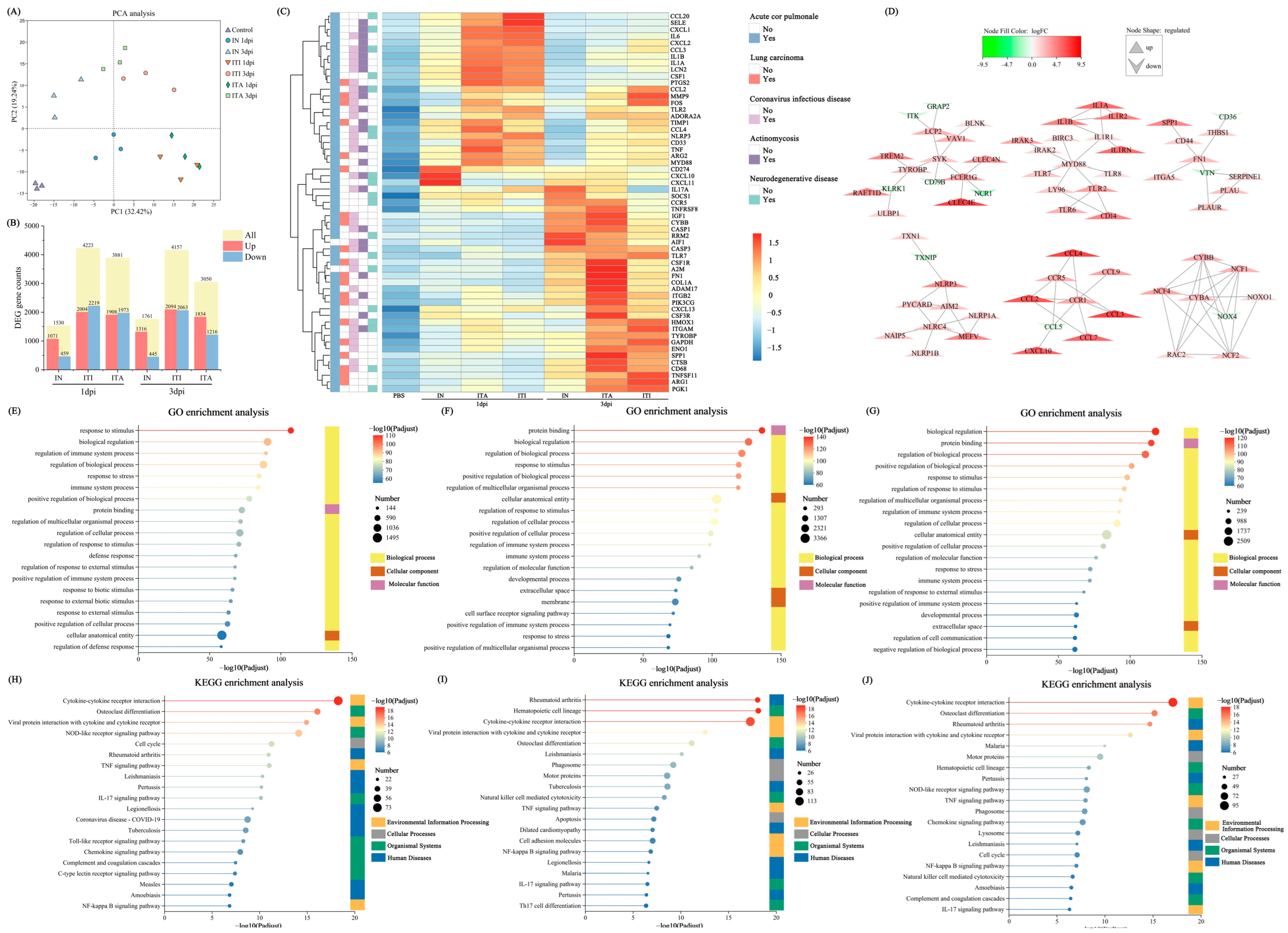
2.9. RNA Extraction, Library Preparation, Sequencing and Analysis
2.10. Statistical Analysis
3. Results
3.1. Mice Infected with N. farcinica via ITA Route Exhibit More Uniform Distribution in Lungs
3.2. Mice Infected with N. farcinica via ITA Route Results in Higher Fatality, More Weight Loss and More Severe Bacterial Burden
3.3. Mice Infected with N. farcinica via ITA Route Exhibit More Severe Clinical Symptoms and Inflammatory Responses
3.4. ITA Route Leads to More Pronounced Histopathological Lesion
3.5. Differential Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IN | Intranasal inoculation |
| ITA | Intratracheal aerosolization |
| ITI | Intratracheal instillation |
| HIV | Human Immunodeficiency Virus |
| BHI | Brain Heart Infusion |
| PBS | Phosphate-buffered saline |
| USA | the United States of America |
| CFU | Colony-forming units |
| BALF | Bronchoalveolar lavage fluid |
| hpi | Hour post infection |
| dpi | Day post infection |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon-gamma |
| CV | Coefficient of variation |
| LMM | Linear mixed effects model |
| H&E | Haematoxylin and Eosin |
| ANOVA | Analysis of variance |
| GO | Gene Ontology |
| PCA | Principal component analysis |
| DEGs | Differentially expressed genes |
| PPI | Protein-protein interaction |
| TLR4 | The toll-like receptor 4 |
References
- Traxler, R.M.; Bell, M.E.; Lasker, B.; Headd, B.; Shieh, W.J.; McQuiston, J.R. Updated Review on Nocardia Species: 2006–2021. Clin. Microbiol. Rev. 2022, 35, e0002721. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.M.; Brown, J.M. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin. Microbiol. Rev. 1994, 7, 357–417. [Google Scholar] [CrossRef]
- Thipmontree, W.; Suputtamonkol, Y. Comparison of clinical outcomes of pulmonary nocardiosis between AIDS and non-AIDS patients. BMC Infect. Dis. 2024, 28, 649. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Han, L.; Zhang, W.; Sun, L.; Xu, S.; Qiu, X.; Fan, S.; Li, Z. Molecular, cellular and neurological consequences of infection by the neglected human pathogen Nocardia. BMC Biol. 2022, 20, 251. [Google Scholar] [CrossRef]
- Shen, J.; Du, B.; Liu, Z.; Song, Z.; Yuan, M.; Qiu, X.; Li, Z. Multicenter systematic review of clinical characteristics, diagnostic optimization, and personalized treatment for brain Nocardia infections. Microb. Pathog. 2025, 198, 107147. [Google Scholar] [CrossRef]
- Steinbrink, J.; Leavens, J.; Kauffman, C.A.; Miceli, M.H. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine 2018, 97, e12436. [Google Scholar] [CrossRef]
- Beaman, B.L.; Burnside, J.; Edwards, B.; Causey, W. Nocardial infections in the United States, 1972−1974. J. Infect. Dis. 1976, 134, 286–289. [Google Scholar] [CrossRef]
- Fatahi-Bafghi, M. Nocardiosis from 1888 to 2017. Microb. Pathog. 2018, 114, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Ruoff, K.; Hooper, D.C. Nocardia bacteremia. Report of 4 cases and review of the literature. Medicine 1998, 77, 255–267. [Google Scholar] [CrossRef]
- Beaman, B.L.; Beaman, L. Nocardia species: Host-parasite relationships. Clin. Microbiol. Rev. 1994, 7, 213–264. [Google Scholar] [CrossRef]
- Presant, C.A.; Wiernik, P.H.; Serpick, A.A. Factors affecting survival in nocardiosis. Am. Rev. Respir. Dis. 1973, 108, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Dezfuli, A.A.Z.; Khosravi, A.D.; Savari, M.; Jahangirimehr, F. Genotypic and phenotypic prevalence of Nocardia species in Iran: First systematic review and meta-analysis of data accumulated over years 1992–2021. PLoS ONE 2021, 16, e0254840. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Nagakura, T.; Fujita, H.; Fukusima, S.; Gonoi, T. Nocardia elegans infection: A case report and literature review. Int. J. Infect. Dis. 2017, 54, 15–17. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Xu, H.; Sun, L.; Li, C.; Guo, W.; Xiang, L.; Luo, G.; Cui, Y.; Lu, B. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009–2017. Diagn. Microbiol. Infect. Dis. 2019, 94, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Han, L.; Yao, J.; Qiu, X.; Xu, S.; Liu, X.; Li, F.; Li, Z. Infection route influence the consequences of Nocardia farcinica infection in BALB/c mice. BMC Infect. Dis. 2024, 24, 1016. [Google Scholar] [CrossRef]
- Beaman, B.L.; Goldstein, E.; Gershwin, M.E.; Maslan, S.; Lippert, W. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect. Immun. 1978, 22, 867–877. [Google Scholar] [CrossRef]
- Bergamini, G.; Perico, M.E.; Di Palma, S.; Sabatini, D.; Andreetta, F.; Defazio, R.; Felici, A.; Ferrari, L.; Subbian, S. Mouse pneumonia model by Acinetobacter baumannii multidrug resistant strains: Comparison between intranasal inoculation, intratracheal instillation and oropharyngeal aspiration techniques. PLoS ONE 2021, 16, e0260627. [Google Scholar] [CrossRef]
- Jin, X.-Y.; Yang, H.-Y.; Zhao, G.-Y.; Dai, C.-X.; Zhang, Z.-Q.; Zhou, D.-S.; Yin, Q.; Dai, E.-H. Comparative pathogenicity of influenza virus-induced pneumonia mouse model following intranasal and aerosolized intratracheal inoculation. Virol. J. 2024, 21, 240. [Google Scholar] [CrossRef]
- Verma, K.; Garg, T.; Singh, S.; Deivreddy, V.S.R.; Raman, S.K.; Bharti, R.; Sofi, H.S.; Singh, K.; Shaik, M.; Dasgupta, A.; et al. Preclinical model of Mycobacteroides abscessus lung disease by nose-only exposure of mice to bacterial powder aerosol. Tuberculosis 2025, 151, 102606. [Google Scholar] [CrossRef]
- Ishikawa, J.; Yamashita, A.; Mikami, Y.; Hoshino, Y.; Kurita, H.; Hotta, K.; Shiba, T.; Hattori, M. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 2004, 101, 14925–14930. [Google Scholar] [CrossRef]
- Mann, P.C.; Vahle, J.; Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; et al. International harmonization of toxicologic pathology nomenclature: An overview and review of basic principles. Toxicol. Pathol. 2012, 40 (Suppl. S4), 7S–13S. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. Imeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Wang, J.; Hu, L.; Zhang, Z.; Sui, C.; Zhu, X.; Wu, C.; Zhang, L.; Lv, M.; Yang, W.; Zhou, D.; et al. Mice fatal pneumonia model induced by less-virulent Streptococcus pneumoniae via intratracheal aerosolization. Future Microbiol. 2024, 19, 1055–1070. [Google Scholar] [CrossRef]
- Han, L.; Ji, X.; Fan, S.; Shen, J.; Liang, B.; Li, Z. Secreted protein NFA47630 from Nocardia farcinica IFM10152 induces immunoprotective effects in mice. Trop. Dis. Travel Med. Vaccines 2024, 10, 21. [Google Scholar] [CrossRef]
- Lam, J.C.; Chan, W.W.; Walsh, J.F. Disseminated nocardiosis in an immunocompetent host with occupational exposure. IDCases. 2022, 30, e01620. [Google Scholar] [CrossRef]
- Shen, J.; Ji, X.Z.; Han, L.; Yao, J.; Liang, Y.; Yuan, M.; Xu, S.; Li, Z.; Feng, Y. Activation of NF-κB/MAPK signaling and induction of apoptosis by salicylate synthase NbtS in Nocardia farcinica promotes neuroinflammation development. mSystems 2024, 9, e0089324. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Nie, Y.; Wang, J.; Feng, G.; Hao, L.; Huang, W.; Li, Y.; Liu, Z. Functional Characterization of Largemouth Bass (Micropterus salmoides) Soluble FcγR Homolog in Response to Bacterial Infection. Int. J. Mol. Sci. 2022, 23, 13788. [Google Scholar] [CrossRef]
- Crowley, M.T.; Costello, P.S.; Fitzer-Attas, C.J.; Turner, M.; Meng, F.; Lowell, C.; Tybulewicz, V.L.J.; DeFranco, A.L. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J. Exp. Med. 1997, 186, 1027–1039. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Song, Z.; Shen, J.; Yao, J.; Xu, S.; Qiu, X.; Yuan, M.; Li, Z. Intratracheal Aerosolization of Nocardia farcinica in Mice Optimizes Bacterial Distribution and Enhances Pathogenicity Compared to Intranasal Inoculation and Intratracheal Instillation. Biomolecules 2025, 15, 950. https://doi.org/10.3390/biom15070950
Du B, Song Z, Shen J, Yao J, Xu S, Qiu X, Yuan M, Li Z. Intratracheal Aerosolization of Nocardia farcinica in Mice Optimizes Bacterial Distribution and Enhances Pathogenicity Compared to Intranasal Inoculation and Intratracheal Instillation. Biomolecules. 2025; 15(7):950. https://doi.org/10.3390/biom15070950
Chicago/Turabian StyleDu, Bingqian, Ziyu Song, Jirao Shen, Jiang Yao, Shuai Xu, Xiaotong Qiu, Min Yuan, and Zhenjun Li. 2025. "Intratracheal Aerosolization of Nocardia farcinica in Mice Optimizes Bacterial Distribution and Enhances Pathogenicity Compared to Intranasal Inoculation and Intratracheal Instillation" Biomolecules 15, no. 7: 950. https://doi.org/10.3390/biom15070950
APA StyleDu, B., Song, Z., Shen, J., Yao, J., Xu, S., Qiu, X., Yuan, M., & Li, Z. (2025). Intratracheal Aerosolization of Nocardia farcinica in Mice Optimizes Bacterial Distribution and Enhances Pathogenicity Compared to Intranasal Inoculation and Intratracheal Instillation. Biomolecules, 15(7), 950. https://doi.org/10.3390/biom15070950






