Emerging Molecular Mechanisms in Malaria Pathogenesis and Novel Therapeutic Approaches: A Focus on P. falciparum Malaria
Abstract
1. Introduction
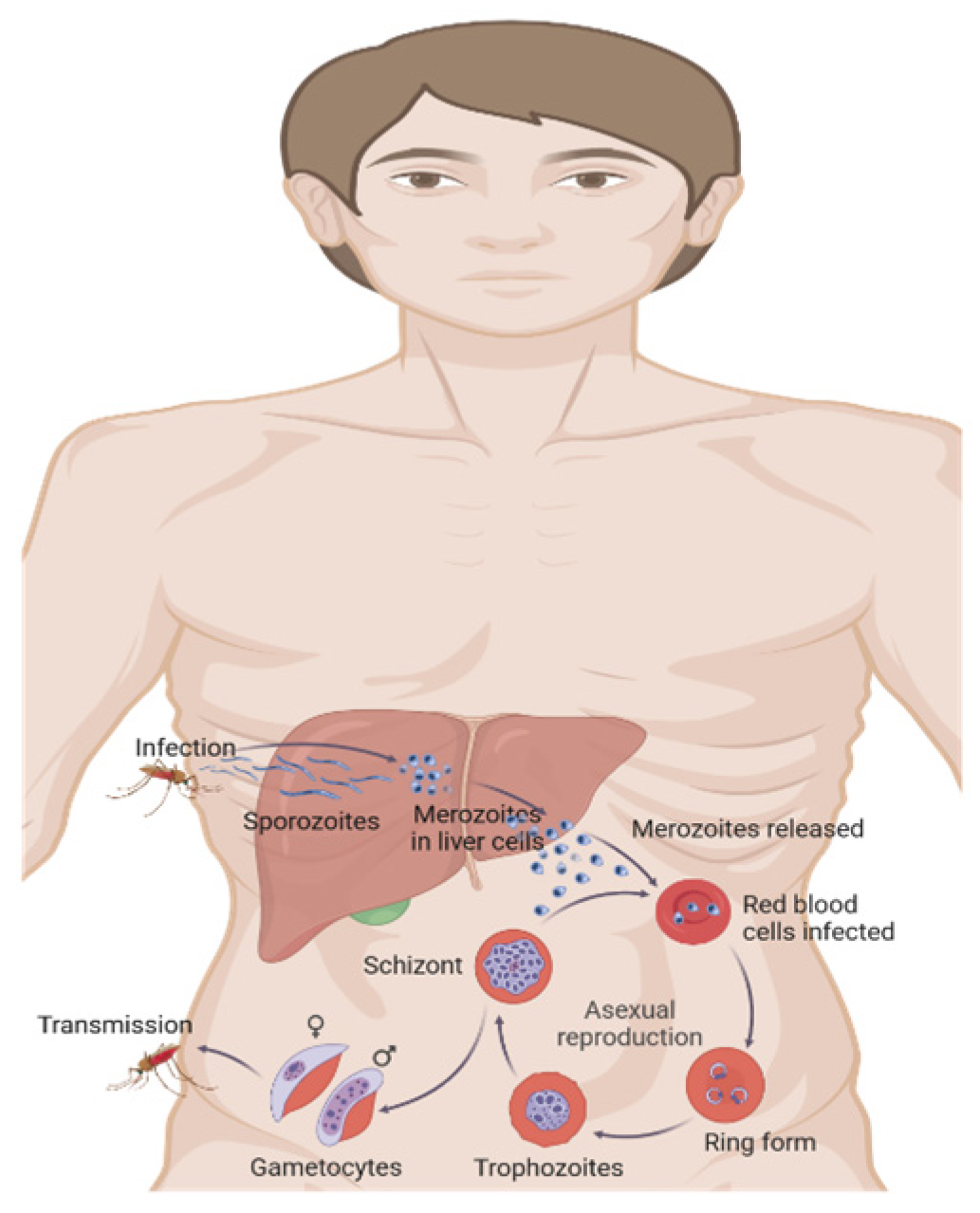
2. Emerging Molecular Mechanisms in Malaria Pathogenesis
2.1. Molecular Mechanisms
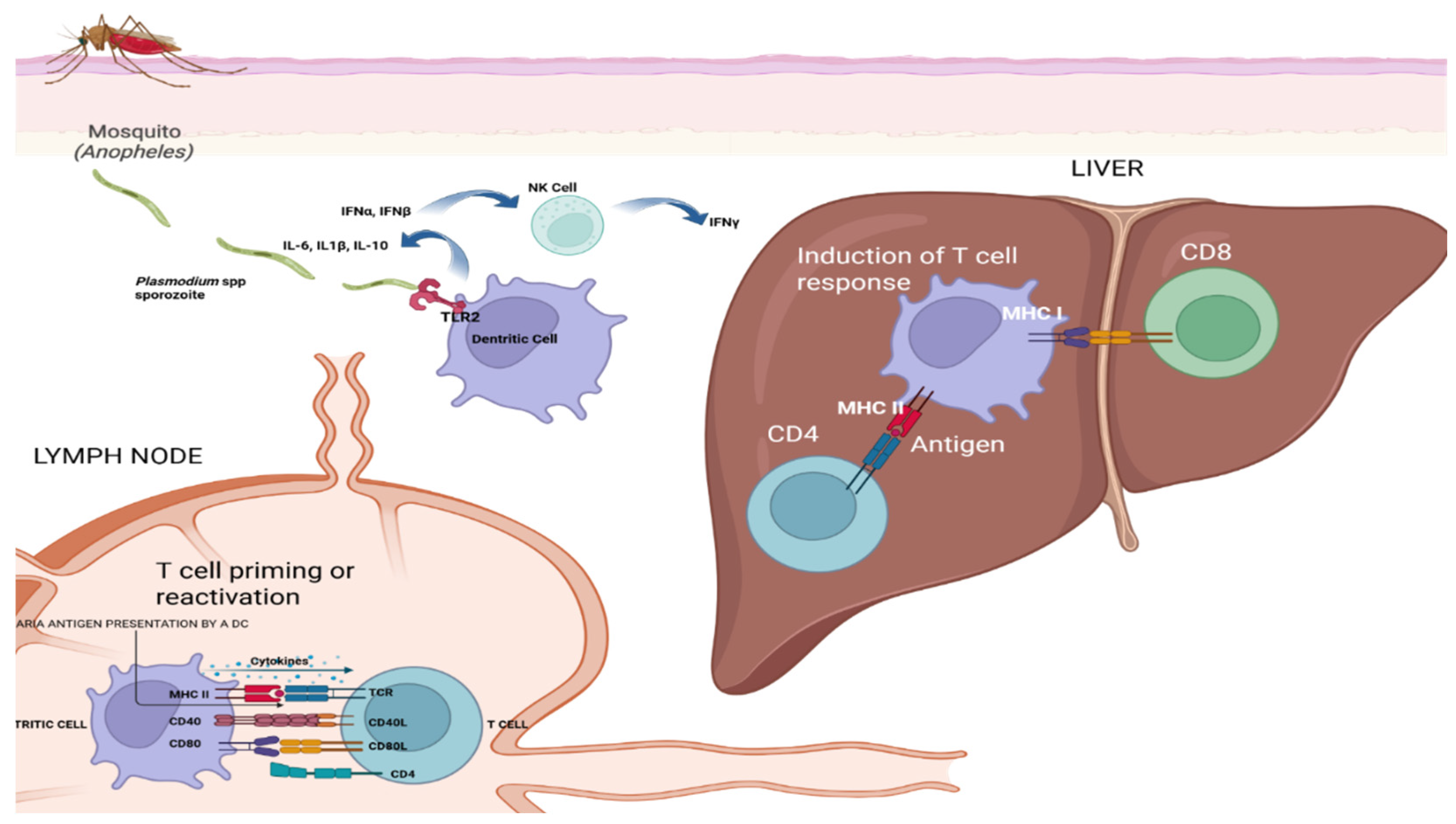
2.2. Host–Pathogen Interaction
2.3. Molecular Mechanisms of Disease Severity
2.4. Molecular Basis of Drug Resistance
2.5. Recent Advances in Malaria Research
2.5.1. Genomic and Transcriptomic Insights
2.5.2. Proteomics and Metabolomics in Malaria
2.5.3. Understanding Antigenic Variation and Immune Evasion
3. Emerging Therapeutic Strategies
3.1. Novel Antimalarial Drugs
3.1.1. Mechanism of Action of New Drug Candidates
3.1.2. The Role of Synthetic Chemistry and Natural Products in Drug Discovery
3.1.3. Targeting the Malaria Life Cycle
Drugs Targeting Specific Stages of the Plasmodium Life Cycle
Inhibiting Parasite Entry and Replication
3.2. Immunotherapies and Vaccines
3.2.1. Recent Advancements in Malaria Vaccines (e.g., RTS,S/AS01)
3.2.2. Monoclonal Antibodies and Immune Modulators as Potential Therapies
3.2.3. Combination Therapies
3.2.4. The Role of Combination Drug Regimens to Overcome Resistance
3.2.5. Synergistic Effects of Existing Drugs with Novel Agents
3.2.6. Molecular Markers for Diagnostics to Monitor Drug Efficacy and Resistance
4. Challenges and Future Directions
4.1. Antimalarial Drug Resistance: Current Threats and Advances in Treatment
4.2. Genetic and Molecular Mechanisms Driving Antimalarial Resistance in Plasmodium falciparum
4.3. The Importance of Continued Drug Discovery and Surveillance
4.4. From Drug Resistance to Vaccine Breakthroughs: Advances in Malaria Control
4.5. Challenges in Creating an Effective Malaria Vaccine
4.6. Immunological Hurdles and the Need for Novel Vaccine Platforms
4.7. Global Health and Equity Considerations
4.7.1. Access to New Therapies in Low-Income Regions
4.7.2. Strategies for Equitable Distribution of Antimalarial Treatments and Vaccines
4.7.3. The Role of Collaborative Global Initiatives
4.7.4. Role of Organizations Like the WHO, Gates Foundation, and Other Entities in Combating Malaria
4.7.5. The Importance of Funding and International Collaboration for Sustained Progress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PfCRT | Plasmodium falciparum chloroquine resistance transporter |
| PfMDR1 | Plasmodium falciparum multidrug resistance protein 1 |
| DHODH | Dihydroorotate dehydrogenase |
| ITNs | Insecticide-treated bed nets |
| RDTs | Rapid Diagnostic Tests |
| WHO | World Health Organization |
| CDC | Centers for Disease Control and Prevention’s |
| ACT | Artemisinin-based combination therapy |
| RBM | Roll Back Malaria |
| USAID | United States Agency for International Development |
| APMEN | Asia Pacific Malaria Elimination Network |
| G6PD | Glucose-6-phosphate dehydrogenase |
References
- WHO Malaria. Questions and Answers 4 April 2025. 2025. Available online: https://www.who.int/news-room/questions-and-answers/item/malaria (accessed on 6 May 2025).
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. The Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef]
- Perdomo Ruiz, A.P.; Jimenez, M.; Carrasquilla, G. Barriers and facilitators for malaria elimination: A narrative literature review. RESPYN J. Public Health Nutr. 2023, 22, 26–41. [Google Scholar] [CrossRef]
- Garcia, L.S. Malaria. Clin. Lab. Med. 2010, 30, 93–129. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2023. 2023. Available online: https://iris.who.int/handle/10665/374472 (accessed on 6 May 2025).
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Eliminating Malaria. 2025. Available online: https://www.who.int/activities/eliminating-malaria#:~:text=Since%202015%2C%2015%20countries%20have,least%2030%20countries%20by%202030 (accessed on 6 May 2025).
- Thellier, M.; Gemegah, A.A.J.; Tantaoui, I. Global Fight against Malaria: Goals and Achievements 1900–2022. J. Clin. Med. 2024, 13, 5680. [Google Scholar] [CrossRef]
- Coetzee, M.; Koekemoer, L.L. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu. Rev. Entomol. 2013, 58, 393–412. [Google Scholar] [CrossRef]
- Wangrawa, D.W.; Odero, J.O.; Baldini, F.; Okumu, F.; Badolo, A. Distribution and insecticide resistance profile of the major malaria vector Anopheles funestus group across the African continent. Med. Vet. Entomol. 2024, 38, 119–137. [Google Scholar] [CrossRef]
- Dacey, D.P.; Chain, F.J.J. The Challenges of Microbial Control of Mosquito-Borne Diseases Due to the Gut Microbiome. Front. Genet. 2020, 11, 504354. [Google Scholar] [CrossRef]
- Van den Berg, H.; Yadav, R.S.; Zaim, M. Setting international standards for the management of public health pesticides. PLoS Med. 2015, 12, e1001824. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO. Framework for Malaria Elimination. 2017. Available online: https://iris.paho.org/handle/10665.2/34172 (accessed on 24 June 2025).
- WHO. Global Technical Strategy for Malaria 2016–2030. 2015. Available online: https://iris.who.int/bitstream/handle/10665/186671/9789243564999_spa.pdf?sequence=1 (accessed on 6 May 2025).
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria parasite biology, pathogenesis, and protection: Insights from molecular studies. Nat. Rev. Microbiol. 2020, 18, 741–755. [Google Scholar] [CrossRef]
- Ashley, E.A.; Phyo, A.P.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Gural, N.; Mancio-Silva, L.; Miller, A.B.; Galstian, A.; Butty, V.L.; Levine, S.S.; Patrapuvich, R.; Desai, S.P.; Mikolajczak, S.A.; Kappe, S.H.I.; et al. In vitro culture, drug sensitivity, and transcriptome of Plasmodium vivax hypnozoites. Cell Host Microbe 2018, 23, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Voorberg-van der Wel, A.; Roma, G.; Gupta, D.K.; Schuierer, S.; Nigsch, F.; Carbone, W.; Zeeman, A.-M.; Lee, B.H.; Hofman, S.O.; Faber, B.W.; et al. A comparative transcriptomic analysis of replicating and dormant liver stages of the relapsing malaria parasite Plasmodium cynomolgi. Elife 2017, 6, e29605. [Google Scholar] [CrossRef]
- Anstey, N.M.; Russell, B.; Yeo, T.W.; Price, R.N. The pathophysiology of vivax malaria. Trends Parasitol. 2009, 25, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.E.; William, T.; Grigg, M.J.; Menon, J.; Auburn, S.; Marfurt, J.; Anstey, N.M.; Yeo, T.W. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: High proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. 2013, 56, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Cox-Singh, J.; Davis, T.M.; Lee, K.S.; Shamsul, S.S.G.; Matusop, A.; Ratnam, S.; Rahman, H.A.; Conway, D.J.; Singh, B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life-threatening. Clin. Infect. Dis. 2008, 46, 165–171. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Cox-Singh, J. Plasmodium knowlesi—An emerging pathogen. ISBT Sci. Ser. 2015, 10 (Suppl. 1), 134–140. [Google Scholar] [CrossRef]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I. Plasmodium falciparum rhoptry proteins: Key players in erythrocyte invasion and promising targets for vaccine development. Vaccine 2020, 38, 5237–5244. [Google Scholar] [CrossRef]
- Abdi, A.I.; Hodgson, S.H.; Muthui, M.K.; Kivisi, C.A.; Osier, F.H. Plasmodium falciparum virulence and immune evasion: Insights from genomic and transcriptomic analyses. Nat. Rev. Microbiol. 2020, 18, 100–112. [Google Scholar] [CrossRef]
- Taylor, S.M.; Cerami, C.; Fairhurst, R.M. Host genetic factors influencing malaria outcomes. Annu. Rev. Med. 2019, 70, 167–181. [Google Scholar] [CrossRef]
- Percário, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.S.; Gonçalves, A.C.M.; Laurindo, P.S.O.C.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef]
- Sharma, L.; Kaur, J.; Shukla, G. Role of oxidative stress and apoptosis in the placental pathology of malaria. Parasitology 2017, 144, 1078–1087. [Google Scholar] [CrossRef]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Van Den Ham, K.; Shio, M.T.; Kassa, F.A.; Fougeray, S. Malarial pigment hemozoin and the innate inflammatory response. Front. Immunol. 2014, 5, 25. [Google Scholar] [CrossRef]
- Schwarzer, E.; Skorokhod, O. Post-Translational Modifications of Proteins of Malaria Parasites during the Life Cycle. Int. J. Mol. Sci. 2024, 25, 6145. [Google Scholar] [CrossRef]
- Siddiqui, G.; Srivastava, A.; Russell, A.S.; Creek, D.J. Multi-omics analysis reveals the extent of post-translational modifications and their role in the life cycle of Plasmodium falciparum. Front. Cell Infect. Microbiol. 2020, 10, 393. [Google Scholar] [CrossRef]
- Menard, D.; Dondorp, A.M. Antimalarial drug resistance: A threat to malaria elimination. Nat. Rev. Microbiol. 2022, 20, 183–196. [Google Scholar] [CrossRef]
- Sutherland, C.J.; Henrici, R.C.; Artavanis-Tsakonas, K. Multidrug-resistant malaria: Epidemiology and mechanisms of parasite resistance. Trends Parasitol. 2021, 37, 519–533. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.-C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Florens, L.; Washburn, M.P.; Raine, J.D.; Anthony, R.M.; Grainger, M.; Haynes, J.D.; Moch, J.K.; Muster, N.; Sacci, J.B.; Tabb, D.L.; et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 2002, 419, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Allman, E.L.; Painter, H.J.; Samra, J.; Carrasquilla, M.; Llinás, M. Metabolomic Profiling of the Malaria Box Reveals Antimalarial Target Pathways. Antimicrob. Agents Chemother. 2016, 60, 6635–6649. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.; Ashley, E.A.; Ferreira, P.E.; Zhu, L.; Lin, Z.; Yeo, T.; Chotivanich, K.; Imwong, M.; Pukrittayakamee, S.; Dhorda, M.; et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 2015, 347, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.D.; Gilabert, A.; Crellen, T. Whole-genome sequencing of Plasmodium falciparum. Trends Parasitol. 2018, 34, 230–243. [Google Scholar] [CrossRef]
- Cowell, A.N.; Winzeler, E.A. The genomic architecture of antimalarial drug resistance. Brief. Funct. Genom. 2019, 18, 314–328. [Google Scholar] [CrossRef]
- Lee, H.J.; Georgiadou, A.; Walther, M.; Nwakanma, D. Transcriptomic analysis of malaria parasites. Front. Cell. Infect. Microbiol. 2018, 8, 190. [Google Scholar] [CrossRef]
- Toenhake, C.G.; Bártfai, R. What functional genomics has taught us about transcriptional regulation in malaria parasites. Brief. Funct. Genom. 2019, 18, 290–301. [Google Scholar] [CrossRef]
- Reid, A.J.; Talman, A.M.; Bennett, H.M.; Gomes, A.R.; Sanders, M.J.; Illingworth, C.J.R.; Billker, O.; Berriman, M.; Lawniczak, M.K. Single-cell RNA-seq reveals hidden transcriptional variation in malaria parasites. eLife 2018, 7, e33105. [Google Scholar] [CrossRef]
- Phillips, M.A.; Rathod, P.K.; Prigge, S.T. Targeting the malaria parasite’s pyrimidine biosynthesis pathway for antimalarial drug development. Biochim. Et Biophys. Acta 2015, 1854, 1467–1475. [Google Scholar] [CrossRef]
- Yeh, E.; DeRisi, J.L. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 2011, 9, e1001138. [Google Scholar] [CrossRef]
- Spillman, N.J.; Allen, R.J.W.; McNamara, C.W.; Yeung, B.K.S.; Winzeler, E.A.; Kirk, K. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 2013, 13, 227–237. [Google Scholar] [CrossRef]
- Istvan, E.S.; Dharia, N.V.; Bopp, S.E.; Gluzman, I.Y.; Winzeler, E.A.; Goldberg, D.E. Validation of isoprenoid biosynthesis as an antimalarial drug target through chemical rescue of apicoplast function. Proc. Natl. Acad. Sci. USA 2019, 116, 8601–8606. [Google Scholar] [CrossRef]
- Lindner, S.E.; Swearingen, K.E.; Shears, M.J.; Walker, M.P.; Vrana, E.N. Proteomics and malaria research: Identifying key proteins and pathways for therapeutic intervention. Malar. J. 2019, 18, 242. [Google Scholar] [CrossRef]
- Florens, L.; Washburn, M.P.; Swearingen, K.E. Proteomics approaches to understanding Plasmodium biology and identifying novel drug targets. Expert. Rev. Proteom. 2018, 15, 39–55. [Google Scholar] [CrossRef]
- Paul, A.S.; Egan, E.S.; Duraisingh, M.T. Host-parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 2020, 27, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Doerig, C.; Rayner, J.C.; Scherf, A.; Tobin, A.B. Post-translational protein modifications in malaria parasites. Nat. Rev. Microbiol. 2015, 13, 160–172. [Google Scholar] [CrossRef]
- Yakubu, R.R.; Weiss, L.M.; Silmon de Monerri, N.C. Post-translational modifications as key regulators of apicomplexan biology. Front. Cell Infect. Microbiol. 2018, 8, 265. [Google Scholar] [CrossRef]
- Alam, M.M.; Solyakov, L.; Bottrill, A.R.; Flueck, C.; Siddiqui, F.A.; Singh, S.; Mistry, S.; Viskaduraki, M.; Lee, K.; Hopp, C.S.; et al. Phosphoproteomics reveals malaria parasite protein kinase G as a signalling hub regulating egress and invasion. Nat. Commun. 2015, 6, 7285. [Google Scholar] [CrossRef]
- Treeck, M.; Sanders, J.L.; Elias, J.E.; Boothroyd, J.C. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe 2011, 10, 410–419. [Google Scholar] [CrossRef]
- Gupta, A.P.; Bozdech, Z. Epigenetic landscapes underlying malaria parasite life-cycle progression. J. Cell Sci. 2017, 130, 671–679. [Google Scholar] [CrossRef]
- Batugedara, G.; Lu, X.M.; Saraf, A.; Sardiu, M.E.; Cort, A.; Abel, S.; Prudhomme, J.; Washburn, M.P.; Florens, L.; Le Roch, K.G. The chromatin landscape of the malaria parasite Plasmodium falciparum throughout its life cycle. PLoS Pathog. 2020, 16, e1009105. [Google Scholar] [CrossRef]
- Reverte, M.; Snäkä, T.; Fasel, N. The dangerous liaisons in oxidative stress: Protein lipoxidation in infection. Pathogens 2022, 11, 409. [Google Scholar] [CrossRef]
- King, N.R.; Martins Freire, C.; Touhami, J.; Sitbon, M.; Toye, A.M.; Satchwell, T.J. Basigin mediation of Plasmodium falciparum red blood cell invasion does not require its transmembrane domain or interaction with monocarboxylate transporter 1. PLoS Pathog. 2024, 20, e1011989. [Google Scholar] [CrossRef]
- Collins, C.R.; Hackett, F.; Strath, M.; Penzo, M.; Withers-Martinez, C.; Baker, D.A.; Blackman, M.J. Malaria parasite cGMP-dependent protein kinase regulates host-cell egress. Nat. Commun. 2013, 4, 2261. [Google Scholar] [CrossRef]
- Glennon, E.K.K.; Dankwa, S.; Smith, J.D.; Kaushansky, A. Opportunities for host-targeted therapies for malaria. Trends Parasitol. 2018, 34, 843–860. [Google Scholar] [CrossRef]
- Portugal, S.; Pierce, S.K.; Crompton, P.D. Young lives lost as B cells falter: What we are learning about antibody responses in malaria. J. Immunol. 2013, 190, 3039–3046. [Google Scholar] [CrossRef]
- Wassmer, S.C.; Grau, G.E. Severe malaria: What’s new on the pathogenesis front? Int. J. Parasitol. 2017, 47, 145–152. [Google Scholar] [CrossRef]
- Moxon, C.A.; Grau, G.E.; Craig, A.G. Malaria: Modification of the red blood cell and consequences in the human host. Br. J. Haematol. 2011, 154, 670–679. [Google Scholar] [CrossRef]
- Olszewski, K.L.; Llinás, M. Central carbon metabolism of Plasmodium parasites. Mol. Biochem. Parasitol. 2019, 233, 111221. [Google Scholar] [CrossRef]
- Srivastava, A.; Philip, N.; Hughes, K.R.; Georgiou, K.; MacRae, J.I.; Barrett, M.P. Stage-specific changes in Plasmodium falciparum metabolism required for differentiation and adaptation to human host. Nat. Commun. 2016, 7, 11024. [Google Scholar] [CrossRef]
- Guler, J.L.; Freeman, D.L.; Ahyong, V.; Patrapuvich, R.; White, J.; Gujjar, R.; Fidock, D.A. A proteomic analysis of the Plasmodium falciparum digestive vacuole. Mol. Biochem. Parasitol. 2013, 190, 80–90. [Google Scholar] [CrossRef]
- Li, H.; O’Donoghue, A.J.; van der Linden, W.A.; Xie, S.C.; Yoo, E.; Foe, I.T.; Bogyo, M. Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature 2016, 530, 233–236. [Google Scholar] [CrossRef]
- Cowell, A.; Winzeler, E. Exploration of the Plasmodium falciparum Resistome and Druggable Genome Reveals New Mechanisms of Drug Resistance and Antimalarial Targets. Microbiol. Insights 2018, 11, 1178636118808529. [Google Scholar] [CrossRef]
- Lindner, S.E.; Swearingen, K.E.; Harupa, A.; Vaughan, A.M.; Sinnis, P.; Moritz, R.L.; Kappe, S.H. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol. Cell Proteom. 2013, 12, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Rowe, J.A.; Higgins, M.K.; Lavstsen, T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 2019, 21, e13070. [Google Scholar] [CrossRef]
- Wahlgren, M.; Goel, S.; Akhouri, R.R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 2020, 18, 479–491. [Google Scholar] [CrossRef]
- Wright, G.J.; Rayner, J.C.; Jore, M.M. Structural insights into malaria vaccine targets and mechanisms of immune evasion. PLoS Pathog. 2016, 12, e1005456. [Google Scholar] [CrossRef]
- Coban, C.; Lee, M.S.J.; Ishii, K.J. Tissue-specific immunopathology during malaria infection. Nat. Rev. Immunol. 2018, 18, 266–278. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Pryce, J.; Medley, N.; Choi, L. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst. Rev. 2022, 1, CD012688. [Google Scholar] [CrossRef]
- Coulibaly, D.; Kone, A.K.; Traore, K.; Niangaly, A.; Kouriba, B.; Arama, C.; Zeguime, A.; Dolo, A.; Lyke, K.E.; Plowe, C.V.; et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: A randomized, controlled phase 1 trial. EClinicalMedicine 2022, 52, 101579. [Google Scholar] [CrossRef] [PubMed]
- Lamers, O.A.C.; Franke-Fayard, B.M.D.; Koopman, J.P.R.; Roozen, G.V.T.; Janse, J.J.; Chevalley-Maurel, S.C.; Geurten, F.J.A.; de Bes-Roeleveld, H.M.; Iliopoulou, E.; Colstrup, E.; et al. Safety and Efficacy of Immunization with a Late-Liver-Stage Attenuated Malaria Parasite. N. Engl. J. Med. 2024, 391, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Silk, S.E.; Kalinga, W.F.; Salkeld, J.; Mtaka, I.M.; Ahmed, S.; Milando, F.; Diouf, A.; Bundi, C.K.; Balige, N.; Hassan, O.; et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet 2024, 24, 1105–1117. [Google Scholar] [CrossRef]
- Stewart, A.G.A.; Zimmerman, P.A.; McCarthy, J.S. Genetic Variation of G6PD and CYP2D6: Clinical Implications on the Use of Primaquine for Elimination of Plasmodium vivax. Front. Pharmacol. 2021, 12, 784909. [Google Scholar] [CrossRef] [PubMed]
- Pernaute-Lau, L.; Morris, U.; Msellem, M.; Mårtensson, A.; Björkman, A.; Gil, J.P. Influence of cytochrome P450 (CYP) 2C8 polymorphisms on the efficacy and tolerability of artesunate-amodiaquine treatment of uncomplicated Plasmodium falciparum malaria in Zanzibar. Malar. J. 2021, 20, 90. [Google Scholar] [CrossRef]
- Vanheer, L.N.; Zhang, H.; Lin, G.; Kafsack, B.F.C. Activity of Epigenetic Inhibitors against Plasmodium falciparum Asexual and Sexual Blood Stages. Antimicrob. Agents Chemother. 2020, 64, e02523-19. [Google Scholar] [CrossRef]
- Coetzee, N.; von Grüning, H.; Opperman, D.; van der Watt, M.; Reader, J.; Birkholtz, L.M. Epigenetic inhibitors target multiple stages of Plasmodium falciparum parasites. Sci. Rep. 2020, 10, 2355. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial drug discovery: Progress and approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Yipsirimetee, A.; Chiewpoo, P.; Tripura, R.; Lek, D.; Day, N.P.J.; Dondorp, A.M.; Pukrittayakamee, S.; White, N.J.; Chotivanich, K. Assessment In Vitro of the Antimalarial and Transmission-Blocking Activities of Cipargamin and Ganaplacide in Artemisinin-Resistant Plasmodium falciparum. Antimicrob. Agents Chemother. 2022, 66, e0148121. [Google Scholar] [CrossRef] [PubMed]
- Huskey, S.E.; Zhu, C.Q.; Fredenhagen, A.; Kühnöl, J.; Luneau, A.; Jian, Z.; Yang, Z.; Miao, Z.; Yang, F.; Jain, J.P.; et al. KAE609 (Cipargamin), a New Spiroindolone Agent for the Treatment of Malaria: Evaluation of the Absorption, Distribution, Metabolism, and Excretion of a Single Oral 300-mg Dose of [14C]KAE609 in Healthy Male Subjects. Drug Metab. Dispos. Biol. Fate Chem. 2016, 44, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Gal, I.R.; Demarta-Gatsi, C.; Fontinha, D.; Arez, F.; Wicha, S.G.; Rottmann, M.; Nunes-Cabaço, H.; Blais, J.; Jain, J.P.; Lakshminarayana, S.B.; et al. Drug Interaction Studies of Cabamiquine:Ganaplacide Combination against Hepatic Plasmodium berghei. ACS Infect. Dis. 2025, 11, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mahajan, A.; Chibale, K. Synthetic medicinal chemistry of selected antimalarial natural products. Bioorganic Med. Chem. 2009, 17, 2236–2275. [Google Scholar] [CrossRef]
- Kingston, D.G.I.; Cassera, M.B. Antimalarial Natural Products; Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2022; Volume 117, pp. 1–106. [Google Scholar] [CrossRef]
- Persico, M.; Fattorusso, R.; Taglialatela-Scafati, O.; Chianese, G.; de Paola, I.; Zaccaro, L.; Rondinelli, F.; Lombardo, M.; Quintavalla, A.; Trombini, C.; et al. The interaction of heme with plakortin and a synthetic endoperoxide analogue: New insights into the heme-activated antimalarial mechanism. Sci. Rep. 2017, 7, 45485. [Google Scholar] [CrossRef]
- Aguiar, A.C.C.; Parisi, J.R.; Granito, R.N.; de Sousa, L.R.F.; Renno, A.C.M.; Gazarini, M.L. Metabolites from Marine Sponges and Their Potential to Treat Malarial Protozoan Parasites Infection: A Systematic Review. Mar. Drugs 2021, 19, 134. [Google Scholar] [CrossRef]
- Belete, T.M. Recent Progress in the Development of New Antimalarial Drugs with Novel Targets. Drug Des. Dev. Ther. 2020, 14, 3875–3889. [Google Scholar] [CrossRef]
- Rodrigo, C.; Rajapakse, S.; Fernando, D. Tafenoquine for preventing relapse in people with Plasmodium vivax malaria. Cochrane Database Syst. Rev. 2020, 9, CD010458. [Google Scholar] [CrossRef]
- Wiser, M.F. The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome. Pathogens 2024, 13, 182. [Google Scholar] [CrossRef]
- Hirako, I.C.; Antunes, M.M.; Rezende, R.M.; Hojo-Souza, N.S.; Figueiredo, M.M.; Dias, T.; Nakaya, H.; Menezes, G.B.; Gazzinelli, R.T. Uptake of Plasmodium chabaudi hemozoin drives Kupffer cell death and fuels superinfections. Sci. Rep. 2022, 12, 19805. [Google Scholar] [CrossRef]
- Dumarchey, A.; Lavazec, C.; Verdier, F. Erythropoiesis and Malaria, a Multifaceted Interplay. Int. J. Mol. Sci. 2022, 23, 12762. [Google Scholar] [CrossRef] [PubMed]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: A comparative study with human and rodent parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [PubMed]
- Rishikesh, K.; Saravu, K. Primaquine treatment and relapse in Plasmodium vivax malaria. Pathog. Glob. Health 2016, 110, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; O’Neil, M.; Xie, L.; Caridha, D.; Zeng, Q.; Zhang, J.; Pybus, B.; Hickman, M.; Melendez, V. Assessment of the prophylactic activity and pharmacokinetic profile of oral tafenoquine compared to primaquine for inhibition of liver stage malaria infections. Malar. J. 2014, 13, 141. [Google Scholar] [CrossRef]
- Aleshnick, M.; Florez-Cuadros, M.; Martinson, T.; Wilder, B.K. Monoclonal antibodies for malaria prevention. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 1810–1821. [Google Scholar] [CrossRef]
- Dacon, C.; Moskovitz, R.; Swearingen, K.; Da Silva Pereira, L.; Flores-Garcia, Y.; Aleshnick, M.; Kanatani, S.; Flynn, B.; Molina-Cruz, A.; Wollenberg, K.; et al. Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science 2025, 387, eadr0510. [Google Scholar] [CrossRef]
- Marques-da-Silva, C.; Peissig, K.; Kurup, S.P. Pre-Erythrocytic Vaccines against Malaria. Vaccines 2020, 8, 400. [Google Scholar] [CrossRef]
- Burns, A.L.; Dans, M.G.; Balbin, J.M.; de Koning-Ward, T.F.; Gilson, P.R.; Beeson, J.G.; Boyle, M.J.; Wilson, D.W. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol. Rev. 2019, 43, 223–238. [Google Scholar] [CrossRef]
- Srinivasan, P.; Yasgar, A.; Luci, D.K.; Beatty, W.L.; Hu, X.; Andersen, J.; Narum, D.L.; Moch, J.K.; Sun, H.; Haynes, J.D.; et al. Disrupting malaria parasite AMA1-RON2 interaction with a small molecule prevents erythrocyte invasion. Nat. Commun. 2013, 4, 2261. [Google Scholar] [CrossRef]
- Cowman, A.F.; Crabb, B.S. Invasion of red blood cells by malaria parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef]
- Cohen, J.; Nussenzweig, V.; Nussenzweig, R.; Vekemans, J.; Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Human Vaccines 2010, 6, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert. Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Stockdale, L.; Sagara, I.; Zongo, I.; Yerbanga, R.S.; Mahamar, A.; Nikièma, F.; Tapily, A.; Sompougdou, F.; Diarra, M.; et al. The anti-circumsporozoite antibody response to repeated, seasonal booster doses of the malaria vaccine RTS,S/AS01E. NPJ Vaccines 2025, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Seidlein, L.V. Roll out and prospects of the malaria vaccine R21/Matrix-M. PLoS Med. 2025, 22, e1004515. [Google Scholar] [CrossRef]
- Guerra Mendoza, Y.; Garric, E.; Leach, A.; Lievens, M.; Ofori-Anyinam, O.; Pirçon, J.-Y.; Stegmann, J.-U.; Vandoolaeghe, P.; Otieno, L.; Otieno, W.; et al. Safety profile of the RTS,S/AS01 malaria vaccine in infants and children: Additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum. Vaccines Immunother. 2019, 15, 2386–2398. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Emran, T.B.; Amelia, F.; Islam, A.; Syafira, I.; et al. A Viral-Vectored Multi-Stage Malaria Vaccine Regimen With Protective and Transmission-Blocking Efficacies. Front. Immunol. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Tsoumani, M.E.; Voyiatzaki, C.; Efstathiou, A. Malaria Vaccines: From the Past towards the mRNA Vaccine Era. Vaccines 2023, 11, 1452. [Google Scholar] [CrossRef]
- Alanine, D.G.W.; Quinkert, D.; Kumarasingha, R.; Mehmood, S.; Donnellan, F.R.; Minkah, N.K.; Dadonaite, B.; Diouf, A.; Galaway, F.; Silk, S.E.; et al. Human Antibodies That Slow Erythrocyte Invasion Potentiate Malaria-Neutralizing Antibodies. Cell 2019, 178, 216–228.e21. [Google Scholar] [CrossRef]
- Frosch, A.E.; John, C.C. Immunomodulation in Plasmodium falciparum malaria: Experiments in nature and their conflicting implications for potential therapeutic agents. Expert. Rev. Anti-Infect. Ther. 2012, 10, 1343–1356. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Berkowitz, N.M.; Idris, A.H.; Coates, E.E.; Holman, L.A.; Mendoza, F.; Gordon, I.J.; Plummer, S.H.; Trofymenko, O.; Hu, Z.; et al. A Monoclonal Antibody for Malaria Prevention. N. Engl. J. Med. 2021, 385, 803–814. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Gao, B.; Amaratunga, C.; Dhorda, M.; Tran, T.N.; White, N.J.; Dondorp, A.M.; Boni, M.F.; Aguas, R. Preventing antimalarial drug resistance with triple artemisinin-based combination therapies. Nat. Commun. 2023, 14, 4568. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.Q.; Cunha, N.; Varela, E.L.P.; Brígido, H.P.C.; Vale, V.V.; Dolabela, M.F.; De Carvalho, E.P.; Percário, S. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int. J. Mol. Sci. 2022, 23, 5949. [Google Scholar] [CrossRef]
- Loveridge, K.M.; Sigala, P.A. Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites. Proc. Natl. Acad. Sci. USA 2024, 121, e2411631121. [Google Scholar] [CrossRef] [PubMed]
- Varo, R.; Crowley, V.M.; Mucasse, H.; Sitoe, A.; Bramugy, J.; Serghides, L.; Weckman, A.M.; Erice, C.; Bila, R.; Vitorino, P.; et al. Adjunctive rosiglitazone treatment for severe pediatric malaria: A randomized placebo-controlled trial in Mozambican children. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Diseases 2024, 139, 34–40. [Google Scholar] [CrossRef]
- Rasmussen, C.; Alonso, P.; Ringwald, P. Current and emerging strategies to combat antimalarial resistance. Expert. Rev. Anti-Infect. Ther. 2022, 20, 353–372. [Google Scholar] [CrossRef]
- Wellems, T.E.; Plowe, C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001, 184, 770–776. [Google Scholar] [CrossRef]
- Hastings, I.M.; Watkins, W.M. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005, 94, 218–229. [Google Scholar] [CrossRef]
- Ogutu, B.; Yeka, A.; Kusemererwa, S.; Thompson, R.; Tinto, H.; Toure, A.O.; Uthaisin, C.; Verma, A.; Kibuuka, A.; Lingani, M.; et al. Ganaplacide (KAF156) plus lumefantrine solid dispersion formulation combination for uncomplicated Plasmodium falciparum malaria: An open-label, multicentre, parallel-group, randomised, controlled, phase 2 trial. Lancet. Infect. Dis. 2023, 23, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. Antimalarial drug synergism and antagonism: Mechanistic and clinical significance. FEMS Microbiol. Lett. 2005, 253, 171–184. [Google Scholar] [CrossRef]
- Gupta, S.; Thapar, M.M.; Wernsdorfer, W.H.; Björkman, A. In vitro interactions of artemisinin with atovaquone, quinine, and mefloquine against Plasmodium falciparum. Antimicrob. Agents Chemother. 2002, 46, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, I.; Soebandrio, A.; Ekawati, L.L.; Chand, K.; Noviyanti, R.; Satyagraha, A.W.; Subekti, D.; Santy, Y.W.; Crenna-Darusallam, C.; Instiaty, I.; et al. Tafenoquine co-administered with dihydroartemisinin-piperaquine for the radical cure of Plasmodium vivax malaria (INSPECTOR): A randomised, placebo-controlled, efficacy and safety study. Lancet Infect. Dis. 2023, 23, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Harmonis, J.A.; Kusuma, S.A.F.; Rukayadi, Y.; Hasanah, A.N. Exploring Biomarkers for Malaria: Advances in Early Detection and Asymptomatic Diagnosis. Biosensors 2025, 15, 106. [Google Scholar] [CrossRef]
- Pickard, A.L.; Wongsrichanalai, C.; Purfield, A.; Kamwendo, D.; Emery, K.; Zalewski, C.; Kawamoto, F.; Miller, R.S.; Meshnick, S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 2003, 47, 2418–2423. [Google Scholar] [CrossRef]
- Ndiaye, D.; Dieye, B.; Ndiaye, Y.D.; Van Tyne, D.; Daniels, R.; Bei, A.K.; Mbaye, A.; Valim, C.; Lukens, A.; Mboup, S.; et al. Polymorphism in dhfr/dhps genes, parasite density and ex vivo response to pyrimethamine in Plasmodium falciparum malaria parasites in Thies, Senegal. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 135–142. [Google Scholar] [CrossRef]
- Nain, M.; Dhorda, M.; Flegg, J.A.; Gupta, A.; Harrison, L.E.; Singh-Phulgenda, S.; Otienoburu, S.D.; Harriss, E.; Bharti, P.K.; Behera, B.; et al. Systematic Review and Geospatial Modeling of Molecular Markers of Resistance to Artemisinins and Sulfadoxine-Pyrimethamine in Plasmodium falciparum in India. Am. J. Trop. Med. Hyg. 2024, 110, 910–920. [Google Scholar] [CrossRef]
- Lenharo, M. Resistance to crucial malaria drug detected in severely ill kids in Africa. Nature 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Small-Saunders, J.L.; Sinha, A.; Bloxham, T.S.; Hagenah, L.M.; Sun, G.; Preiser, P.R.; Dedon, P.C.; Fidock, D.A. tRNA modification reprogramming contributes to artemisinin resistance in Plasmodium falciparum. Nat. Microbiol. 2024, 9, 1483–1498. [Google Scholar] [CrossRef]
- Okwu, D.G.; Manego, R.Z.; Duparc, S.; Kremsner, P.G.; Ramharter, M.; Mombo-Ngoma, G. The non-artemisinin antimalarial drugs under development: A review. Clin. Microbiol. Infect. 2025, 31, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Medhi, B.; Sehgal, R. Challenges of drug-resistant malaria. Parasite 2014, 21, 61. [Google Scholar] [CrossRef]
- Luth, M.R.; Godinez-Macias, K.P.; Chen, D.; Okombo, J.; Thathy, V.; Cheng, X.; Daggupati, S.; Davies, H.; Dhingra, S.K.; Economy, J.M.; et al. Systematic in vitro evolution in Plasmodium falciparum reveals key determinants of drug resistance. Science 2024, 386, eadk9893. [Google Scholar] [CrossRef]
- Ndwiga, L.; Kimenyi, K.M.; Wamae, K.; Osoti, V.; Akinyi, M.; Omedo, I.; Ishengoma, D.S.; Duah-Quashie, N.; Andagalu, B.; Ghansah, A.; et al. A review of the frequencies of Plasmodium falciparum Kelch 13 artemisinin resistance mutations in Africa. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Stokes, B.H.; Dhingra, S.K.; Rubiano, K.; Mok, S.; Straimer, J.; Gnädig, N.F.; Deni, I.; Schindler, K.A.; Bath, J.R.; Ward, K.E.; et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. eLife 2021, 10, e66277. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.-L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.-B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef]
- Kümpornsin, K.; Loesbanluechai, D.; De Cozar, C.; Kotanan, N.; Chotivanich, K.; White, N.J.; Wilairat, P.; Gomez-Lorenzo, M.G.; Gamo, F.J.; Sanz, L.M.; et al. Lumefantrine attenuates Plasmodium falciparum artemisinin resistance during the early ring stage. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 186–190. [Google Scholar] [CrossRef]
- Oleinikov, A.V. Malaria Parasite Plasmodium falciparum Proteins on the Surface of Infected Erythrocytes as Targets for Novel Drug Discovery. Biochemistry 2022, 87 (Suppl. 1), S192–S202. [Google Scholar] [CrossRef]
- Jacob, C.G.; Thuy-Nhien, N.; Mayxay, M.; Maude, R.J.; Quang, H.H.; Hongvanthong, B.; Vanisaveth, V.; Duc, T.N.; Rekol, H.; Van Der Pluijm, R.; et al. Genetic surveillance in the Greater Mekong subregion and South Asia to support malaria control and elimination. eLife 2021, 10, e62997. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N.C.; Van Huijsduijnen, R.H.; Van Voorhis, W.C. Malaria medicines: A glass half full? Nat. Rev. Drug Discov. 2015, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.B.S.; Verdier-Pinard, D.; Fidock, D.A. Chloroquine Resistance in Plasmodium falciparum Malaria Parasites Conferred by pfcrt Mutations. Science 2002, 298, 210–213. [Google Scholar] [CrossRef]
- Alker, A.P.; Kazadi, W.M.; Kutelemeni, A.K.; Bloland, P.B.; Tshefu, A.K.; Meshnick, S.R. dhfr and dhps genotype and sulfadoxine-pyrimethamine treatment failure in children with falciparum malaria in the Democratic Republic of Congo. Trop. Med. Int. Health 2008, 13, 1384–1391. [Google Scholar] [CrossRef]
- Crompton, P.D.; Pierce, S.K.; Miller, L.H. Advances and challenges in malaria vaccine development. J. Clin. Investig. 2010, 120, 4168–4178. [Google Scholar] [CrossRef]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. MosquirixTM RTS, S/AS01 Vaccine Development, immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef]
- Laurens, M.B. RTS,S/AS01 vaccine (MosquirixTM): An overview. Hum. Vaccines Immunother. 2019, 16, 480–489. [Google Scholar] [CrossRef]
- Haine, V.; Oneko, M.; Debois, M.; Ndeketa, L.; Agyapong, P.D.; Boahen, O.; Harrison, S.B.E.; Adeniji, E.; Kaali, S.; Kayan, K.; et al. Safety of RTS,S/AS01E malaria vaccine up to 1 year after the third dose in Ghana, Kenya, and Malawi (EPI-MAL-003): A phase 4 cohort event monitoring study. Lancet Glob. Health 2025, 13, e995–e1005. [Google Scholar] [CrossRef]
- Schmit, N.; Topazian, H.M.; Natama, H.M.; Bellamy, D.; Traoré, O.; Somé, M.A.; Rouamba, T.; Tahita, M.C.; Bonko, M.D.A.; Sourabié, A.; et al. The public health impact and cost-effectiveness of the R21/Matrix-M malaria vaccine: A mathematical modelling study. Lancet Infect. Dis. 2024, 24, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Lopez, F.R.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Osoro, C.B.; Ochodo, E.; Kwambai, T.K.; Otieno, J.A.; Were, L.; Sagam, C.K.; Owino, E.J.; Kariuki, S.; Ter Kuile, F.O.; Hill, J. Policy uptake and implementation of the RTS,S/AS01 malaria vaccine in sub-Saharan African countries: Status 2 years following the WHO recommendation. BMJ Glob. Health 2024, 9, e014719. [Google Scholar] [CrossRef]
- Genton, B. R21/Matrix-MTM malaria vaccine: A new tool to achieve WHO’s goal to eliminate malaria in 30 countries by 2030? J. Travel Med. 2023, 30, taad140. [Google Scholar] [CrossRef] [PubMed]
- Richie, T.L.; Saul, A. Progress and challenges for malaria vaccines. Nature 2002, 415, 694–701. [Google Scholar] [CrossRef]
- Dieng, C.C.; Ford, C.T.; Lerch, A.; Doniou, D.; Vegesna, K.; Janies, D.; Cui, L.; Amoah, L.; Afrane, Y.; Lo, E. Genetic variations of Plasmodium falciparum circumsporozoite protein and the impact on interactions with human immunoproteins and malaria vaccine efficacy. Infect. Genet. Evol. 2023, 110, 105418. [Google Scholar] [CrossRef]
- Oyong, D.A.; Duffy, F.J.; Neal, M.L.; Du, Y.; Carnes, J.; Schwedhelm, K.V.; Hertoghs, N.; Jun, S.-H.; Miller, H.; Aitchison, J.D.; et al. Distinct immune responses associated with vaccination status and protection outcomes after malaria challenge. PLoS Pathog. 2023, 19, e1011051. [Google Scholar] [CrossRef]
- Jun, H.; Mazigo, E.; Lee, W.-J.; Louis, J.M.; Syahada, J.H.; Fitriana, F.; Heo, J.; Kim, Y.; Kwon, B.; Muh, F.; et al. Estimation of PfRh5-based vaccine efficacy in asymptomatic Plasmodium falciparum patients from high-endemic areas of Tanzania using genetic and antigenicity variation screening. Front. Immunol. 2024, 15, 1495513. [Google Scholar] [CrossRef]
- Brod, F.; Miura, K.; Taylor, I.; Li, Y.; Marini, A.; Salman, A.M.; Spencer, A.J.; Long, C.A.; Biswas, S. Combination of RTS,S and PFS25-IMX313 induces a functional antibody response against malaria infection and transmission in mice. Front. Immunol. 2018, 9, 2780. [Google Scholar] [CrossRef]
- Ilani, P.; Nyarko, P.B.; Camara, A.; Amenga-Etego, L.N.; Aniweh, Y. PfRH5 vaccine; from the bench to the vial. npj Vaccines 2025, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Thiam, L.G.; McHugh, K.; Ba, A.; Li, R.; Guo, Y.; Pouye, M.N.; Cisse, A.; Pipini, D.; Diallo, F.; Sene, S.D.; et al. Vaccine-induced human monoclonal antibodies to PfRH5 show broadly neutralizing activity against P. falciparum clinical isolates. npj Vaccines 2024, 9, 198. [Google Scholar] [CrossRef]
- Zeeshan, M.; Alam, M.T.; Vinayak, S.; Bora, H.; Tyagi, R.K.; Alam, M.S.; Choudhary, V.; Mittra, P.; Lumb, V.; Bharti, P.K.; et al. Genetic Variation in the Plasmodium falciparum Circumsporozoite Protein in India and Its Relevance to RTS,S Malaria Vaccine. PLoS ONE 2012, 7, e43430. [Google Scholar] [CrossRef]
- Feehan, J.; Plebanski, M.; Apostolopoulos, V. Recent perspectives in clinical development of malaria vaccines. Nat. Commun. 2025, 16, 3565. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Al-Khatib, A.O.; Al-Mahzoum, K.S.; Abdelaziz, D.H.; Sallam, M. Current Developments in Malaria Vaccination: A concise review on implementation, challenges, and future directions. Clin. Pharmacol. Adv. Appl. 2025, 17, 29–47. [Google Scholar] [CrossRef]
- Ross, L.S.; Fidock, D.A. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef]
- Calle, C.L.; Mordmüller, B.; Singh, A. Immunosuppression in malaria: Do Plasmodium falciparum parasites hijack the host? Pathogens 2021, 10, 1277. [Google Scholar] [CrossRef]
- Augustijn, K.D.; Kleemann, R.; Thompson, J.; Kooistra, T.; Crawford, C.E.; Reece, S.E.; Pain, A.; Siebum, A.H.G.; Janse, C.J.; Waters, A.P. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect. Immun. 2007, 75, 1116–1128. [Google Scholar] [CrossRef]
- Walther, M.; Tongren, J.E.; Andrews, L.; Korbel, D.; King, E.; Fletcher, H.; Andersen, R.F.; Bejon, P.; Thompson, F.; Dunachie, S.J.; et al. Upregulation of TGF-β, FOXP3, and CD4+ CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 2005, 23, 287–296. [Google Scholar] [CrossRef]
- Portugal, S.; Tipton, C.M.; Sohn, H.; Kone, Y.; Wang, J.; Li, S.; Skinner, J.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; et al. Malar-ia-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. eLife 2015, 4, e07218. [Google Scholar] [CrossRef]
- Che, J.N.; Nmorsi, O.P.G.; Nkot, B.P.; Isaac, C.; Okonkwo, B.C. Chemokines responses to Plasmodium falciparum malaria and co-infections among rural Cameroonians. Parasitol. Int. 2015, 64, 139–144. [Google Scholar] [CrossRef]
- Urban, B.C.; Ferguson, D.J.P.; Pain, A.; Willcox, N.; Plebanski, M.; Austyn, J.M.; Roberts, D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999, 400, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Hajime, H.; Yasutomo, K.; Himeno, K. Malaria: Immune evasion by parasites. Int. J. Biochem. Cell Biol. 2005, 37, 700–706. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef]
- Wang, R.; Smith, J.D.; Kappe, S.H.I. Advances and challenges in malaria vaccine development. Expert Rev. Mol. Med. 2009, 11, e39. [Google Scholar] [CrossRef][Green Version]
- Locke, E.; Flores-Garcia, Y.; Mayer, B.T.; MacGill, R.S.; Borate, B.; Salgado-Jimenez, B.; Gerber, M.W.; Mathis-Torres, S.; Shapiro, S.; King, C.R.; et al. Establishing RTS,S/AS01 as a benchmark for comparison to next-generation malaria vaccines in a mouse model. npj Vaccines 2024, 9, 29. [Google Scholar] [CrossRef]
- Miura, K.; Flores-Garcia, Y.; Long, C.A.; Zavala, F. Vaccines and monoclonal antibodies: New tools for malaria control. Clin. Microbiol. Rev. 2024, 37, e00071-23. [Google Scholar] [CrossRef] [PubMed]
- Kazmin, D.; Nakaya, H.I.; Lee, E.K.; Johnson, M.J.; Van Der Most, R.; Van Den Berg, R.A.; Ballou, W.R.; Jongert, E.; Wille-Reece, U.; Ockenhouse, C.; et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.L.; Duffy, F.J.; Du, Y.; Aitchison, J.D.; Stuart, K.D. Preimmunization correlates of protection shared across malaria vaccine trials in adults. npj Vaccines 2022, 7, 5. [Google Scholar] [CrossRef]
- Hayashi, C.T.H.; Cao, Y.; Clark, L.C.; Tripathi, A.K.; Zavala, F.; Dwivedi, G.; Knox, J.; Alameh, M.-G.; Lin, P.J.C.; Tam, Y.K.; et al. mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. npj Vaccines 2022, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Kayentao, K.; Ongoiba, A.; Preston, A.C.; Healy, S.A.; Doumbo, S.; Doumtabe, D.; Traore, A.; Traore, H.; Djiguiba, A.; Li, S.; et al. Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali. N. Engl. J. Med. 2022, 387, 1833–1842. [Google Scholar] [CrossRef]
- Suscovich, T.J.; Fallon, J.K.; Das, J.; Demas, A.R.; Crain, J.; Linde, C.H.; Michell, A.; Natarajan, H.; Arevalo, C.; Broge, T.; et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci. Transl. Med. 2020, 12, eabb4757. [Google Scholar] [CrossRef]
- World Malaria Report 2024: Addressing Inequity in the Global Response; Available online: https://policycommons.net/artifacts/17877248/world-malaria-report-2024/18773006/World Health Organization: Geneva, Switzerland, 2024; (accessed on 7 May 2025).
- Venkatesan, P. WHO World Malaria Report 2024. Lancet Microbe 2025, 6, 101073. [Google Scholar] [CrossRef]
- Global Technical Strategy for Malaria 2016–2030; Available online: https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdfWorld Health Organization: Geneva, Switzerland, 2015; (accessed on 7 May 2025).
- Degarege, A.; Fennie, K.; Degarege, D.; Chennupati, S.; Madhivanan, P. Improving socioeconomic status may reduce the burden of malaria in sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0211205. [Google Scholar] [CrossRef]
- Tusting, L.S.; Rek, J.C.; Arinaitwe, E.; Staedke, S.G.; Kamya, M.R.; Bottomley, C.; Johnston, D.; Lines, J.; Dorsey, G.; Lindsay, S.W. Measuring socioeconomic inequalities in relation to malaria risk: A comparison of metrics in rural Uganda. Am. J. Trop. Med. Hyg. 2016, 94, 650–658. [Google Scholar] [CrossRef]
- Chiziba, C.; Mercer, L.D.; Diallo, O.; Bertozzi-Villa, A.; Weiss, D.J.; Gerardin, J.; Ozodiegwu, I.D. Socioeconomic, demographic, and environmental factors may inform malaria intervention prioritization in urban Nigeria. Int. J. Environ. Res. Public Health 2024, 21, 78. [Google Scholar] [CrossRef]
- Kooko, R.; Wafula, S.T.; Orishaba, P. Socioeconomic determinants of malaria prevalence among under five children in Uganda: Evidence from 2018-19 Uganda Malaria Indicator Survey. J. Vector Borne Dis. 2022, 60, 38. [Google Scholar] [CrossRef] [PubMed]
- Apeagyei, A.E.; Patel, N.K.; Cogswell, I.; O’Rourke, K.; Tsakalos, G.; Dieleman, J. Examining geographical inequalities for malaria outcomes and spending on malaria in 40 malaria-endemic countries, 2010–2020. Malar. J. 2024, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Obeagu, G.U. Adapting to the shifting landscape: Implications of climate change for malaria control: A review. Medicine 2024, 103, e39010. [Google Scholar] [CrossRef]
- Patouillard, E.; Griffin, J.; Bhatt, S.; Ghani, A.; Cibulskis, R. Global investment targets for malaria control and elimination between 2016 and 2030. BMJ Glob. Health 2017, 2, e000176. [Google Scholar] [CrossRef]
- Hill, J.; Bange, T.; Hoyt, J.; Kariuki, S.; Jalloh, M.F.; Webster, J.; Okello, G. Integration of the RTS,S/AS01 malaria vaccine into the Essential Programme on Immunisation in western Kenya: A qualitative longitudinal study from the health system perspective. Lancet Glob. Health 2024, 12, e672–e684. [Google Scholar] [CrossRef]
- De Boeck, K.; Decouttere, C.; Jónasson, J.O.; Vandaele, N. Vaccine supply chains in resource-limited settings: Mitigating the impact of rainy season disruptions. Eur. J. Oper. Res. 2021, 301, 300–317. [Google Scholar] [CrossRef]
- Okesanya, O.J.; Atewologun, F.; Lucero-Prisno, D.E.; Adigun, O.A.; Oso, T.A.; Manirambona, E.; Olabode, N.O.; Eshun, G.; Agboola, A.O.; Okon, I.I. Bridging the gap to malaria vaccination in Africa: Challenges and opportunities. J. Med. Surg. Public Health 2024, 2, 100059. [Google Scholar] [CrossRef]
- Dimala, C.A.; Kika, B.T.; Kadia, B.M.; Blencowe, H. Current challenges and proposed solutions to the effective implementation of the RTS, S/AS01 Malaria Vaccine Program in sub-Saharan Africa: A systematic review. PLoS ONE 2018, 13, e0209744. [Google Scholar] [CrossRef]
- WHO. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk: Historic RTS,S/AS01 Recommendation Can Reinvigorate the Fight Against Malaria. 2021. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 6 May 2025).
- Amimo, F. Malaria vaccination: Hurdles to reach high-risk children. BMC Med. 2024, 22, 111. [Google Scholar] [CrossRef]
- Mboussou, F.; Ndoula, S.T.; Nembot, R.; Baonga, S.F.; Njinkeu, A.; Njoh, A.A.; Biey, J.N.-M.; Kaba, M.; Amani, A.; Farham, B.; et al. Setting up a data system for monitoring malaria vaccine introduction readiness and uptake in 42 health districts in Cameroon. BMJ Glob. Health 2024, 9, e015312. [Google Scholar] [CrossRef]
- Kokwaro, G. Ongoing challenges in the management of malaria. Malar. J. 2009, 8, S2. [Google Scholar] [CrossRef] [PubMed]
- Von Seidlein, L.; Hanboonkunupakarn, B.; Jittamala, P.; Pongsuwan, P.; Chotivanich, K.; Tarning, J.; Hoglund, R.M.; Winterberg, M.; Mukaka, M.; Peerawaranun, P.; et al. Combining antimalarial drugs and vaccine for malaria elimination campaigns: A randomized safety and immunogenicity trial of RTS,S/AS01 administered with dihydroartemisinin, piperaquine, and primaquine in healthy Thai adult volunteers. Hum. Vaccines Immunother. 2019, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fambirai, T.; Chimbari, M.J.; Ndarukwa, P. Global Cross-Border Malaria Control Collaborative Initiatives: A Scoping review. Int. J. Environ. Res. Public Health 2022, 19, 12216. [Google Scholar] [CrossRef]
- Sikaala, C.H.; Dlamini, B.; Lungu, A.; Fakudze, P.; Chisenga, M.; Siame, C.L.; Mwendera, N.; Shaba, D.; Chimumbwa, J.M.; Kleinschmidt, I. Malaria elimination and the need for intensive inter-country cooperation: A critical evaluation of regional technical co-operation in Southern Africa. Malar. J. 2024, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Agaba, B.B.; Travis, J.; Smith, D.; Rugera, S.P.; Zalwango, M.G.; Opigo, J.; Katureebe, C.; Mpirirwe, R.; Bakary, D.; Antonio, M.; et al. Emerging threat of artemisinin partial resistance markers (pfk13 mutations) in Plasmodium falciparum parasite populations in multiple geographical locations in high transmission regions of Uganda. Malar. J. 2024, 23, 330. [Google Scholar] [CrossRef]
- Gates Foundation Commits $258.3 Million for Malaria Research and Development. Available online: https://www.gatesfoundation.org/ideas/media-center/press-releases/2005/10/gates-foundation-commits-2583-million-for-malaria-research (accessed on 6 May 2025).
- Malaria. Eradication, Prevention, Through Innovation & Data. Available online: https://www.gatesfoundation.org/our-work/programs/global-health/malaria (accessed on 6 May 2025).
- Jakubowski, A.; Stearns, S.C.; Kruk, M.E.; Angeles, G.; Thirumurthy, H. The US President’s Malaria Initiative and under-5 child mortality in sub-Saharan Africa: A difference-in-differences analysis. PLoS Med. 2017, 14, e1002319. [Google Scholar] [CrossRef]
- Impacts of the ExxonMobil Malaria Initiative. ExxonMobil. Available online: https://corporate.exxonmobil.com/community/the-fight-against-malaria/impacts-of-the-exxonmobil-malaria-initiative (accessed on 6 May 2025).
- Moonen, B.; Cohen, J.M.; Snow, R.W.; Slutsker, L.; Drakeley, C.; Smith, D.L.; Abeyasinghe, R.R.; Rodriguez, M.H.; Maharaj, R.; Tanner, M.; et al. Operational strategies to achieve and maintain malaria elimination. Lancet 2010, 376, 1592–1603. [Google Scholar] [CrossRef]
- Tougher, S.; Ye, Y.; Amuasi, J.H.; Kourgueni, I.A.; Thomson, R.; Goodman, C.; Mann, A.G.; Ren, R.; Willey, B.A.; Adegoke, C.A.; et al. Effect of the Affordable Medicines Facility—Malaria (AMFm) on the availability, price, and market share of quality-assured artemisinin-based combination therapies in seven countries: A before-and-after analysis of outlet survey data. Lancet 2012, 380, 1916–1926. [Google Scholar] [CrossRef]
- Malaria. The Global Fund to Fight AIDS, Tuberculosis and Malaria. Available online: https://www.theglobalfund.org/en/malaria/ (accessed on 6 May 2025).
- The Impact of Global Fund Investment in Fighting Malaria. Available online: https://dp.malariaconsortium.org/the-impact-of-global-fund-investment-in-fighting-malaria/index.html (accessed on 6 May 2025).
- Rannan-Eliya, R.P. Financing malaria. PLOS Glob. Public Health 2022, 2, e0000609. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanyaolu, A.; Marinkovic, A.; Prakash, S.; Balendra, V.; Shazley, O.; Gardellini, T.; Jan, A.; Younis, K.; Okorie, C.; Izurieta, R. Emerging Molecular Mechanisms in Malaria Pathogenesis and Novel Therapeutic Approaches: A Focus on P. falciparum Malaria. Biomolecules 2025, 15, 1038. https://doi.org/10.3390/biom15071038
Sanyaolu A, Marinkovic A, Prakash S, Balendra V, Shazley O, Gardellini T, Jan A, Younis K, Okorie C, Izurieta R. Emerging Molecular Mechanisms in Malaria Pathogenesis and Novel Therapeutic Approaches: A Focus on P. falciparum Malaria. Biomolecules. 2025; 15(7):1038. https://doi.org/10.3390/biom15071038
Chicago/Turabian StyleSanyaolu, Adekunle, Aleksandra Marinkovic, Stephanie Prakash, Vyshnavy Balendra, Omar Shazley, Tatiana Gardellini, Abdul Jan, Kokab Younis, Chuku Okorie, and Ricardo Izurieta. 2025. "Emerging Molecular Mechanisms in Malaria Pathogenesis and Novel Therapeutic Approaches: A Focus on P. falciparum Malaria" Biomolecules 15, no. 7: 1038. https://doi.org/10.3390/biom15071038
APA StyleSanyaolu, A., Marinkovic, A., Prakash, S., Balendra, V., Shazley, O., Gardellini, T., Jan, A., Younis, K., Okorie, C., & Izurieta, R. (2025). Emerging Molecular Mechanisms in Malaria Pathogenesis and Novel Therapeutic Approaches: A Focus on P. falciparum Malaria. Biomolecules, 15(7), 1038. https://doi.org/10.3390/biom15071038








