A Review Discussing Synthesis and Translational Studies of Medicinal Agents Targeting Sphingolipid Pathways
Abstract
1. Introduction
2. Ceramide Biosynthesis and Metabolism
2.1. De Novo Biosynthesis Pathway
2.2. Sphingomyelinase (SMase) Pathway
2.3. The Salvage Pathway
3. Biological Significance of Sphingolipids and Derivatized Sphingolipids
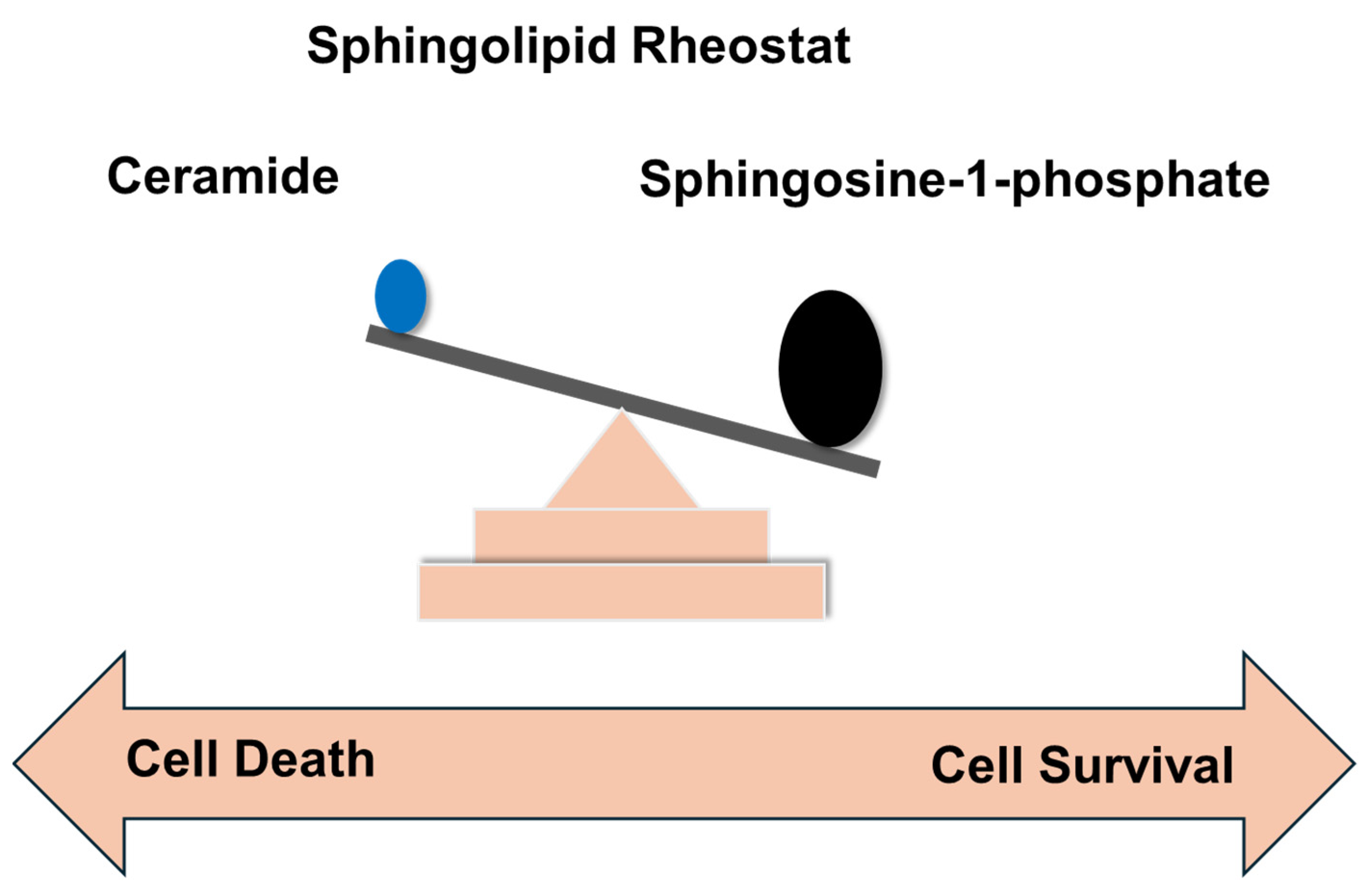
4. Sphingolipid Rheostat and Its Significance in Cancer
5. Sphingolipid-Based Therapeutic Agents
5.1. Myriocin
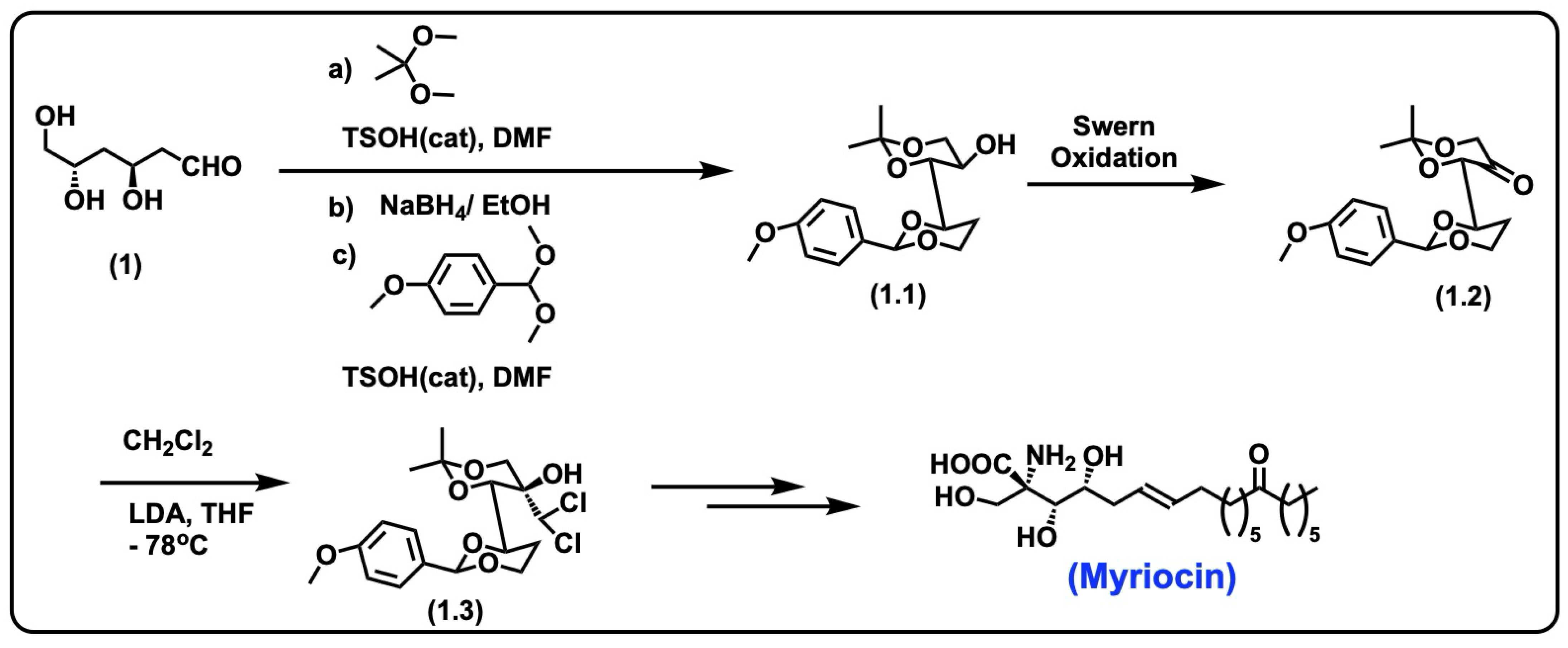
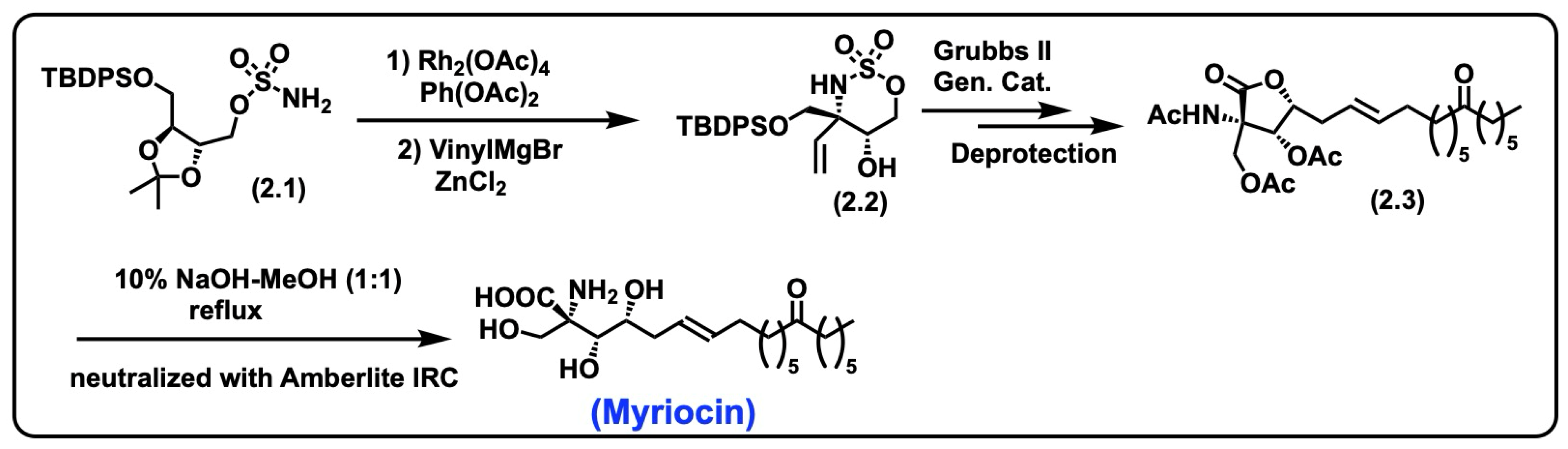
5.1.1. Synthesis of Myriocin
5.1.2. Biological Significance of Myriocin
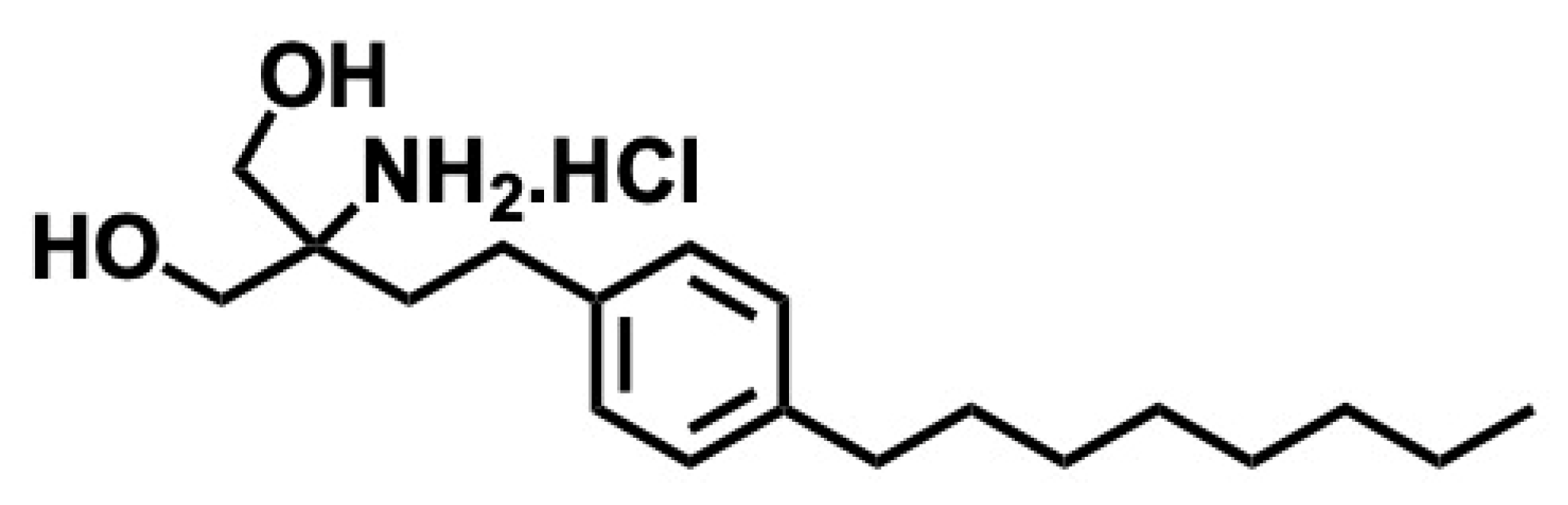
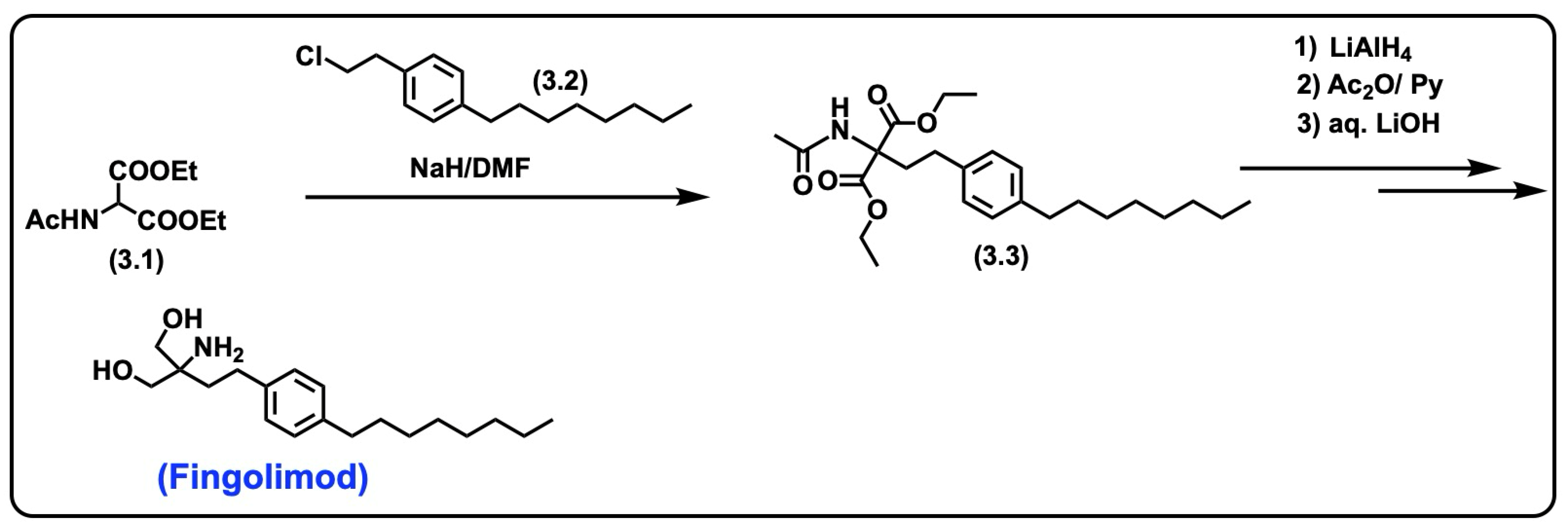
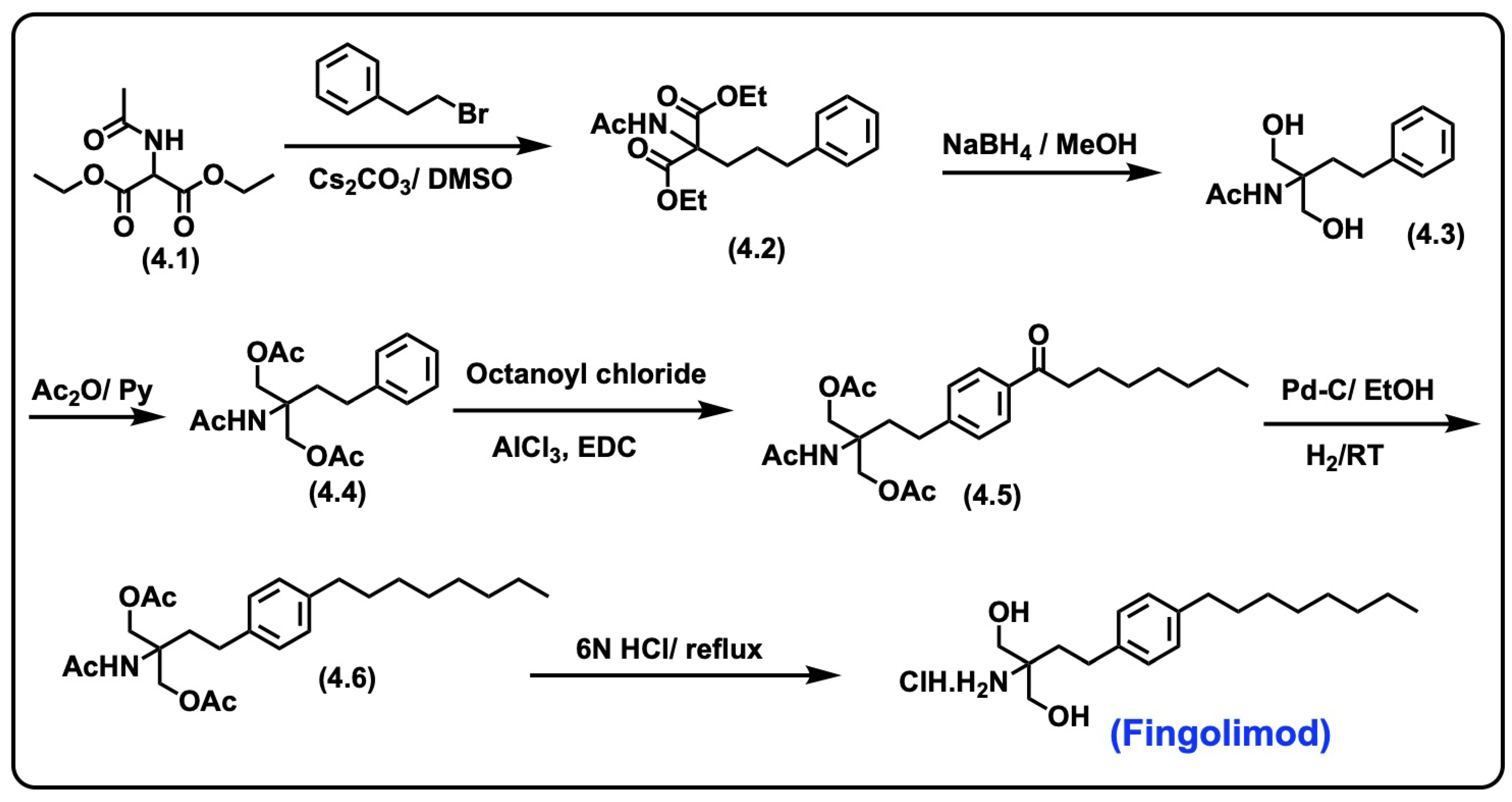
5.2. Fingolimod
5.2.1. Synthesis of Fingolimod
5.2.2. Pharmacological Significance of Fingolimod
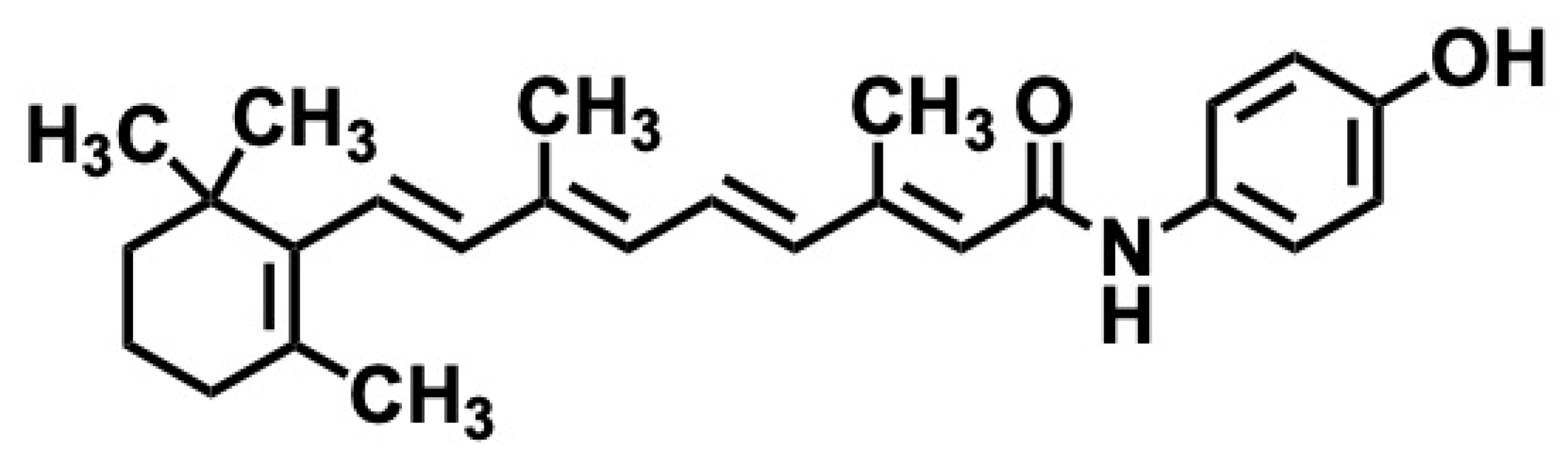
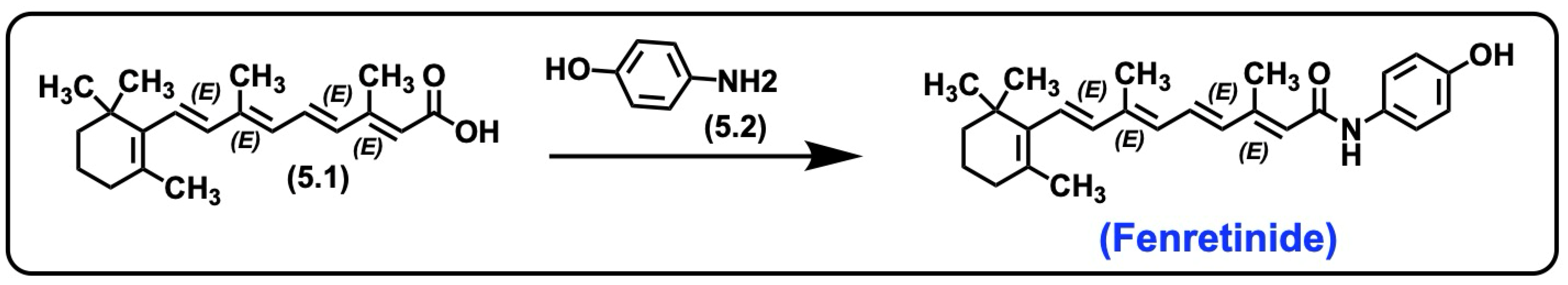
5.3. Fenretinide
5.3.1. Synthesis of Fenretinide
5.3.2. Pharmacological Significance of Fenretinide
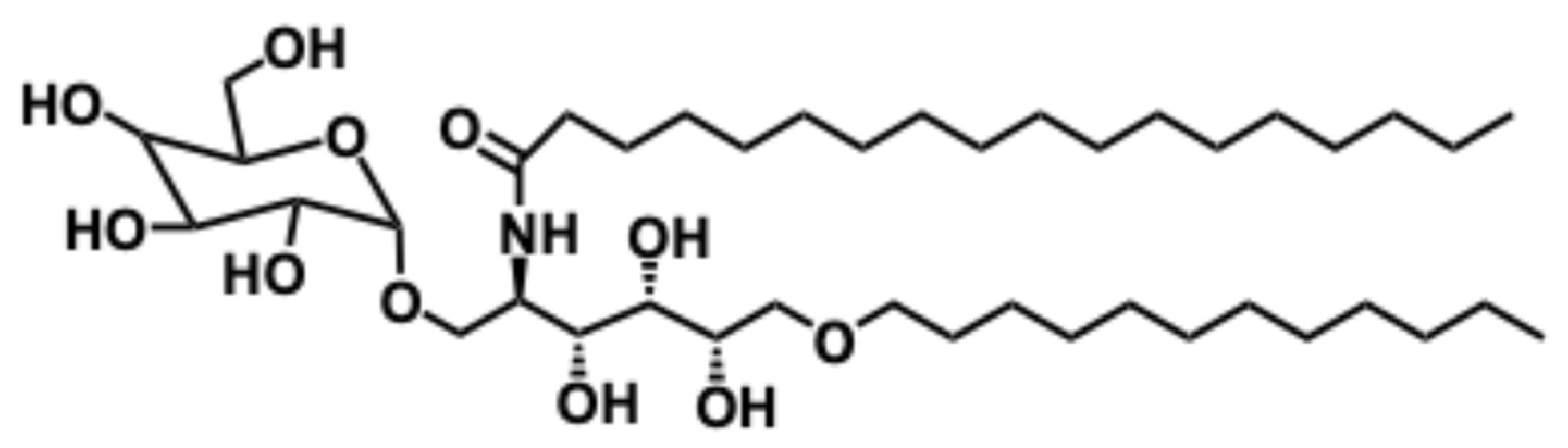
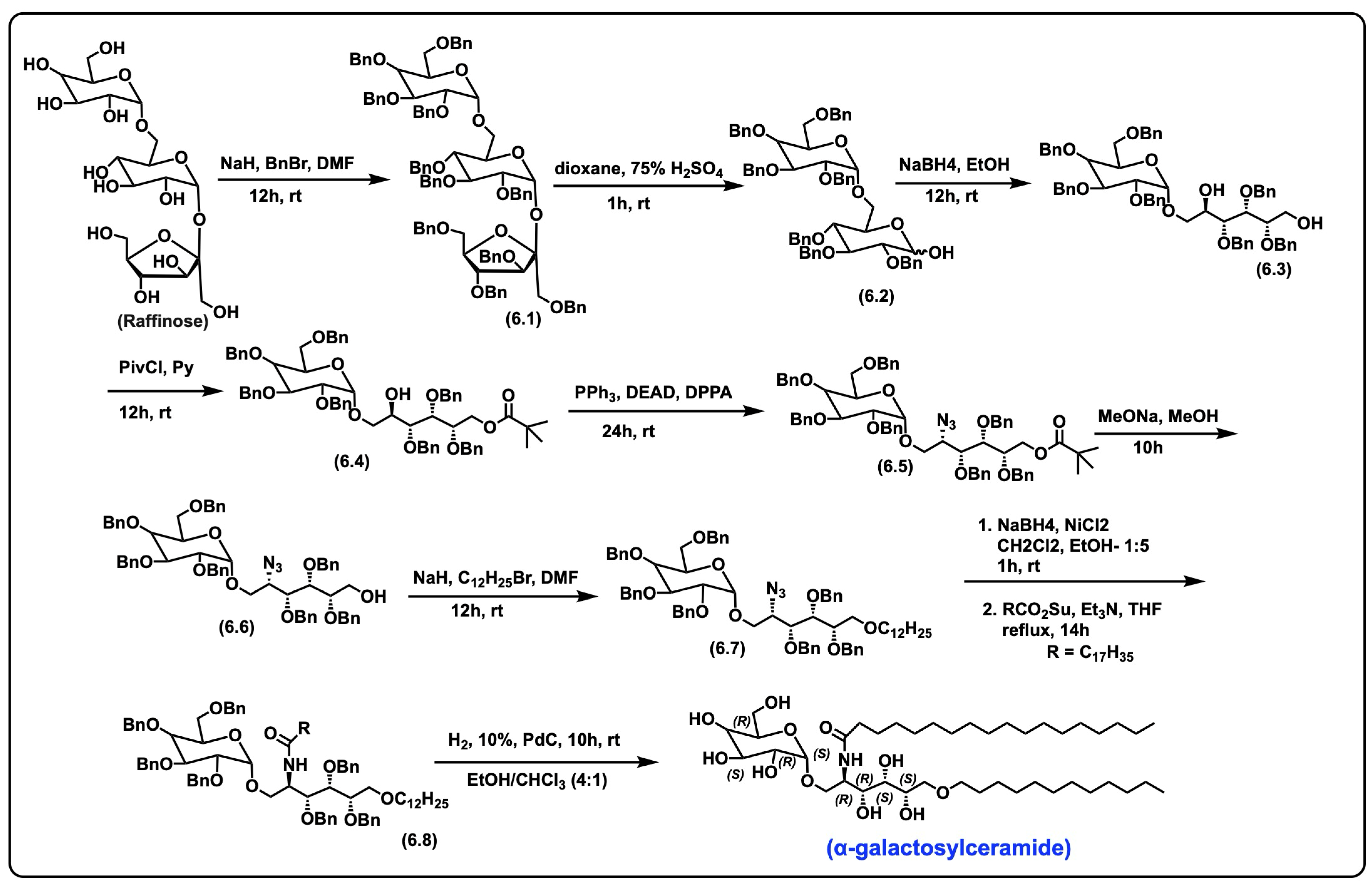
5.4. α-Galactosylceramide
5.4.1. Synthesis of α-Galactosylceramide
5.4.2. Pharmacological Relevance and Mode of Action of α-Galactosyl Ceramide
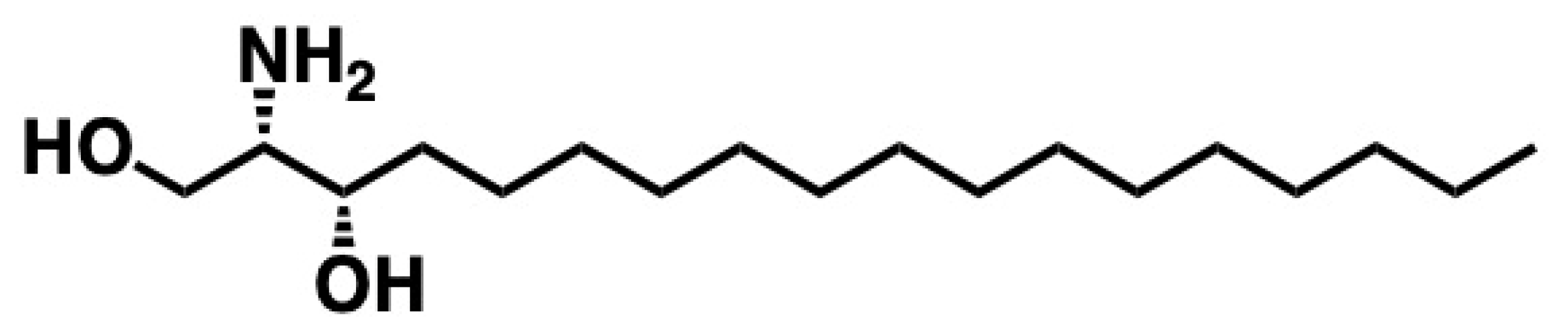
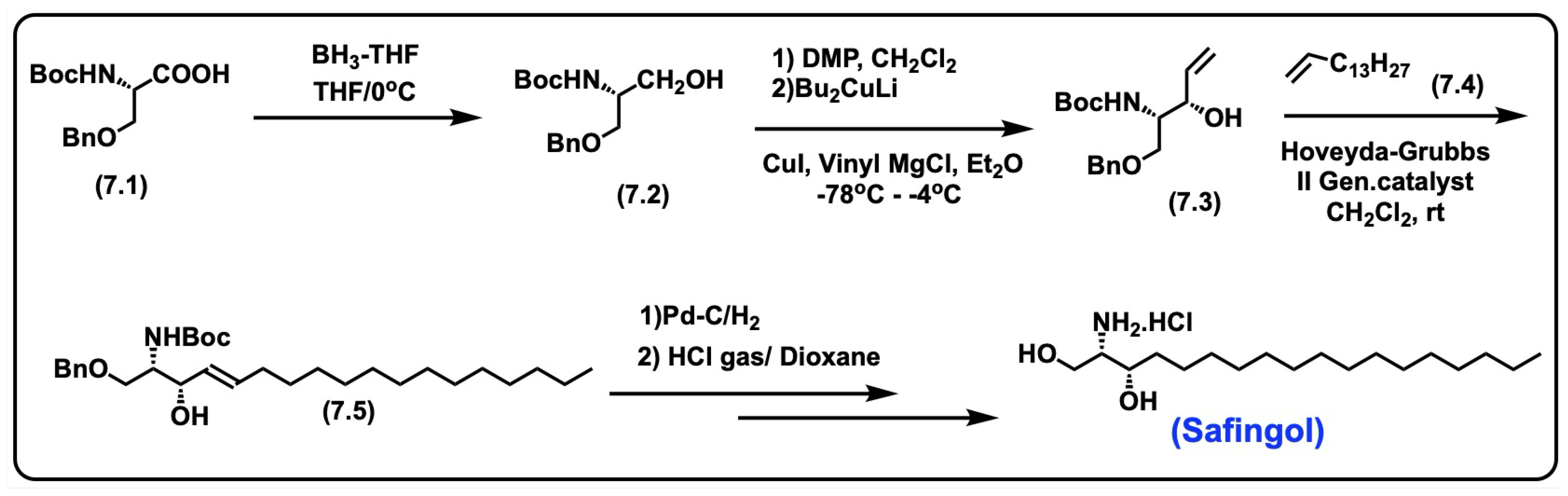
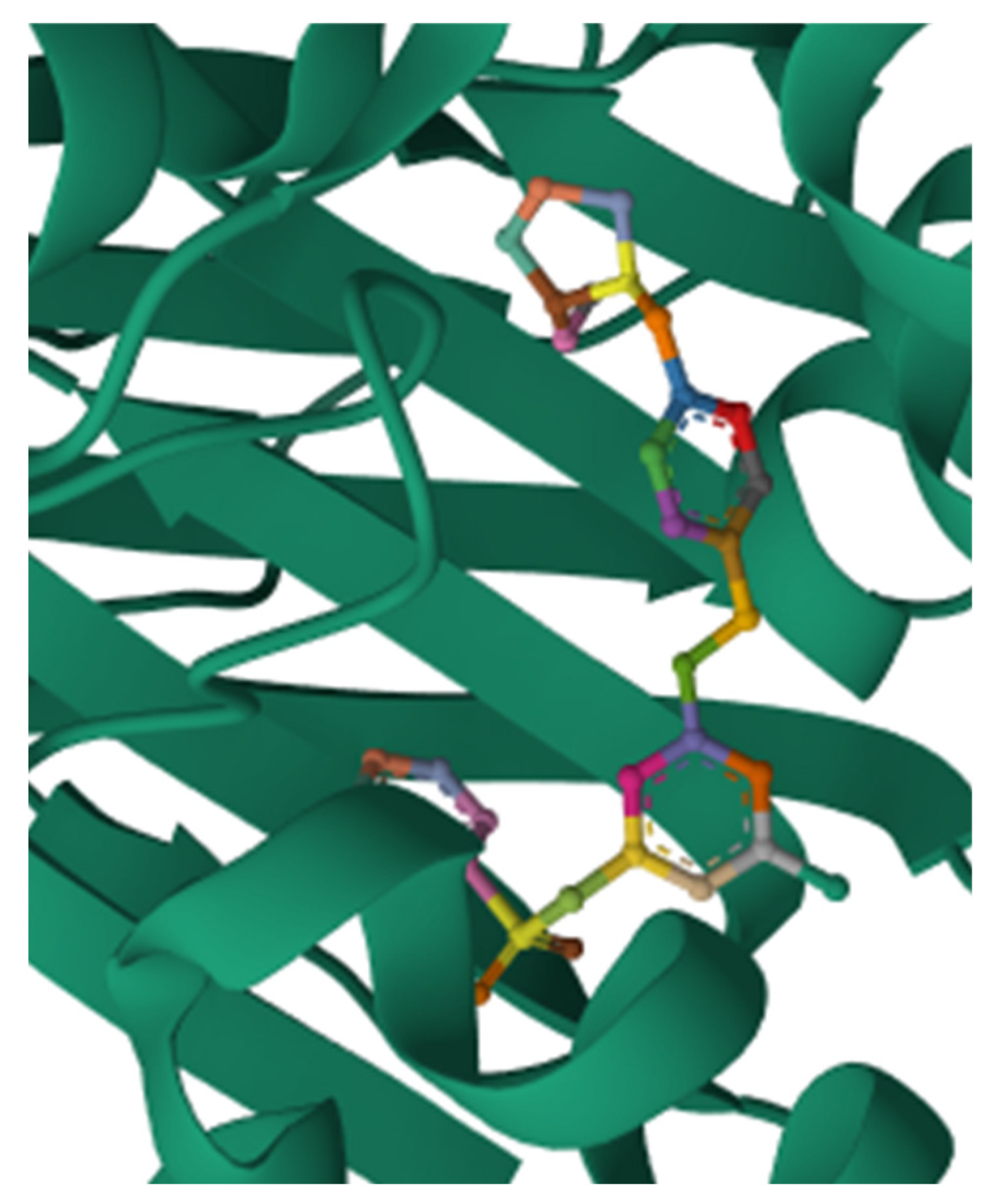
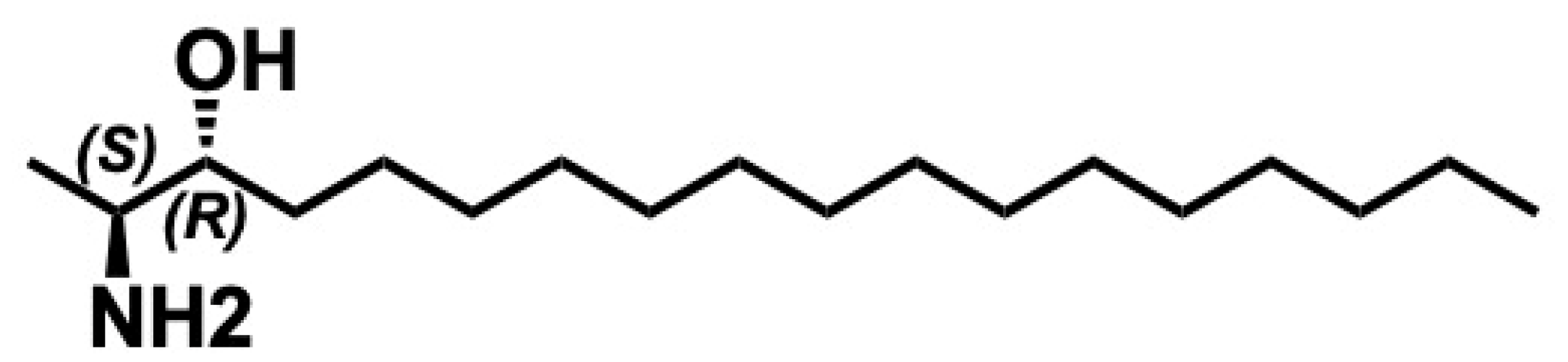
5.5. Safingol
5.5.1. Synthesis of Safingol
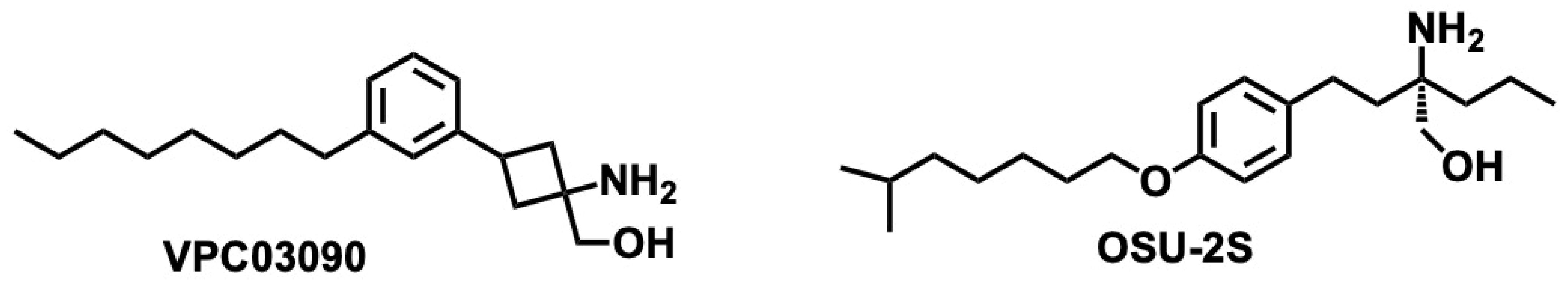
5.5.2. Pharmacological Relevance and Mode of Action of Safingol
5.6. Spisulosine
5.6.1. Synthesis of Spisulosine
5.6.2. Pharmacological Relevance and Mode of Action of Spisulosine
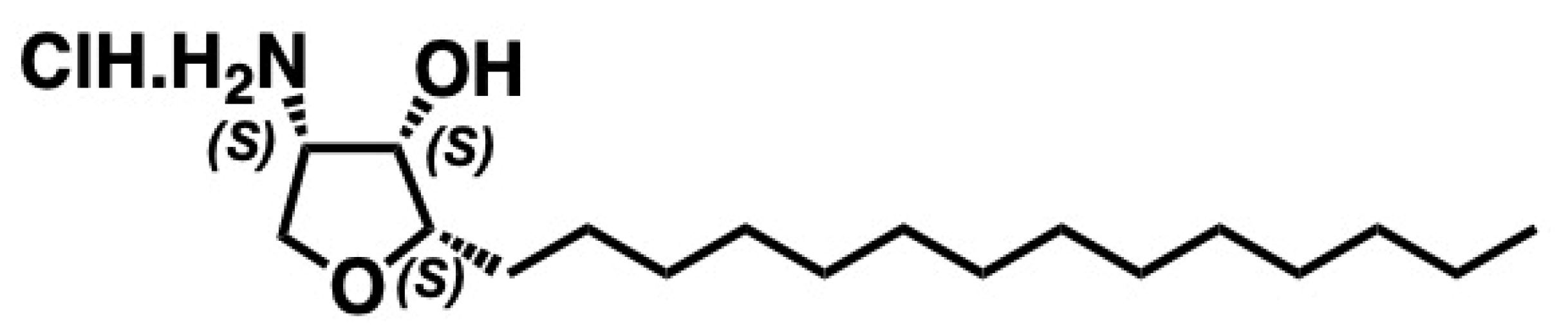
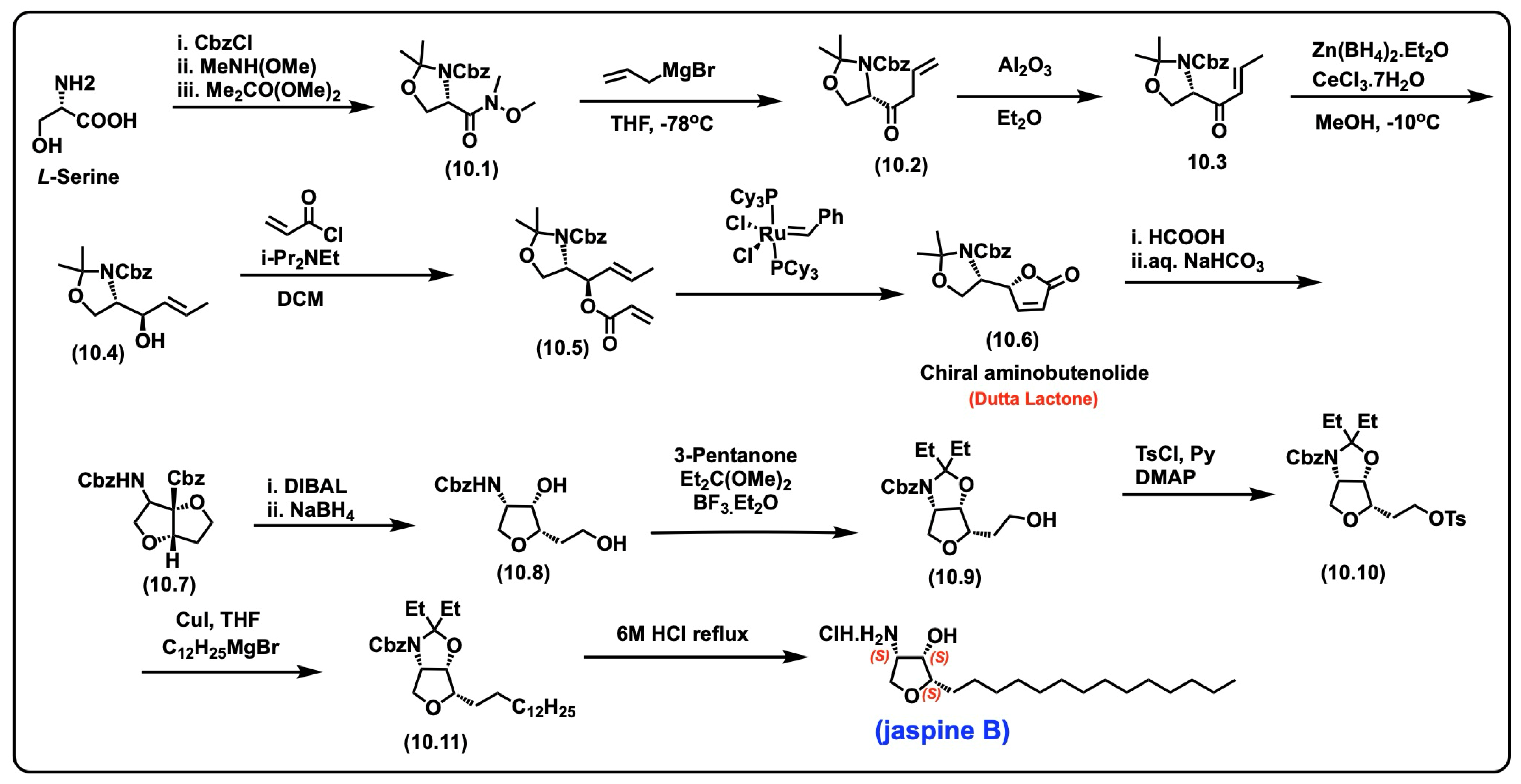
5.7. Jaspine B
5.7.1. Synthesis of Jaspine B
5.7.2. Pharmacological Relevance and Mode of Action of Jaspine B
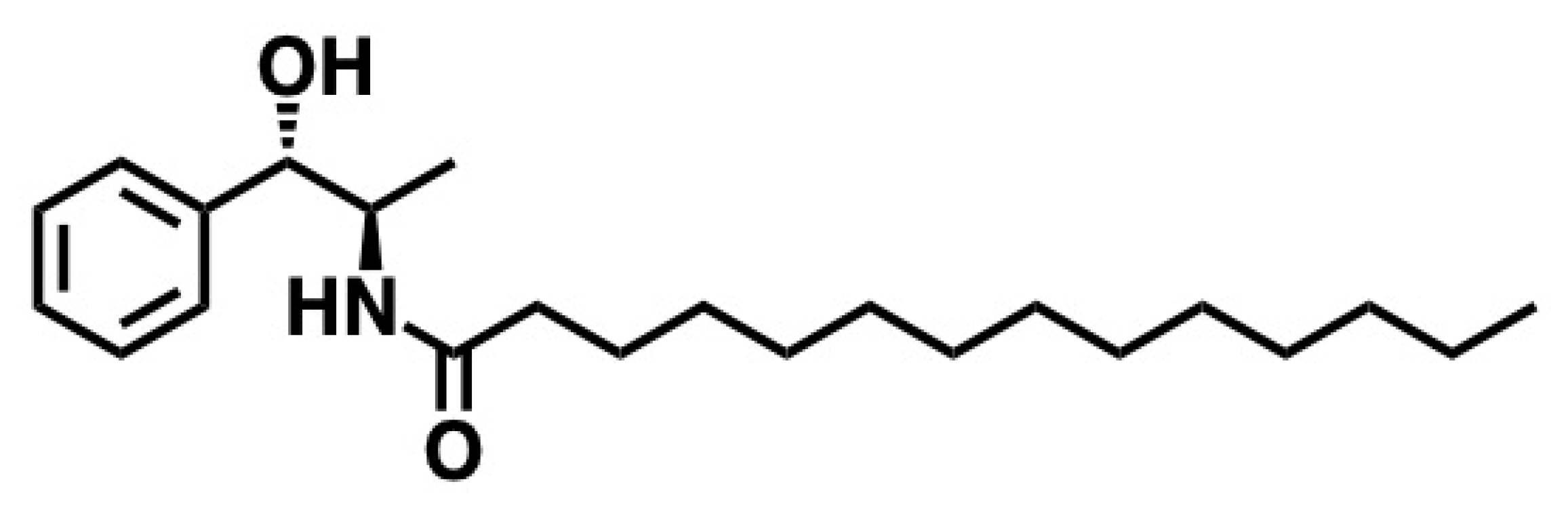
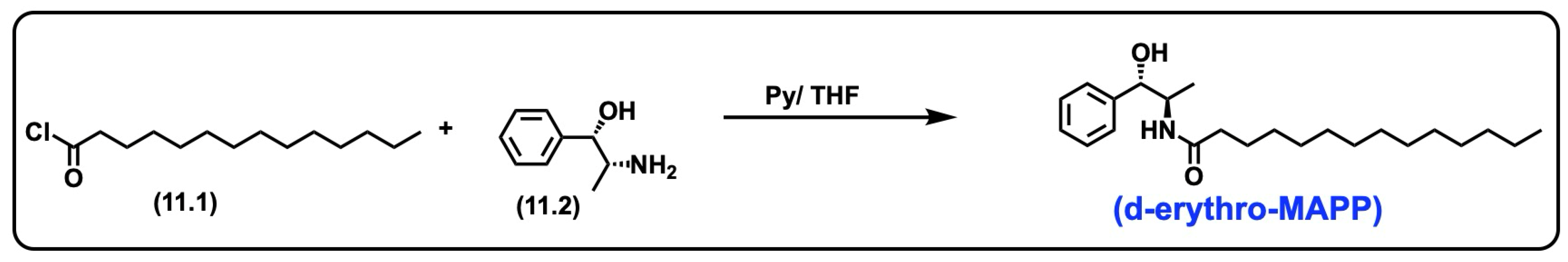
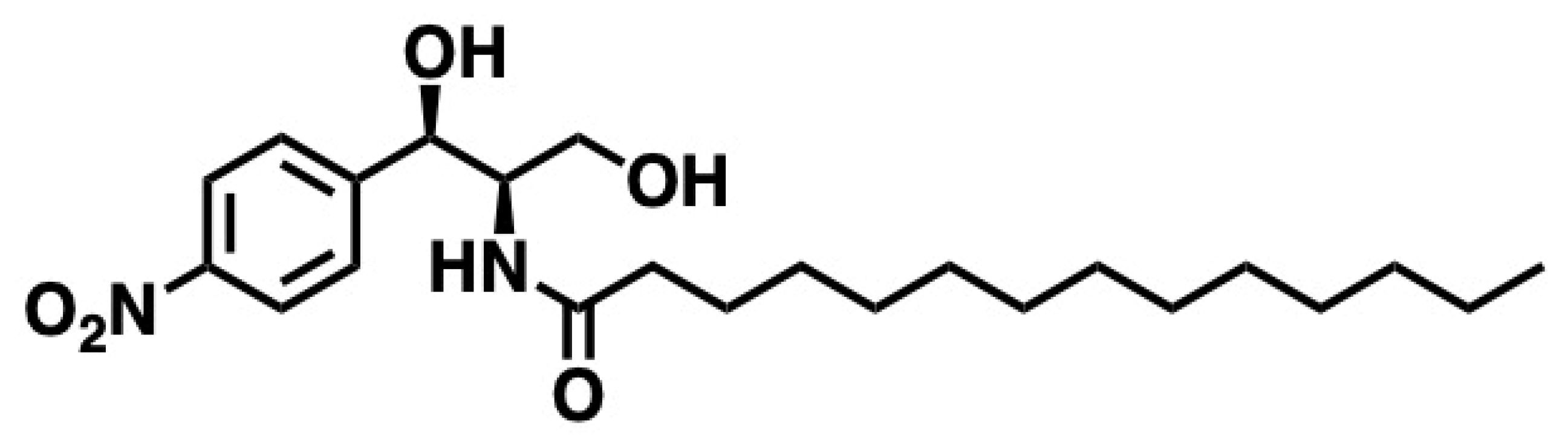
5.8. d-Erythro-MAPP(D-e-MAPP)
5.8.1. Synthesis of d-Erythro-MAPP(D-e-MAPP)
5.8.2. Pharmacological Significance of d-Erythro-MAPP
5.9. B13
5.9.1. Synthesis of B13
5.9.2. Pharmacological Significance of B13
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLs | Sphingolipids |
| Sph | Sphingosine |
| Cer | Ceramide |
| GCer | Glucosylceramide |
| C1P | Ceramide-1-phosphate |
| SA | Sphinganine |
| S1P | Sphingosine-1-phosphate |
| L-Ser | L-Serine |
| L-Ala | L-Alanine |
| SMase | Sphingomyelinase |
| SPT | Serine palmitoyl transferase |
| DhCer | Dihydroceramide |
| RDS | Rate-determining step |
| PLP | pyridoxal 5′-phosphate |
| TNF alpha | Tumor necrosis factor alpha |
| SAR | Structural activity relationship |
| RCSB | Research Collaboratory for Structural Bioinformatics |
| SMPD | Sphingomyelin phosphodiesterase |
| PLC | Phospholipase C |
| Mg | Magnesium |
| p-JNK | p-phosphorylated c-Jun N-terminal kinase |
| PKCs | Classical protein kinase C |
| JNK | Jun N-terminal kinase |
| RCM | Ring-closing metathesis |
| DMG | Dimethylglycine |
| P-p38 | Phosphorylated p38 mitogen-activated protein kinase |
| p70 S6K | p70 ribosomal S6 kinase |
| IND | Investigational new drug |
| FDA | Food and Drug Administration |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein kinase B |
| mTOR | Mechanistic target of rapamycin |
| cPLA2 | Cytosolic phospholipase A2 |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| NBD | N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-ethylenediamine |
References
- Merrill, A.H., Jr.; Schmelz, E.; Dillehay, D.L.; Dillehay, D.; Spiegel, S.; Spiegel, S.; Shayman, J.A.; Shayman, J.; Schroeder, J.J.; Schroeder, J.; et al. Sphingolipids—The enigmatic lipid class: Biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 1997, 142, 208–225. [Google Scholar] [CrossRef]
- Schöffski, P.; Dumez, H.; Ruijter, R.; Miguel-Lillo, B.; Soto-Matos, A.; Alfaro, V.; Giaccone, G. Spisulosine (ES-285) given as a weekly three-hour intravenous infusion: Results of a phase I dose-escalating study in patients with advanced solid malignancies. Cancer Chemother. Pharmacol. 2011, 68, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Bouscary, A.; Quessada, C.; René, F.; Spedding, M.; Turner, B.J.; Henriques, A.; Ngo, S.T.; Loeffler, J.P. Sphingolipids metabolism alteration in the central nervous system: Amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Chalfant, C., Poeta, M.D., Eds.; Springer: New York, NY, USA, 2010; pp. 1–23. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H., Jr. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Myers, S.; McGowan, C.; Henstridge, D.; Eri, R.; Sonda, S.; Caruso, V. 1-Deoxysphingolipids, Early Predictors of Type 2 Diabetes, Compromise the Functionality of Skeletal Myoblasts. Front. Endocrinol. 2021, 12, 772925. [Google Scholar] [CrossRef]
- González-Ramírez, E.J.; García-Arribas, A.B.; Artetxe, I.; Shaw, W.A.; Goñi, F.M.; Alonso, A.; Jiménez-Rojo, N. (1-Deoxy)ceramides in bilayers containing sphingomyelin and cholesterol. Colloids Surf. B Biointerfaces 2024, 243, 114155. [Google Scholar] [CrossRef]
- Carreira, A.C.; Santos, T.C.; Lone, M.A.; Zupančič, E.; Lloyd-Evans, E.; de Almeida, R.F.M.; Hornemann, T.; Silva, L.C. Mammalian sphingoid bases: Biophysical, physiological and pathological properties. Prog. Lipid Res. 2019, 75, 100988. [Google Scholar] [CrossRef]
- Pradas, I.; Jové, M.; Huynh, K.; Ingles, M.; Borras, C.; Mota-Martorell, N.; Galo-Licona, J.D.; Puig, J.; Viña, J.; Meikle, P.J.; et al. Long-lived Humans Have a Unique Plasma Sphingolipidome. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 728–735. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X. A review of lipidomic technologies applicable to sphingolipidomics and their relevant applications. Eur. J. Lipid Sci. Technol. 2009, 111, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Stith, J.L.; Velazquez, F.N.; Obeid, L.M. Advances in determining signaling mechanisms of ceramide and role in disease. J. Lipid Res. 2019, 60, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Mandon, E.C.; Ehses, I.; Rother, J.; Rother, J.; van Echten, G.; van Echten, G.; Sandhoff, K.; Sandhoff, K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J. Biol. Chem. 1992, 267, 11144–11148. [Google Scholar] [CrossRef]
- Moskot, M.; Bocheńska, K.; Jakóbkiewicz-Banecka, J.; Banecki, B.; Gabig-Cimińska, M. Abnormal Sphingolipid World in Inflammation Specific for Lysosomal Storage Diseases and Skin Disorders. Int. J. Mol. Sci. 2018, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Quadri, Z.; Pu, J.; Zhang, L.; Ferko, B.; Berkes, D.; et al. Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12233. [Google Scholar] [CrossRef]
- Perry, D.K. Serine palmitoyltransferase: Role in apoptotic de novo ceramide synthesis and other stress responses. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2002, 1585, 146–152. [Google Scholar] [CrossRef]
- Yard, B.A.; Carter, L.G.; Johnson, K.A.; Overton, I.M.; Dorward, M.; Liu, H.; McMahon, S.A.; Oke, M.; Puech, D.; Barton, G.J.; et al. The Structure of Serine Palmitoyltransferase; Gateway to Sphingolipid Biosynthesis. J. Mol. Biol. 2007, 370, 870–886. [Google Scholar] [CrossRef]
- Zemski Berry, K.A.-O.X.; Garfield, A.A.-O.; Jambal, P.; Zarini, S.A.-O.; Perreault, L.A.-O.; Bergman, B.A.-O. Oxidised phosphatidylcholine induces sarcolemmal ceramide accumulation and insulin resistance in skeletal muscle. Diabetologia 2024, 67, 2819–2832. [Google Scholar] [CrossRef]
- Tahia, F.; Ma, D.A.-O.; Stephenson, D.J.; Basu, S.K.; Del Mar, N.A.; Lenchik, N.; Kochat, H.; Brown, K.; Chalfant, C.A.-O.; Mandal, N.A.-O. Inhibiting De Novo Biosynthesis of Ceramide by L-Cycloserine Can Prevent Light-Induced Retinal Degeneration in Albino BALB/c Mice. Int. J. Mol. Sci. 2024, 25, 13389. [Google Scholar] [CrossRef]
- Hengst, J.A.; Ruiz-Velasco, V.J.; Raup-Konsavage, W.A.-O.; Vrana, K.A.-O.; Yun, J.A.-O. Cannabinoid-Induced Immunogenic Cell Death of Colorectal Cancer Cells Through De Novo Synthesis of Ceramide Is Partially Mediated by CB2 Receptor. Cancers 2024, 16, 3973. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.M.; Cowart, L.A.; Signorelli, P.; Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Acute Activation of de Novo Sphingolipid Biosynthesis upon Heat Shock Causes an Accumulation of Ceramide and Subsequent Dephosphorylation of SR Proteins. J. Biol. Chem. 2002, 277, 42572–42578. [Google Scholar] [CrossRef]
- Jamjoum, R.; Majumder, S.; Issleny, B.; Stiban, J. Mysterious sphingolipids: Metabolic interrelationships at the center of pathophysiology. Front. Physiol. 2023, 14, 1229108. [Google Scholar] [CrossRef]
- Thurm, A.; Chlebowski, C.; Joseph, L.; Farmer, C.; Adedipe, D.; Weiss, M.; Wiggs, E.; Farhat, N.; Bianconi, S.; Berry-Kravis, E.; et al. Neurodevelopmental Characterization of Young Children Diagnosed with Niemann-Pick Disease, Type C1. J. Dev. Behav. Pediatr. 2020, 41, 388–396. [Google Scholar] [CrossRef]
- Kim, M.Y.; Linardic, C.; Obeid, L.; Hannun, Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J. Biol. Chem. 1991, 266, 484–489. [Google Scholar] [CrossRef]
- Yi, J.; Qi, B.; Yin, J.; Li, R.; Chen, X.; Hu, J.; Li, G.; Zhang, S.; Zhang, Y.; Yang, M. Molecular basis for the catalytic mechanism of human neutral sphingomyelinases 1 (hSMPD2). Nat. Commun. 2023, 14, 7755. [Google Scholar] [CrossRef] [PubMed]
- Tomiuk, S.; Hofmann, K.; Nix, M.; Nix, M.; Zumbansen, M.; Zumbansen, M.; Stoffel, W.; Stoffel, W. Cloned mammalian neutral sphingomyelinase: Functions in sphingolipid signaling? Proc. Natl. Acad. Sci. USA 1998, 95, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Monturiol-Gross, L.; Villalta-Romero, F.; Flores-Díaz, M.; Alape-Girón, A. Bacterial phospholipases C with dual activity: Phosphatidylcholinesterase and sphingomyelinase. FEBS Open Bio 2021, 11, 3262–3275. [Google Scholar] [CrossRef]
- Bikman, B.T.; Summers, S.A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Investig. 2011, 121, 4222–4230. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Z.; Wang, X.R.; Wang, J.; Xie, C.; Wang, X.X.; Pan, H.D.; Meng, W.Y.; Liang, T.L.; Li, J.X.; Yan, P.Y.; et al. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front. Oncol. 2022, 12, 941643. [Google Scholar] [CrossRef]
- Wajapeyee, N.; Beamon, T.C.; Gupta, R. Roles and therapeutic targeting of ceramide metabolism in cancer. Mol. Metab. 2024, 83, 101936. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y. Ceramide signaling in mammalian epidermis. Biochim. Biophys. Acta 2014, 1841, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef]
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef]
- Nilsson, O.; Svennerholm, L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J. Neurochem. 1982, 39, 709–718. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Barnes, S.; Sun, Y.; Grabowski, G.A. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid Res. 2010, 51, 1643–1675. [Google Scholar] [CrossRef]
- Schnaar, R.L. The Biology of Gangliosides. Adv. Carbohydr. Chem. Biochem. 2019, 76, 113–148. [Google Scholar] [CrossRef]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gómez-Muñoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Yamada, A.; Aoyagi, T.; Allegood, J.; Wakai, T.; Spiegel, S.; Takabe, K. Sphingosine-1-phosphate in the lymphatic fluid determined by novel methods. Heliyon 2016, 2, e00219. [Google Scholar] [CrossRef]
- Santos, W.L.; Lynch, K.R. Drugging sphingosine kinases. ACS Chem. Biol. 2015, 10, 225–233. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Vaena, S.G.; Thyagarajan, K.; Chatterjee, S.; Al-Khami, A.; Selvam, S.P.; Nguyen, H.; Kang, I.; Wyatt, M.W.; Baliga, U.; et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep. 2019, 28, 1879–1893.e7. [Google Scholar] [CrossRef]
- Giussani, P.; Tringali, C.; Riboni, L.; Viani, P.; Venerando, B. Sphingolipids: Key Regulators of Apoptosis and Pivotal Players in Cancer Drug Resistance. Int. J. Mol. Sci. 2014, 15, 4356–4392. [Google Scholar] [CrossRef]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar] [CrossRef]
- Torres, O.; Matute, J.; Gelineau-van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Voss, K.A.; Riley, R.T. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 2015, 8, 143–160. [Google Scholar] [CrossRef]
- Oskouian, B.; Saba, J.D. Cancer Treatment Strategies Targeting Sphingolipid Metabolism. In Sphingolipids as Signaling and Regulatory Molecules; Chalfant, C., Poeta, M.D., Eds.; Springer: New York, NY, USA, 2010; pp. 185–205. [Google Scholar]
- Zaibaq, F.; Dowdy, T.; Larion, M. Targeting the Sphingolipid Rheostat in Gliomas. Int. J. Mol. Sci. 2022, 23, 9255. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, K.M.; Choi, M.H.; Ji, S.Y.; Lee, S.; Sin, D.M.; Oh, K.W.; Lee, Y.M.; Hong, J.T.; Yun, Y.P.; et al. Serine palmitoyltransferase inhibitor myriocin induces growth inhibition of B16F10 melanoma cells through G2/M phase arrest. Cell Prolif. 2011, 44, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Hada, N.; Katsume, A.; Kenichi, K.; Endo, C.; Horiba, N.; Sudoh, M. Novel oral SPT inhibitor CH5169356 inhibits hepatic stellate cell activation and ameliorates hepatic fibrosis in mouse models of non-alcoholic steatohepatitis (NASH). Pharmacol. Res. Perspect. 2023, 11, e01094. [Google Scholar] [CrossRef]
- Tahia, F.; Basu, S.K.; Prislovsky, A.; Mondal, K.; Ma, D.; Kochat, H.; Brown, K.; Stephenson, D.J.; Chalfant, C.E.; Mandal, N. Sphingolipid biosynthetic inhibitor L-Cycloserine prevents oxidative-stress-mediated death in an in vitro model of photoreceptor-derived 661W cells. Exp. Eye Res. 2024, 242, 109852. [Google Scholar] [CrossRef]
- De Vita, T.; Albani, C.; Realini, N.; Migliore, M.; Basit, A.; Ottonello, G.; Cavalli, A. Inhibition of Serine Palmitoyltransferase by a Small Organic Molecule Promotes Neuronal Survival after Astrocyte Amyloid Beta 1–42 Injury. ACS Chem. Neurosci. 2019, 10, 1627–1635. [Google Scholar] [CrossRef]
- Lowther, J.; Beattie, A.E.; Langridge-Smith, P.R.R.; Clarke, D.J.; Campopiano, D.J. l-Penicillamine is a mechanism-based inhibitor of serine palmitoyltransferase by forming a pyridoxal-5′-phosphate-thiazolidine adduct. MedChemComm 2012, 3, 1003–1008. [Google Scholar] [CrossRef]
- Hanada, K.; Nishijima, M.; Fujita, T.; Kobayashi, S. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells: A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem. Pharmacol. 2000, 59, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Ji, C.; Zhou, Y.; Huang, W.; Ni, W.; Tong, X.; Wei, J.-F. Sphingosine kinase inhibitors: A patent review. Int. J. Mol. Med. 2018, 41, 2450–2460. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; ME, L.L. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. [Google Scholar] [CrossRef]
- Adams, D.R.; Pyne, S.; Pyne, N.J. Structure-function analysis of lipid substrates and inhibitors of sphingosine kinases. Cell. Signal. 2020, 76, 109806. [Google Scholar] [CrossRef]
- Bonica, J.; Clarke, C.J.; Obeid, L.M.; Luberto, C.; Hannun, Y.A. Upregulation of sphingosine kinase 1 in response to doxorubicin generates an angiogenic response via stabilization of Snail. FASEB J. 2023, 37, e22787. [Google Scholar] [CrossRef]
- Hammer, S.; Sauer, B.; Spika, I.; Schraut, C.; Kleuser, B.; Schäfer-Korting, M. Glucocorticoids mediate differential anti-apoptotic effects in human fibroblasts and keratinocytes via sphingosine-1-phosphate formation. J. Cell. Biochem. 2004, 91, 840–851. [Google Scholar] [CrossRef]
- Hara-Yokoyama, M.; Terasawa, K.; Ichinose, S.; Watanabe, A.; Podyma-Inoue, K.A.; Akiyoshi, K.; Igarashi, Y.; Yanagishita, M. Sphingosine kinase 2 inhibitor SG-12 induces apoptosis via phosphorylation by sphingosine kinase 2. Bioorg. Med. Chem. Lett. 2013, 23, 2220–2224. [Google Scholar] [CrossRef]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tang, X.; Li, T.; Chen, L.; He, H.; Wu, X.; Xiang, C.; Cao, M.; Wang, Z.; Wang, Y.; et al. Therapeutic potential of the sphingosine kinase 1 inhibitor, PF-543. Biomed. Pharmacother. 2023, 163, 114401. [Google Scholar] [CrossRef]
- Gehin, M.; Melchior, M.; Welford, R.W.D.; Sidharta, P.N.; Dingemanse, J. Assessment of Target Engagement in a First-in-Human Trial with Sinbaglustat, an Iminosugar to Treat Lysosomal Storage Disorders. Clin. Transl. Sci. 2021, 14, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Deng, Y.; Yasgar, A.; Yadav, R.; Talley, D.; Zakharov, A.V.; Jain, S.; Rai, G.; Noinaj, N.; Simeonov, A.; et al. Venglustat Inhibits Protein N-Terminal Methyltransferase 1 in a Substrate-Competitive Manner. J. Med. Chem. 2022, 65, 12334–12345. [Google Scholar] [CrossRef] [PubMed]
- Shayman, J.A.; Lee, L.; Abe, A.; Shu, L. Inhibitors of glucosylceramide synthase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2000; pp. 373–387. [Google Scholar]
- Stocker, B.L.; Dangerfield, E.M.; Win-Mason, A.L.; Haslett, G.W.; Timmer, M.S.M. Recent Developments in the Synthesis of Pyrrolidine-Containing Iminosugars. Eur. J. Org. Chem. 2010, 2010, 1615–1637. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W.; Sun, J.; Huang, Y.; Wang, P.; Venkataramanan, R.; Yang, D.; Ma, X.; Rana, A.; Li, S. Novel glucosylceramide synthase inhibitor based prodrug copolymer micelles for delivery of anticancer agents. J. Control. Release 2018, 288, 212–226. [Google Scholar] [CrossRef]
- Tanaka, Y.; Seto, M.; Kakegawa, K.; Takami, K.; Kikuchi, F.; Yamamoto, T.; Nakamura, M.; Daini, M.; Murakami, M.; Ohashi, T.; et al. Discovery of Brain-Penetrant Glucosylceramide Synthase Inhibitors with a Novel Pharmacophore. J. Med. Chem. 2022, 65, 4270–4290. [Google Scholar] [CrossRef]
- Delgado, A.; Casas, J.; Llebaria, A.; Abad, J.L.; Fabriás, G. Chemical Tools to Investigate Sphingolipid Metabolism and Functions. ChemMedChem 2007, 2, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Ottenhoff, R.; Powlson, A.S.; Grefhorst, A.; van Eijk, M.; Dubbelhuis, P.F.; Aten, J.; Kuipers, F.; Serlie, M.J.; Wennekes, T.; et al. Pharmacological Inhibition of Glucosylceramide Synthase Enhances Insulin Sensitivity. Diabetes 2007, 56, 1341–1349. [Google Scholar] [CrossRef]
- Artola, M.; Kuo, C.-L.; Lelieveld, L.T.; Rowland, R.J.; van der Marel, G.A.; Codée, J.D.C.; Boot, R.G.; Davies, G.J.; Aerts, J.M.F.G.; Overkleeft, H.S. Functionalized Cyclophellitols Are Selective Glucocerebrosidase Inhibitors and Induce a Bona Fide Neuropathic Gaucher Model in Zebrafish. J. Am. Chem. Soc. 2019, 141, 4214–4218. [Google Scholar] [CrossRef] [PubMed]
- Rempel, B.P.; Withers, S.G. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 2008, 18, 570–586. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Kallemeijn, W.W.; Lelieveld, L.T.; Mirzaian, M.; Zoutendijk, I.; Vardi, A.; Futerman, A.H.; Meijer, A.H.; Spaink, H.P.; Overkleeft, H.S.; et al. In vivo inactivation of glycosidases by conduritol B epoxide and cyclophellitol as revealed by activity-based protein profiling. FEBS J. 2019, 286, 584–600. [Google Scholar] [CrossRef]
- Watanabe, S.; Oyama, M.; Iwai, T.; Tanabe, M. Glucosylceramide synthase inhibitor ameliorates chronic inflammatory pain. J. Pharmacol. Sci. 2024, 156, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.A.; Yuyama, K.; Murai, Y.; Igarashi, Y.; Mikami, D.; Sivasothy, Y.; Awang, K.; Monde, K. Malabaricone C as Natural Sphingomyelin Synthase Inhibitor against Diet-Induced Obesity and Its Lipid Metabolism in Mice. ACS Med. Chem. Lett. 2019, 10, 1154–1158. [Google Scholar] [CrossRef]
- Meng, A.; Luberto, C.; Meier, P.; Bai, A.; Yang, X.; Hannun, Y.A.; Zhou, D. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Exp. Cell Res. 2004, 292, 385–392. [Google Scholar] [CrossRef]
- Deng, X.; Lin, F.; Zhang, Y.; Li, Y.; Zhou, L.; Lou, B.; Li, Y.; Dong, J.; Ding, T.; Jiang, X.; et al. Identification of small molecule sphingomyelin synthase inhibitors. Eur. J. Med. Chem. 2014, 73, 1–7. [Google Scholar] [CrossRef]
- Lou, B.; Dong, J.; Li, Y.; Ding, T.; Bi, T.; Li, Y.; Deng, X.; Ye, D.; Jiang, X.-C. Pharmacologic Inhibition of Sphingomyelin Synthase (SMS) Activity Reduces Apolipoprotein-B Secretion from Hepatocytes and Attenuates Endotoxin-Mediated Macrophage Inflammation. PLoS ONE 2014, 9, e102641. [Google Scholar] [CrossRef]
- Pavek, A.; Afrin, F.; Meldrum, T.; Pashikanti, S.; Barrott, J. Sphingomyelin synthase inhibition in sarcomas results in ceramide accumulation and apoptosis. The FASEB Journal. 2019, 33, 471.16. [Google Scholar] [CrossRef]
- JaffrÉZou, J.-P.; Bruno, A.P.; Moisand, A.; Levade, T.; Laurent, G.U.Y. Activation of a nuclear sphingomyelinase in radiation induced apoptosis. FASEB J. 2001, 15, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kakoi, H.; Maeda, S.; Shinohara, N.; Matsuyama, K.; Imamura, K.; Kawamura, I.; Nagano, S.; Setoguchi, T.; Yokouchi, M.; Ishidou, Y.; et al. Bone Morphogenic Protein (BMP) Signaling Up-regulates Neutral Sphingomyelinase 2 to Suppress Chondrocyte Maturation via the Akt Protein Signaling Pathway as a Negative Feedback Mechanism. J. Biol. Chem. 2014, 289, 8135–8150. [Google Scholar] [CrossRef]
- Keresztes, A.; Streicher, J.M. Synergistic interaction of the cannabinoid and death receptor systems—A potential target for future cancer therapies? FEBS Lett. 2017, 591, 3235–3251. [Google Scholar] [CrossRef]
- Klutzny, S.; Lesche, R.; Keck, M.; Kaulfuss, S.; Schlicker, A.; Christian, S.; Sperl, C.; Neuhaus, R.; Mowat, J.; Steckel, M.; et al. Functional inhibition of acid sphingomyelinase by Fluphenazine triggers hypoxia-specific tumor cell death. Cell Death Dis. 2017, 8, e2709. [Google Scholar] [CrossRef] [PubMed]
- Risner, M.L.; Ribeiro, M.; McGrady, N.R.; Kagitapalli, B.S.; Chamling, X.; Zack, D.J.; Calkins, D.J. Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo. Cell Commun. Signal. 2023, 21, 305. [Google Scholar] [CrossRef]
- Arenz, C. Small-molecule inhibitors of acid sphingomyelinase. Cell Physiol Biochem. 2010, 26, 1–8. [Google Scholar] [CrossRef]
- Skácel, J.; Slusher, B.S.; Tsukamoto, T. Small Molecule Inhibitors Targeting Biosynthesis of Ceramide, the Central Hub of the Sphingolipid Network. J. Med. Chem. 2021, 64, 279–297. [Google Scholar] [CrossRef]
- Inoue, M.; Yokota, W.; Katoh, T. Enantioselective Total Synthesis of (+)-Scyphostatin, a Potent and Specific Inhibitor of Neutral Sphingomyelinase. Synthesis 2007, 2007, 622–637. [Google Scholar] [CrossRef]
- Cheng, J.C.; Bai, A.; Beckham, T.H.; Marrison, S.T.; Yount, C.L.; Young, K.; Lu, P.; Bartlett, A.M.; Wu, B.X.; Keane, B.J.; et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Investig. 2013, 123, 4344–4358. [Google Scholar] [CrossRef]
- Ghandour, B.; Dbaibo, G.; Darwiche, N. The unfolding role of ceramide in coordinating retinoid-based cancer therapy. Biochem. J. 2021, 478, 3621–3642. [Google Scholar] [CrossRef]
- El Bawab, S.; Birbes, H.; Roddy, P.; Szulc, Z.M.; Bielawska, A.; Hannun, Y.A. Biochemical Characterization of the Reverse Activity of Rat Brain Ceramidase: A CoA-independent and fumonisin B1-insensitive ceramide synthase. J. Biol. Chem. 2001, 276, 16758–16766. [Google Scholar] [CrossRef] [PubMed]
- Draper, J.M.; Xia, Z.; Smith, R.A.; Zhuang, Y.; Wang, W.; Smith, C.D. Discovery and Evaluation of Inhibitors of Human Ceramidase. Mol. Cancer Ther. 2011, 10, 2052–2061. [Google Scholar] [CrossRef]
- Kus, G.; Kabadere, S.; Uyar, R.; Kutlu, H.M. Induction of apoptosis in prostate cancer cells by the novel ceramidase inhibitor ceranib-2. In Vitro Cell. Dev. Biol.—Anim. 2015, 51, 1056–1063. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Small Molecule Inhibitors of Ceramidases. Cell. Physiol. Biochem. 2014, 34, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, A.; Bielawski, J.; Szulc, Z.M.; Mayroo, N.; Liu, X.; Bai, A.; Elojeimy, S.; Rembiesa, B.; Pierce, J.; Norris, J.S.; et al. Novel analogs of d-e-MAPP and B13. Part 2: Signature effects on bioactive sphingolipids. Bioorganic Med. Chem. 2008, 16, 1032–1045. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Inhibitors of Ceramidases. Chem. Phys. Lipids 2016, 197, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jarocki, M.; Turek, K.; Saczko, J.; Tarek, M.; Kulbacka, J.A.-O. Lipids associated with autophagy: Mechanisms and therapeutic targets. Cell Death Discov. 2024, 10, 460. [Google Scholar] [CrossRef]
- Ruzzi, F.; Cappello, C.; Semprini, M.S.; Scalambra, L.; Angelicola, S.; Pittino, O.M.; Landuzzi, L.; Palladini, A.; Nanni, P.; Lollini, P.L. Lipid rafts, caveolae, and epidermal growth factor receptor family: Friends or foes? Cell Commun. Signal. 2024, 22, 489. [Google Scholar] [CrossRef]
- Jia, W.; Yuan, J.; Zhang, J.; Li, S.; Lin, W.; Cheng, B. Bioactive sphingolipids as emerging targets for signal transduction in cancer development. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189176. [Google Scholar] [CrossRef]
- Lai, M.K.P.; Chew, W.S.; Torta, F.; Rao, A.; Harris, G.L.; Chun, J.; Herr, D.R. Biological Effects of Naturally Occurring Sphingolipids, Uncommon Variants, and Their Analogs. NeuroMolecular Med. 2016, 18, 396–414. [Google Scholar] [CrossRef]
- Campisi, G.M.; Signorelli, P.; Rizzo, J.; Ghilardi, C.; Antognetti, J.; Caretti, A.; Lazarević, J.S.; Strettoi, E.; Novelli, E.; Ghidoni, R.; et al. Determination of the serine palmitoyl transferase inhibitor myriocin by electrospray and Q-trap mass spectrometry. Biomed. Chromatogr. 2017, 31, e4026. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Yokokawa, Y.; Okuno, Y.; Murakami, N. ChemInform Abstract: Total Syntheses of Myriocin and Z-Myriocin, Two Potent Immunosuppressants, from 2-Deoxy-D-glucose. ChemInform 1995, 26, 6209–6228. [Google Scholar] [CrossRef]
- Noda, N.; Nambu, H.; Ubukata, K.; Fujiwara, T.; Tsuge, K.; Yakura, T. Total synthesis of myriocin and mycestericin D employing Rh(II)-catalyzed CH amination followed by stereoselective alkylation. Tetrahedron 2017, 73, 868–878. [Google Scholar] [CrossRef]
- Choi, K.E.; Jung, Y.S.; Kim, D.H.; Song, J.K.; Kim, J.Y.; Jung, Y.Y.; Eum, S.Y.; Kim, J.H.; Yoon, N.Y.; Yoo, H.S.; et al. Myriocin induces apoptotic lung cancer cell death via activation of DR4 pathway. Arch. Pharmacal Res. 2014, 37, 501–511. [Google Scholar] [CrossRef]
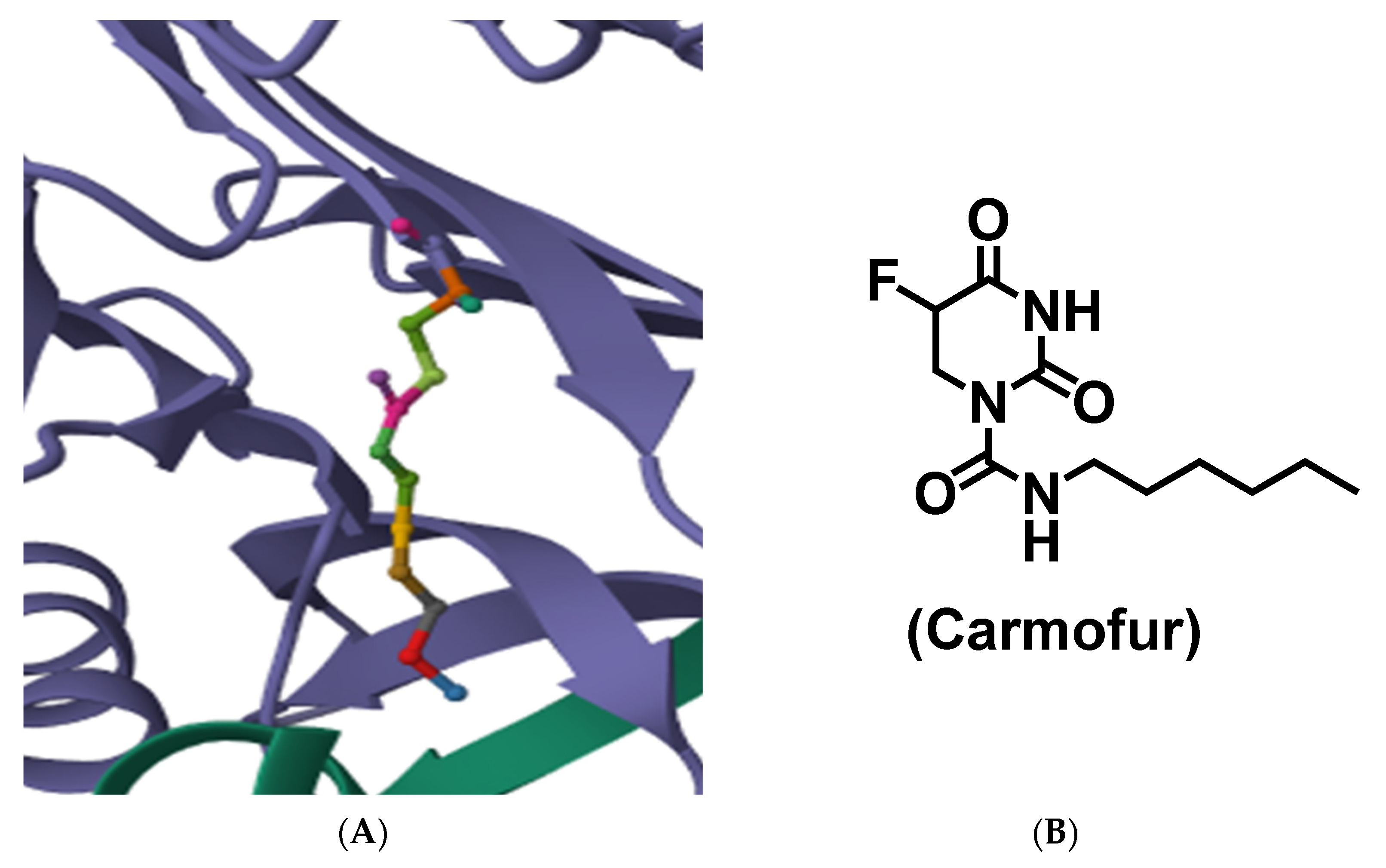
- Dementiev, A.; Joachimiak, A.; Nguyen, H.; Gorelik, A.; Illes, K.; Shabani, S.; Gelsomino, M.; Ahn, E.Y.E.; Nagar, B.; Doan, N. Molecular mechanism of inhibition of acid ceramidase by carmofur. J. Med. Chem. 2018, 62, 987–992. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A.; Vurro, M.; Mathieu, V.; Cimmino, A.; Evidente, M.; van Otterlo, W.A.L.; Dasari, R.; Lefranc, F.; Kiss, R. Toward a Cancer Drug of Fungal Origin. Med. Res. Rev. 2015, 35, 937–967. [Google Scholar] [CrossRef]
- Sharma, S.; Mathur, A.G.; Pradhan, S.; Singh, D.B.; Gupta, S. Fingolimod (FTY720): First approved oral therapy for multiple sclerosis. J. Pharmacol. Pharmacother. 2011, 2, 49–51. [Google Scholar] [CrossRef]
- Adachi, K.; Kohara, T.; Nakao, N.; Arita, M.; Chiba, K.; Mishina, T.; Sasaki, S.; Fujita, T. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: Discovery of a novel immunosuppressant, FTY720. Bioorganic Med. Chem. Lett. 1995, 5, 853–856. [Google Scholar] [CrossRef]
- Kandagatla, B.; Prasada Raju, V.V.N.K.V.; Kumar, N.S.; Reddy, G.M.; Srinivas, K.; Iqbal, J.; Bandichhor, R.; Oruganti, S. Practical synthesis of fingolimod from diethyl acetamidomalonate. RSC Adv. 2013, 3, 9687–9689. [Google Scholar] [CrossRef]
- Hunter, S.F.; Bowen, J.D.; Reder, A.T. The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis. CNS Drugs 2015, 30, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Chan, E.S.L. 87—Immunomodulating Pharmaceuticals. In Clinical Immunology, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1177–1184.e1. [Google Scholar]
- Rolland, W.B.; Lekic, T.; Krafft, P.R.; Krafft, P.; Hasegawa, Y.; Hasegawa, Y.; Altay, O.; Altay, O.; Hartman, R.; Hartman, R.; et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp. Neurol. 2013, 241, 45–55. [Google Scholar] [CrossRef]
- Billich, A.; Bornancin, F.; Dévay, P.; Mechtcheriakova, D.; Urtz, N.; Baumruker, T. Phosphorylation of the Immunomodulatory Drug FTY720 by Sphingosine Kinases. J. Biol. Chem. 2003, 278, 47408–47415. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhu, J.; Zhu, J.; Ding, K.; Ding, K.; Xu, J.; Xu, J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumor Biol. 2014, 35, 10707–10714. [Google Scholar] [CrossRef]
- Mahajan-Thakur, S.; Bien-Möller, S.; Marx, S.; Schroeder, H.; Rauch, B.H. Sphingosine 1-Phosphate (S1P) Signaling in Glioblastoma Multiforme—A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2448. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.G.; Oskeritzian, C.; Griffiths, R.; Griffiths, R.; Subramanian, P.; Subramanian, P.; Barbour, S.E.; Barbour, S.; Chalfant, C.E.; Chalfant, C.; et al. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood 2007, 109, 1077–1085. [Google Scholar] [CrossRef]
- Berdyshev, E.V.; Gorshkova, I.; Skobeleva, A.; Bittman, R.; Lu, X.; Dudek, S.M.; Mirzapoiazova, T.; Garcia, J.G.N.; Natarajan, V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem. 2009, 284, 5467–5477. [Google Scholar] [CrossRef] [PubMed]
- Zununi Vahed, S.; Ghiyasvand, S.; Hosseiniyan Khatibi, S.M.; Patel, B.; Mohajel Shoja, M.; Tolouian, R.; Ardalan, M. Sphingosine 1 phosphate agonists (SPI); a potential agent to prevent acute lung injury in COVID-19. Immunopathol. Persa 2021, 7, e03. [Google Scholar] [CrossRef]
- Teymouri, S.; Pourbayram Kaleybar, S.; Hejazian, S.S.; Hejazian, S.M.; Ansarin, K.; Ardalan, M.; Zununi Vahed, S. The effect of Fingolimod on patients with moderate to severe COVID-19. Pharmacol. Res. Perspect. 2023, 11, e01039. [Google Scholar] [CrossRef]
- Thompson, D.; Mahmood, S.; Morrice, N.; Kamli-Salino, S.; Dekeryte, R.; Hoffmann, P.A.; Doherty, M.K.; Whitfield, P.D.; Delibegović, M.; Mody, N. Fenretinide inhibits obesity and fatty liver disease but induces Smpd3 to increase serum ceramides and worsen atherosclerosis in LDLR−/− mice. Sci. Rep. 2023, 13, 3937. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Attempts to improve lipophilic drugs’ solubility and bioavailability: A focus on Fenretinide. Pharmaceutics 2024, 16, 579. [Google Scholar] [CrossRef] [PubMed]
- Patruno, I.; Thompson, D.; Dall’Angelo, S.; Windhorst, A.D.; Vugts, D.J.; Poot, A.J.; Mody, N.; Zanda, M. Design, synthesis, radiosynthesis and biological evaluation of fenretinide analogues as anticancer and metabolic syndrome-preventive agents. ChemMedChem. 2020, 15, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Hunsu, V.O.; Facey, C.O.B.; Fields, J.Z.; Boman, B.M. Retinoids as Chemo-Preventive and Molecular-Targeted Anti-Cancer Therapies. Int. J. Mol. Sci. 2021, 22, 7731. [Google Scholar] [CrossRef] [PubMed]
- Cuello, M.; Coats, A.; Darko, I.; Ettenberg, S.A.; Gardner, G.J.; Nau, M.M.; Liu, J.R.; Birrer, M.J.; Lipkowitz, S. N-(4-hydroxyphenyl) retinamide (4HPR) enhances TRAIL-mediated apoptosis through enhancement of a mitochondrial-dependent amplification loop in ovarian cancer cell lines. Cell Death Differ. 2004, 11, 527–541. [Google Scholar] [CrossRef]
- Yang, X.; Hao, Y.; Ding, Z.; Pater, A. BAG-1 Promotes Apoptosis Induced by N-(4-hydroxyphenyl)retinamide in Human Cervical Carcinoma Cells. Exp. Cell Res. 2000, 256, 491–499. [Google Scholar] [CrossRef]
- Fields, A.L.; Soprano, D.R.; Soprano, K.J. Retinoids in biological control and cancer. J. Cell. Biochem. 2007, 102, 886–898. [Google Scholar] [CrossRef]
- Veronesi, U.; Mariani, L.; Decensi, A.; Formelli, F.; Camerini, T.; Miceli, R.; Di Mauro, M.G.; Costa, A.; Marubini, E.; Sporn, M.B.; et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006, 17, 1065–1071. [Google Scholar] [CrossRef]
- Potenza, R.L.; Lodeserto, P.; Orienti, I. Fenretinide in Cancer and Neurological Disease: A Two-Face Janus Molecule. Int. J. Mol. Sci. 2022, 23, 7426. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Varricchio, C.; Montefrancesco, C.; Feroce, I.; Guerrieri-Gonzaga, A. Fenretinide (4-HPR): A Preventive Chance for Women at Genetic and Familial Risk? BioMed Res. Int. 2012, 2012, 172897. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A new bioavailable fenretinide formulation with antiproliferative, antimetabolic, and cytotoxic effects on solid tumors. Cell Death Dis. 2019, 10, 529. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Francescangeli, F.; Aricò, E.; Verachi, P.; Zucchetti, M.; Matteo, C.; Petricci, E.; Pilozzi, E.; Orienti, I.; Boe, A.; et al. A nanoencapsulated oral formulation of fenretinide promotes local and metastatic breast cancer dormancy in HER2/neu transgenic mice. J. Exp. Clin. Cancer Res. 2024, 43, 296. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.-O.X.; El-Khoueiry, A.B.; Maurer, B.J.; Groshen, S.; Pinski, J.K.; Cobos, E.; Gandara, D.R.; Lenz, H.J.; Kang, M.H.; Reynolds, C.P.; et al. A phase I study of intravenous fenretinide (4-HPR) for patients with malignant solid tumors. Cancer Chemother. Pharmacol. 2021, 87, 525–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Springfield, R.; Chen, S.; Li, X.; Feng, X.; Moshirian, R.; Yang, R.; Yuan, W. α-GalCer and iNKT Cell-Based Cancer Immunotherapy: Realizing the Therapeutic Potentials. Front. Immunol. 2019, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, W.; Wang, B.; Xia, C.; Zhang, W.; Wang, P.G. The Total Synthesis of Immunostimulant α-Galactosylceramides from Naturally Configured α-Galactoside Raffinose. Org. Lett. 2011, 13, 4530–4533. [Google Scholar] [CrossRef]
- Sullivan, B.A.; Kronenberg, M. Activation or anergy: NKT cells are stunned by α- galactosylceramide. J. Clin. Investig. 2005, 115, 2328–2329. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Nieda, M.; Schmidt, C.; Koezuka, Y.; Ishihara, S.; Ishikawa, Y.; Tadokoro, K.; Durrant, S.; Boyd, A.; Juji, T.; et al. In vitro anti-tumour activity of α-galactosylceramide-stimulated human invariant Vα24+NKT cells against melanoma. Br. J. Cancer 2001, 85, 741–746. [Google Scholar] [CrossRef]
- Toyoda, T.; Kamata, T.; Tanaka, K.; Ihara, F.; Takami, M.; Suzuki, H.; Nakajima, T.; Ikeuchi, T.; Kawasaki, Y.; Hanaoka, H.; et al. Phase II study of α-galactosylceramidepulsed antigen-presenting cells in patients with advanced or recurrent non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e000316. [Google Scholar] [CrossRef]
- Coward, J.; Ambrosini, G.; Musi, E.; Truman, J.P.; Haimovitz-Friedman, A.; Allegood, J.C.; Wang, E.; Merrill, A.H., Jr.; Schwartz, G.K. Safingol (l-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy 2009, 5, 184–193. [Google Scholar] [CrossRef]
- Michael, E.; Jung, S.W.Y. Synthesis of threo-β-aminoalcohols from aminoaldehydes via chelation-controlled additions. Total synthesis of l-threo sphingosine and safingol2012. Tetrahedron Lett. 2012, 53, 4216–4220. [Google Scholar] [CrossRef]
- Dickson, M.A.; Carvajal, R.D.; Merrill, A.H., Jr.; Gonen, M.; Cane, L.M.; Schwartz, G.K. A Phase I Clinical Trial of Safingol in Combination with Cisplatin in Advanced Solid Tumors. Clin. Cancer Res. 2011, 17, 2484–2492. [Google Scholar] [CrossRef]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Knapp, S.; Pyne, N.J.; Pyne, S.; Elkins, J.M. Crystal structure of sphingosine kinase 1 with PF-543. ACS Med. Chem. Lett. 2014, 5, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Fabišíková, M.; Martinková, M.; Hirková, S.; Gonda, J.; Pilátová, M.B.; Gönciová, G. Total synthesis and the anticancer activity of (+)-spisulosine. Carbohydr. Res. 2016, 435, 26–36. [Google Scholar] [CrossRef]
- Amarante, G.W.; Cavallaro, M.; Coelho, F. Highly diastereoselective total synthesis of the anti-tumoral agent (±)-Spisulosine (ES285) from a Morita–Baylis–Hillman adduct. Tetrahedron Lett. 2010, 51, 2597–2599. [Google Scholar] [CrossRef]
- Salcedo, M.; Cuevas, C.; Alonso, J.L.; Otero, G.; Faircloth, G.; Fernandez-Sousa, J.M.; Avila, J.; Wandosell, F. The marine sphingolipid-derived compound ES 285 triggers an atypical cell death pathway. Apoptosis 2007, 12, 395–409. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Malagarie-Cazenave, S.; Olea, N.; Vara, D.; Cuevas, C.; Díaz-Laviada, I. Spisulosine (ES-285) induces prostate tumor PC-3 and LNCaP cell death by de novo synthesis of ceramide and PKCζ activation. Eur. J. Pharmacol. 2008, 584, 237–245. [Google Scholar] [CrossRef]
- Cuadros, R.; Montejo de Garcini, E.; Wandosell, F.; Faircloth, G.; Fernández-Sousa, J.M.; Avila, J. The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett. 2000, 152, 23–29. [Google Scholar] [CrossRef]
- Vilar, E.; Grünwald, V.; Schöffski, P.; Singer, H.; Salazar, R.; Iglesias, J.L.; Casado, E.; Cullell-Young, M.; Baselga, J.; Tabernero, J. A phase I dose-escalating study of ES-285, a marine sphingolipid-derived compound, with repeat dose administration in patients with advanced solid tumors. Investig. New Drugs 2012, 30, 299–305. [Google Scholar] [CrossRef]
- Massard, C.; Salazar, R.; Armand, J.P.; Majem, M.; Deutsch, E.; García, M.; Oaknin, A.; Fernández-García, E.M.; Soto, A.; Soria, J.C. Phase I dose-escalating study of ES-285 given as a three-hour intravenous infusion every three weeks in patients with advanced malignant solid tumors. Investig. New Drugs 2012, 30, 2318–2326. [Google Scholar] [CrossRef]
- Ledroit, V.; Debitus, C.; Lavaud, C.; Massiot, G. Jaspines A and B: Two new cytotoxic sphingosine derivatives from the marine sponge Jaspis sp. Tetrahedron Lett. 2003, 44, 225–228. [Google Scholar] [CrossRef]
- Cingolani, F.; Simbari, F.; Abad, J.L.; Casasampere, M.; Fabrias, G.; Futerman, A.H.; Casas, J. Jaspine B induces nonapoptotic cell death in gastric cancer cells independently of its inhibition of ceramide synthase. J. Lipid Res. 2017, 58, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Pashikanti, S.; Ukani, R.; David, S.A.; Datta, A. Total Synthesis and Structure-Activity Relationship Studies of the Cytotoxic Anhydrophytosphingosine Jaspine B (Pachastrissamine). Synthesis 2017, 49, 2088–2100. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Jin, Y.; Zhang, M.; Jin, Y.; Cao, J.; Dong, H.; Fu, X.; Jin, C.Y. The RIP3 activator C8 regulates the autophagy flux mediated by p62 and promotes the immunogenic form of cell death in human gastric cancer cells. Bioorganic Chem. 2024, 153, 107937. [Google Scholar] [CrossRef] [PubMed]
- Bhaket, P.; Morris, K.; Stauffer, C.S.; Datta, A. Total synthesis of cytotoxic anhydrophytosphingosine pachastrissamine (jaspine B). Org. Lett. 2005, 7, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Khajeh Pour, S.; Mateen, S.; Pashikanti, S.; Barrott, J.A.-O.; Aghazadeh-Habashi, A.A.-O. Formulation, Characterization, and In Vitro/In Vivo Efficacy Studies of a Novel Liposomal Drug Delivery System of Amphiphilic Jaspine B for Treatment of Synovial Sarcoma. Mar. Drugs 2022, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.; Giri, P.; Mateen, S.; Pashikanti, S.; Aghazadeh-Habashi, A. Comparative Bioavailability Study of Jaspine B: Impact of Nanoliposomal Drug Delivery System on Pharmacokinetics. Pharmaceutics 2025, 17, 807. [Google Scholar] [CrossRef]
- Choi, M.-K.; Lee, J.; Nam, S.J.; Kang, Y.J.; Han, Y.; Choi, K.; Choi, Y.A.; Kwon, M.; Lee, D.; Song, I.-S. Pharmacokinetics of Jaspine B and Enhancement of Intestinal Absorption of Jaspine B in the Presence of Bile Acid in Rats. Mar. Drugs 2017, 15, 279. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, Q.; Chen, Y.; Yang, J.; Xia, Y.; Rao, B.; Li, S.; Shen, Y.; Cao, M.; Lu, H.; et al. Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis. Nat. Struct. Mol. Biol. 2024, 31, 884–895. [Google Scholar] [CrossRef]
- Bielawska, A.; Greenberg, M.S.; Perry, D.; Jayadev, S.; Shayman, J.A.; McKay, C.; Hannun, Y.A. (1S,2R)-D-erythro-2-(N-Myristoylamino)-1-phenyl-1-propanol as an Inhibitor of Ceramidase. J. Biol. Chem. 1996, 271, 12646–12654. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Choi, J.; Ding, S.; Prieschl, E.E.; Baumruker, T.; Lee, J.-M.; Chung, S.-K.; Schultz, P.G. The Synthesis and Biological Characterization of a Ceramide Library. J. Am. Chem. Soc. 2002, 124, 1856–1857. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, J.; Dong, M.; Qiu, Z.; Jin, J. Human alkaline ceramidase 2 promotes the growth, invasion, and migration of hepatocellular carcinoma cells via sphingomyelin phosphodiesterase acid-like 3B. Cancer Sci. 2020, 111, 2259–2274. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Larrauri, A.; Das Adhikari, U.; Aramburu-Nuñez, M.; Custodia, A.; Ouro, A. Ceramide Metabolism Enzymes—Therapeutic Targets against Cancer. Medicina 2021, 57, 729. [Google Scholar] [CrossRef]
- Xu, R.; Sun, W.; Jin, J.; Obeid, L.M.; Mao, C. Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J. 2010, 24, 2507–2515. [Google Scholar] [CrossRef]
- Bai, A.; Szulc, Z.M.; Bielawski, J.; Pierce, J.S.; Rembiesa, B.; Terzieva, S.; Mao, C.; Xu, R.; Wu, B.; Clarke, C.J.; et al. Targeting (cellular) lysosomal acid ceramidase by B13: Design, synthesis and evaluation of novel DMG-B13 ester prodrugs. Bioorg. Med. Chem. Lett. 2014, 22, 6933–6944. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.H.; Turner, L.S.; El-Zawahry, A.; Elojeimy, S.; Liu, X.; Bielawski, J.; Szulc, Z.M.; Norris, K.; Zeidan, Y.H.; Hannun, Y.A. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother. Pharmacol. 2008, 61, 231–242. [Google Scholar] [CrossRef]
- Hojjati, M.R.; Li, Z.; Zhou, H.; Tang, S.; Huan, C.; Ooi, E.; Lu, S.; Jiang, X.-C. Effect of Myriocin on Plasma Sphingolipid Metabolism and Atherosclerosis in apoE-deficient Mice. J. Biol. Chem. 2005, 280, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Trial of Fenretinide in Combination with Paclitaxel and Cisplatin. Available online: https://clinicaltrials.gov/study/NCT00005819 (accessed on 11 June 2010).
- Phase II Trial of Fenretinide (NSC-374551; IND-40, 294) in Patients with Relapsed Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT00009971 (accessed on 2 November 2013).
- A Phase Two Study of Fenretinide in Renal Cell Cancer. Available online: https://clinicaltrials.gov/study/NCT00011973 (accessed on 23 March 2005).
- Phase II Trial of Fenretinide (NSC 374551) in Recurrent Ovarian Cancer and Primary Peritoneal Carcinoma. Available online: https://clinicaltrials.gov/study/NCT00026091 (accessed on 25 March 2013).
- An Exploratory Evaluation of Fenretinide (4-HPR) as a Chemopreventive Agent for Ovarian Carcinoma. Available online: https://clinicaltrials.gov/study/NCT00017134 (accessed on 10 June 2013).
- A Phase I Study of Fenretinide Combined with Paclitaxel and Cisplatin for the Treatment of Refractory Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT00009932 (accessed on 15 May 2013).
- A Phase II Study of Fenretinide (NSC# 374551, IND# 40294) in Children with Recurrent/Resistant High Risk Neuroblastoma. Available online: https://clinicaltrials.gov/study/NCT00053326 (accessed on 8 October 2013).
- A Phase II Evaluation with Correlative Studies of Fenretinide (NSC 374551-4HPR) as a Single Agent in the Treatment of Adult Patients with Recurrent Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/study/NCT00075491 (accessed on 24 January 2013).
- A Phase 2 Study of Fenretinide in Patients with Hormone Refractory Prostate Cancer. Available online: https://clinicaltrials.gov/study/NCT00077402 (accessed on 15 May 2013).
- A Multicenter Randomized Double-Blinded Trial for Chemoprevention of Ovarian Cancer: Modulation of Biomarkers and Spectral Properties Using Contrast Enhanced Ultrasound in High-Risk Women Using Fenretinide (4-HPR). Available online: https://clinicaltrials.gov/study/NCT00098800 (accessed on 24 March 2010).
- A Phase II Trial of Fenretinide (4-HPR) in Biochemically Recurrent, Hormone Naive Prostate Cancer. Available online: https://clinicaltrials.gov/study/NCT00080899 (accessed on 6 March 2015).
- Phase I Trial of Intravenous Fenretinide (4-HPR) for Patients with Hematologic Malignancies. Available online: https://clinicaltrials.gov/study/NCT00104923 (accessed on 19 July 2017).
- A Phase IB Randomized Translational Study of Fenretinide (4-HPR) in Combination with SCH66336, a Farnesyl Transferase Inhibitor, in Patients with Advanced or Recurrent Head and Neck Cancer. Available online: https://clinicaltrials.gov/study/NCT00102635 (accessed on 15 November 2018).
- A Phase I-II Trial of Fenretinide (4-HPR) + Rituximab in Patients with B-cell Lymphoma. Available online: https://clinicaltrials.gov/study/NCT00288067 (accessed on 6 October 2014).
- Villablanca, J.G.; Krailo, M.D.; Ames, M.M.; Reid, J.M.; Reaman, G.H.; Reynolds, C.P. Phase I trial of oral fenretinide in children with high-risk solid tumors: A report from the Children’s Oncology Group (CCG 09709). J. Clin. Oncol. 2006, 24, 3423–3430. [Google Scholar] [CrossRef]
- Phase I Study of Fenretinide (4-HPR, NSC 374551) Lym-X-Sorb™ (LXS) Oral Powder in Patients with Recurrent or Resistant Neuroblastoma. Available online: https://clinicaltrials.gov/study/NCT00295919 (accessed on 7 April 2023).
- A Phase II Multicenter, Randomized, Double-Masked, Placebo-Controlled, Dose-Comparison Study of the Safety and Efficacy of Fenretinide in the Treatment of Geographic Atrophy in Subjects with Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/study/NCT00429936 (accessed on 22 June 2010).
- Phase 2 Study of the Assessment of the Insulin Sensitizing Activity of Fenretinide in Subjects with Insulin Resistance with BMI > 30 Kg/m2, and Liver Inflammation Related to Non-alcoholic Fatty Liver. Available online: https://clinicaltrials.gov/study/NCT00546455 (accessed on 10 February 2021).
- Phase I Trial of Fenretinide (4-HPR, NSC 374551) Lym-X-Sorb™ (LXS) Oral Powder (4-HPR/LXS Oral Powder) (4-HPR) in Adults with Solid Tumors and Lymphomas. Available online: https://clinicaltrials.gov/study/NCT00589381 (accessed on 8 March 2012).
- Comparison Efficacy of Bexarotene and Fenretinide as Addition to Antipsychotic Treatment in Schizophrenia Patients: Double-blind Placebo Controlled Study. Available online: https://clinicaltrials.gov/study/NCT00534898 (accessed on 29 June 2010).
- A Phase I Study of Intravenous (Emulsion) Fenretinide in Children with Recurrent or Resistant Neuroblastoma. Available online: https://clinicaltrials.gov/study/NCT00646230 (accessed on 7 April 2023).
- A Phase I Study of Intravenous (Emulsion) Fenretinide (4-HPR, NSC 374551) in Children with Recurrent or Resistant Acute Lymphoblastic Leukemia (ALL), Acute Myelogenous Leukemia (AML), and Non-Hodgkin’s Lymphoma (NHL) IND #70,058”. Available online: https://clinicaltrials.gov/study/NCT01187810 (accessed on 31 March 2022).
- Breast Cancer Prevention with Fenretinide in Young Women at Genetic and Familil Risk. A Phase III Randomized Clinical Trial. Available online: https://clinicaltrials.gov/study/NCT01479192 (accessed on 5 November 2018).
- Phase I Trial of Intravenous Fenretinide (4-HPR) Plus Intravenous Safingol for Patients with Relapsed Malignancies. Available online: https://clinicaltrials.gov/study/NCT01553071 (accessed on 31 March 2021).
- Phase I/II Trial of Fenretinide/LXS Oral Powder (NSC 374551) Plus Ketoconazole in Recurrent Ovarian Cancer and Primary Peritoneal Carcinoma. Available online: https://clinicaltrials.gov/study/NCT01535157 (accessed on 27 August 2020).
- An Adaptive Phase I Intra-patient Dose Escalation Study of Fenretinide in Adult Cystic Fibrosis Patients. Available online: https://clinicaltrials.gov/study/NCT02141958 (accessed on 12 July 2016).
- Lopez-Barcons, L.; Kang, M.H.; Maurer, B.J.; Reynolds, C.P. Enhanced activity of fenretinide/-LYM-X-SORBTM (4-HPR/LXS) oral powder in combination with ketoconazole and vincristine against recurrent neuroblastoma xenografts. Cancer Res. 2012, 72, 2493. [Google Scholar] [CrossRef]
- Expanded Access Study of Fenretinide (4-HPR, NSC 374551) Lym-X-Sorb (LXS) Oral Powder Plus Ketoconazole in Patients with Recurrent or Resistant Neuroblastoma (IND#68,254). Available online: https://clinicaltrials.gov/study/NCT02075177 (accessed on 21 March 2022).
- Phase II Trial of Intravenous Fenretinide (N-(4-hydroxyphenyl) Retinamide, 4-HPR) Emulsion for Patients with Relapsed/Refractory Peripheral T-cell Lymphomas (PTCL). Available online: https://clinicaltrials.gov/study/NCT02495415 (accessed on 23 July 2019).
- APPLAUD: A Double-Blind, Randomized, Placebo-Controlled, Phase II Study of the Efficacy and Safety of LAU-7b in the Treatment of Cystic Fibrosis in Adults. Available online: https://clinicaltrials.gov/study/NCT03265288 (accessed on 9 October 2024).
- A Phase 1a/1b Trial in Relapsed/Refractory T-cell Non-Hodgkin Lymphoma to Determine the Safety Profile, Pharmacology, and Maximum Tolerated Dose of ST-001, a Fenretinide Phospholipid Suspension (12.5 mg/mL) for Intravenous Infusion. Available online: https://clinicaltrials.gov/study/NCT04234048 (accessed on 26 March 2025).
- RESOLUTION: A Double-blind, Randomized, Placebo-controlled, Phase II/III Study of the Efficacy and Safety of LAU-7b in the Treatment of Adult Hospitalized Patients with COVID-19 Disease. Available online: https://clinicaltrials.gov/study/NCT04417257 (accessed on 30 July 2024).
- Why Antiprogestrone (Mifepristone) and Cyp 26 Inhibitor Must be Combined with Tamoxifen or (Tamoxifen and Retinoic Acid) for Treating Early Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT05016349 (accessed on 23 August 2021).
- A Phase 1a, Randomized, Double-Blind Placebo-Controlled Study to Evaluate Safety and Tolerability and to Characterize the Pharmacokinetic Profile of Single Ascending Doses of Fenretinide Oral Capsules in Healthy Adult Volunteers. Available online: https://clinicaltrials.gov/study/NCT06181760 (accessed on 26 March 2025).
- A Double-Blind, Randomized, Placebo-Controlled, Adaptive Phase 2/3 Study of the Efficacy of Lau-7B in the Treatment of Adults with Long Covid and Moderate to Severe Symptoms. Available online: https://clinicaltrials.gov/study/NCT05999435 (accessed on 28 August 2024).
- A Phase 2, Randomized, Double Blind, Placebo-Controlled Study to Examine the Prophylactic and Treatment Effects of a Single Dose Level of Oral Fenretinide in a Dengue Virus Challenge Model. Available online: https://clinicaltrials.gov/study/NCT06528457 (accessed on 21 February 2025).
- A Phase 1a/1b Trial in Relapsed/Refractory Small Cell Lung Cancer to Determine the Safety Profile, Pharmacology, and Maximum Tolerated Dose of ST-001, a Fenretinide Phospholipid Suspension (12.5 mg/mL) for Intravenous Infusion. Available online: https://clinicaltrials.gov/study/NCT06922539 (accessed on 13 April 2025).
- Phase I/II Trial of KRN7000 in Patients with Chronic Hepatitis C Infection. Available online: https://clinicaltrials.gov/study/NCT00352235 (accessed on 14 July 2006).
- Phase I/II Trial of Combination of Lenalidomide (Revlimid, LEN) and Autologous Mature Dendritic Cells Pulsed with α-Galactosyl Ceramide (α-GalCer; KRN7000) in Myeloma. Available online: https://clinicaltrials.gov/study/NCT00698776 (accessed on 21 June 2016).
- Lysosomal Storage Disease: Health, Development, and Functional Outcome Surveillance in Preschool Children. Available online: https://clinicaltrials.gov/study/NCT01938014 (accessed on 4 October 2019).
- The Institute for Myelin and Glia Exploration’s Clinical Database of Patients with Krabbe Disease, A World-Wide Registry. Available online: https://clinicaltrials.gov/study/NCT02993796 (accessed on 10 February 2025).


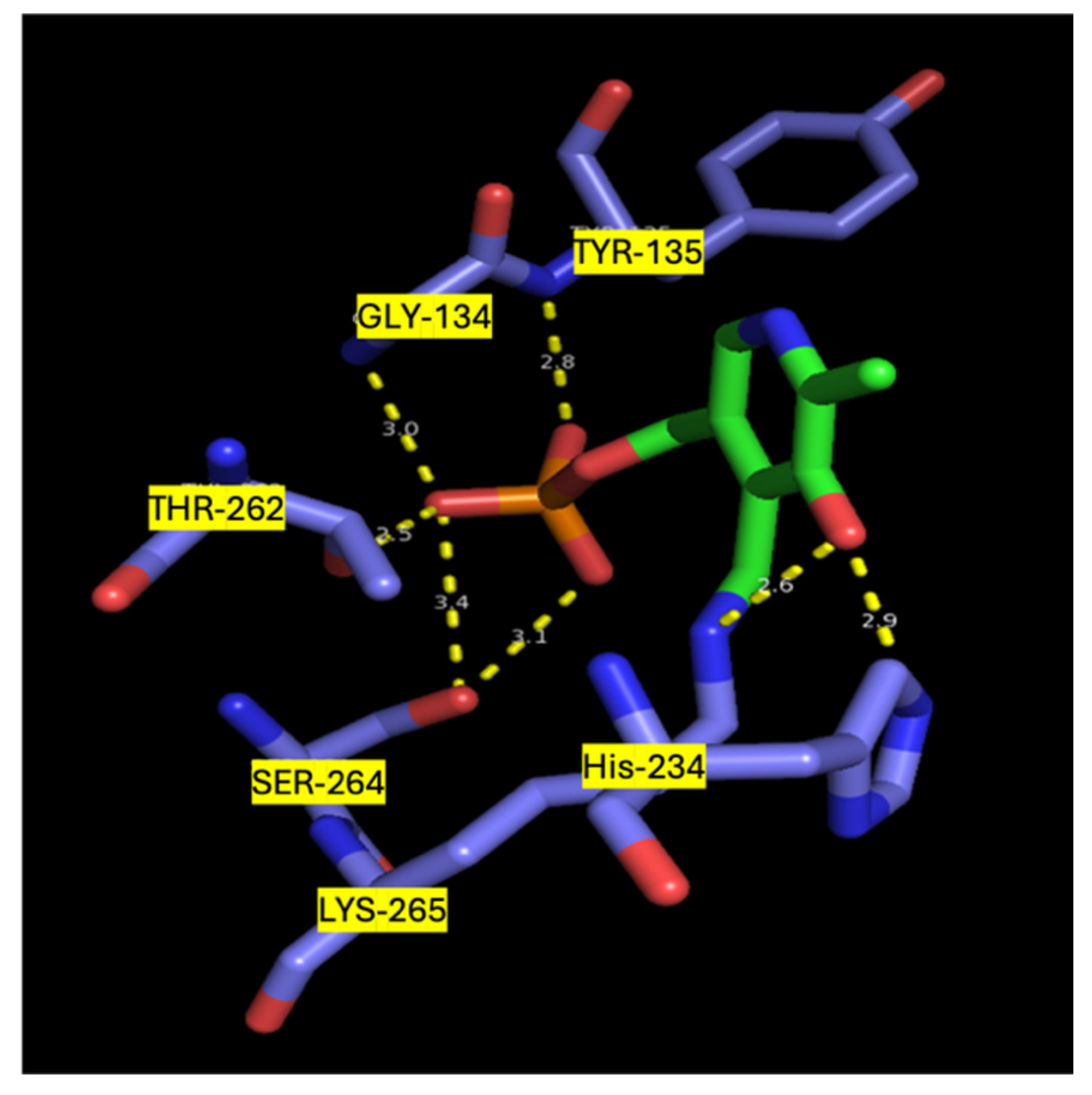






| Sphingolipid | Structure | Location | Biological Roles | References |
|---|---|---|---|---|
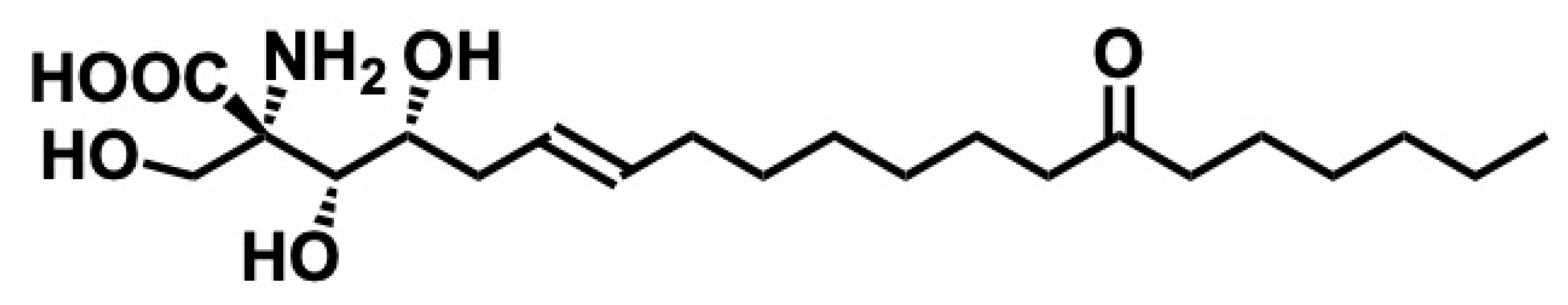
| Ceramide | Sphingosine + fatty acid | Cell membranes, especially lipid rafts | Apoptosis, cell cycle arrest, skin barrier, inflammation, insulin resistance | [35,36,37] |
| Sphingomyelin | Ceramide + phosphocholine | Plasma membrane (outer leaflet) | Myelin and membrane structure integrity by forming lipid rafts, and participating in cell signaling, acts as secondary messenger. | [35,38,39] |
| Glucosylceramide | Ceramide + glucose | Golgi, lysosomes, plasma membrane | Precursor to glycosphingolipids, aids in cell adhesion, lipid raft structure, improved lipid metabolism, skin barrier function, and reduced inflammation, accumulation of glucosylceramide leads to Gaucher disease. | [39,40] |
| Lactosyl ceramide | Ceramide + lactose | Plasma membrane | Immune signaling, pathogen recognition, inflammation | [41] |
| Ceramide-1-phosphate (C1P) | Ceramide + phosphate group | Cytoplasm, Golgi | Cell survival, inflammation control, macrophage migration | [42] |
| Sphingosine-1-phosphate (S1P) | Sphingosine + phosphate group | Extracellular (blood, lymph), cytosol | Immune cell trafficking, vascular integrity, anti-apoptosis, angiogenesis, proliferatory. | [43,44] |
| Enzyme | Small Molecule | Function | References |
|---|---|---|---|
| Serine palmitoyl transferase | Myriocin/ISP-1 | Inhibition | [54] |
| NA808 | [55] | ||
| L-cycloserine | [56] | ||
| ARN14494 | [57] | ||
| L-penicillamine | [58] | ||
| β-halo-l-alanines | [59] | ||
| Sphingosine kinase | SKI-I-V | Inhibition | [60] |
| Safingol | [61,62] | ||
| Doxorubicin | [63] | ||
| Actinomycin D | [64] | ||
| SG-12 | [65] | ||
| ABC294640 | [66] | ||
| PF-543 | [67] | ||
| Glucosylceramide synthase | Sinbaglustat(OGT2378) | Inhibition | [68] |
| Ibiglustat HCl/Venglustat | [69] | ||
| PDMP.HCl | [70] | ||
| L-Malic acid | [71] | ||
| PPMP.HCl | [72] | ||
| T-036 | [73] | ||
| Glucosylceramidase | D-gluconolactone | Inhibition | [74] |
| AMP-DNM-(5′-adamantane-1′-yl-methoxy)-pentyl-1-deoxynojirimycin) | [75] | ||
| Cyclophellitol | [76] | ||
| Conduritol B aziridine (CBA), Conduritol B epoxide (CBE) | [77,78] | ||
| butyldeoxynojirimycin (NB-DNJ) | [79] | ||
| Sphingomyelin synthase | Malabaricone C | Inhibition | [80] |
| D609 | [81] | ||
| MS-209 | [82] | ||
| D609 Prodrug | [81] | ||
| Dy105 | [83] | ||
| Jaspine B | [84] | ||
| Sphingomyelinase | Radiation | Activation | [85] |
| BMP | [86] | ||
| Win5 | [87] | ||
| Sphingomyelinase | Fluphenazine | Inhibition | [88] |
| GW4869 | [89] | ||
| AD2765 | [90] | ||
| Cowanin | [91] | ||
| Scyphostatin | [92] | ||
| Ceramidase | Radiation | Activation | [93] |
| Daunorubicin | [94] | ||
| Ceramidase | Fumonisin B1 Ceranib-1 Ceranib-2 Cystatin SA D-e-MAPP Carmofur SABRAC B13/D-NAPPD LCL204 | Inhibition | [95] [96] [97] [98] [99] [100] [100] [100] |
| Compound | Pharmacological Potential | Preclinical/Clinical Trials | References |
|---|---|---|---|
| Myriocin | Serine Palmitoyl Transfer (SPT) Inhibitor | Only mouse model studies have relevance to neurodegenerative disease; atherosclerotic lesion (AL) progression was studied so far. The main conclusion of AL study was a significant decrease in liver SPT activity preventing progression of atherosclerotic lesion. | [171] |
| Fingolimod (Gilenya)® and Cycle Vita™ | Sphingosine-1-phosphate modulator | FDA approved in 2010 to treat relapsing–remitting multiple sclerosis. Recently, 2023, an easy to swallow, orally disintegrating tablet (ODT)–Cycle Vita™ was approved to treat multiple sclerosis (www.tascenso.com, accessed on 29 March 2025). | [111] |
| Fenretinide | Disruption of normal lipid metabolism within cancer cells leading to apoptosis | From 1999 to 2025, 48 studies were performed with 1 currently active in different types of cancers. Several formulations were also explored in these studies. | [172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208] |
| Safingol | Modulates the immune system by binding to the CD1molecule on antigen-presenting cells to release cytokines. | There were four clinical studies from 2006 to 2016 to combat infections, autoimmune diseases, and cancer | [209,210,211,212] |
| Spisulosine | Caspase-mediated apoptosis in cancer cells. | Two clinical trials but withdrawn due to adverse events. | [2] |
| Jaspine B | Mitochondrial membrane depolarization by targeting ceramide metabolism. | Preclinical studies in mouse models to study the anticancer potential in synovial sarcoma. | [160,212] |
| D-e-MAPP | Alkaline ceramidase inhibition resulting in increase in endogenous ceramide levels. | [164,166,167] | |
| B13 | Inhibits acid ceramidase resulting in increase in ceramide levels. | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateen, S.; Oman, J.; Haniyyah, S.; Sharma, K.; Aghazadeh-Habashi, A.; Pashikanti, S. A Review Discussing Synthesis and Translational Studies of Medicinal Agents Targeting Sphingolipid Pathways. Biomolecules 2025, 15, 1022. https://doi.org/10.3390/biom15071022
Mateen S, Oman J, Haniyyah S, Sharma K, Aghazadeh-Habashi A, Pashikanti S. A Review Discussing Synthesis and Translational Studies of Medicinal Agents Targeting Sphingolipid Pathways. Biomolecules. 2025; 15(7):1022. https://doi.org/10.3390/biom15071022
Chicago/Turabian StyleMateen, Sameena, Jordan Oman, Soha Haniyyah, Kavita Sharma, Ali Aghazadeh-Habashi, and Srinath Pashikanti. 2025. "A Review Discussing Synthesis and Translational Studies of Medicinal Agents Targeting Sphingolipid Pathways" Biomolecules 15, no. 7: 1022. https://doi.org/10.3390/biom15071022
APA StyleMateen, S., Oman, J., Haniyyah, S., Sharma, K., Aghazadeh-Habashi, A., & Pashikanti, S. (2025). A Review Discussing Synthesis and Translational Studies of Medicinal Agents Targeting Sphingolipid Pathways. Biomolecules, 15(7), 1022. https://doi.org/10.3390/biom15071022










