Long-Term Exercise Mitigates Energy Expenditure and Inflammatory Responses Induced by Sleep Deprivation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Physical Exercise Training Protocol
2.3. Experimental Design and Sleep Deprivation Protocol
2.4. Body Weight and Food Intake
2.5. Measurement of BMR
2.6. Tissue Collection and Body Fat Contents
2.7. Measurement of LPS, IL-6 Concentration in Serum
2.8. Measurements of Gene Expression in BAT, Muscle by RT-qPCR
2.9. Measurements of TLR4, NF-κB, IL-6, and GAPDH by Western Blot
2.10. Microbiota 16S rRNA Gene Sequencing
2.11. Determination of Intestinal SCFA Concentration
2.12. Statistical Analysis
3. Results
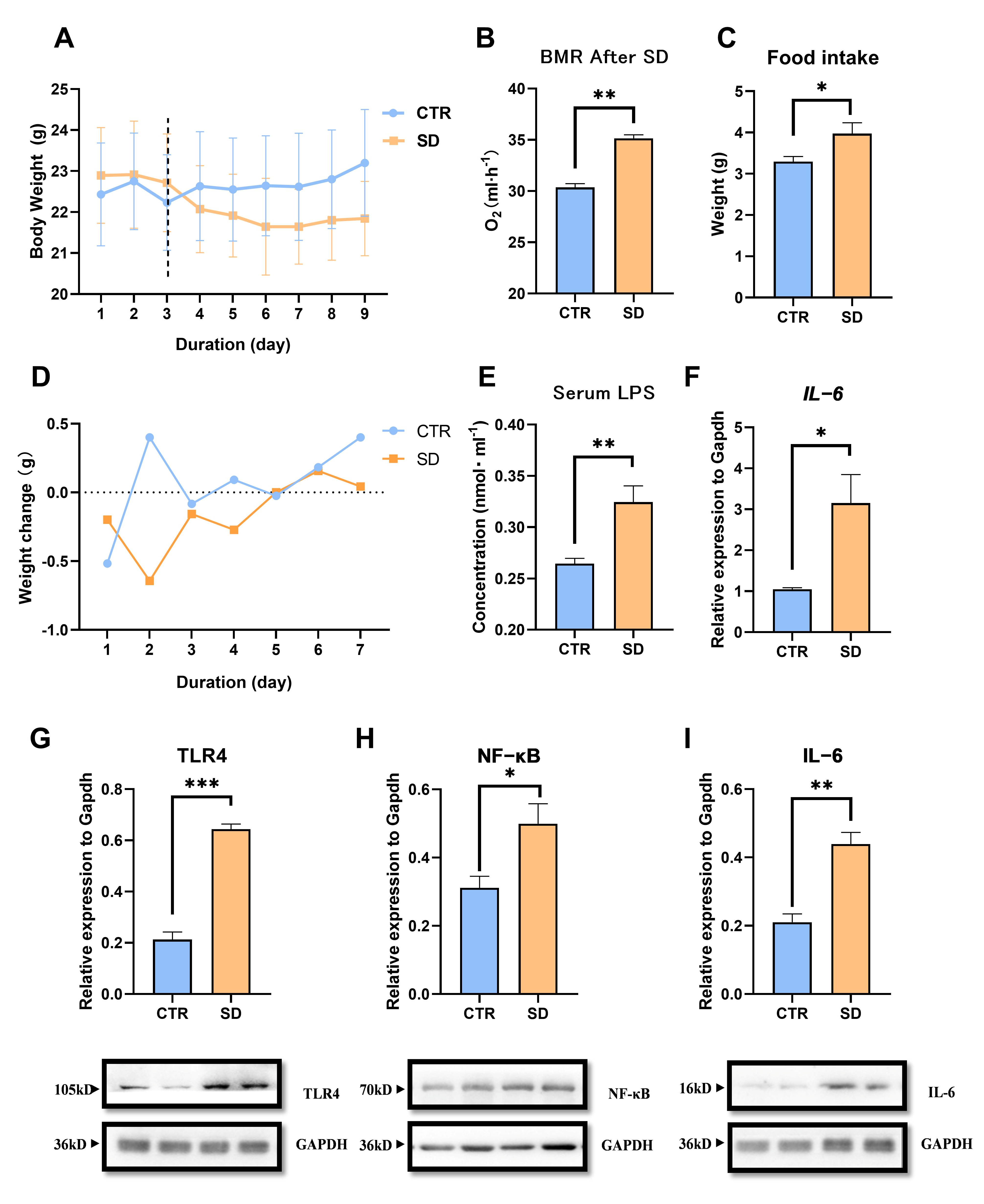
3.1. The Impact of SD on Energy Metabolism in Mice
3.2. The Inhibitory Effect of MICT on SD-Induced Detrimental Impacts
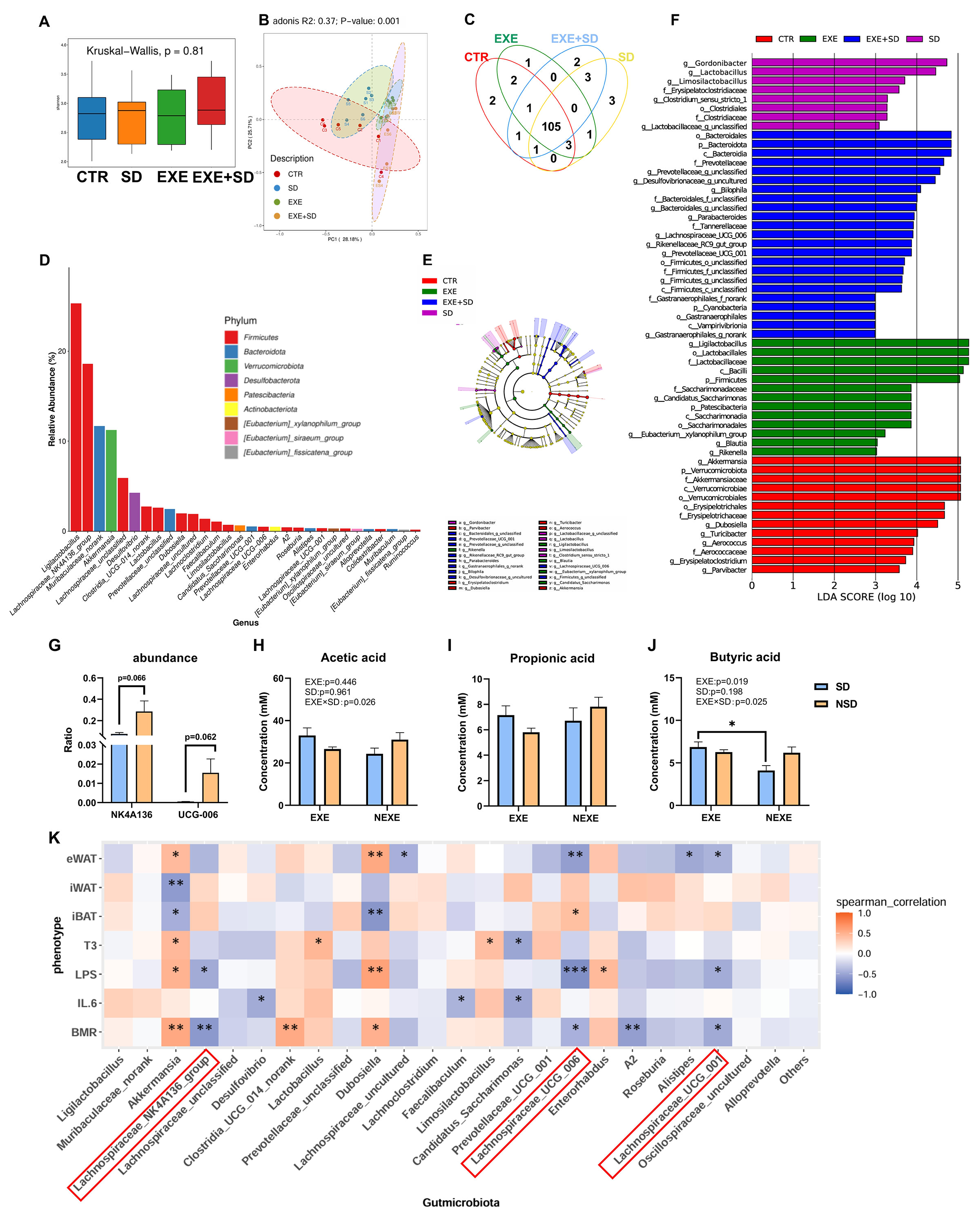
3.3. The Modulation of MICT on the Gut Microbiota of SD Mice
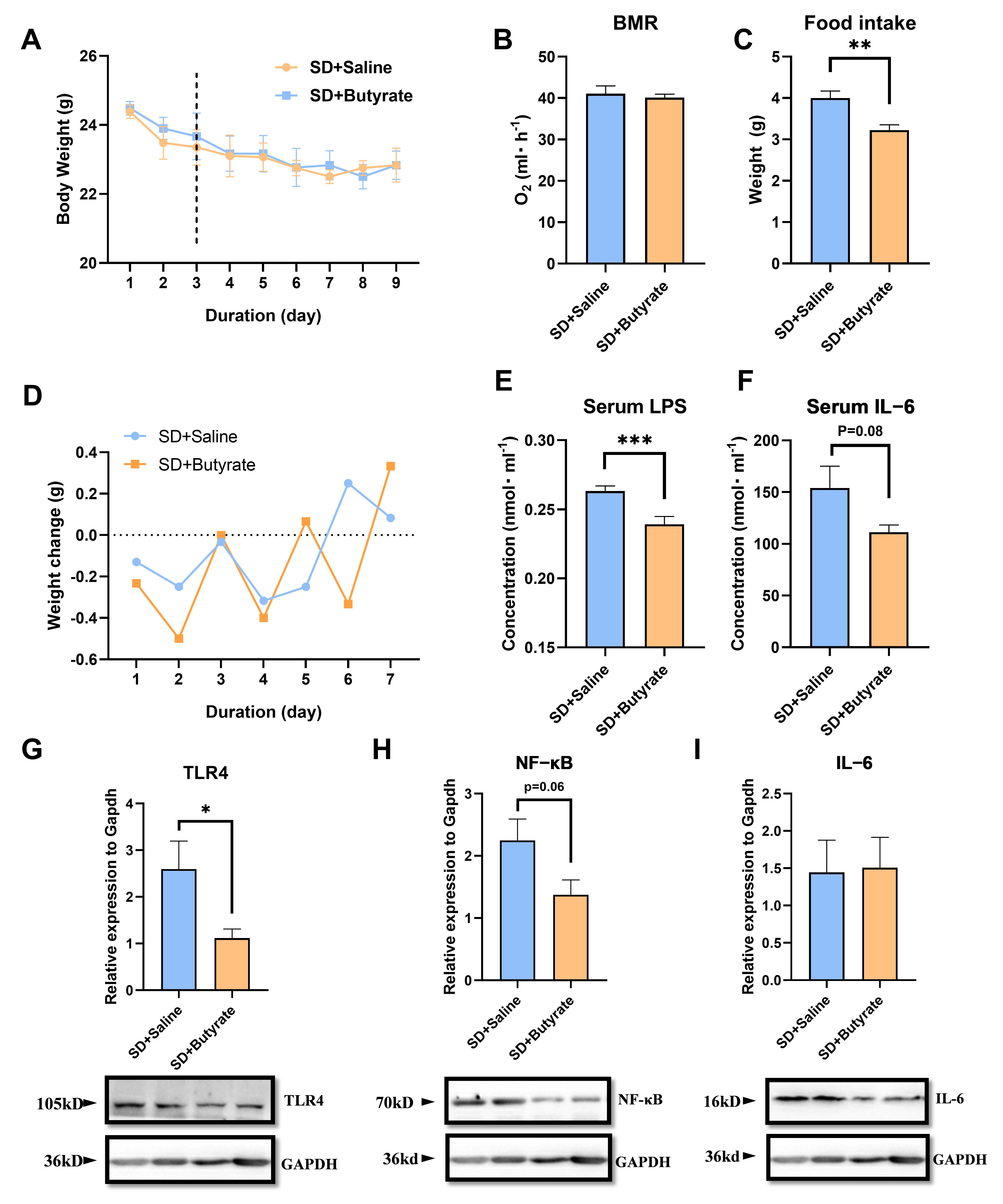
3.4. Butyrate Supplementation’s Inhibitory Effect of Metabolism and Inflammation on SD
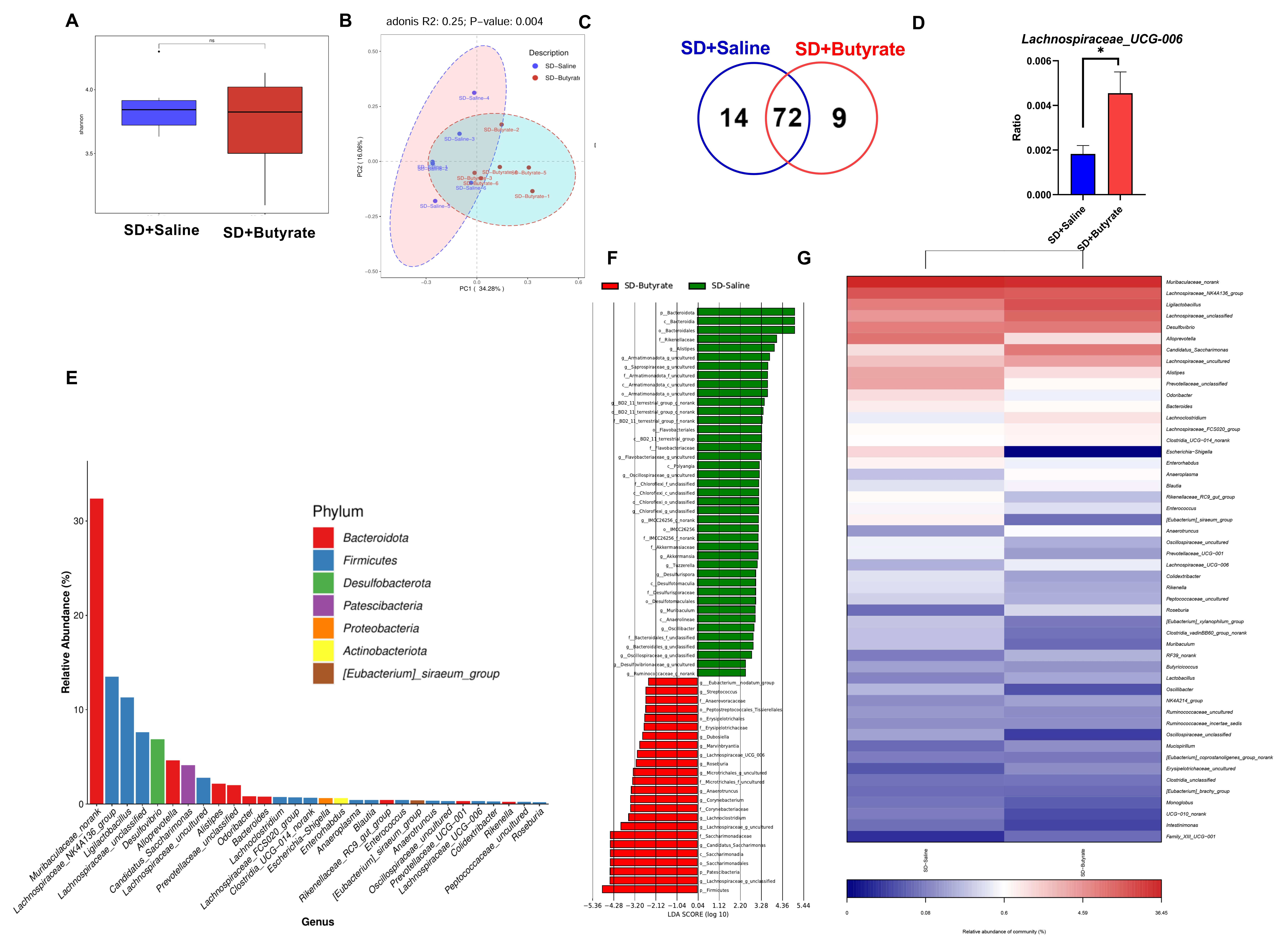
3.5. The Effects of Butyrate Supplementation on the Gut Microbiota of SD Mice
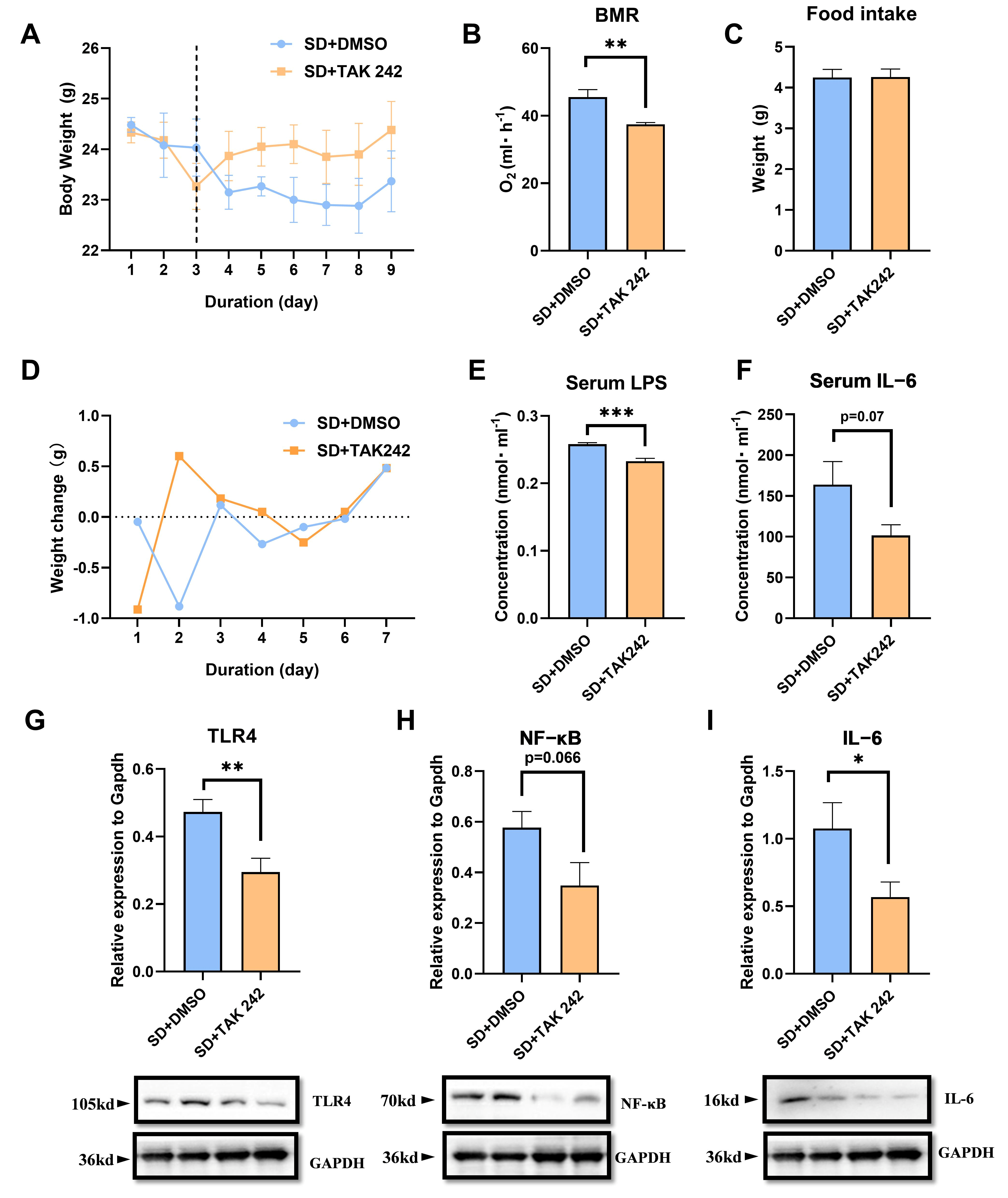
3.6. The Protective Effect of Inhibiting LPS in SD Mice
4. Discussion
- SD Increased energy expenditure in Mice
- 2.
- Exercise alleviates harmful effects induced by SD
- 3.
- The stability of the gut microbiota directly influences the body’s energy metabolism and immune function
5. Conclusions
6. Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Foti, K.E.; Eaton, D.K.; Lowry, R.; McKnight-Ely, L.R. Sufficient sleep, physical activity, and sedentary behaviors. Am. J. Prev. Med. 2011, 41, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T. Chronobiology: The human sleep project. Nature 2013, 498, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.D.; Tumminia, M.J.; Price, S.; Sefidgar, Y.; Cohen, S.; Ren, Y.; Brown, J.; Dey, A.K.; Dutcher, J.M.; Villalba, D.; et al. Nightly sleep duration predicts grade point average in the first year of college. Proc. Natl. Acad. Sci. USA 2023, 120, e2209123120. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, K.; Ji, Y.B.; Li, S.X.; Shi, L.; Wang, Z.; Zhou, X.Y.; Bao, Y.P.; Xie, W.; Han, Y.; et al. Alterations of the Gut Microbiota in Response to Total Sleep Deprivation and Recovery Sleep in Rats. Nat. Sci. Sleep 2022, 14, 121–133. [Google Scholar] [CrossRef]
- Suardiaz-Muro, M.; Ortega-Moreno, M.; Morante-Ruiz, M.; Monroy, M.; Ruiz, M.A.; Martin-Plasencia, P.; Vela-Bueno, A. Sleep quality and sleep deprivation: Relationship with academic performance in university students during examination period. Sleep. Biol. Rhythms 2023, 21, 377–383. [Google Scholar] [CrossRef]
- Magnavita, N.; Garbarino, S. Sleep, Health and Wellness at Work: A Scoping Review. Int. J. Environ. Res. Public Health 2017, 14, 1347. [Google Scholar] [CrossRef]
- Craven, J.; McCartney, D.; Desbrow, B.; Sabapathy, S.; Bellinger, P.; Roberts, L.; Irwin, C. Effects of Acute Sleep Loss on Physical Performance: A Systematic and Meta-Analytical Review. Sports Med. 2022, 52, 2669–2690. [Google Scholar] [CrossRef]
- Nedeltcheva, A.V.; Scheer, F.A. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 293–298. [Google Scholar] [CrossRef]
- Chaput, J.P.; Pereira, S.; Katzmarzyk, P.T.; Hedeker, D.; Barreira, T.V.; Garganta, R.; Farias, C.; Garbeloto, F.; Tani, G.; Stodden, D.F.; et al. Sleep and fundamental movement skills in primary schoolchildren: The REACT project. Am. J. Hum. Biol. 2024, 36, e24019. [Google Scholar] [CrossRef]
- Leproult, R.; Van Cauter, E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr. Dev. 2010, 17, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Vanrobaeys, Y.; Peterson, Z.J.; Walsh, E.N.; Chatterjee, S.; Lin, L.C.; Lyons, L.C.; Nickl-Jockschat, T.; Abel, T. Spatial transcriptomics reveals unique gene expression changes in different brain regions after sleep deprivation. Nat. Commun. 2023, 14, 7095. [Google Scholar] [CrossRef]
- Duan, D.; Kim, L.J.; Jun, J.C.; Polotsky, V.Y. Connecting insufficient sleep and insomnia with metabolic dysfunction. Ann. N. Y Acad. Sci. 2023, 1519, 94–117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.S.; Gao, X.R.; Xu, R.P.; Zhao, Y.F.; Yang, Z.T.; Wang, D.H. Sleep deprivation stimulates adaptive thermogenesis by activating AMPK pathway in mice. J. Comp. Physiol. B 2025, 195, 141–153. [Google Scholar] [CrossRef]
- Penev, P.D. Sleep deprivation and energy metabolism: To sleep, perchance to eat? Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Sun, L.; Yang, X.; Yuan, Z.; Wang, H. Metabolic Reprogramming in Immune Response and Tissue Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; Deoliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014, 20, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Veler, H. Sleep and Inflammation: Bidirectional Relationship. Sleep. Med. Clin. 2023, 18, 213–218. [Google Scholar] [CrossRef]
- Sang, D.; Lin, K.; Yang, Y.; Ran, G.; Li, B.; Chen, C.; Li, Q.; Ma, Y.; Lu, L.; Cui, X.Y.; et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 2023, 186, 5500–5516.e21. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Whitney, C.C.; Wilson, M.A.; Fagnant, H.S.; Radcliffe, P.N.; Chakraborty, N.; Campbell, R.; Hoke, A.; Gautam, A.; Hammamieh, R.; et al. Severe, short-term sleep restriction reduces gut microbiota community richness but does not alter intestinal permeability in healthy young men. Sci. Rep. 2023, 13, 213. [Google Scholar] [CrossRef]
- Wankhede, N.L.; Kale, M.B.; Kyada, A.; M, R.M.; Chaudhary, K.; Naidu, K.S.; Rahangdale, S.; Shende, P.V.; Taksande, B.G.; Khalid, M.; et al. Sleep deprivation-induced shifts in gut microbiota: Implications for neurological disorders. Neuroscience 2025, 565, 99–116. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schurmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Sun, J.; Fang, D.; Wang, Z.; Liu, Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int. J. Mol. Sci. 2023, 24, 9603. [Google Scholar] [CrossRef] [PubMed]
- Saadati, H.; Esmaeili-Mahani, S.; Esmaeilpour, K.; Nazeri, M.; Mazhari, S.; Sheibani, V. Exercise improves learning and memory impairments in sleep deprived female rats. Physiol. Behav. 2015, 138, 285–291. [Google Scholar] [CrossRef]
- He, Q.; Ji, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, J.; Zhu, Q.; Xie, M.; Ou, W.; Liu, J.; et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 2024, 36, 1998–2014.e15. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, C.; Yi, C. Physical Activity and Sleep Quality Association in Different Populations: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1864. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Hadjigeorgiou, G.M.; Manconi, M.; Bargiotas, P. Unfolding the role of exercise in the management of sleep disorders. Eur. J. Appl. Physiol. 2024, 124, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Atoui, S.; Chevance, G.; Romain, A.J.; Kingsbury, C.; Lachance, J.P.; Bernard, P. Daily associations between sleep and physical activity: A systematic review and meta-analysis. Sleep. Med. Rev. 2021, 57, 101426. [Google Scholar] [CrossRef]
- Alnawwar, M.A.; Alraddadi, M.I.; Algethmi, R.A.; Salem, G.A.; Salem, M.A.; Alharbi, A.A. The Effect of Physical Activity on Sleep Quality and Sleep Disorder: A Systematic Review. Cureus 2023, 15, e43595. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Powell, K.E.; Sarmiento, O.L.; Hallal, P.C. Even a small dose of physical activity can be good medicine. Nat. Med. 2025, 31, 376–378. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, X.; Feng, M.; Li, M.; Wang, J.; Yang, H.; He, S.; Xia, Z.; Shang, L.; Jiang, X.; et al. Early-life exercise induces immunometabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice. Nat. Commun. 2024, 15, 3103. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Loyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Lu, H.; Xu, X.; Fu, D.; Gu, Y.; Fan, R.; Yi, H.; He, X.; Wang, C.; Ouyang, B.; Zhao, P.; et al. Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-kappaB axis. Cell Host Microbe 2022, 30, 1139–1150.e7. [Google Scholar] [CrossRef]
- Huang, W.C.; Tung, C.L.; Yang, Y.S.H.; Lin, I.H.; Ng, X.E.; Tung, Y.T. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci. Rep. 2022, 12, 3612. [Google Scholar] [CrossRef]
- Zhao, Z.; Tong, Y.; Kang, Y.; Qiu, Z.; Li, Q.; Xu, C.; Wu, G.; Jia, W.; Wang, P. Sodium butyrate (SB) ameliorated inflammation of COPD induced by cigarette smoke through activating the GPR43 to inhibit NF-kappaB/MAPKs signaling pathways. Mol. Immunol. 2023, 163, 224–234. [Google Scholar] [CrossRef]
- Kullberg, R.F.J.; Wikki, I.; Haak, B.W.; Kauko, A.; Galenkamp, H.; Peters-Sengers, H.; Butler, J.M.; Havulinna, A.S.; Palmu, J.; McDonald, D.; et al. Association between butyrate-producing gut bacteria and the risk of infectious disease hospitalisation: Results from two observational, population-based microbiome studies. Lancet Microbe 2024, 5, 100864. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Wood, N.R.; Garritson, J.; Mathias, A.; Haughian, J.M.; Hayward, R. Moderate Intensity Endurance and Resistance Exercise Attenuates Cachexia in Tumor-bearing Mice. Anticancer. Res. 2022, 42, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.; Wang, C.; Deng, X.; Li, R.; Guo, Z.; Quan, H.; Li, S. Treadmill exercise ameliorates chronic REM sleep deprivation-induced anxiety-like behavior and cognitive impairment in C57BL/6J mice. Brain Res. Bull. 2020, 164, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, L.; Li, M.; Lam, S.M.; Wang, G.; Wu, Y.; Zhang, H.; Niu, C.; Zhang, X.; Liu, X.; et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019, 26, 2720–2737.e5. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Cheng, C.; Ding, G.; Zhao, P.; Tan, K.; Chen, L.; Perrimon, N.; Veenstra, J.A.; Zhang, L.; et al. Gut AstA mediates sleep deprivation-induced energy wasting in Drosophila. Cell Discov. 2023, 9, 49. [Google Scholar] [CrossRef]
- Monico-Neto, M.; Antunes, H.K.; Lee, K.S.; Phillips, S.M.; Giampa, S.Q.; Souza Hde, S.; Dattilo, M.; Medeiros, A.; de Moraes, W.M.; Tufik, S.; et al. Resistance training minimizes catabolic effects induced by sleep deprivation in rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1143–1150. [Google Scholar] [CrossRef]
- Tiba, P.A.; Oliveira, M.G.; Rossi, V.C.; Tufik, S.; Suchecki, D. Glucocorticoids are not responsible for paradoxical sleep deprivation-induced memory impairments. Sleep 2008, 31, 505–515. [Google Scholar] [CrossRef]
- Galvao Mde, O.; Sinigaglia-Coimbra, R.; Kawakami, S.E.; Tufik, S.; Suchecki, D. Paradoxical sleep deprivation activates hypothalamic nuclei that regulate food intake and stress response. Psychoneuroendocrinology 2009, 34, 1176–1183. [Google Scholar] [CrossRef]
- Ringgold, K.M.; Barf, R.P.; George, A.; Sutton, B.C.; Opp, M.R. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J. Neurosci. Methods 2013, 219, 104–112. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Du, C.; Hao, H.; Liu, S.; Liu, G.; Zhang, G.; Fan, K.; Ma, J. Up-regulated succinylation modifications induce a senescence phenotype in microglia by altering mitochondrial energy metabolism. J. Neuroinflam. 2024, 21, 296. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- da Silva, B.R.D.; Nunes, P.I.G.; Santos, F.A.; de Bruin, P.F.C.; de Bruin, V.M.S. Exercise modifies lipid and glucose metabolism alterations induced by sleep deprivation in mice. Sleep. Sci. 2022, 15, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Sahin, L.; Cevik, O.S.; Cevik, K.; Guven, C.; Taskin, E.; Kocahan, S. Mild regular treadmill exercise ameliorated the detrimental effects of acute sleep deprivation on spatial memory. Brain Res. 2021, 1759, 147367. [Google Scholar] [CrossRef]
- Saner, N.J.; Bishop, D.J.; Bartlett, J.D. Is exercise a viable therapeutic intervention to mitigate mitochondrial dysfunction and insulin resistance induced by sleep loss? Sleep. Med. Rev. 2018, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Modinos, G.; McLaughlin, A.; Egerton, A.; McMullen, K.; Kumari, V.; Barker, G.J.; Keysers, C.; Williams, S.C. Corticolimbic hyper-response to emotion and glutamatergic function in people with high schizotypy: A multimodal fMRI-MRS study. Transl. Psychiatry 2017, 7, e1083. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.; Braddock, A.; Roberts, E.; Ellis, D.; Chan, V. Identification of sleep fragmentation-induced gut microbiota alteration and prediction of functional impact in Sprague Dawley rats harboring microbiome derived from multiple human donors. Sleep. Sci. 2022, 15, 07–19. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Nagarajan, A.; Scoggin, K.; Gupta, J.; Threadgill, D.W.; Andrews-Polymenis, H.L. Using the collaborative cross to identify the role of host genetics in defining the murine gut microbiome. Microbiome 2023, 11, 149. [Google Scholar] [CrossRef]
- Szentirmai, E.; Buckley, K.; Massie, A.R.; Kapas, L. Lipopolysaccharide-mediated effects of the microbiota on sleep and body temperature. Sci. Rep. 2024, 14, 27378. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, J.; Li, S.; Zhang, D.; Bai, X. Progress of research on short-chain fatty acids, metabolites of gut microbiota, and acute ischemic stroke. Clin. Neurol. Neurosurg. 2025, 249, 108725. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hsieh, Y.R.; Lai, W.D.; Tung, T.H.; Chen, Y.X.; Yang, C.H.; Fang, Y.C.; Huang, S.Y. Melatonin Ameliorates Neuropsychiatric Behaviors, Gut Microbiome, and Microbiota-Derived Metabolites in Rats with Chronic Sleep Deprivation. Int. J. Mol. Sci. 2023, 24, 16820. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S. The Inflammation Induced by Lipopolysaccharide can be Mitigated by Short-chain Fatty Acid, Butyrate, through Upregulation of IL-10 in Septic Shock. Scand. J. Immunol. 2017, 85, 258–263. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.D.; Li, H.F.; Tian, M.L.; Pan, J.Q.; Shu, G.; Jiang, Q.Y.; Yin, Y.L.; Zhang, L. LSD1 mediates microbial metabolite butyrate-induced thermogenesis in brown and white adipose tissue. Metabolism 2020, 102, 154011. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Domagalska, D.; Borycka-Kiciak, K.; Rydzewska, G. Determination of butyric acid dosage based on clinical and experimental studies—A literature review. Prz. Gastroenterol. 2020, 15, 119–125. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.-S.; Gao, X.-R.; Gu, C.; Ru, Y.-N.; Xu, R.-P.; Yang, Y.-H.; Wang, D.-H. Long-Term Exercise Mitigates Energy Expenditure and Inflammatory Responses Induced by Sleep Deprivation in Mice. Biomolecules 2025, 15, 862. https://doi.org/10.3390/biom15060862
Zheng T-S, Gao X-R, Gu C, Ru Y-N, Xu R-P, Yang Y-H, Wang D-H. Long-Term Exercise Mitigates Energy Expenditure and Inflammatory Responses Induced by Sleep Deprivation in Mice. Biomolecules. 2025; 15(6):862. https://doi.org/10.3390/biom15060862
Chicago/Turabian StyleZheng, Tian-Shu, Xin-Ran Gao, Chen Gu, Yu-Ning Ru, Rui-Ping Xu, Yu-Hang Yang, and De-Hua Wang. 2025. "Long-Term Exercise Mitigates Energy Expenditure and Inflammatory Responses Induced by Sleep Deprivation in Mice" Biomolecules 15, no. 6: 862. https://doi.org/10.3390/biom15060862
APA StyleZheng, T.-S., Gao, X.-R., Gu, C., Ru, Y.-N., Xu, R.-P., Yang, Y.-H., & Wang, D.-H. (2025). Long-Term Exercise Mitigates Energy Expenditure and Inflammatory Responses Induced by Sleep Deprivation in Mice. Biomolecules, 15(6), 862. https://doi.org/10.3390/biom15060862






