Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
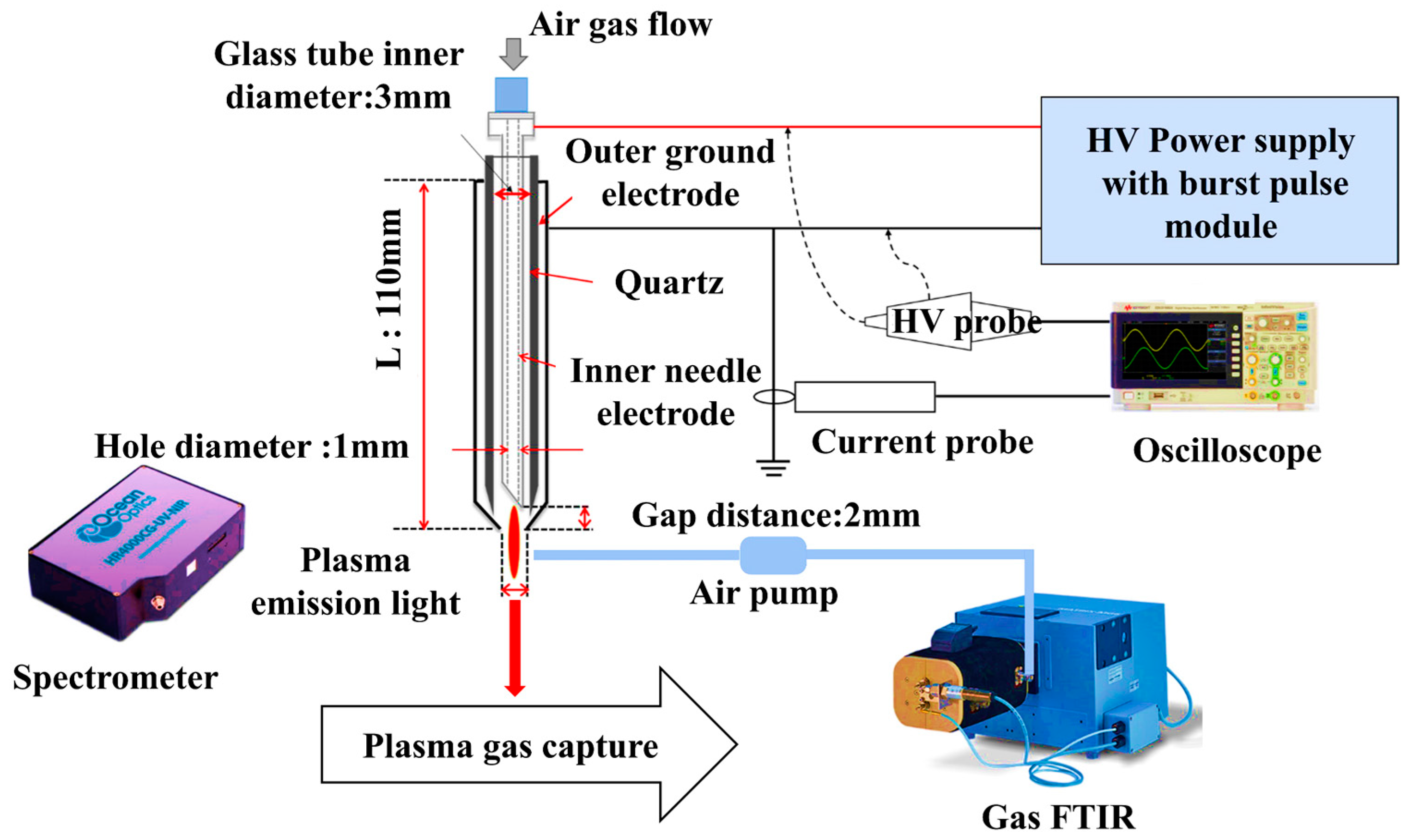
2.3. Plasma Device Setup
2.4. Physicochemical Properties of the Reactive Species
2.5. Cell Viability
2.6. Cell Movement Assay
2.7. Clonogenic Assay
2.8. Intracellular ROS/RNS Detection
2.9. Cell Death Analysis by Propidium Iodide (PI) Staining
2.10. Flow Cytometry
2.11. qRT-PCR
2.12. Live/Dead Cell Staining
2.13. Cancer Sphere Formation
2.14. Immunostaining
2.15. Intracellular Chelate Iron
2.16. Determination of Labile Iron Pool (LIP)
2.17. Lipid Peroxidation Assay
2.18. GSH Assay
2.19. Western Blot Analysis
2.20. Statistical Analysis
3. Results
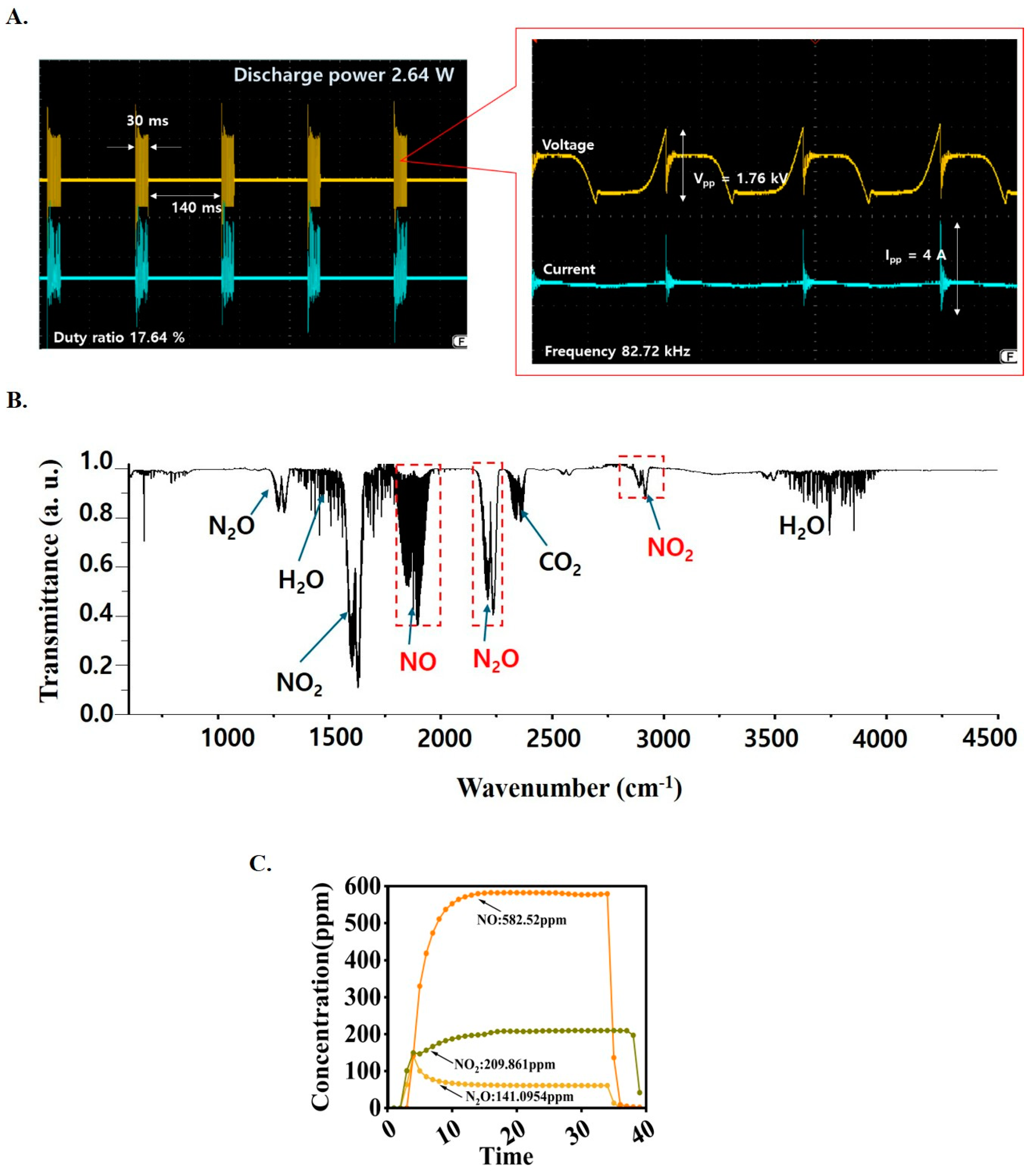
3.1. Measurement of the Electrical Discharge Power
3.2. Measurement of the Plasma Temperature and Density
3.3. Measurement of the Plasma Reactive Species Concentration
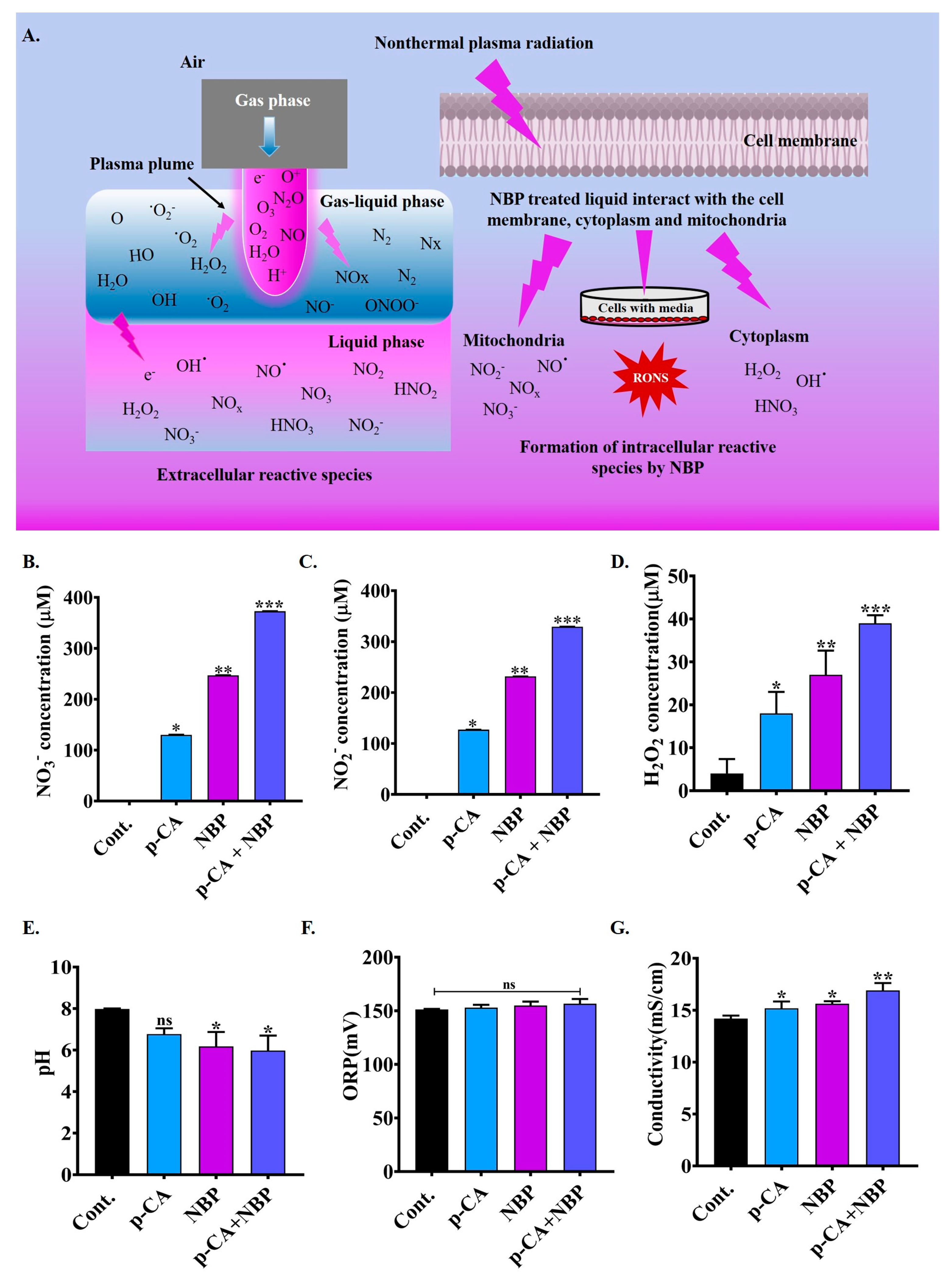
3.4. Physiochemical Properties of NBP-Jet Plasma-Treated Media
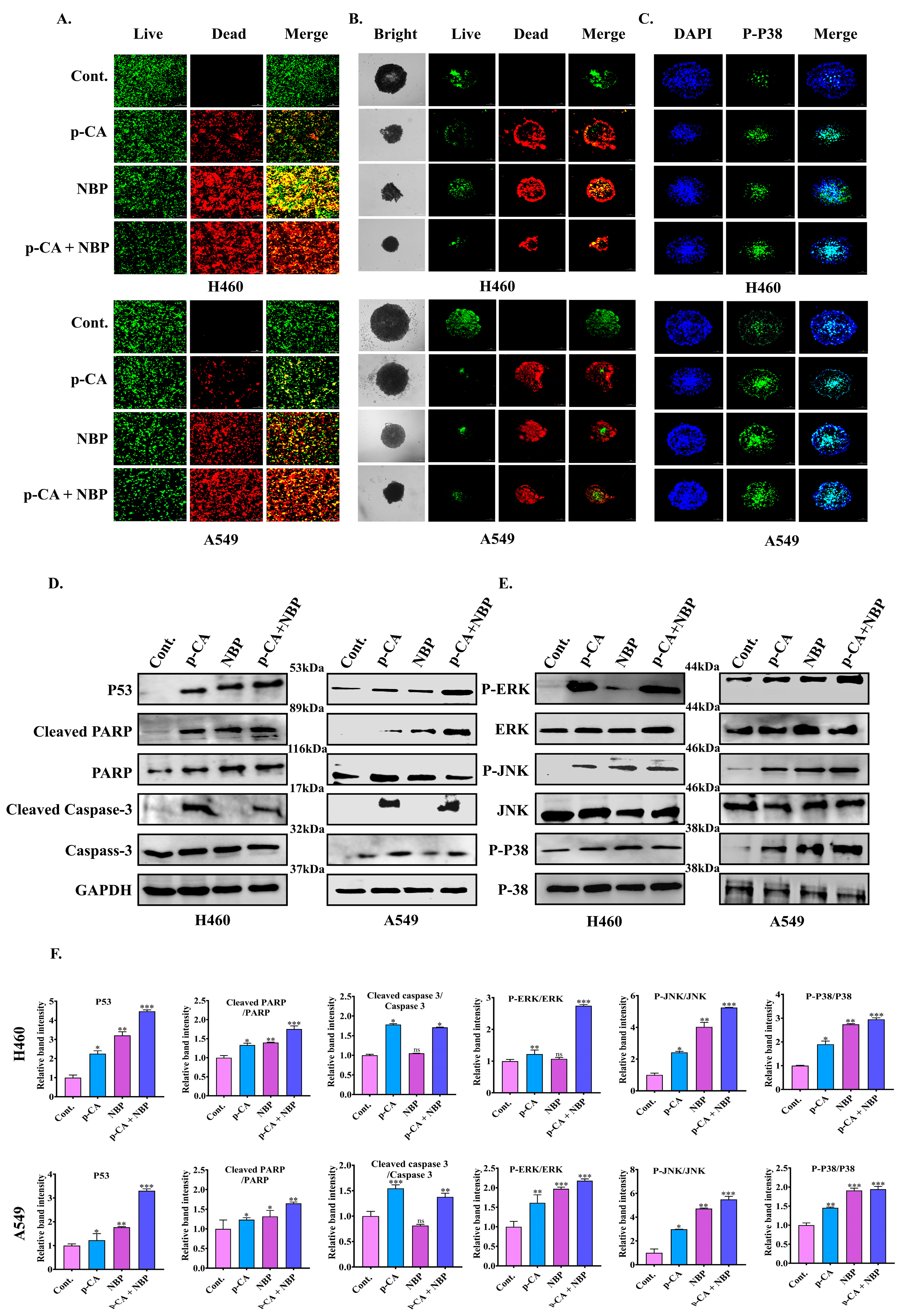
3.5. NBP-Jet Plasma and Para-Coumaric Acid Treated Media Decreased Cell Viability, Colony Formation and Movement Ability of Lung Adenocarcinoma
3.6. NBP-Jet Plasma and Para-Coumaric Acid Induce Apoptosis by Inhibiting Cancer Spheroid Formation Through the MAPK and PARP Pathways
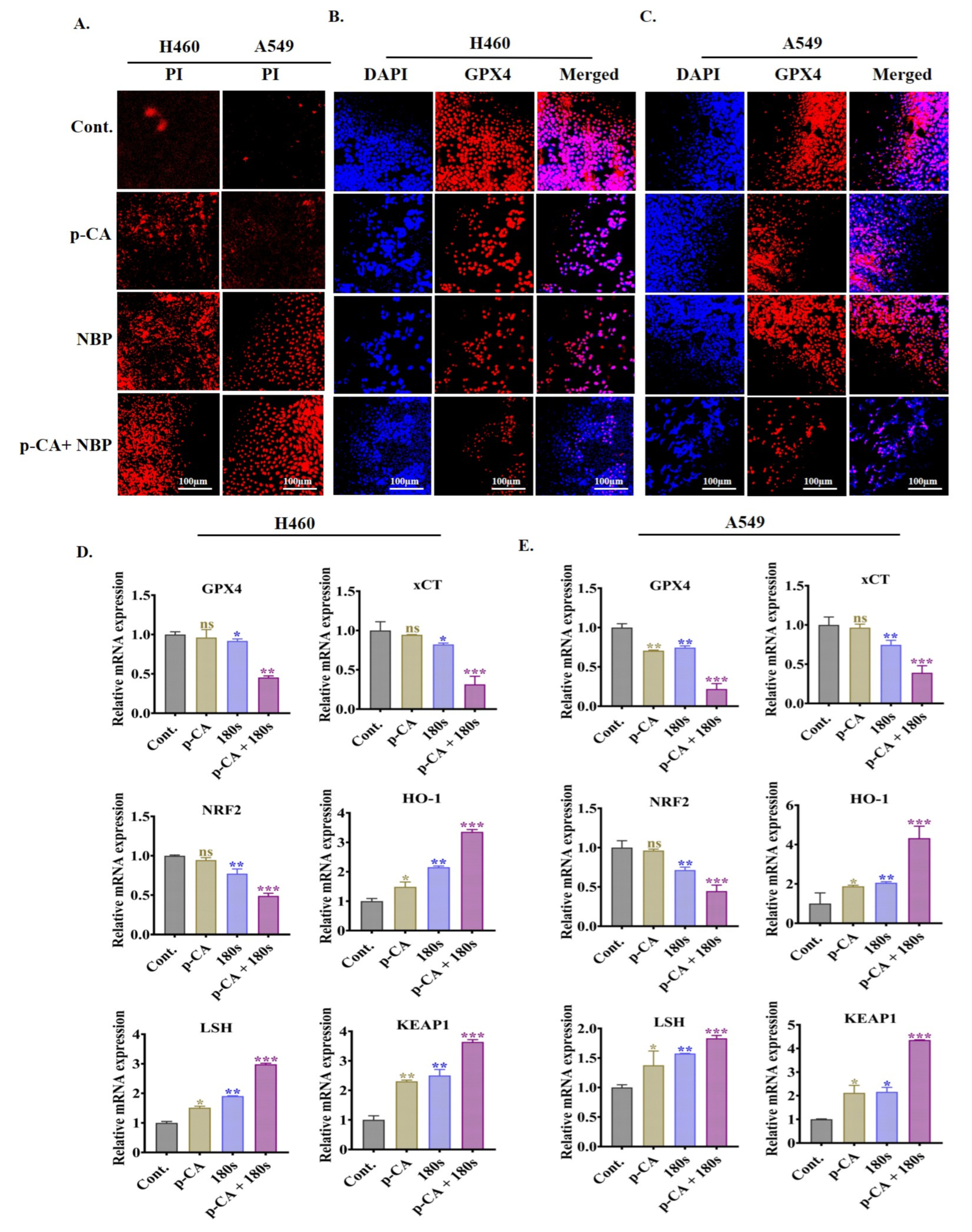
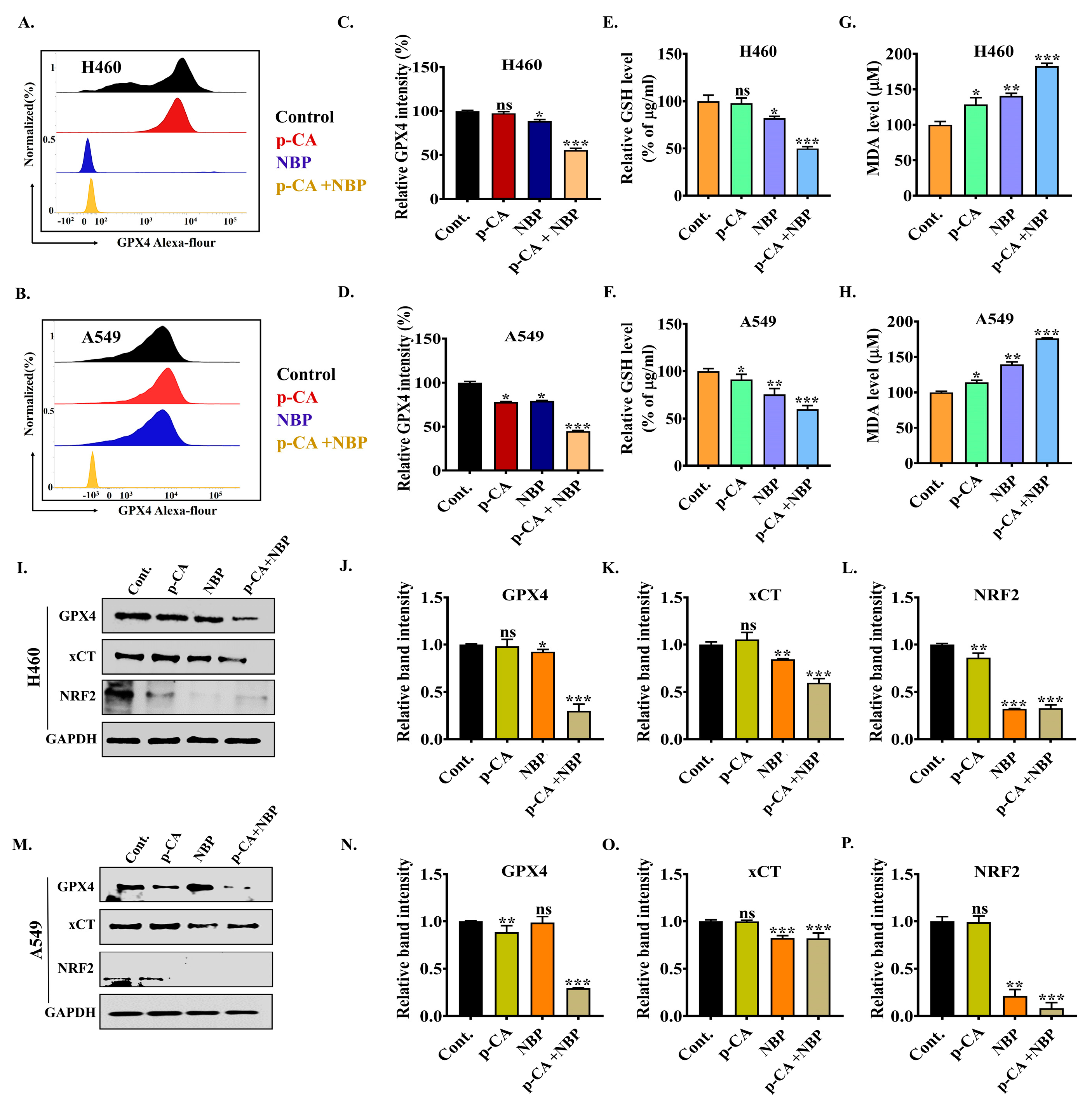
3.7. Combination Treatment Induced Ferroptotic Cell Death via the Downregulation of Ferroptosis Markers in Lung Adenocarcinoma Cell Line
3.8. NBP-Jet Plasma and Para-Coumaric Acid Trigger Ferroptosis by Modulating the GPX4, xCT and NRF2 Pathways
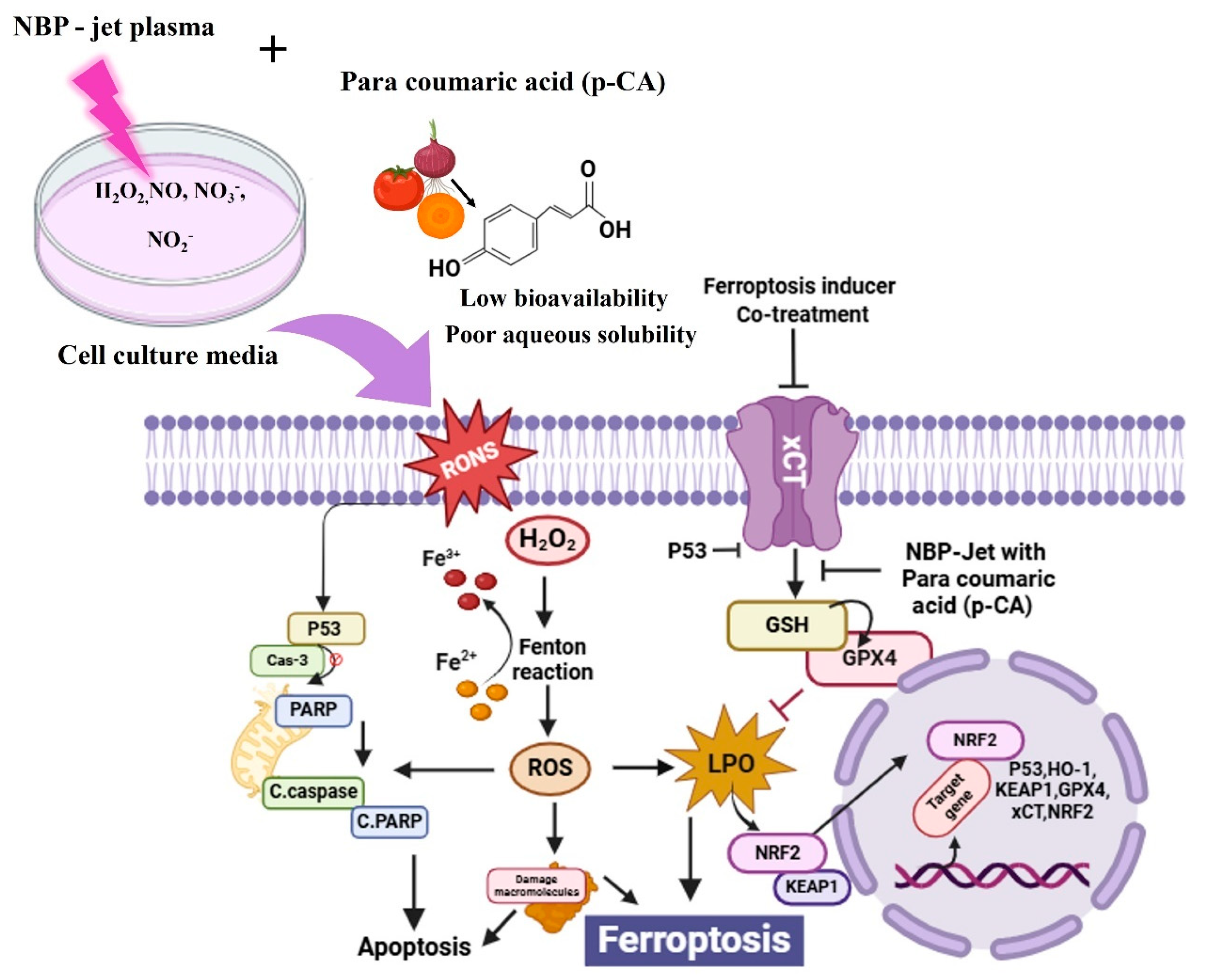
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer Statistics. Lung Cancer Pers. Med. Curr. Knowl. Ther. 2016, 893, 1–19. [Google Scholar]
- Cao, W.; Sun, M.; Yu, K.N.; Zhao, L.; Feng, Y.; Tan, C.; Yang, M.; Wang, Y.; Zhu, F.; Chen, L.; et al. Exogenous Carbon Monoxide Promotes GPX4-Dependent Ferroptosis Through ROS/GSK3β Axis in Non-Small Cell Lung Cancer. Cell Death Discov. 2024, 10, 42. [Google Scholar] [CrossRef]
- Bogart, J.A.; Waqar, S.N.; Mix, M.D. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Du, Q.; Guo, S.; Xiang, G.; Zhang, Y.; Meng, X.; Xiang, L.; Wang, S. Ferroptosis in Lung Cancer: A Novel Pathway Regulating Cell Death and a Promising Target for Drug Therapy. Cell Death Discov. 2023, 9, 110. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X.; Huang, P. XCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine Transporter SLC7A11/XCT in Cancer: Ferroptosis, Nutrient Dependency, and Cancer Therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a P53-Mediated Activity during Tumour Suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a New Form of Cell Death, and Its Relationships with Tumourous Diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Jiang, L.; Hickman, J.H.; Wang, S.-J.; Gu, W. Dynamic Roles of P53-Mediated Metabolic Activities in ROS-Induced Stress Responses. Cell Cycle 2015, 14, 2881–2885. [Google Scholar] [CrossRef]
- Doll, S.; Conrad, M. Iron and Ferroptosis: A Still Ill-Defined Liaison. IUBMB Life 2017, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Kaushik, N.; Mitra, S.; Baek, E.J.; Nguyen, L.N.; Bhartiya, P.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. The Inactivation and Destruction of Viruses by Reactive Oxygen Species Generated through Physical and Cold Atmospheric Plasma Techniques: Current Status and Perspectives. J. Adv. Res. 2023, 43, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Lee, S.J.; Choi, T.G.; Baik, K.Y.; Uhm, H.S.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Non-Thermal Plasma with 2-Deoxy-D-Glucose Synergistically Induces Cell Death by Targeting Glycolysis in Blood Cancer Cells. Sci. Rep. 2015, 5, 8726. [Google Scholar] [CrossRef]
- Akter, M.; Jangra, A.; Choi, S.A.; Choi, E.H.; Han, I. Non-Thermal Atmospheric Pressure Bio-Compatible Plasma Stimulates Apoptosis via P38/MAPK Mechanism in U87 Malignant Glioblastoma. Cancers 2020, 12, 245. [Google Scholar] [CrossRef]
- Jo, A.; Bae, J.H.; Yoon, Y.J.; Chung, T.H.; Lee, E.-W.; Kim, Y.-H.; Joh, H.M.; Chung, J.W. Plasma-Activated Medium Induces Ferroptosis by Depleting FSP1 in Human Lung Cancer Cells. Cell Death Dis. 2022, 13, 212. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, J.H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; et al. Nonthermal Plasma Induces Head and Neck Cancer Cell Death: The Potential Involvement of Mitogen-Activated Protein Kinase-Dependent Mitochondrial Reactive Oxygen Species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef]
- Akter, M.; Lim, J.S.; Choi, E.H.; Han, I. Non-Thermal Biocompatible Plasma Jet Induction of Apoptosis in Brain Cancer Cells. Cells 2021, 10, 236. [Google Scholar] [CrossRef]
- Negi, M.; Kaushik, N.; Lamichhane, P.; Patel, P.; Jaiswal, A.; Choi, E.H.; Kaushik, N.K. Nitric Oxide Water-Driven Immunogenic Cell Death: Unfolding Mitochondrial Dysfunction’s Role in Sensitizing Lung Adenocarcinoma to Ferroptosis and Autophagic Cell Death. Free Radic. Biol. Med. 2024, 222, 1–15. [Google Scholar] [CrossRef]
- Yang, X.; Chen, G.; Yu, K.N.; Yang, M.; Peng, S.; Ma, J.; Qin, F.; Cao, W.; Cui, S.; Nie, L.; et al. Cold Atmospheric Plasma Induces GSDME-Dependent Pyroptotic Signaling Pathway via ROS Generation in Tumor Cells. Cell Death Dis. 2020, 11, 295. [Google Scholar] [CrossRef]
- Hara, H.; Kobayashi, M.; Shiiba, M.; Kamiya, T.; Adachi, T. Sublethal Treatment with Plasma-Activated Medium Induces Senescence-like Growth Arrest of A549 Cells: Involvement of Intracellular Mobile Zinc. J. Clin. Biochem. Nutr. 2019, 65, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Cespedes-Acuña, C.L.; Oh, J.-W. Spiraeoside Extracted from Red Onion Skin Ameliorates Apoptosis and Exerts Potent Antitumor, Antioxidant and Enzyme Inhibitory Effects. Food Chem. Toxicol. 2021, 154, 112327. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The Anti-Tumor Effects of p-Coumaric Acid on Melanoma A375 and B16 Cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.H.; El-Mahdy, M.A.; Abd-Ellah, M.F.; Helal, G.K.; Khalifa, F.; Hamada, F.M.A. Influence of P-Coumaric Acid on Doxorubicin-Induced Oxidative Stress in Rat’s Heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart Drug Delivery of P-Coumaric Acid Loaded Aptamer Conjugated Starch Nanoparticles for Effective Triple-Negative Breast Cancer Therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of Phytochemicals and Its Enhancement by Drug Delivery Systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Zhu, W.; Lee, S.-J.; Castro, N.J.; Yan, D.; Keidar, M.; Zhang, L.G. Synergistic Effect of Cold Atmospheric Plasma and Drug Loaded Core-Shell Nanoparticles on Inhibiting Breast Cancer Cell Growth. Sci. Rep. 2016, 6, 21974. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Wende, K.; Williams, P.; Dalluge, J.; Van Gaens, W.; Aboubakr, H.; Bischof, J.; Von Woedtke, T.; Goyal, S.M.; Weltmann, K.-D.; Bogaerts, A.; et al. Identification of the Biologically Active Liquid Chemistry Induced by a Nonthermal Atmospheric Pressure Plasma Jet. Biointerphases 2015, 10, 029518. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lamichhane, P.; Jaiswal, A.; Amsalu, K.; Hong, Y.J.; Kaushik, N.; Kaushik, N.K.; Choi, E.H. The Potential of Multicylindrical Dielectric Barrier Discharge Plasma for Diesel-Contaminated Soil Remediation and Biocompatibility Assessment. Environ. Res. 2024, 240, 117398. [Google Scholar] [CrossRef]
- Bhartiya, P.; Jaiswal, A.; Negi, M.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Unlocking Melanoma Suppression: Insights from Plasma-Induced Potent MiRNAs through PI3K-AKT-ZEB1 Axis. J. Adv. Res. 2024, 68, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Morjani, H.; Chénais, B. Free Radical Production and Labile Iron Pool Decrease Triggered by Subtoxic Concentration of Aclarubicin in Human Leukemia Cell Lines. Leuk. Res. 2002, 26, 927–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.-M.; Pu, Y.-K. Determining the Electron Temperature in Inductively Coupled Nitrogen Plasmas by Optical Emission Spectroscopy with Molecular Kinetic Effects. Phys. Plasmas 2005, 12, 103501. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Pu, Y.-K. A Simple Collisional—Radiative Model for Low-Temperature Argon Discharges with Pressure Ranging from 1 Pa to Atmospheric Pressure: Kinetics of Paschen 1s and 2p Levels. J. Phys. D Appl. Phys. 2009, 43, 15204. [Google Scholar] [CrossRef]
- Zhu, X.M.; Pu, Y.K. Optical Emission Spectroscopy in Low-Temperature Plasmas Containing Argon and Nitrogen: Determination of the Electron Temperature and Density by the Line-Ratio Method. J. Phys. D Appl. Phys. 2010, 43, 403001. [Google Scholar] [CrossRef]
- Choi, E.H.; Kaushik, N.K.; Hong, Y.J.; Lim, J.S.; Choi, J.S.; Han, I. Plasma Bioscience for Medicine, Agriculture and Hygiene Applications. J. Korean Phys. Soc. 2022, 80, 817–851. [Google Scholar] [CrossRef]
- Lee, T.; Keidar, M. Adaptive Plasma and Machine Learning. Plasma Cancer Ther. 2020, 115, 223–250. [Google Scholar]
- Gudmundsson, J.T. Electron Excitation Rate Coefficients for the Nitrogen Discharge; University of Iceland: Reykjavik, Iceland, 2005. [Google Scholar]
- Lofthus, A.; Krupenie, P.H. The Spectrum of Molecular Nitrogen. J. Phys. Chem. Ref. Data 1977, 6, 113–307. [Google Scholar] [CrossRef]
- Pipa, A.V.; Ropcke, J. Analysis of the Mid-Infrared Spectrum of the Exhaust Gas from an Atmospheric Pressure Plasma Jet (APPJ) Working with an Argon—Air Mixture. IEEE Trans. Plasma Sci. 2009, 37, 1000–1003. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Rauen, U. Determination of the Chelatable Iron Pool of Single Intact Cells by Laser Scanning Microscopy. Arch. Biochem. Biophys. 2000, 376, 74–81. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-Mediated Ferroptosis as a Target for Protection against Retinal Pigment Epithelium Degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
- Wen, R.; Dong, X.; Zhuang, H.; Pang, F.; Ding, S.; Li, N.; Mai, Y.; Zhou, S.; Wang, J.; Zhang, J. Baicalin Induces Ferroptosis in Osteosarcomas through a Novel Nrf2/XCT/GPX4 Regulatory Axis. Phytomedicine 2023, 116, 154881. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Su, K.; Wang, L.; Feng, B.; You, X.; Deng, M.; Toh, W.S.; Wu, J.; Cheng, B.; Xia, J. Poly (p-Coumaric Acid) Nanoparticles Alleviate Temporomandibular Joint Osteoarthritis by Inhibiting Chondrocyte Ferroptosis. Bioact. Mater. 2024, 40, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, P.; Muthusami, S.; Arumugam, R. In Vitro Evaluation of P-Coumaric Acid and Naringin Combination in Human Epidermoid Carcinoma Cell Line (A431). Med. Oncol. 2023, 41, 4. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, G.; Yu, K.N.; Feng, Y.; Zhao, L.; Yang, M.; Cao, W.; Almahi, W.A.A.; Sun, M.; Xu, Y.; et al. Synergism of Non-Thermal Plasma and Low Concentration RSL3 Triggers Ferroptosis via Promoting XCT Lysosomal Degradation through ROS/AMPK/MTOR Axis in Lung Cancer Cells. Cell Commun. Signal. 2024, 22, 112. [Google Scholar] [CrossRef]
- Bulusu, R.K.M.; Wandell, R.J.; Gallan, R.O.; Locke, B.R. Nitric Oxide Scavenging of Hydroxyl Radicals in a Nanosecond Pulsed Plasma Discharge Gas—Liquid Reactor. J. Phys. D Appl. Phys. 2019, 52, 504002. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; de Medeiros Machado, M.; dos Santos, J.R.; Zanco, J.J.; Ribeiro, D.H.B.; André, A.S.; Debacher, N.A.; Moecke, E.H.S. Effect of Chemical Species Generated by Different Geometries of Air and Argon Non-Thermal Plasma Reactors on Bacteria Inactivation in Water. Sep. Purif. Technol. 2019, 222, 68–74. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Dezhpour, A.; Ghafouri, H.; Jafari, S.; Nilkar, M. Effects of Cold Atmospheric-Pressure Plasma in Combination with Doxorubicin Drug against Breast Cancer Cells in Vitro and in Vivo. Free Radic. Biol. Med. 2023, 209, 202–210. [Google Scholar] [CrossRef]
- Golpour, M.; Alimohammadi, M.; Sohbatzadeh, F.; Fattahi, S.; Bekeschus, S.; Rafiei, A. Cold Atmospheric Pressure Plasma Treatment Combined with Starvation Increases Autophagy and Apoptosis in Melanoma in Vitro and in Vivo. Exp. Dermatol. 2022, 31, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Wattananapakasem, I.; Panjan, W.; Kiattisin, K. Optimizing Anthocyanins Extraction and the Effect of Cold Plasma Treatment on the Anti-Aging Potential of Purple Glutinous Rice (Oryza sativa L.) Extract. Cereal Chem. 2021, 98, 571–582. [Google Scholar] [CrossRef]
- Du, L.; Ming, H.; Yan, Z.; Chen, J.; Song, W.; Dai, H. Decitabine Combined with Cold Atmospheric Plasma Induces Pyroptosis via the ROS/Caspase-3/GSDME Signaling Pathway in Ovcar5 Cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2024, 1868, 130602. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, K.; Chen, Y.; Wang, W.; Chen, H.; Zou, Z.; Li, Y.; Dai, M. Scutellaria Barbata Inhibits Hepatocellular Carcinoma Tumorigenicity by Inducing Ferroptosis of Hepatocellular Carcinoma Cells. Front. Oncol. 2022, 12, 693395. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin Yellow Improves Cerebral Ischemia-Reperfusion Injury by Attenuating Inflammation and Ferroptosis in Rats. Int. J. Mol. Med. 2021, 47, 1. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, H.; Shaikh, A.B.; Han, W.; Hou, H.; Zhang, Z.; Wang, S.; Shang, P. Directly Targeting Glutathione Peroxidase 4 May Be More Effective than Disrupting Glutathione on Ferroptosis-Based Cancer Therapy. Biochim. Biophys. Acta BBA-Gen. Subj. 2020, 1864, 129539. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| LSH | GATTTTGGATCGAATGCTGCCAG | ATGGACCCATCAAGCCTGCTGA |
| SLC7A11 | TCCTGCTTTGGCTCCATGAACG | AGAGGAGTGTGCTTGCGGACAT |
| GPX4 | ACAAGAACGGCTGCGTGGTGAA | GCCACACACTTGTGGAGCTAGA |
| HMOX1 | CCAGCGGGCCAGCAACAAAGTGC | AAGCCTTCAGTGCCCACGGTAAGG |
| NRF2 | TTCCCGGTCACATCGAGAG | TCCTGTTGCATACCGTCTAAACT |
| KEAP1 | TGCTAACCTCTATACATGCAACT | GCAACGGTCAAAGAAGACT |
| P53 | AGGAAATTTGCGTGTGGAGTAT | TCCGTCCCAGTAGATTACCACT |
| PARP | GCTCCCAGGAGTCAAGAGTG | TCAGGTCGTTCTGAGCCTTT |
| Caspase3 | CATACTCCACAGCACCTGGTTA | ACTCAAATTCTGTTGCCACCTT |
| BAX | GAGAGGTCTTCCGAGTGG | GGAGGAAGTCCAATGTCCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, S.; Hong, Y.J.; Kim, Y.; Choi, E.H.; Han, I. Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound. Biomolecules 2025, 15, 691. https://doi.org/10.3390/biom15050691
Khanam S, Hong YJ, Kim Y, Choi EH, Han I. Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound. Biomolecules. 2025; 15(5):691. https://doi.org/10.3390/biom15050691
Chicago/Turabian StyleKhanam, Sabnaj, Young June Hong, Youngsun Kim, Eun Ha Choi, and Ihn Han. 2025. "Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound" Biomolecules 15, no. 5: 691. https://doi.org/10.3390/biom15050691
APA StyleKhanam, S., Hong, Y. J., Kim, Y., Choi, E. H., & Han, I. (2025). Enhancing Ferroptosis in Lung Adenocarcinoma Cells via the Synergistic Action of Nonthermal Biocompatible Plasma and a Bioactive Phenolic Compound. Biomolecules, 15(5), 691. https://doi.org/10.3390/biom15050691








