BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Kinase Expression Determination
2.3. Differential Expression Analysis
2.4. BUB1 mRNA Interaction Predictions
2.5. Sarcoma Cell Lines and Non-Tumoral Mesenchymal Cells Culture
2.6. RNA Purification and RT-qPCR
2.7. Total Protein Extraction and Western Blot Analysis
2.8. BUB1 Pharmacological Inhibition
2.9. Colony Formation Assay
2.10. Proliferation Assay
2.11. Overall Survival Analysis
2.12. Statistical Analysis
3. Results
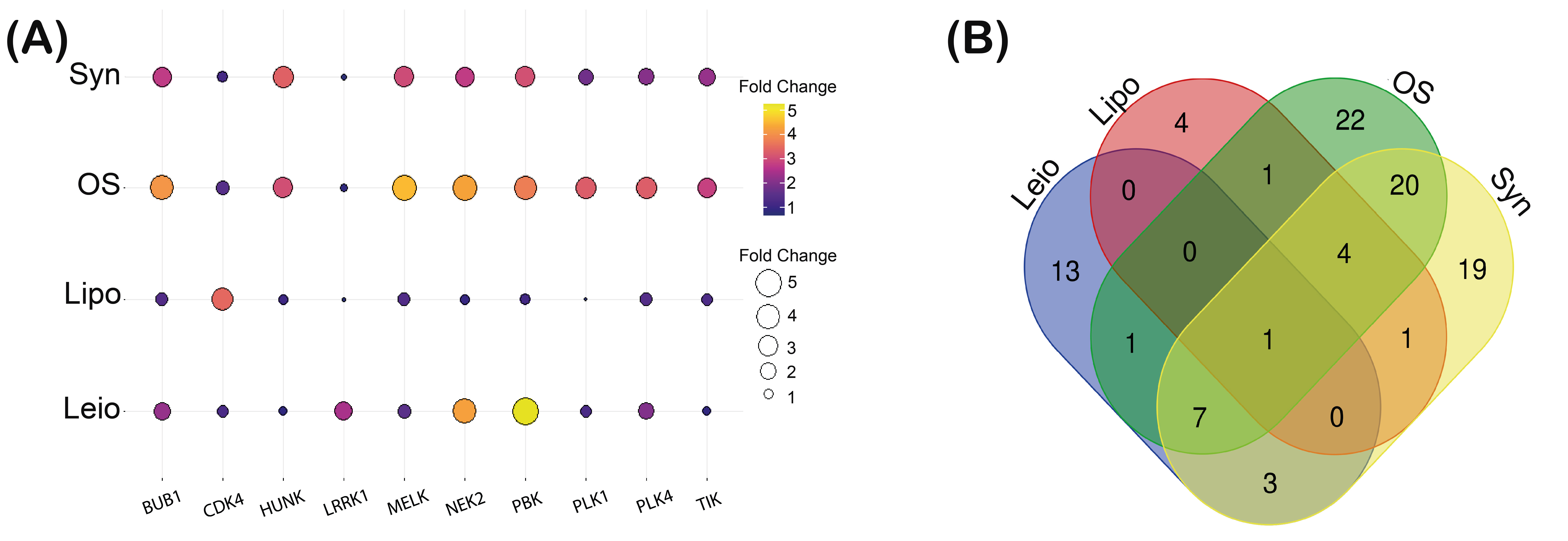
3.1. Kinase Expression Profile Establishment in Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma
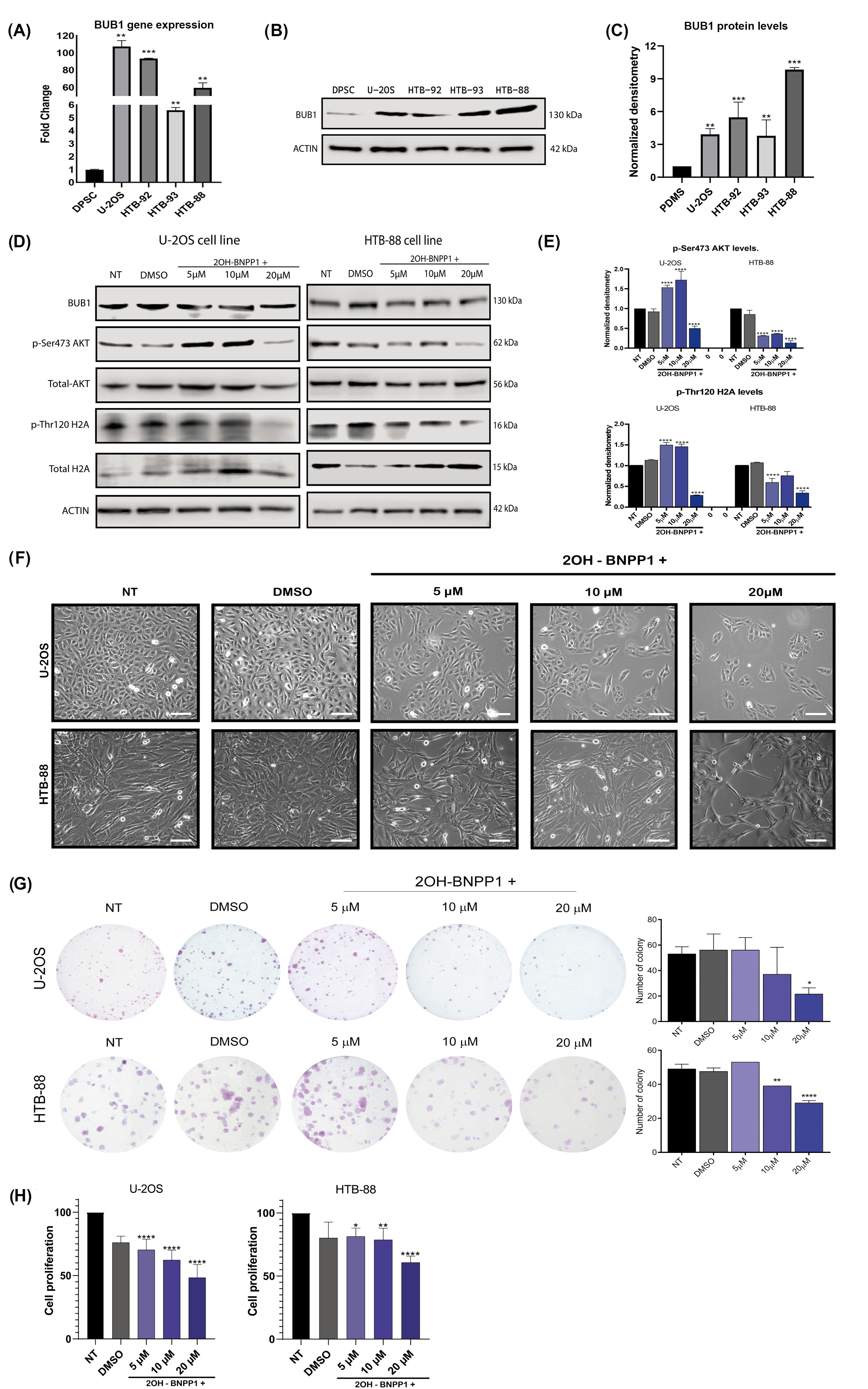
3.2. BUB1 Kinase Overexpression Correlates in Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma
3.3. The Network of Regulation of BUB1 Is Associated with Cancer Progression Signaling Pathways
3.4. High Levels of BUB1 Kinase Are Shown in Several Types of Sarcomas, and Its Inhibition Precludes Cell Proliferation
3.5. The Overall Survival of Sarcoma Patients Is Predicted by BUB1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, A.; Dirksen, U.; Bielack, S. Sarcomas of Soft Tissue and Bone. Prog. Tumor Res. 2016, 43, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Nakata, E.; Fujiwara, T.; Kunisada, T.; Ito, T.; Takihira, S.; Ozaki, T. Immunotherapy for Sarcomas. Jpn. J. Clin. Oncol. 2021, 51, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft Tissue Sarcoma across the Age Spectrum: A Population-Based Study from the Surveillance Epidemiology and End Results Database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef]
- Strauss, S.J.; Whelan, J.S. Current Questions in Bone Sarcomas. Curr. Opin. Oncol. 2018, 30, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.F.; Schiavone, K.; Heymann, D. Bone Sarcomas in the Immunotherapy Era. Br. J. Pharmacol. 2021, 178, 1955–1972. [Google Scholar] [CrossRef]
- Hui, J.Y.C. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef]
- Larrier, N.A.; Czito, B.G.; Kirsch, D.G. Radiation Therapy for Soft Tissue Sarcoma: Indications and Controversies for Neoadjuvant Therapy, Adjuvant Therapy, Intraoperative Radiation Therapy, and Brachytherapy. Surg. Oncol. Clin. N. Am. 2016, 25, 841–860. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin Alone versus Intensified Doxorubicin plus Ifosfamide for First-Line Treatment of Advanced or Metastatic Soft-Tissue Sarcoma: A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets for Bone and Soft-Tissue Sarcomas. Int. J. Mol. Sci. 2019, 20, 170. [Google Scholar] [CrossRef]
- Tang, Q.-X.; Wang, L.-C.; Wang, Y.; Gao, H.-D.; Hou, Z.-L. Efficacy of Methotrexate, Doxorubicin, and Cisplatin for Osteosarcoma Study Protocol for a Systematic Review of Randomized Controlled Trial. Medicine 2019, 98, e14442. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, Z.; Zhang, X.; Wan, J.; Liu, T. Cellular Plasticity and Drug Resistance in Sarcoma. Life Sci. 2020, 263, 118589. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Wang, H.; Zuo, D.; Hua, Y.; Cai, Z. Research Progress on the Multidrug Resistance Mechanisms of Osteosarcoma Chemotherapy and Reversal. Tumor Biol. 2015, 36, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Gray, N.S. Kinase Inhibitors: The Road Ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; Ingham, M.; Spraker, M.B.; Schwartz, G.K. Emerging Targeted and Immune-Based. Ther. Sarcoma J. Clin Oncol. 2018, 36, 125–135. [Google Scholar] [CrossRef]
- Patwardhan, P.P.; Surriga, O.; Beckman, M.J.; De Stanchina, E.; Dematteo, R.P.; Tap, W.D.; Schwartz, G.K. Sustained Inhibition of Receptor Tyrosine Kinases and Macrophage Depletion by PLX3397 and Rapamycin as a Potential New Approach for the Treatment of MPNSTs. Clin. Cancer Res. 2014, 20, 3146–3158. [Google Scholar] [CrossRef]
- Patwardhan, P.P.; Ivy, K.S.; Musi, E.; de Stanchina, E.; Schwartz, G.K.; Patwardhan, P.P.; Ivy, K.S.; Musi, E.; de Stanchina, E.; Schwartz, G.K. Significant Blockade of Multiple Receptor Tyrosine Kinases by MGCD516 (Sitravatinib), a Novel Small Molecule Inhibitor, Shows Potent Anti-Tumor Activity in Preclinical Models of Sarcoma. Oncotarget 2015, 7, 4093–4109. [Google Scholar] [CrossRef] [PubMed]
- Klebig, C.; Korinth, D.; Meraldi, P. Bub1 Regulates Chromosome Segregation in a Kinetochore-Independent Manner. J. Cell Biol. 2009, 185, 841–858. [Google Scholar] [CrossRef]
- Col On-Marrero, S.; Jusino, S.; Rivera-Rivera, Y.; Saavedra, H.I. Minireview Mitotic Kinases as Drivers of the Epithelial-to-Mesenchymal Transition and as Therapeutic Targets against Breast Cancers. Exp. Biol. Med. 2021, 246, 1036–1044. [Google Scholar] [CrossRef]
- Wang, M.; You, L.; Su, Z.; He, Y.; Li, D.; Liu, Z. BUB1 Induces AKT/MTOR Pathway Activity to Promote EMT Induction in Human Small Cell Lung Cancer. Sci. Rep. 2024, 14, 20654. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Wei, H.; Chen, H.; Shen, R.; Lin, R.; Wang, X.; Lan, W.; Lin, R.; Lin, J.; et al. Inhibition of BUB1 Suppresses Tumorigenesis of Osteosarcoma via Blocking of PI3K/Akt and ERK Pathways. J. Cell Mol. Med. 2021, 25, 8442–8453. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Open Access Bioconductor: Open Software Development for Computational Biology and Bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Eid, S.; Turk, S.; Volkamer, A.; Rippmann, F.; Fulle, S. KinMap: A Web-Based Tool for Interactive Navigation through Human Kinome Data. BMC Bioinform. 2017, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A Package for the Generation of Highly-Customizable Venn and Euler Diagrams in R. BMC Bioinform. 2011, 11, 35. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. MiRTarBase 2020: Updates to the Experimentally Validated MicroRNA-Target Interaction Database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, Q.; He, J.; Li, L.; Yang, N.; Yu, S.; Wang, M.; Zhang, Y.; Lin, J.; Cui, T.; et al. RNAInter v4.0: RNA Interactome Repository with Redefined Confidence Scoring System and Improved Accessibility. Nucleic Acids Res. 2022, 50, D326–D332. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Hadaegh, Y.; Niknam, M.; Attar, A.; Maharlooei, M.; Tavangar, M.; Aarabi, A.; Monabati, A. Characterization of Stem Cells from the Pulp of Unerupted Third Molar Tooth. Indian J. Dent. Res. 2014, 25, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Thompson, J.A. An R Package for Survival-Based Gene Set Enrichment Analysis. Res. Sq. 2023, rs.3, rs-3367968. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, C.; Wang, L.; Jiang, H.; Zhai, Y.; Wang, Y.; Fang, J.; Zhang, G. Zombies Never Die: The Double Life Bub1 Lives in Mitosis. Front. Cell Dev. Biol. 2022, 10, 870745. [Google Scholar] [CrossRef]
- Nyati, S.; Young, G.; Speers, C.; Nyati, M.K. Rehemtulla A.Budding Uninhibited by Benzimidazoles-1 (BUB1) Regulates EGFR Signaling by Reducing EGFR Internalization. Aging 2023, 15, 6011–6030. [Google Scholar] [CrossRef] [PubMed]
- Amalina, I.; Bennett, A.; Whalley, H.; Perera, D.; McGrail, J.C.; Tighe, A.; Procter, D.J.; Taylor, S.S. Inhibitors of the Bub1 Spindle Assembly Checkpoint Kinase: Synthesis of BAY-320 and Comparison with 2OH-BNPP1. R. Soc. Open Sci. 2021, 8, 210854. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Lajeunesse, A.; Dulla, K.; Combes, G.; Thebault, P.; Nigg, E.A.; Elowe, S. Bub1 Autophosphorylation Feeds Back to Regulate Kinetochore Docking and Promote Localized Substrate Phosphorylation. Nat. Commun. 2015, 6, 8364. [Google Scholar] [CrossRef]
- Kawashima, S.A.; Yamagishi, Y.; Honda, T.; Lshiguro, K.I.; Watanabe, Y. Phosphorylation of H2A by Bub1 Prevents Chromosomal Instability through Localizing Shugoshin. Science 2010, 327, 172–177. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, C.; Chen, Q.; Yan, H.; Xu, J.; Zhao, H.; Yuan, X.; Liu, J.; Lin, S.; Lu, W.; et al. Histone H2A Phosphorylation Recruits Topoisomerase II α to Centromeres to Safeguard Genomic Stability. EMBO J. 2020, 39, e101863. [Google Scholar] [CrossRef]
- He, Q.; Jia, L.; Wang, X.; Feng, D.; Mao, T. Knockdown of BUB1 Inhibits Tumor Necrosis Factor-α-induced Proliferation and Migration of Rheumatoid Arthritis Synovial Fibroblasts by Regulating PI3K/Akt Pathway. Int. J. Rheum. Dis. 2023, 26, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Esperança-Martins, M.; F.Duarte, I.; Rodrigues, M.; Soares do Brito, J.; López-Presa, D.; Costa, L.; Fernandes, I.; Dias, S. On the Relevance of Soft Tissue Sarcomas Metabolic Landscape Mapping. Int. J. Mol. Sci. 2022, 23, 11430. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone Sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Calderillo-Ruíz, G.; Pérez-Yepez, E.A.; García-Gámez, M.A.; Millan-Catalan, O.; Díaz-Romero, C.; Ugalde-Silva, P.; Salas-Benavides, R.; Pérez-Plasencia, C.; Carbajal-López, B. Genomic Profiling in GIST: Implications in Clinical Outcome and Future Challenges. Neoplasia 2024, 48, 100959. [Google Scholar] [CrossRef]
- Van Der Graaf, W.T.A.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a Multikinase Angiogenesis Inhibitor, in Patients with Relapsed or Refractory Advanced Soft Tissue Sarcoma: A Phase II Study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.; Fang, B.; Edwards, A.; Zhang, G.; Bui, M.; Eschrich, S.; Altiok, S.; Koomen, J.; Haura, E.B. Phosphoproteomics Identifies Driver Tyrosine Kinases in Sarcoma Cell Lines and Tumors. Cancer Res. 2012, 72, 2501–2511. [Google Scholar] [CrossRef]
- Hoyt, M.A.; Totis, L.; Roberts, B.T.S. Cerevisiae Genes Required for Cell Cycle Arrest in Response to Loss of Microtubule Function. Cell 1991, 66, 507–517. [Google Scholar] [CrossRef]
- Grabsch, H.; Takeno, S.; Parsons, W.J.; Pomjanski, N.; Boecking, A.; Gabbert, H.E.; Mueller, W. Overexpression of the Mitotic Checkpoint Genes BUB1, BUBR1, and BUB3 in Gastric Cancer--Association with Tumour Cell Proliferation. J. Pathol. 2003, 200, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Kong, L.; Pan, Z.; Chen, G. BUB1 Promotes Gemcitabine Resistance in Pancreatic Cancer Cells by Inhibiting Ferroptosis. Cancers 2024, 16, 1540. [Google Scholar] [CrossRef] [PubMed]
- Cicirò, Y.; Ragusa, D.; Sala, A. Expression of the Checkpoint Kinase BUB1 Is a Predictor of Response to Cancer Therapies. Sci. Rep. 2024, 14, 4461. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Bandara, N.; Morgan, S.; Nowell, C.; Mehdi, A.M.; Lyu, R.; McCarthy, D.; Anderson, D.; Creek, D.J.; Achen, M.G.; et al. Key Signaling Networks Are Dysregulated in Patients with the Adipose Tissue Disorder, Lipedema. Int. J. Obes. 2021, 46, 502–514. [Google Scholar] [CrossRef]
- Sun, Y.; Man, Y.N.; Cheng, J.H.; Li, J.T.; Liu, Y.Y. FAM60A Promotes Osteosarcoma Development and Progression. Cancer Med. 2023, 12, 17491–17503. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Siemeister, G.; Mengel, A.; Fernandez-Montalv, A.E.; Bone, W.; Schroder, J.; Zitzmann-Kolbe, S.; Briem, H.; Prechtl, S.; Holton, S.J.; Monning, U.; et al. Inhibition of BUB1 Kinase by Bay 1816032 Sensitizes Tumor Cells toward Taxanes, ATR, and PARP Inhibitors in Vitro and in Vivo. Clin. Cancer Res. 2019, 25, 1404–1414. [Google Scholar] [CrossRef]
- Jiang, N.; Liao, Y.; Wang, M.; Wang, Y.; Wang, K.; Guo, J.; Wu, P.; Zhong, B.; Guo, T.; Wu, C. BUB1 Drives the Occurrence and Development of Bladder Cancer by Mediating the STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2021, 40, 378. [Google Scholar] [CrossRef]
- Investigation of Crucial Genes and MicroRNAs in Conventional Osteosarcoma Using Gene Expression Profiling Analysis. Available online: https://www.spandidos-publications.com/mmr/16/5/7617 (accessed on 27 November 2024).
- Li, X.; Li, R. Exploration of Biomarkers for Nursing Physical Examination Early Screening of Multiple Tumors. Medicine 2024, 103, e39231. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, M.; Li, M.; Peng, Y.; Zhang, X. BUB1 as a Novel Marker for Predicting the Immunotherapy Efficacy and Prognosis of Breast Cancer. Transl. Cancer Res. 2024, 13, 4534–4554. [Google Scholar] [CrossRef]



| Type of Sarcoma | GEO Accession | Sequencing Technology | Samples |
|---|---|---|---|
| Osteosarcoma | GSE218035 | Expression profiling by high-throughput sequencing: Oxford Nanopore Technologies (ONT) long-read RNA-Seq | Tumor and adjacent normal tissues from 23 osteosarcoma patients paired with adjacent healthy tissue. |
| Liposarcoma | GSM6049698 | Expression profiling by high-throughput sequencing: Illumina NovaSeq 6000 | Expression data from 18 liposarcoma and 20 adjacent healthy tissue samples. |
| Synovial sarcoma | GSE144190 | Expression profiling by high-throughput sequencing: Illumina NovaSeq 6000 | Tumor and adjacent normal tissues from 10 synovial sarcoma patients paired with adjacent healthy tissue. |
| Leiomyosarcoma | GSE45510 | Expression profiling by high-throughput sequencing: Illumina Genome Analyzer IIx | Expression data from 99 leiomyosarcoma tissue samples. |
| GSE54511 | Illumina Genome Analyzer IIx | Expression data of 4 healthy tissue samples from myometrium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera-Valencia, M.; Luna-Maldonado, F.; Juarez-Reyes, J.; Lopez-Saavedra, A.; Coronel-Hernandez, J.; Millan-Catalan, O.; Guzman-Gomez, D.; Rodríguez-Izquierdo, F.; Herrera, L.A.; Cantú-De León, D.F.; et al. BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma. Biomolecules 2025, 15, 1046. https://doi.org/10.3390/biom15071046
Olvera-Valencia M, Luna-Maldonado F, Juarez-Reyes J, Lopez-Saavedra A, Coronel-Hernandez J, Millan-Catalan O, Guzman-Gomez D, Rodríguez-Izquierdo F, Herrera LA, Cantú-De León DF, et al. BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma. Biomolecules. 2025; 15(7):1046. https://doi.org/10.3390/biom15071046
Chicago/Turabian StyleOlvera-Valencia, Mercedes, Fernando Luna-Maldonado, Joselyn Juarez-Reyes, Alejandro Lopez-Saavedra, Jossimar Coronel-Hernandez, Oliver Millan-Catalan, Daniel Guzman-Gomez, Frida Rodríguez-Izquierdo, Luis A. Herrera, David Francisco Cantú-De León, and et al. 2025. "BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma" Biomolecules 15, no. 7: 1046. https://doi.org/10.3390/biom15071046
APA StyleOlvera-Valencia, M., Luna-Maldonado, F., Juarez-Reyes, J., Lopez-Saavedra, A., Coronel-Hernandez, J., Millan-Catalan, O., Guzman-Gomez, D., Rodríguez-Izquierdo, F., Herrera, L. A., Cantú-De León, D. F., Perez-Plasencia, C., & Pérez-Yepez, E.-A. (2025). BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma. Biomolecules, 15(7), 1046. https://doi.org/10.3390/biom15071046







