Chromoanagenesis in Osteosarcoma
Abstract
1. Introduction
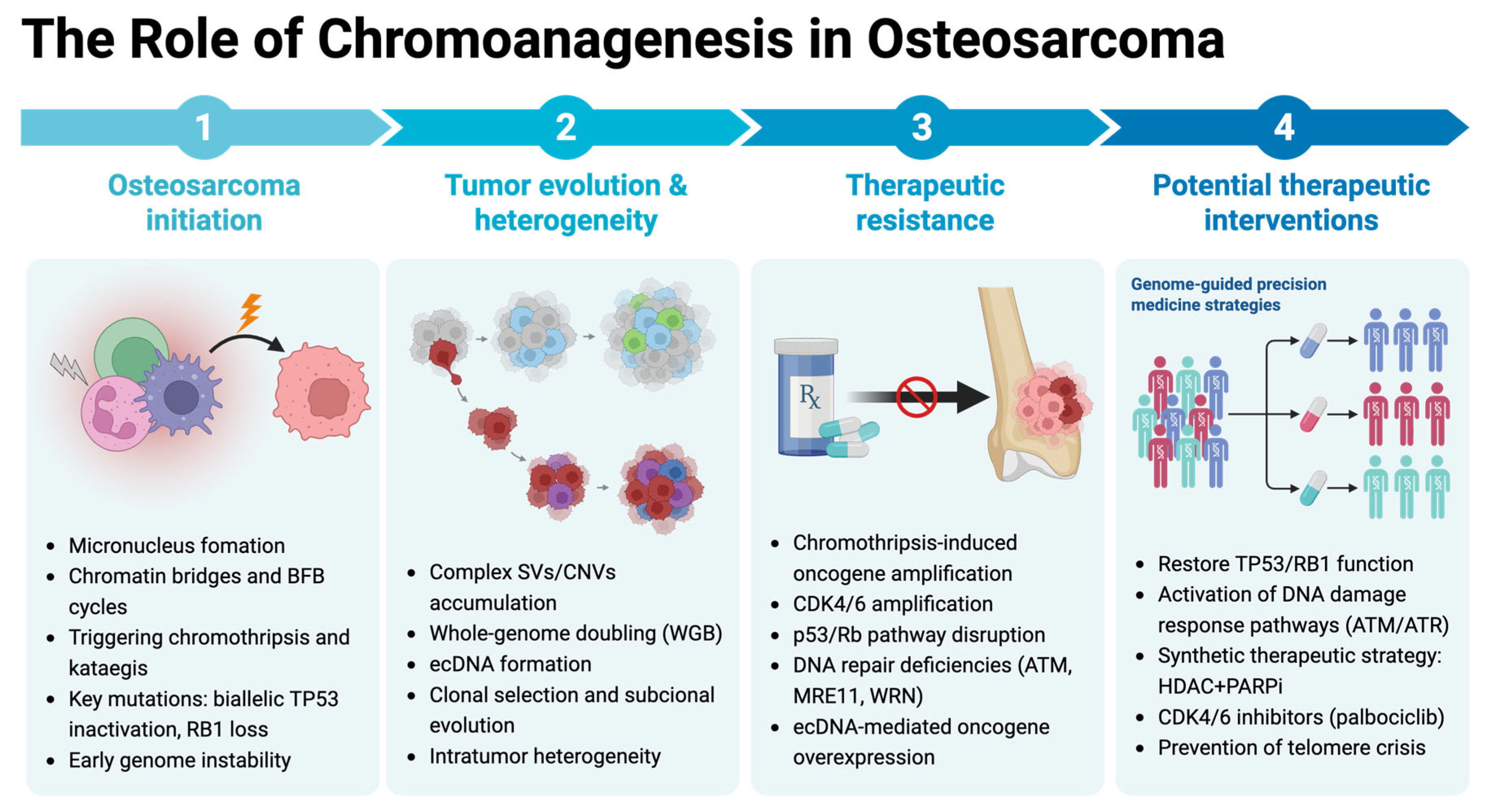
2. Impact of Chromoanagenesis in Osteosarcoma
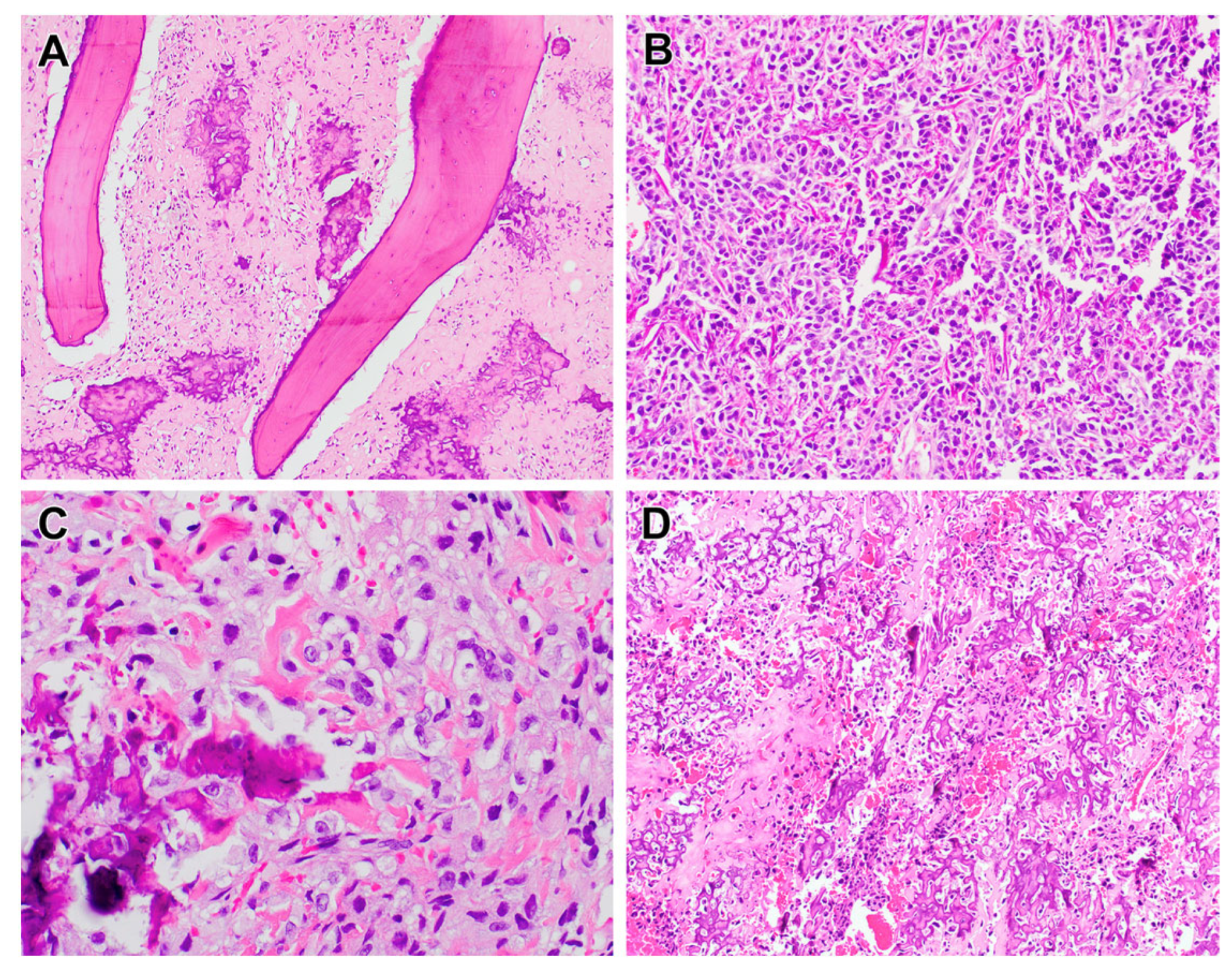
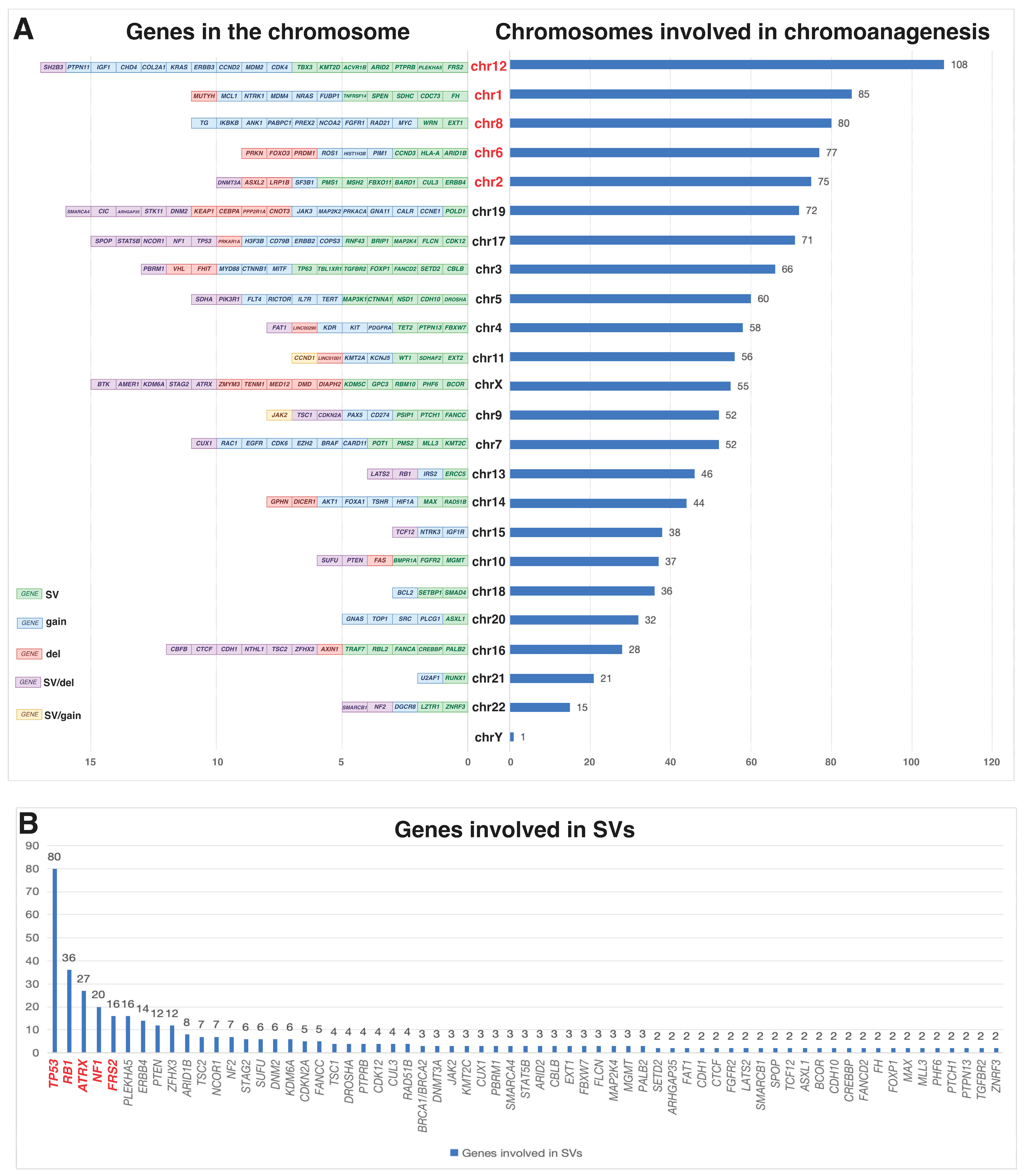
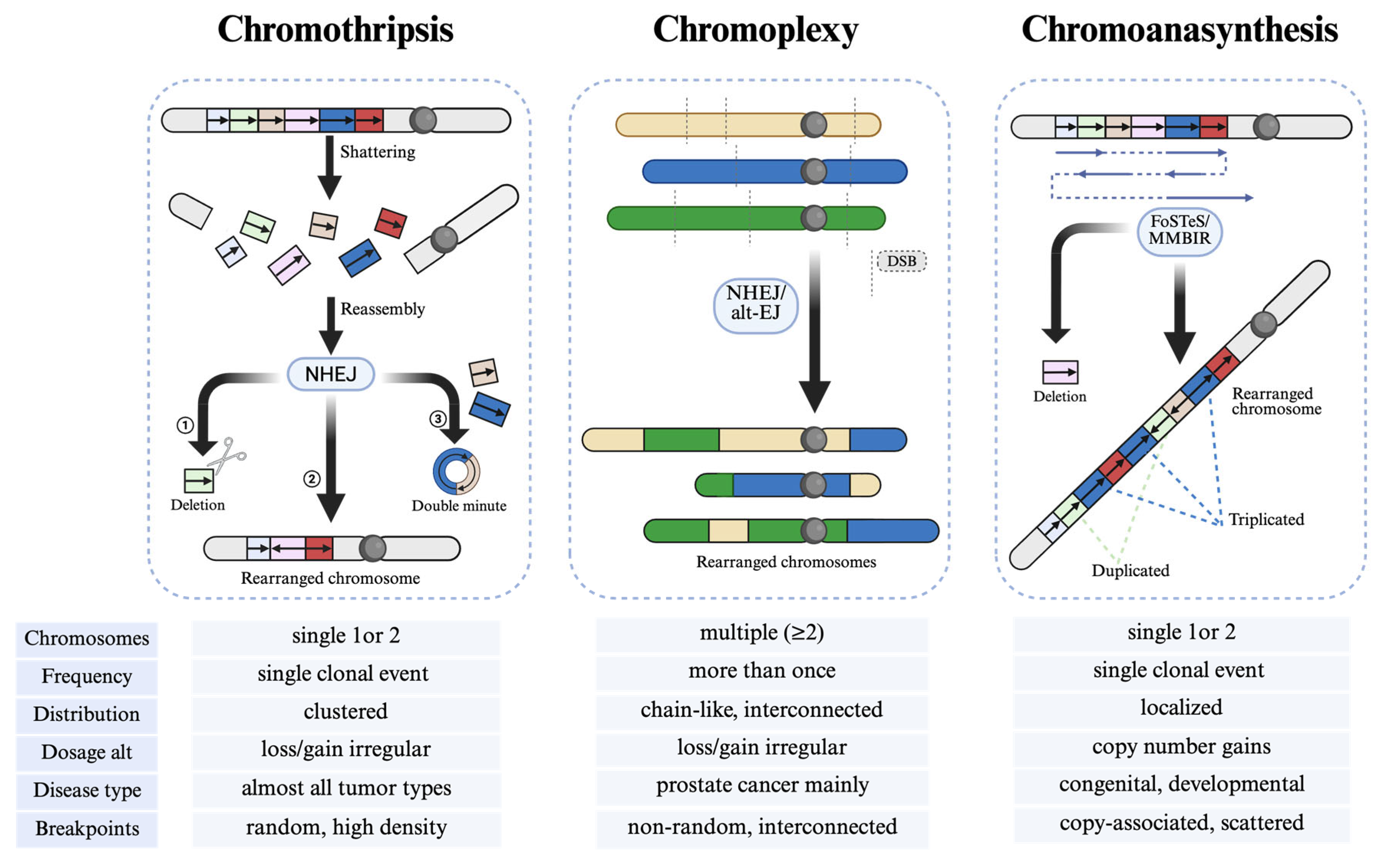
2.1. Complex Genomic Rearrangements and Genetic Instability
2.1.1. Chromothripsis in Osteosarcoma
2.1.2. Other Types of Chromoanagenesis
Chromoanasynthesis in Osteosarcoma
Chromoplexy in Osteosarcoma
2.2. Tumor Heterogeneity and Evolutionary Dynamics
2.3. Prognostic Significance and Clinical Outcomes
2.4. Therapeutic Strategies and Targeted Interventions
3. Molecular Basis and Detection of Chromoanagenesis
3.1. Molecular Basis of Chromoanagenesis
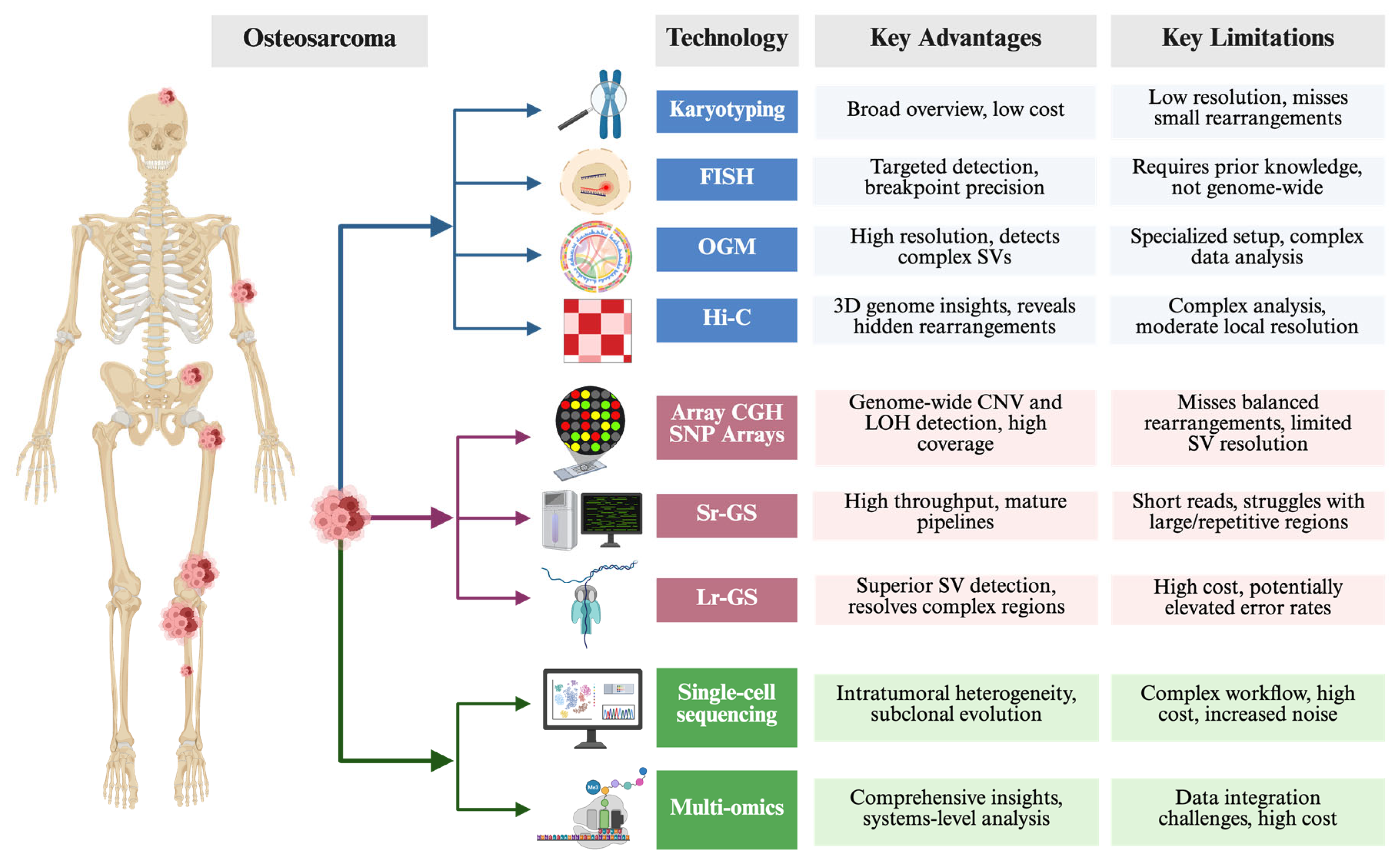
3.2. Traditional, Advanced, Emergering Technologies to Study Chromoanagenesis
3.2.1. Traditional Cytogenetic Methods
3.2.2. Array-Based and Sequencing Approaches
3.2.3. Single-Cell Sequencing Coupled with Multi-Omics Profiling
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFB | Breakage–fusion–bridge |
| CGR | Complex genomic rearrangement |
| CNVs | Copy number variants |
| DBS | Double-strand break |
| ecDNA | Extrachromosomal circular DNA |
| FISH | Fluorescence in situ hybridization |
| FoSTeS | Fork stalling and template switching |
| HGOS | High-grade osteosarcoma |
| HR | Homologous recombination |
| LOH | Loss of heterozygosity |
| Lr-GS | Long-read genome sequencing |
| MMBIR | Microhomology-mediated break-induced replication |
| NGS | Next-generation sequencing |
| NHEJ | Non-homologous end joining |
| OGM | Optical genome mapping |
| PDTX | Patient-derived tumor xenograft |
| SNVs | Single-nucleotide variants |
| Sr-GS | Short-read genome sequencing |
| SVs | Structural variants |
| WGD | Whole-genome doubling |
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Bertoni, F.; Bacchini, P. Classification of bone tumors. Eur. J. Radiol. 1998, 27 (Suppl. 1), S74–S76. [Google Scholar] [CrossRef]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef]
- Holland, A.J.; Cleveland, D.W. Chromoanagenesis and cancer: Mechanisms and consequences of localized, complex chromosomal rearrangements. Nat. Med. 2012, 18, 1630–1638. [Google Scholar] [CrossRef]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; MacDonald, T.Y.; Ghandi, M.; et al. Punctuated evolution of prostate cancer genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Cuppen, E. Chromothripsis in congenital disorders and cancer: Similarities and differences. Curr. Opin. Cell Biol. 2013, 25, 341–348. [Google Scholar] [CrossRef]
- Zepeda-Mendoza, C.J.; Morton, C.C. The Iceberg under Water: Unexplored Complexity of Chromoanagenesis in Congenital Disorders. Am. J. Hum. Genet. 2019, 104, 565–577. [Google Scholar] [CrossRef]
- de Pagter, M.S.; van Roosmalen, M.J.; Baas, A.F.; Renkens, I.; Duran, K.J.; van Binsbergen, E.; Tavakoli-Yaraki, M.; Hochstenbach, R.; van der Veken, L.T.; Cuppen, E.; et al. Chromothripsis in healthy individuals affects multiple protein-coding genes and can result in severe congenital abnormalities in offspring. Am. J. Hum. Genet. 2015, 96, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Espejo Valle-Inclan, J.; De Noon, S.; Trevers, K.; Elrick, H.; van Belzen, I.; Zumalave, S.; Sauer, C.M.; Tanguy, M.; Butters, T.; Muyas, F.; et al. Ongoing chromothripsis underpins osteosarcoma genome complexity and clonal evolution. Cell 2025, 188, 352–370.E32. [Google Scholar] [CrossRef]
- Wu, C.C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.L.; Ju, Z.; et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Pires, S.F.; Barros, J.S.; Costa, S.S.D.; Carmo, G.B.D.; Scliar, M.O.; Lengert, A.V.H.; Boldrini, É.; Silva, S.; Vidal, D.O.; Maschietto, M.; et al. Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development. Int. J. Mol. Sci. 2023, 24, 10463. [Google Scholar] [CrossRef]
- Tvrdik, T.; Gjeorgjievski, S.G.; Wong, P.; Oskouei, S.; Read, W.; Bahrami, A. Genomic Insights Into High-Grade Infarct-Associated Bone Sarcomas. Mod. Pathol. 2024, 37, 100572. [Google Scholar] [CrossRef]
- Saba, K.H.; Difilippo, V.; Styring, E.; Nilsson, J.; Magnusson, L.; van den Bos, H.; Wardenaar, R.; Spierings, D.C.J.; Foijer, F.; Nathrath, M.; et al. CDK4 is co-amplified with either TP53 promoter gene fusions or MDM2 through distinct mechanisms in osteosarcoma. NPJ Genom. Med. 2024, 9, 42. [Google Scholar] [CrossRef]
- Ratnaparkhe, M.; Hlevnjak, M.; Kolb, T.; Jauch, A.; Maass, K.K.; Devens, F.; Rode, A.; Hovestadt, V.; Korshunov, A.; Pastorczak, A.; et al. Genomic profiling of Acute lymphoblastic leukemia in ataxia telangiectasia patients reveals tight link between ATM mutations and chromothripsis. Leukemia 2017, 31, 2048–2056. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Bolhaqueiro, A.C.F.; Ponsioen, B.; Bakker, B.; Klaasen, S.J.; Kucukkose, E.; van Jaarsveld, R.H.; Vivié, J.; Verlaan-Klink, I.; Hami, N.; Spierings, D.C.J.; et al. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet. 2019, 51, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Kovac, M.; Blattmann, C.; Ribi, S.; Smida, J.; Mueller, N.S.; Engert, F.; Castro-Giner, F.; Weischenfeldt, J.; Kovacova, M.; Krieg, A.; et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat. Commun. 2015, 6, 8940. [Google Scholar] [CrossRef]
- Pellestor, F.; Gaillard, J.B.; Schneider, A.; Puechberty, J.; Gatinois, V. Chromoanagenesis, the mechanisms of a genomic chaos. Semin. Cell Dev. Biol. 2022, 123, 90–99. [Google Scholar] [CrossRef]
- Cortés-Ciriano, I.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.Z.; Pellman, D.S.; et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef]
- Voronina, N.; Wong, J.K.L.; Hübschmann, D.; Hlevnjak, M.; Uhrig, S.; Heilig, C.E.; Horak, P.; Kreutzfeldt, S.; Mock, A.; Stenzinger, A.; et al. The landscape of chromothripsis across adult cancer types. Nat. Commun. 2020, 11, 2320. [Google Scholar] [CrossRef]
- Umbreit, N.T.; Zhang, C.Z.; Lynch, L.D.; Blaine, L.J.; Cheng, A.M.; Tourdot, R.; Sun, L.; Almubarak, H.F.; Judge, K.; Mitchell, T.J.; et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 2020, 368, eaba0712. [Google Scholar] [CrossRef]
- Gong, Y.; Zou, S.; Deng, D.; Wang, L.; Hu, H.; Qiu, Z.; Wei, T.; Yang, P.; Zhou, J.; Zhang, Y.; et al. Loss of RanGAP1 drives chromosome instability and rapid tumorigenesis of osteosarcoma. Dev. Cell 2023, 58, 192–210.e111. [Google Scholar] [CrossRef]
- Scotto di Carlo, F.; Russo, S.; Muyas, F.; Mangini, M.; Garribba, L.; Pazzaglia, L.; Genesio, R.; Biamonte, F.; De Luca, A.C.; Santaguida, S.; et al. Profilin 1 deficiency drives mitotic defects and reduces genome stability. Commun. Biol. 2023, 6, 9. [Google Scholar] [CrossRef]
- Willis, N.A.; Rass, E.; Scully, R. Deciphering the Code of the Cancer Genome: Mechanisms of Chromosome Rearrangement. Trends Cancer 2015, 1, 217–230. [Google Scholar] [CrossRef]
- Liu, P.; Erez, A.; Nagamani, S.C.; Dhar, S.U.; Kołodziejska, K.E.; Dharmadhikari, A.V.; Cooper, M.L.; Wiszniewska, J.; Zhang, F.; Withers, M.A.; et al. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell 2011, 146, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.D.; de Borja, R.; Young, M.D.; Fuligni, F.; Rosic, A.; Roberts, N.D.; Hajjar, S.; Layeghifard, M.; Novokmet, A.; Kowalski, P.E.; et al. Rearrangement bursts generate canonical gene fusions in bone and soft tissue tumors. Science 2018, 361, eaam8419. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tang, H.; Huang, P.; Guo, J.; Shi, Y.; Yuan, C.; Liang, T.; Tang, K. Single-Cell Profiling of Tumor Microenvironment Heterogeneity in Osteosarcoma Identifies a Highly Invasive Subcluster for Predicting Prognosis. Front. Oncol. 2022, 12, 732862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Yang, L.; Lee, M.S.; Lu, H.; Oh, D.Y.; Kim, Y.J.; Park, D.; Park, G.; Ren, X.; Bristow, C.A.; Haseley, P.S.; et al. Analyzing Somatic Genome Rearrangements in Human Cancers by Using Whole-Exome Sequencing. Am. J. Hum. Genet. 2016, 98, 843–856. [Google Scholar] [CrossRef]
- Funnell, T.; O’Flanagan, C.H.; Williams, M.J.; McPherson, A.; McKinney, S.; Kabeer, F.; Lee, H.; Salehi, S.; Vázquez-García, I.; Shi, H.; et al. Single-cell genomic variation induced by mutational processes in cancer. Nature 2022, 612, 106–115. [Google Scholar] [CrossRef]
- Shoshani, O.; Brunner, S.F.; Yaeger, R.; Ly, P.; Nechemia-Arbely, Y.; Kim, D.H.; Fang, R.; Castillon, G.A.; Yu, M.; Li, J.S.Z.; et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 2021, 591, 137–141, Correction in Nature 2021, 591, E19. [Google Scholar] [CrossRef]
- Rajan, S.; Zaccaria, S.; Cannon, M.V.; Cam, M.; Gross, A.C.; Raphael, B.J.; Roberts, R.D. Structurally Complex Osteosarcoma Genomes Exhibit Limited Heterogeneity Within Individual Tumors and across Evolutionary Time. Cancer Res. Commun. 2023, 3, 564–575. [Google Scholar] [CrossRef]
- Gordon, N.; Kleinerman, E.S. The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat. Res. 2009, 152, 497–508. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Khanna, C.; Mendoza, A.; Guan, H.; DeLauter, L.; Kleinerman, E.S. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: The role of the Fas pathway in the metastatic process of osteosarcoma. Mol. Cancer Res. 2007, 5, 991–999. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.H.; Sun, Z.Z.; Rui, Y.J.; Yin, Q.D.; Song, S.; Wei, X.M.; Liu, J.; Liu, X.G.; Hu, K.J. Effect of c-erbB2 overexpression on prognosis in osteosarcoma: Evidence from eight studies. Tumor Biol. 2014, 35, 8939–8943. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R.; Huvos, A.G.; Heller, G.; Aledo, A.; Beardsley, G.P.; Healey, J.H.; Meyers, P.A. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J. Clin. Oncol. 1999, 17, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, S.E.; Geisinger, K.R.; King, T.S.; Sciarrotta, J.; Ward, W.G.; Gold, S.H.; Bos, G.D. Clinicopathologic analysis of HER-2/neu immunoexpression among various histologic subtypes and grades of osteosarcoma. Mod. Pathol. 2001, 14, 1277–1283. [Google Scholar] [CrossRef]
- Link, M.P.; Goorin, A.M.; Miser, A.W.; Green, A.A.; Pratt, C.B.; Belasco, J.B.; Pritchard, J.; Malpas, J.S.; Baker, A.R.; Kirkpatrick, J.A.; et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. New Engl. J. Med. 1986, 314, 1600–1606. [Google Scholar] [CrossRef]
- Ye, J.C.; Horne, S.; Zhang, J.Z.; Jackson, L.; Heng, H.H. Therapy Induced Genome Chaos: A Novel Mechanism of Rapid Cancer Drug Resistance. Front. Cell Dev. Biol. 2021, 9, 676344. [Google Scholar] [CrossRef]
- Franceschini, N.; Gaeta, R.; Krimpenfort, P.; Briaire-de Bruijn, I.; Kruisselbrink, A.B.; Szuhai, K.; Palubeckaitė, I.; Cleton-Jansen, A.M.; Bovée, J. A murine mesenchymal stem cell model for initiating events in osteosarcomagenesis points to CDK4/CDK6 inhibition as a therapeutic target. Lab. Investig. 2022, 102, 391–400. [Google Scholar] [CrossRef]
- Poot, M. Genes, Proteins, and Biological Pathways Preventing Chromothripsis. Methods Mol. Biol. 2018, 1769, 231–251. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Ratnaparkhe, M.; Wong, J.K.L.; Wei, P.C.; Hlevnjak, M.; Kolb, T.; Simovic, M.; Haag, D.; Paul, Y.; Devens, F.; Northcott, P.; et al. Defective DNA damage repair leads to frequent catastrophic genomic events in murine and human tumors. Nat. Commun. 2018, 9, 4760. [Google Scholar] [CrossRef]
- Khalid, U.; Simovic, M.; Hammann, L.A.; Iskar, M.; Wong, J.K.L.; Kumar, R.; Jugold, M.; Sill, M.; Bolkestein, M.; Kolb, T.; et al. A synergistic interaction between HDAC- and PARP inhibitors in childhood tumors with chromothripsis. Int. J. Cancer 2022, 151, 590–606. [Google Scholar] [CrossRef]
- Suehara, Y.; Alex, D.; Bowman, A.; Middha, S.; Zehir, A.; Chakravarty, D.; Wang, L.; Jour, G.; Nafa, K.; Hayashi, T.; et al. Clinical Genomic Sequencing of Pediatric and Adult Osteosarcoma Reveals Distinct Molecular Subsets with Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6346–6356. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.; Heng, H.H. Genome Chaos, Information Creation, and Cancer Emergence: Searching for New Frameworks on the 50th Anniversary of the “War on Cancer”. Genes 2021, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.; Heng, H.H. Two-phased evolution: Genome chaos-mediated information creation and maintenance. Prog. Biophys. Mol. Biol. 2021, 165, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.; Heng, H.H. Genome chaos: Creating new genomic information essential for cancer macroevolution. Semin. Cancer Biol. 2022, 81, 160–175. [Google Scholar] [CrossRef]
- Abou-El-Ardat, K.; Seifert, M.; Becker, K.; Eisenreich, S.; Lehmann, M.; Hackmann, K.; Rump, A.; Meijer, G.; Carvalho, B.; Temme, A.; et al. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro-Oncol. 2017, 19, 546–557. [Google Scholar] [CrossRef]
- Bochtler, T.; Granzow, M.; Stölzel, F.; Kunz, C.; Mohr, B.; Kartal-Kaess, M.; Hinderhofer, K.; Heilig, C.E.; Kramer, M.; Thiede, C.; et al. Marker chromosomes can arise from chromothripsis and predict adverse prognosis in acute myeloid leukemia. Blood 2017, 129, 1333–1342. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Hoogstraat, M.; Paling, O.; Tavakoli-Yaraki, M.; Renkens, I.; Vermaat, J.S.; van Roosmalen, M.J.; van Lieshout, S.; Nijman, I.J.; Roessingh, W.; et al. Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer. Genome Biol. 2011, 12, R103. [Google Scholar] [CrossRef]
- Fontana, M.C.; Marconi, G.; Feenstra, J.D.M.; Fonzi, E.; Papayannidis, C.; Ghelli Luserna di Rorá, A.; Padella, A.; Solli, V.; Franchini, E.; Ottaviani, E.; et al. Chromothripsis in acute myeloid leukemia: Biological features and impact on survival. Leukemia 2018, 32, 1609–1620. [Google Scholar] [CrossRef]
- Kodama, T.; Motoi, N.; Ninomiya, H.; Sakamoto, H.; Kitada, K.; Tsukaguchi, T.; Satoh, Y.; Nomura, K.; Nagano, H.; Ishii, N.; et al. A novel mechanism of EML4-ALK rearrangement mediated by chromothripsis in a patient-derived cell line. J. Thorac. Oncol. 2014, 9, 1638–1646. [Google Scholar] [CrossRef]
- Ramos, S.; Navarrete-Meneses, P.; Molina, B.; Cervantes-Barragán, D.E.; Lozano, V.; Gallardo, E.; Marchetti, F.; Frias, S. Genomic chaos in peripheral blood lymphocytes of Hodgkin’s lymphoma patients one year after ABVD chemotherapy/radiotherapy. Environ. Mol. Mutagen. 2018, 59, 755–768. [Google Scholar] [CrossRef]
- Zou, Y.S.; Klausner, M.; Ghabrial, J.; Stinnett, V.; Long, P.; Morsberger, L.; Murry, J.B.; Beierl, K.; Gocke, C.D.; Xian, R.R.; et al. A comprehensive approach to evaluate genetic abnormalities in multiple myeloma using optical genome mapping. Blood Cancer J. 2024, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp, S.; van Heesch, S.; Braat, A.K.; de Ligt, J.; van Iterson, M.; Simonis, M.; van Roosmalen, M.J.; Kelder, M.J.; Kruisselbrink, E.; Hochstenbach, R.; et al. Molecular dissection of germline chromothripsis in a developmental context using patient-derived iPS cells. Genome Med. 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Schöpflin, R.; Melo, U.S.; Moeinzadeh, H.; Heller, D.; Laupert, V.; Hertzberg, J.; Holtgrewe, M.; Alavi, N.; Klever, M.K.; Jungnitsch, J.; et al. Integration of Hi-C with short and long-read genome sequencing reveals the structure of germline rearranged genomes. Nat. Commun. 2022, 13, 6470. [Google Scholar] [CrossRef] [PubMed]
- Cretu Stancu, M.; van Roosmalen, M.J.; Renkens, I.; Nieboer, M.M.; Middelkamp, S.; de Ligt, J.; Pregno, G.; Giachino, D.; Mandrile, G.; Espejo Valle-Inclan, J.; et al. Mapping and phasing of structural variation in patient genomes using nanopore sequencing. Nat. Commun. 2017, 8, 1326. [Google Scholar] [CrossRef]
- Rausch, T.; Snajder, R.; Leger, A.; Simovic, M.; Giurgiu, M.; Villacorta, L.; Henssen, A.G.; Fröhling, S.; Stegle, O.; Birney, E.; et al. Long-read sequencing of diagnosis and post-therapy medulloblastoma reveals complex rearrangement patterns and epigenetic signatures. Cell Genom. 2023, 3, 100281. [Google Scholar] [CrossRef]
- Rodriguez, I.; Rossi, N.M.; Keskus, A.G.; Xie, Y.; Ahmad, T.; Bryant, A.; Lou, H.; Paredes, J.G.; Milano, R.; Rao, N.; et al. Insights into the mechanisms and structure of breakage-fusion-bridge cycles in cervical cancer using long-read sequencing. Am. J. Hum. Genet. 2024, 111, 544–561. [Google Scholar] [CrossRef]
- Ding, L.; Wendl, M.C.; McMichael, J.F.; Raphael, B.J. Expanding the computational toolbox for mining cancer genomes. Nat. Rev. Genet. 2014, 15, 556–570. [Google Scholar] [CrossRef]
- Garsed, D.W.; Marshall, O.J.; Corbin, V.D.; Hsu, A.; Di Stefano, L.; Schröder, J.; Li, J.; Feng, Z.P.; Kim, B.W.; Kowarsky, M.; et al. The architecture and evolution of cancer neochromosomes. Cancer Cell 2014, 26, 653–667. [Google Scholar] [CrossRef]
- Leppä, A.M.; Grimes, K.; Jeong, H.; Huang, F.Y.; Andrades, A.; Waclawiczek, A.; Boch, T.; Jauch, A.; Renders, S.; Stelmach, P.; et al. Single-cell multiomics analysis reveals dynamic clonal evolution and targetable phenotypes in acute myeloid leukemia with complex karyotype. Nat. Genet. 2024, 56, 2790–2803. [Google Scholar] [CrossRef]
- Smirnov, P.; Przybilla, M.J.; Simovic-Lorenz, M.; Parra, R.G.; Susak, H.; Ratnaparkhe, M.; Wong, J.K.; Körber, V.; Mallm, J.P.; Philippos, G.; et al. Multi-omic and single-cell profiling of chromothriptic medulloblastoma reveals genomic and transcriptomic consequences of genome instability. Nat. Commun. 2024, 15, 10183. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Grimes, K.; Rauwolf, K.K.; Bruch, P.M.; Rausch, T.; Hasenfeld, P.; Benito, E.; Roider, T.; Sabarinathan, R.; Porubsky, D.; et al. Functional analysis of structural variants in single cells using Strand-seq. Nat. Biotechnol. 2023, 41, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Cusanovich, D.A.; Ramani, V.; Aghamirzaie, D.; Pliner, H.A.; Hill, A.J.; Daza, R.M.; McFaline-Figueroa, J.L.; Packer, J.S.; Christiansen, L.; et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 2018, 361, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Stoeckius, M.; Hafemeister, C.; Stephenson, W.; Houck-Loomis, B.; Chattopadhyay, P.K.; Swerdlow, H.; Satija, R.; Smibert, P. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 2017, 14, 865–868. [Google Scholar] [CrossRef]
- Lee, I.; Razaghi, R.; Gilpatrick, T.; Molnar, M.; Gershman, A.; Sadowski, N.; Sedlazeck, F.J.; Hansen, K.D.; Simpson, J.T.; Timp, W. Simultaneous profiling of chromatin accessibility and methylation on human cell lines with nanopore sequencing. Nat. Methods 2020, 17, 1191–1199. [Google Scholar] [CrossRef]
- Li, W.; Lu, J.; Lu, P.; Gao, Y.; Bai, Y.; Chen, K.; Su, X.; Li, M.; Liu, J.; Chen, Y.; et al. scNanoHi-C: A single-cell long-read concatemer sequencing method to reveal high-order chromatin structures within individual cells. Nat. Methods 2023, 20, 1493–1505. [Google Scholar] [CrossRef]
- Yang, D.; Jones, M.G.; Naranjo, S.; Rideout, W.M., 3rd; Min, K.H.J.; Ho, R.; Wu, W.; Replogle, J.M.; Page, J.L.; Quinn, J.J.; et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution. Cell 2022, 185, 1905–1923.e1925. [Google Scholar] [CrossRef]
- Tang, H.; Cai, Y.; Yang, M.; Tang, S.; Huang, Q.; Li, H.; Liu, S.; Teng, H.; Xie, T.; He, M.; et al. Single-cell and spatial transcriptomics reveals the key role of MCAM(+) tip-like endothelial cells in osteosarcoma metastasis. NPJ Precis. Oncol. 2025, 9, 104. [Google Scholar] [CrossRef]
- A focus on single-cell omics. Nat. Rev. Genet. 2023, 24, 485. [CrossRef]
- L’Yi, S.; Maziec, D.; Stevens, V.; Manz, T.; Veit, A.; Berselli, M.; Park, P.J.; Głodzik, D.; Gehlenborg, N. Chromoscope: Interactive multiscale visualization for structural variation in human genomes. Nat. Methods 2023, 20, 1834–1835. [Google Scholar] [CrossRef]
- Gill, J.; Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021, 18, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Blattmann, C.; Thiemann, M.; Stenzinger, A.; Roth, E.K.; Dittmar, A.; Witt, H.; Lehner, B.; Renker, E.; Jugold, M.; Eichwald, V.; et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J. Transl. Med. 2015, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.D.; Hayashi, M.; Albert, C.M.; Jackson, K.W.; Loeb, D.M. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin. Exp. Metastasis 2015, 32, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Siolas, D.; Hannon, G.J. Patient-derived tumor xenografts: Transforming clinical samples into mouse models. Cancer Res. 2013, 73, 5315–5319. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wu, N.; Ghabrial, J.; Stinnett, V.; Klausner, M.; Morsberger, L.; Long, P.; Baraban, E.; Gross, J.M.; Zou, Y.S. Chromoanagenesis in Osteosarcoma. Biomolecules 2025, 15, 833. https://doi.org/10.3390/biom15060833
Li G, Wu N, Ghabrial J, Stinnett V, Klausner M, Morsberger L, Long P, Baraban E, Gross JM, Zou YS. Chromoanagenesis in Osteosarcoma. Biomolecules. 2025; 15(6):833. https://doi.org/10.3390/biom15060833
Chicago/Turabian StyleLi, Guozhuang, Nan Wu, Jen Ghabrial, Victoria Stinnett, Melanie Klausner, Laura Morsberger, Patty Long, Ezra Baraban, John M. Gross, and Ying S. Zou. 2025. "Chromoanagenesis in Osteosarcoma" Biomolecules 15, no. 6: 833. https://doi.org/10.3390/biom15060833
APA StyleLi, G., Wu, N., Ghabrial, J., Stinnett, V., Klausner, M., Morsberger, L., Long, P., Baraban, E., Gross, J. M., & Zou, Y. S. (2025). Chromoanagenesis in Osteosarcoma. Biomolecules, 15(6), 833. https://doi.org/10.3390/biom15060833




