Sesquiterpenoid Hormones Farnesoic Acid and Methyl Farnesoate Regulate Different Gene Sets in Shrimp Neocaridina davidi Hepatopancreas
Abstract
1. Introduction
2. Methodology
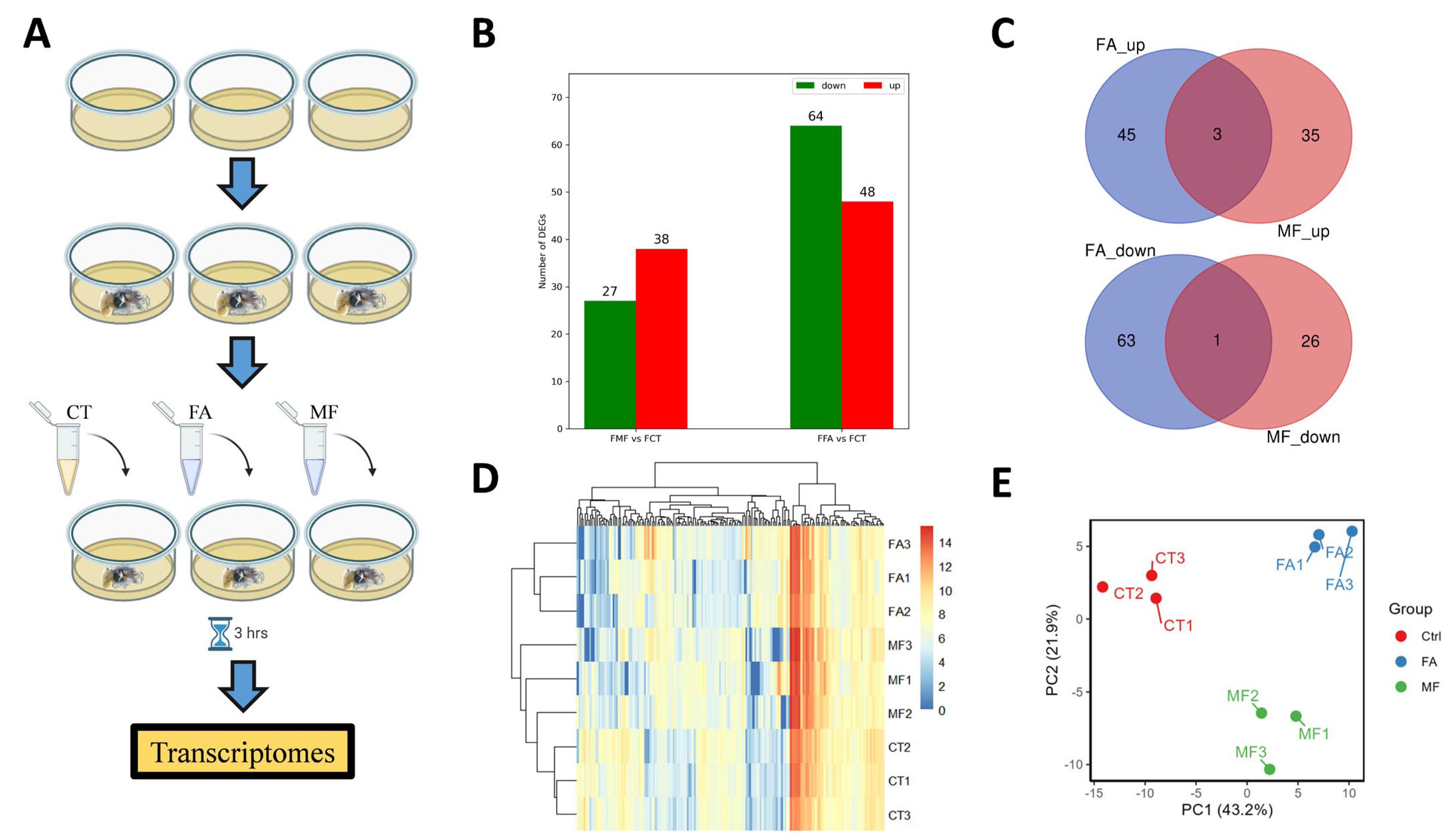
2.1. Animal Sampling and Culture
2.2. In Vitro Sesquiterpenoid Hormones Treatment
2.3. Transcriptome Sequencing and Analyses
2.4. KEGG and Gene Ontology (GO) Enrichment Analyses
2.5. Weighted Gene Co-Expression Network Analyses (WGCNA)
2.6. Real-Time PCR Validation
3. Results
3.1. Differential Gene Expression in Hepatopancreas upon FA or MF Treatment
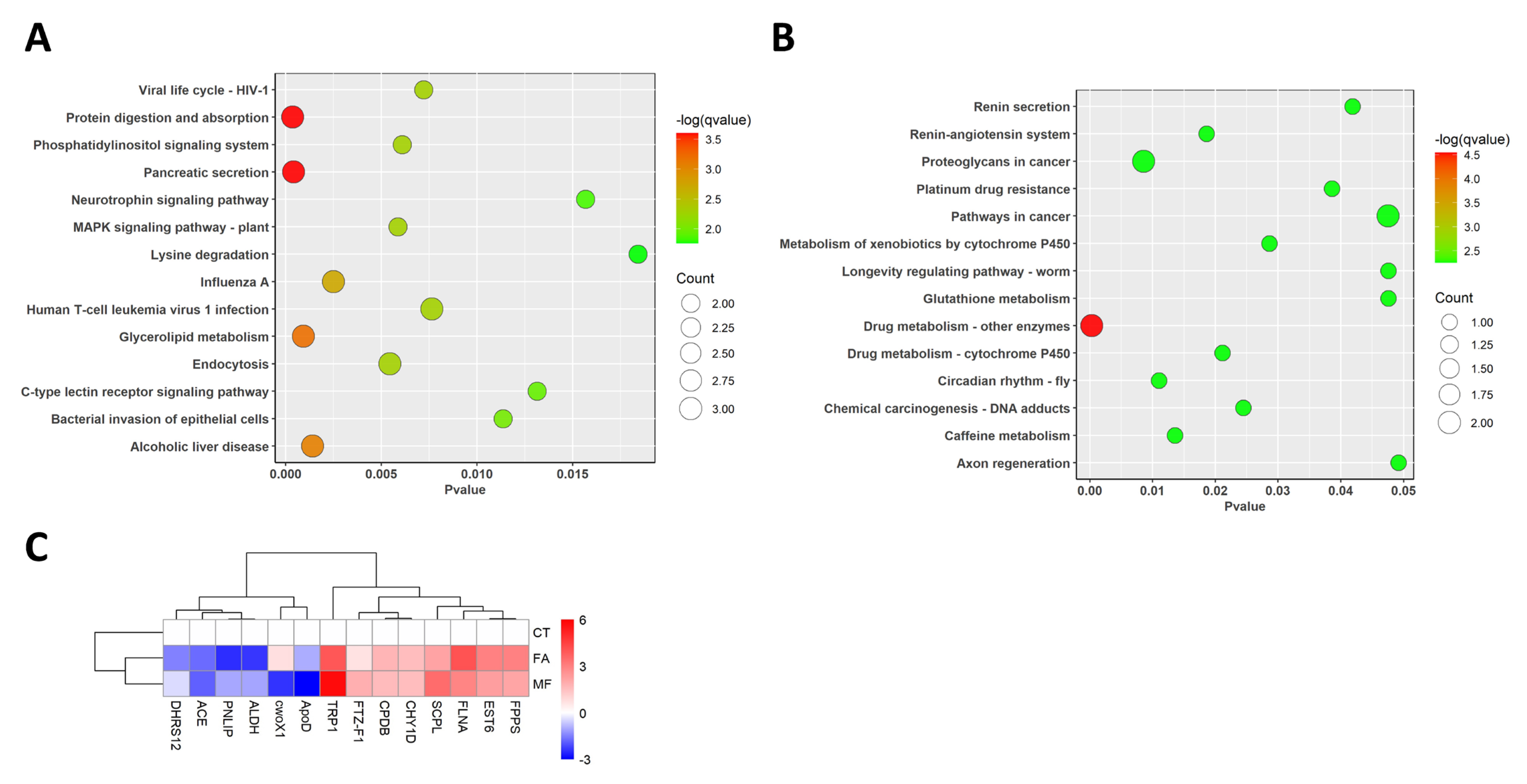
3.2. KEGG Gene Pathway Enrichment Analyses
3.3. Gene Ontology (GO) Enrichment Analysis
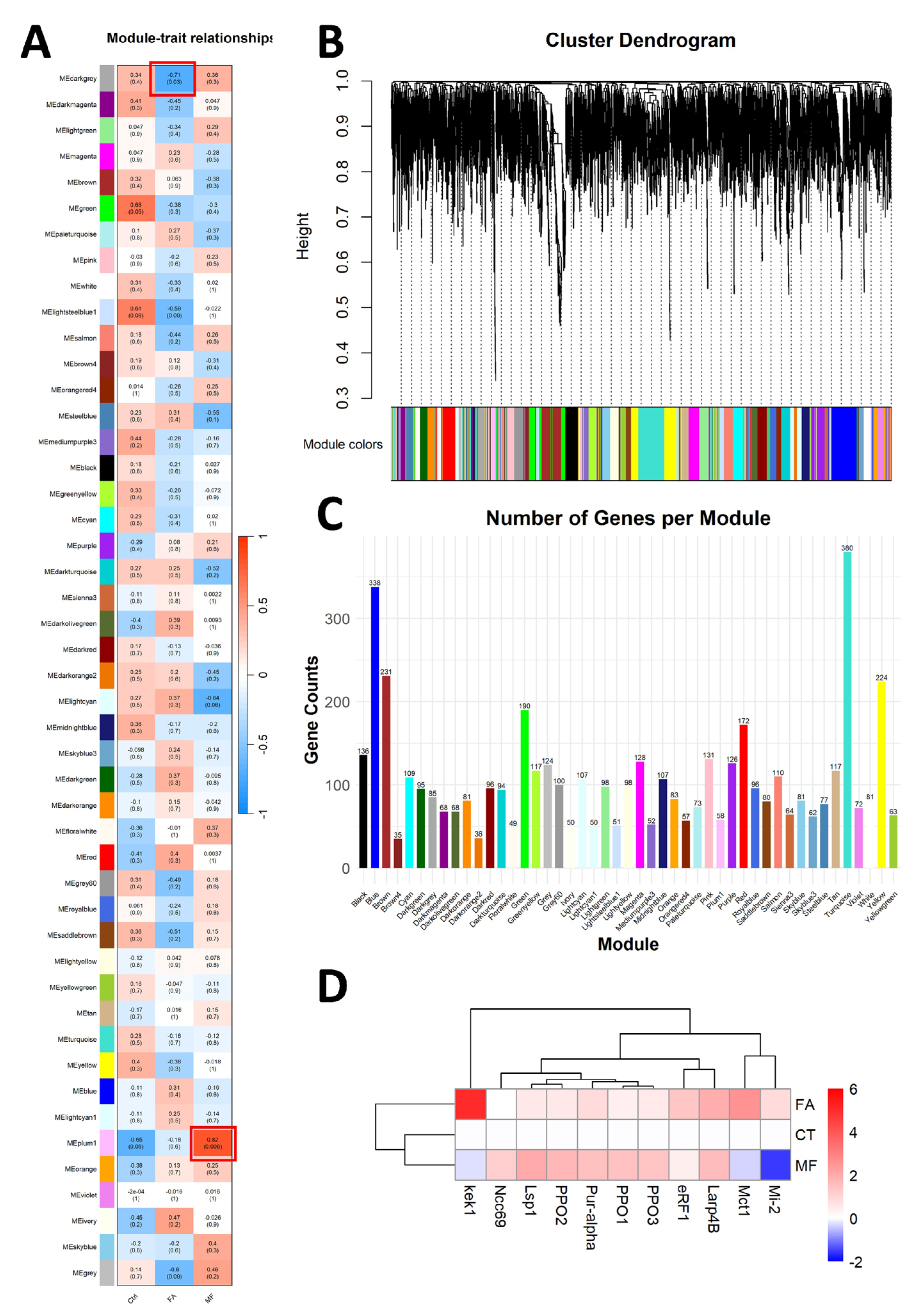
3.4. WGCNA for Identification of Hub Genes Responsible for FA and MF Treatment
3.5. Validation of the Transcriptome Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowe, A. Should Scientific Research Involving Decapod Crustaceans Require Ethical Review? J. Agric. Environ. Ethics 2018, 31, 625–634. [Google Scholar] [CrossRef]
- García-López, M.; Pérez-Martín, R.I.; Sotelo, C.G. Carotenoid Pigments Composition of Two Commonly Discarded Decapod Crustaceans in Grand Sole and the Galician-Northern Portugal Coast Fisheries. J. Aquat. Food Prod. T 2016, 25, 114–121. [Google Scholar] [CrossRef]
- Nates, S.F.; McKenney, C.L. Growth and variations in lipid class and fatty acid composition during larval development of the stone crab Menippe adina Williams and Felder, 1986. Invertebr. Reprod. Dev. 2000, 37, 157–165. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Tsang, S.S.K.; Law, S.T.S.; Li, C.; Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Diversity of Insect Sesquiterpenoid Regulation. Front. Genet 2020, 11, 1027. [Google Scholar] [CrossRef]
- Cheong, S.P.S.; Huang, J.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Evolution of Ecdysis and Metamorphosis in Arthropods: The Rise of Regulation of Juvenile Hormone. Integr. Comp. Biol. 2015, 55, 878–890. [Google Scholar] [CrossRef]
- Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Juvenile hormone and sesquiterpenoids in arthropods: Biosynthesis, signaling, and role of MicroRNA. J. Steroid Biochem. Mol. Biol. 2018, 184, 69–76. [Google Scholar] [CrossRef]
- Sin, Y.W.; Kenny, N.J.; Qu, Z.; Chan, K.W.; Chan, K.W.S.; Cheong, S.P.S.; Leung, R.W.T.; Chan, T.F.; Bendena, W.G.; Chu, K.H.; et al. Identification of putative ecdysteroid and juvenile hormone pathway genes in the shrimp Neocaridina denticulata. Gen. Comp. Endocr. 2015, 214, 167–176. [Google Scholar] [CrossRef]
- Schenk, S.; Krauditsch, C.; Frühauf, P.; Gerner, C.; Raible, F. Discovery of methylfarnesoate as the annelid brain hormone reveals an ancient role of sesquiterpenoids in reproduction. Elife 2016, 5, e17126. [Google Scholar] [CrossRef]
- Nong, W.Y.; Cao, J.Q.; Li, Y.Q.; Qu, Z.; Sun, J.; Swale, T.; Yip, H.Y.; Qian, P.Y.; Qiu, J.W.; Kwan, H.S.; et al. Jellyfish genomes reveal distinct homeobox gene clusters and conservation of small RNA processing. Nat. Commun. 2020, 11, 3051. [Google Scholar] [CrossRef]
- Nong, W.Y.; Yu, Y.F.; Aase-Remedios, M.E.; Xie, Y.C.; So, W.L.; Li, Y.Q.; Wong, C.F.; Baril, T.; Law, S.T.S.; Lai, S.Y.; et al. Genome of the ramshorn snail Biomphalaria straminea—An obligate intermediate host of schistosomiasis. GigaScience 2022, 11, giac012. [Google Scholar] [CrossRef]
- So, W.L.; Nong, W.Y.; Xie, Y.C.; Baril, T.; Ma, H.Y.; Qu, Z.; Haimovitz, J.; Swale, T.; Gaitan-Espitia, J.D.; Lau, K.F.; et al. Myriapod genomes reveal ancestral horizontal gene transfer and hormonal gene loss in millipedes. Nat. Commun. 2022, 13, 3010. [Google Scholar] [CrossRef] [PubMed]
- Laufer, H.; Borst, D.; Baker, F.C.; Reuter, C.C.; Tsai, L.W.; Schooley, D.A.; Carrasco, C.; Sinkus, M. Identification of a Juvenile Hormone-Like Compound in a Crustacean. Science 1987, 235, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Laufer, H.; Biggers, W.J. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Am. Zool. 2001, 41, 442–457. [Google Scholar] [CrossRef]
- Qu, Z.; Kenny, N.J.; Lam, H.M.; Chan, T.F.; Chu, K.H.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. How Did Arthropod Sesquiterpenoids and Ecdysteroids Arise? Comparison of Hormonal Pathway Genes in Noninsect Arthropod Genomes. Genome Biol. Evol. 2015, 7, 1951–1959. [Google Scholar] [CrossRef]
- Aliah, N.A.N.; Ab-Rahim, S.; Moore, H.E.; Heo, C.C. Juvenile hormone: Production, regulation, current application in vector control and its future applications. Trop. Biomed 2021, 38, 254–264. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile Hormone Signaling Pathway in Insect Development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Mak, A.; Choi, C.; Tiu, S.; Hui, J.; He, J.-G.; Tobe, S.; Chan, S.-M. Vitellogenesis in the red crabCharybdis feriatus: Hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression. Mol. Reprod. Dev. 2005, 70, 288–300. [Google Scholar] [CrossRef]
- Tiu, S.H.-K.; Chan, S.-M.; Tobe, S.S. The effects of farnesoic acid and 20-hydroxyecdysone on vitellogenin gene expression in the lobster, Homarus americanus, and possible roles in the reproductive process. Gen. Comp. Endocr. 2010, 166, 337–345. [Google Scholar] [CrossRef]
- Mykles, D.L.; Hui, J.H.L. Neocaridina denticulata: A Decapod Crustacean Model for Functional Genomics. Integr. Comp. Biol. 2015, 55, 891–897. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xing, K.F.; Wu, Z.X.; Yan, C.C.; Sun, Y.Y.; Zhang, J.Q. Construction of a cytidine base editor based on Exopalaemon carinicauda cytidine deaminase and its application in crustacean genome editing. Aquacult Rep. 2022, 27, 101366. [Google Scholar] [CrossRef]
- Kenny, N.J.; Sin, Y.W.; Shen, X.; Qu, Z.; Wang, W.; Chan, T.F.; Tobe, S.S.; Shimeld, S.M.; Chu, K.H.; Hui, J.H.L. Genomic sequencing and experimental tractability of a new decapod shrimp model Neocaridina denticulata. Mar. Drugs. 2014, 12, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.-H.; Davis, F.G.; et al. A Tissue-Mapped Axolotl De Novo Transcriptome Enables Identification of Limb Regeneration Factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Nguyen, C.; Nguyen, T.G.; Van Nguyen, L.; Pham, H.Q.; Nguyen, T.H.; Pham, H.T.; Nguyen, H.T.; Ha, T.T.; Dau, T.H.; Vu, H.T. De novo assembly and transcriptome characterization of major growth-related genes in various tissues of Penaeus monodon. Aquaculture 2016, 464, 545–553. [Google Scholar] [CrossRef]
- McVicar, L.K.; Shivers, R.R. Gap junctions and intercellular communication in the hepatopancreas of the crayfish (Orconectes propinquus) during molt. Cell Tissue Res. 1985, 240, 261–269. [Google Scholar] [CrossRef]
- Homola, E.; Chang, E.S. Methyl Farnesoate: Crustacean Juvenile Hormone in Search of Functions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 347–356. [Google Scholar] [CrossRef]
- Dhar, M.K.; Koul, A.; Kaul, S. Farnesyl pyrophosphate synthase: A key enzyme in isoprenoid biosynthetic pathway and potential molecular target for drug development. N. Biotechnol. 2013, 30, 114–123. [Google Scholar] [CrossRef]
- Liu, M.; Wu, Z.; Yan, C.; Liu, Y.; Xing, K.; Zhang, J.; Sun, Y. Ovarian transcriptome and metabolic responses of RNAi-mediated farnesyl pyrophosphate synthase knockdown in Neocaridina denticulata sinensis. Genomics 2022, 114, 110484. [Google Scholar] [CrossRef]
- Vogt, G.; Stöcker, W.; Storch, V.; Zwilling, R. Biosynthesis of Astacus protease, a digestive enzyme from crayfish. Histochemistry 1989, 91, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-J.; Leung, P.-C. Food digestion by cathepsin L and digestion-related rapid cell differentiation in shrimp hepatopancreas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Lai, Y.; Zhu, F. Molecular characterization of carboxypeptidase B-like (CPB) in Scylla paramamosain and its role in white spot syndrome virus and Vibrio alginolyticus infection. Fish. Shellfish. Immun. 2019, 94, 434–446. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque-Cavalcanti, C.; GarcÍA-CarreÑO, F.L.; Del Toro, M.A.N. Trypsin and trypsin inhibitors from penaeid shrimp. J. Food Biochem. 2002, 26, 233–251. [Google Scholar] [CrossRef]
- Sun, S.; Chen, L.; Qin, J.; Ye, J.; Qin, C.; Jiang, H.; Li, E. Molecular cloning, characterization and mRNA expression of copper-binding protein hemocyanin subunit in Chinese mitten crab, Eriocheir sinensis. Fish. Shellfish. Immun. 2012, 33, 1222–1228. [Google Scholar] [CrossRef]
- Decker, H.; Rimke, T. Tarantula Hemocyanin Shows Phenoloxidase Activity. J. Food Biochem. 1998, 273, 25889–25892. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Huang, W.; Wang, G.X.; Sun, H.M.; Chen, X.Y.; Luo, P.; Liu, J.S.; Hu, C.Q.; Li, H.; Shu, H. Identification and characterization of p38MAPK in response to acute cold stress in the gill of Pacific white shrimp Litopenaeus vannamei. Aquacult Rep. 2020, 17, 100365. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Villalobo, A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 398–435. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, L.; Cheng, Z.; Dong, L.; Guo, L.; Bai, Y.; Wu, Q.; Wang, S.; Yang, X.; Xie, W.; et al. A midgut transcriptional regulatory loop favors an insect host to withstand a bacterial pathogen. Innovation 2024, 5, 100675. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Sasagawa, S.; Nishimura, Y.; Sawada, H.; Zhang, E.; Okabe, S.; Murakami, S.; Ashikawa, Y.; Yuge, M.; Kawaguchi, K.; Kawase, R. Comparative transcriptome analysis identifies CCDC80 as a novel gene associated with pulmonary arterial hypertension. Front. Pharmacol. 2016, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Gong, Q.T.; Fan, J.Q.; Zhang, M.; Abbas, M.; Zhu, W.Y.; Deng, S.F.; Xing, S.P.; Zhang, J.Z. 20-Hydroxyecdysone regulates the prophenoloxidase cascade to immunize Metarhizium anisopliae in Locusta migratoria. Pest. Manag. Sci. 2020, 76, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-J.; Tang, L.; Yu, Y.-Y.; Wang, D.-J.; Liu, C.-L. Identification and expression patterns of 20-hydroxyecdysone-responsive genes from Procambarus clarkii. Genes. Genom. 2017, 39, 601–609. [Google Scholar] [CrossRef]
- Willott, E.; Wang, X.Y.; Wells, M.A. cDNA and gene sequence of Manduca sexta arylphorin, an aromatic amino acid-rich larval serum protein: Homology to arthropod hemocyanins. J. Biol. Chem. 1989, 264, 19052–19059. [Google Scholar] [CrossRef]
- Valzania, L.; Alami, A.; Léopold, P. A temporal allocation of amino acid resources ensures fitness and body allometry in Drosophila. Dev. Cell 2024, 59, 2277–2286.e6. [Google Scholar] [CrossRef]
- Burmester, T.; Antoniewski, C.; Lepesant, J.A. Ecdysone-regulation of synthesis and processing of Fat Body Protein 1, the larval serum protein receptor of Drosophila melanogaster. Eur. J. Biochem. 1999, 262, 49–55. [Google Scholar] [CrossRef]
- Nong, W.Y.; Law, S.T.S.; Wong, A.Y.P.; Baril, T.; Swale, T.; Chu, L.M.; Hayward, A.; Lau, D.T.W.; Hui, J.H.L. Chromosomal-level reference genome of the incense tree Aquilaria sinensis. Mol. Ecol. Resour. 2020, 20, 971–979. [Google Scholar] [CrossRef]

| Sample | Replicates | Clean Reads | Clean Bases | Q30% | Q20% | GC% | Accession Number |
|---|---|---|---|---|---|---|---|
| CT | CT1 | 44,726,374 | 6,708,865,897 | 94.17 | 98.04 | 41.99 | SAMN43294670 |
| CT | CT2 | 35,940,474 | 5,391,000,460 | 94.18 | 98.02 | 42.36 | SAMN43294671 |
| CT | CT3 | 39,464,592 | 5,919,608,543 | 93.97 | 97.94 | 42.85 | SAMN43294672 |
| FA | FA1 | 42,974,740 | 6,446,117,636 | 94.17 | 98.03 | 42.12 | SAMN43294673 |
| FA | FA2 | 40,680,348 | 6,101,970,714 | 93.97 | 97.97 | 41.71 | SAMN43294674 |
| FA | FA3 | 38,650,158 | 5,797,452,168 | 93.58 | 97.79 | 42.56 | SAMN43294675 |
| MF | MF1 | 42,207,424 | 6,331,034,015 | 94.34 | 98.11 | 41.78 | SAMN43294676 |
| MF | MF2 | 37,425,622 | 5,613,776,825 | 94.24 | 98.05 | 42.41 | SAMN43294677 |
| MF | MF3 | 37,352,120 | 5,602,746,352 | 94.14 | 98.04 | 41.68 | SAMN43294678 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Nong, W.; So, W.L.; Hui, J.H.L. Sesquiterpenoid Hormones Farnesoic Acid and Methyl Farnesoate Regulate Different Gene Sets in Shrimp Neocaridina davidi Hepatopancreas. Biomolecules 2025, 15, 815. https://doi.org/10.3390/biom15060815
Luan Y, Nong W, So WL, Hui JHL. Sesquiterpenoid Hormones Farnesoic Acid and Methyl Farnesoate Regulate Different Gene Sets in Shrimp Neocaridina davidi Hepatopancreas. Biomolecules. 2025; 15(6):815. https://doi.org/10.3390/biom15060815
Chicago/Turabian StyleLuan, Yehui, Wenyan Nong, Wai Lok So, and Jerome Ho Lam Hui. 2025. "Sesquiterpenoid Hormones Farnesoic Acid and Methyl Farnesoate Regulate Different Gene Sets in Shrimp Neocaridina davidi Hepatopancreas" Biomolecules 15, no. 6: 815. https://doi.org/10.3390/biom15060815
APA StyleLuan, Y., Nong, W., So, W. L., & Hui, J. H. L. (2025). Sesquiterpenoid Hormones Farnesoic Acid and Methyl Farnesoate Regulate Different Gene Sets in Shrimp Neocaridina davidi Hepatopancreas. Biomolecules, 15(6), 815. https://doi.org/10.3390/biom15060815






