Proton-Sensing G Protein-Coupled Receptors and Their Potential Role in Exercise Regulation of Arterial Function
Abstract
1. Introduction
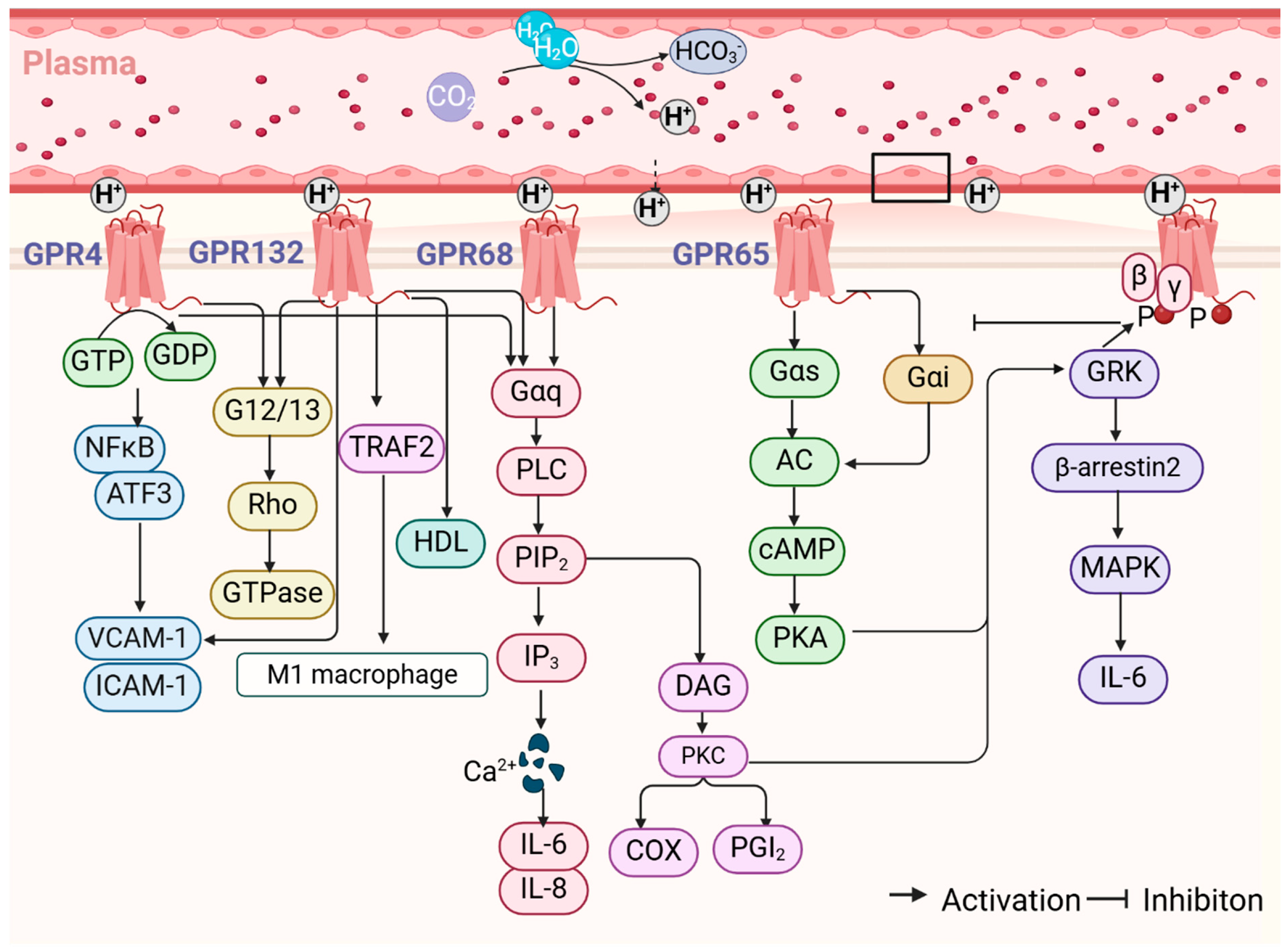
2. Overview of Proton-Sensing GPCRs
2.1. Structure and Function
2.2. Signaling Pathway
3. The Role and Mechanism of Proton-Sensing GPCRs in the Regulation of Arterial Function
3.1. GPR68/OGR1
3.2. GPR4
3.3. GPR132/G2A
3.4. GPR65/TDAG8
4. The Effects of Exercise on the Regulation of Arterial Function
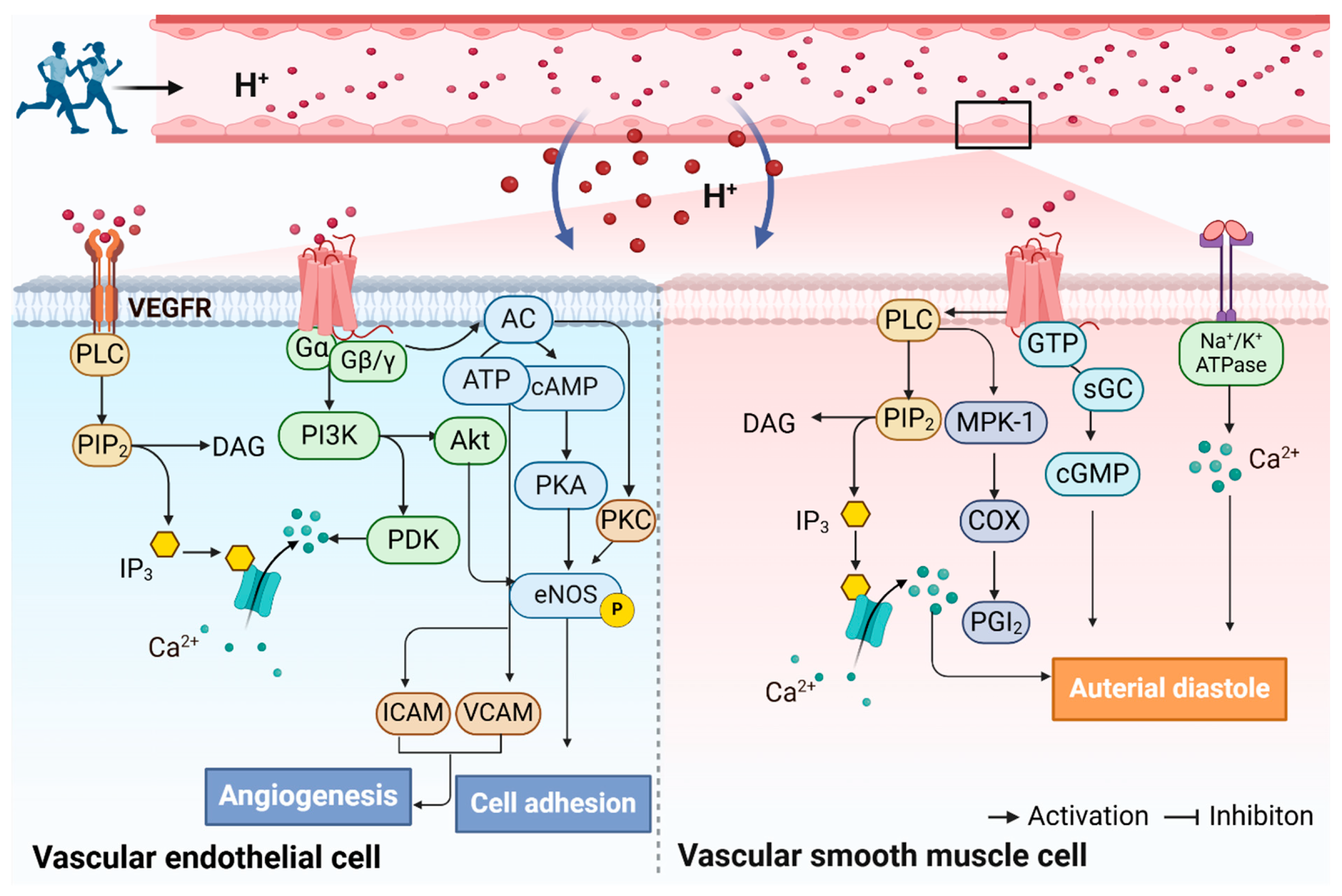
5. Regulation of Arterial Function by Exercise Through Proton-Sensing GPCRs
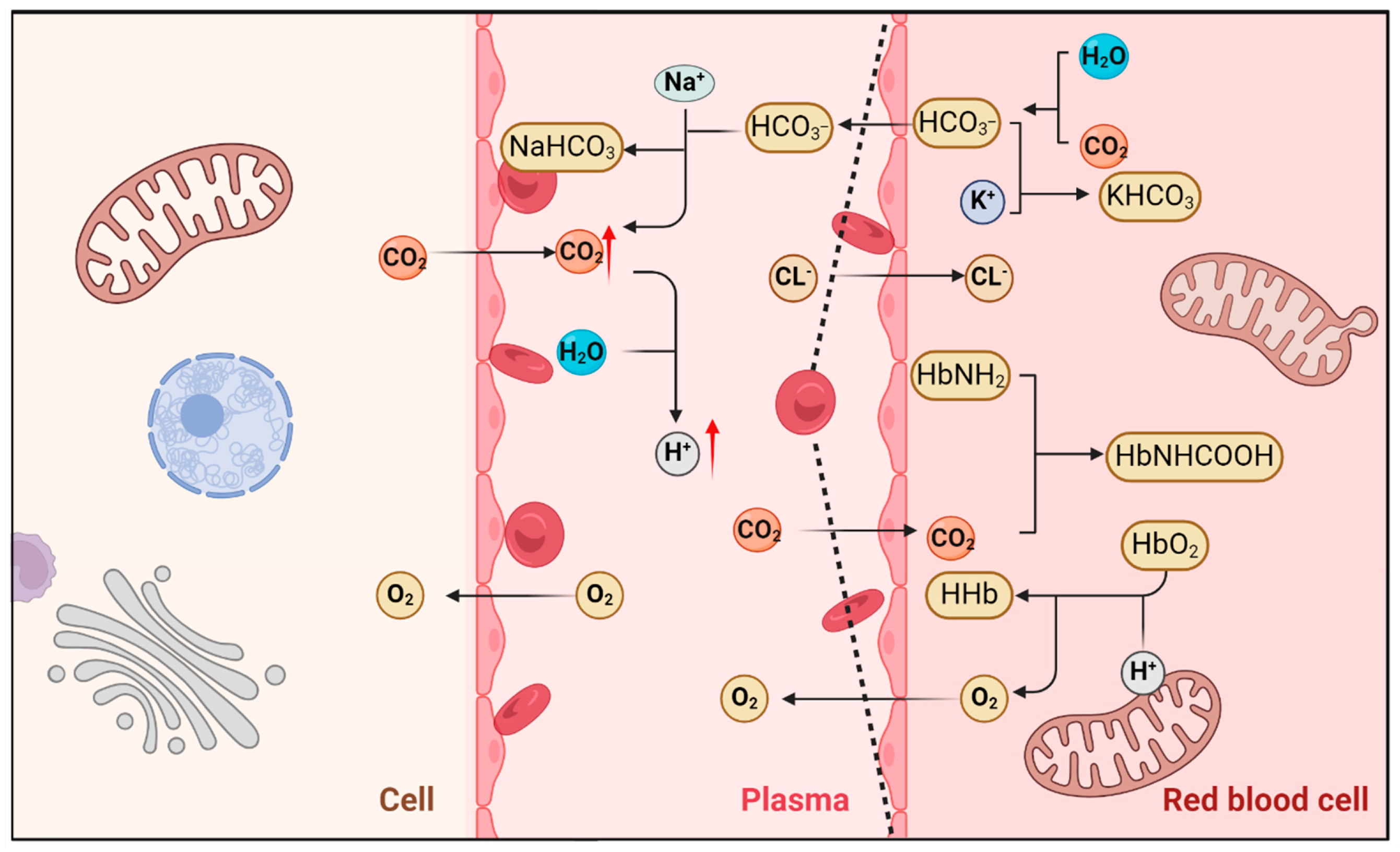
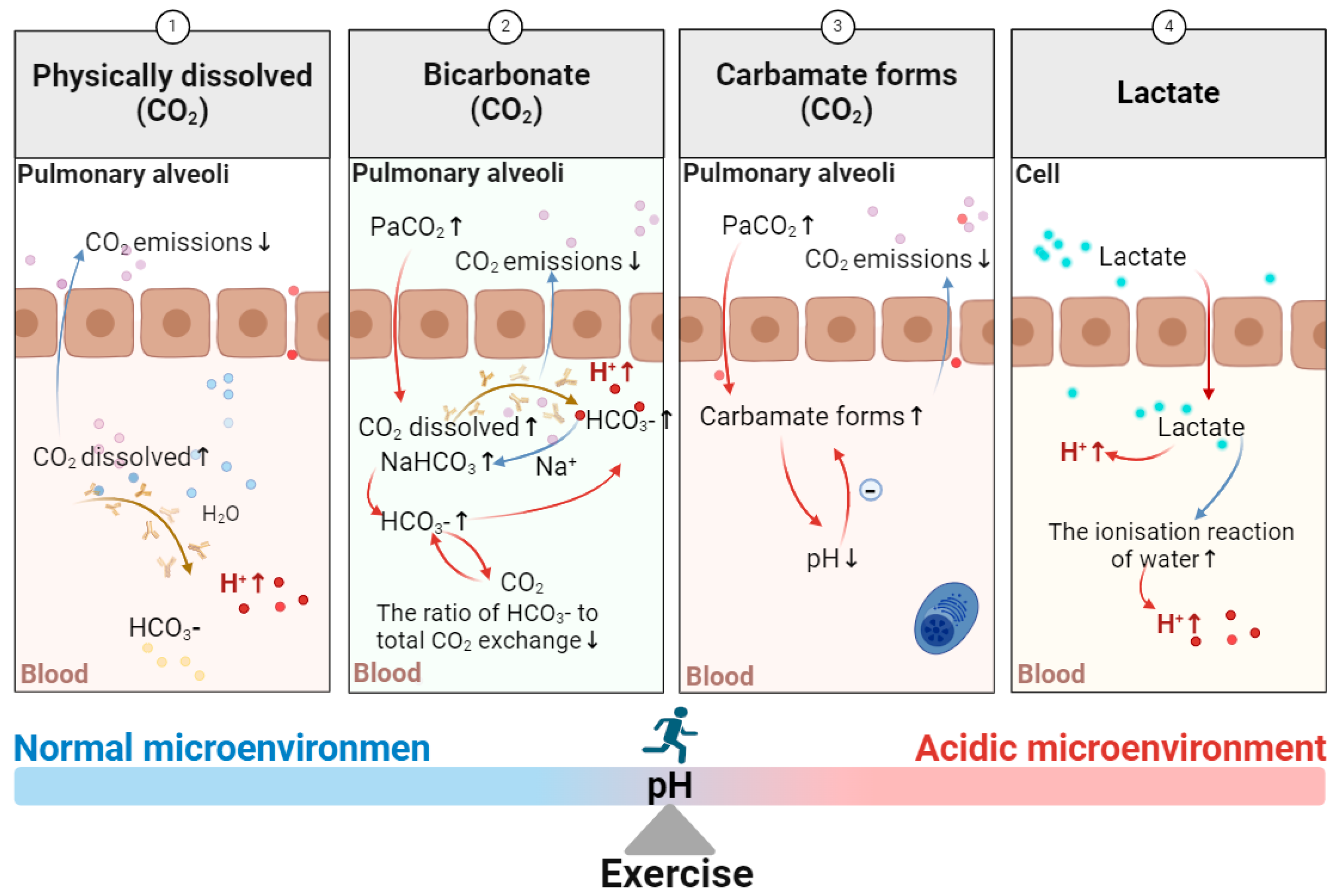
5.1. The Effect of Exercise on the Generation of an Acidic Microenvironment
5.1.1. Regulation of Acid–Base Balance During Respiration and Metabolic Stress
5.1.2. Exercise and the Regulation of Acid–Base Balance
5.2. The Potential Mechanisms by Which Exercise Improves Arterial Function Through the Activation of Proton-Sensing GPCRs
6. Conclusions
| Receptor | Location | Signaling | Protons | Agonist (EC50) | Antagonist (IC50) | Ref. |
|---|---|---|---|---|---|---|
| GPR68 (OGR1) | Human:14q32.11 NM_003485 Mouse: Chr 12 NM_175493 | Gαq; Gαs; Gαi; G12/13 | pH 7.8–5.6, maximum activity at pH 6.8 | CARTPT (3.2 µM) MOsteocrin (0.4 µM); MCorticotropin (1.8 µM); 3,5-disubstituted isoxazoles (µM range); CART (1 µM); Pro-opiomelanocortin-derived peptide (1.3 µM) | Cu2+ (µM range); Zn2+ (µM range) | [44,149,150] |
| GPR4 | Human: 19q13.32 NM_005282 Mouse: Chr 7 NM_175668 | Gαs; Gαq; Gαi; G12/13 | pH 7.6–5.6 | ND | Compound 3b (67 nM); NE 52-QQ 57 (70 nM); NE 52-QQ57 (70 nM); Compound 39c (110 nM) | [44,141,142,143,144,145,146,147,148,149,150,151,152,153,154] |
| GPR132 (G2A) | Human:14q32.33 NM_001278695.2 Mouse: Chr 12 NM_019925 | Gαs; Gαq | pH 8.2–6.6 | 9S-HODE (~0.5 µM); 11-HETE (~1 µM); N -palmitoylglycine (~800 nM); N-linoleoylglycine (~800 nM); ONC212 (~400 nM); 11,12-EET (~10 µM); 9,10-EpOME (~10 µM) | Lysophosphatidylcholine (~10 µM); Telmisartan (~10 µM); GSK1820795A (~1 µM) | [155,156,157,158,159] |
| GPR65 (TDAG8) | Human: 14q31.3 NM_003608 Mouse: Chr 12 NM_ 008152.3 | Gαs | pH 7.2–5.7 | Psychosine (3.4 µM); BTB09089 (active concentration > 5 µM) | ND | [81,160,161,162] |
| Subjects | Exercise Intervention Program | Mode of Action | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Model | Characteristics | Sample Size | Type | Intensity | Duration | ||
| C57BL/6, WT mice; ApoEtm1Unc, ApoE KO mice | 5–6 weeks old | ND | Treadmill running | 15 m/min at a 5° grade (60–80% of VO2max) | 5 days/week; 60 min/session; 15–16 weeks | Endothelial dysfunction ↑ | [103] |
| Patients with amnestic MCI | 29 in SAT/19 in AET | 70 | Moderate-to-vigorous AET or stretching and toning (SAT) | Moderate to vigorous | 12 months | VO2peak ↑; carotid β-stiffness index ↑; CBF pulsatility ↑ | [105] |
| Older sedentary overweight and obese men | 67 ± 2 years, BMI: 30.3 ± 2.8 kg/m2 | 17 males | Progressive, aerobic exercise | 70% maximal power | 3 days/week; 50 min/session; 8 weeks | Endothelial function ↑, retinal arteriolar width ↑, cardiovascular risk ↓ | [163] |
| Recreational athletes | 45.9 ± 9.6 years | 46 females/5 males | Endurance exercise | ND | ND | Coronary artery calcification → | [164] |
| Young men | Tealthy and recreationally active; 23 ± 2 years) | 10 males | Incremental leg cycling exercise | 50, 100, 150 Watts | 30 min | Radial artery mean and anterograde SR ↑ | [165] |
| Overweight men | 21–30 years; BMI: 30 ± 3 kg/m2 | 8 males | Swimming training | 50–80% HRmax | 3 days/week; 55 min/session; 8 weeks | Carotid arterial stiffness, systolic blood pressure, Peripheral resistance ↓; blood flow velocity, flow rate, maximal, mean wall shear stress ↑ | [166] |
| Healthy adults | 22 ± 2 years; BMI: 22 ± 2 kg/m2 | 12 | Cycling | 85 ± 5% HRmax | 30 min | ICA conductance ↑; vasodilation of the ICA ↑ | [167] |
| Healthy adults | 23 ± 4 years | 11 females/9 males | Isometric handgrip training | 30% of maximal voluntary contraction | 3 days/week; four, 2 min unilateral contractions; 8 weeks | Endothelium-dependent vasodilation ↑ | [168] |
| Healthy adults | 61.0 ± 1.3 years | 60 | Aerobic exercise | Medium–high intensity | 8 weeks | Arterial stiffness ↓ | [169] |
| Overweight and obese adults | BMI: 30.5 ± 7.2 | 30 | Aerobic exercise | ND | 8 weeks | Arterial dysfunction ↓ | [170] |
| Healthy adults | 61 ± 2 years | 4 females/7 males | Endurance exercise | 70% VO2max | 60 min/session; 10 days | FMD ↑; CAC ↑ | [171] |
| Healthy adults | 66 ± 1 years | 5 females/6 males | Recumbent cycling | 75–80% HRmax | 30 min | Brachial artery FMD ↑ | [172] |
| Healthy adults | YA: 26 ± 5 years; 23.8 ± 3.3 kg/m2 OA: 60 ± 6 years; 30.0 ± 5.5 kg/m2 | 21 young adults; 25 older adults | Unilateral maximal isokinetic knee flexion/extension exercise | 1RM | 3 sets; 10 reps | CCA strain time ↓ | [173] |
| Patients with metabolic syndrome | 51 ± 12 years | 57 | Endurance exercise | 60–85% 1RM | 8 weeks | cfPWV ↓; artery stiffness → | [107] |
| Healthy adults | 18–30 years | 14 females/12 males | Resistance exercise | 75% 1RM | 3 sets; 10 reps | Arterial stiffness↑ | [174] |
| Healthy adults | 24 ± 1 year | 7 males | Eccentric exercise | High intensity | 1 set; 50 reps | Carotid arterial compliance ↓; endothelial function ↓; β-stiffness index ↑ | [175] |
| Sprague–Dawley rats | 10 weeks old | 40 males | Treadmill running; HIIT | 30 m/min; High intensity | 5 days/week, 60 min/ session; 8 weeks; 4 days/week, 8 weeks, 14 repeats of 20 s/session, 10 s pause between sessions | PWV ↑; central arterial stiffness ↓ | [176] |
| Patients with CAD | 71.8 ± 10.2 years | 18 | Endurance training; HIIT | 60%; 85–90% HRmax | 30 min; 10 interval training periods | Acute endurance training; AS ↓; resistance training AS ↑ | [177] |
| Healthy adults | 56 ± 5 years | 25 females | Endurance exercise; resistance exercise | Medium to high strength | 150 min/weeks endurance exercise; 2 or more days/weeks strength-based exercise | PCS ↑; PSR ↑ | [178] |
| Patients with peripheral artery disease | 50–80 years | 12 | Walking exercise; resistance exercise; combined exercise | ND | 10 bouts of 2 min walking; 2 sets of 10 reps in 8 resistance exercises; 1 set of 10 reps in 8 resistance exercises + 5 bouts of 2 min walking | Artery stiffness ↓ | [179] |
| Same-sex twins | 31 monozygotic, 14 dizygotic pairs; 25.8 ± 6.0 years | 90 | Endurance exercise; resistance exercise | ND | 3 months | FMD ↑; vascular function ↑ | [180] |
| Patients with chronic kidney disease | 55 years and older; CKD stages 3b–4 | 99 | Endurance exercise; resistance exercise | 40–70% HRmax | 6 days/week, 90 min/session; 12 months | Arterial function ↑ | [106] |
| Patients with bariatric surgery | 8–45 years | 40 females | Endurance exercise; resistance exercise | Moderate intensity; 50–75% 1RM | 3 days/week; 60 min/session; 16 months | Arterial stiffness ↓ | [181] |
| Sedentary older adults | 64 ± 1 years | 64 | MICT; HIIT | 70% HRmax; 4 × 4 min at 90% HRmax interspersed with 3 × 3 min active recovery at 70% HRmax | 4 days/week; 8 weeks | MICT: carotid artery compliance ↑; cfPWV ↑ HIIT: carotid artery compliance →; cfPWV → | [182] |
| Healthy adults | 23.5 ± 1.2 years | 10 | MICT; HIIT | 40% HRmax; 85% HRmax | MICT: 40 min; HIIT: 1 min/session; 2 min between sets; total 26 min | Artery blood flow velocity → | [183] |
| Healthy adults | 21.4 ± 0.8 years; 1.73 ± 0.03 m; 62.1 ± 6.4 kg | 11 males | Interval training; interval exercise of semi-recumbent cycling | 57.6 kJ/exercise session | 12 min | ICA SR↑ | [184] |
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| AMPK | AMP-activated protein kinase |
| AoSMCs | aortic smooth muscle cells |
| ASICs | acid-sensitive ion channels |
| ATF3 | activating transcription factor 3 |
| ATP | adenosine triphosphate |
| ATPase | adenosine triphosphatase |
| Bcl-xL | B-cell lymphoma extra large |
| CA | carbonic anhydrase |
| Calm | calmodulin |
| cAMP | cyclic adenosine monophosphate |
| CBX | carotid body excision |
| CHOP | C/EBP homologous protein |
| CO2 | carbon dioxide |
| COX | cyclooxygenase |
| CT1-α1 | collagen type 1-α1 |
| CVDs | cardiovascular diseases |
| DAG | diacylglycerol |
| DDIT3 | DNA damage-induced transcript 3 |
| eNOs | endothelial nitric oxide synthase |
| Epac | exchange protein activated by 3′–5′-cyclic adenosine monophosphate |
| EPCs | endothelial progenitor cells |
| ERK | extracellular signal-regulated kinase |
| FICO2 | fraction of inspired CO2 |
| G2A | G2 accumulation protein |
| GRK | G protein-coupled receptor kinase |
| GTP | guanosine triphosphate |
| GTPase | guanosine triphosphatase |
| H+ | hydrion |
| H2O | water molecule |
| HbNH2 | aminohemoglobin |
| HbNHCOOH | carbamino hemoglobin |
| HbO2 | oxyhemoglobin |
| HCO3− | bicarbonate radical |
| HDL | high-density lipoprotein |
| HHb | unionized hemoglobin |
| HIIT | high-intensity interval training |
| HRP | high repetition protocol |
| HUVECs | human umbilical vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL-6 | interleukin-6 |
| IP3 | inositol triphosphate |
| KHCO3 | potassium bicarbonate |
| KIM-1 | kidney injury molecule 1 |
| KLF2 | Kruppel-like factor 2 |
| KO | knockout |
| MAPK | mitogen-activated protein kinase |
| MCAv | middle cerebral artery blood velocity |
| MCT1 | monocarboxylate transporter |
| MI | myocardial infarction |
| MKP-1 | MAPK phosphatase 1 |
| MRP | moderate repetition protocol |
| MS | multiple sclerosis |
| Na+/K+-ATPase | Sodium–potassium ATPase |
| NAD+ | nicotinamide adenine dinucleotide |
| NADH | reduced form of nicotinamide–adenine dinucleotide |
| NaHCO3 | sodium hydrogen carbonate |
| NFKB1 | nuclear factor kappa B subunit 1 |
| NF-κB | nuclear factor k-binding |
| NHE1 | sodium–hydrogen antiporter 1 |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| Notch1 | notch receptor 1 |
| OGR1 | ovarian cancer G protein-coupled receptor |
| OSS | oscillatory shear stress |
| PaCO2 | carbon dioxide tension |
| PCNA | proliferating cell nuclear antigen |
| PCr | phosphocreatine |
| PDK | phosphoinositide-dependent protein kinase |
| PGI2 | prostaglandin-I-2 |
| PI3K | phosphatidylinositol 3-hydroxy kinase |
| PIP2 | guanosine triphosphatase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PLC | phospholipase C |
| proton-sensing GPCRs | proton-sensing G protein-coupled receptors |
| Ras | Rat sarcoma |
| RC | respiratory compensation threshold |
| RELB | RELB proto-oncogene, NF-KB subunit |
| Rho | Ras homologous |
| ROCK | Rho-associated protein kinase |
| sGC | soluble guanylyl cyclase |
| STAT3 | signal transducer and activator of transcription 3 |
| TDAG8 | T-cell death-associated gene 8 |
| TNF-α | tumor necrosis factor-α |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VE | pulmonary ventilation |
| VECs | vascular endothelial cells |
| VEGFA | vascular endothelial growth factor A |
| VSMCs | vascular smooth muscle cells |
| 7TMRs | 7-transmembrane structural domain receptors |
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Chen, S.; Wu, Y.; Geng, D.; Sun, G.; Zhang, N.; et al. Advances in the study of exosomes in cardiovascular diseases. J. Adv. Res. 2024, 66, 133–153. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.Y.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, 13, zwae281. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Motau, T.H.; Norton, G.R.; Sareli, P.; Woodiwiss, A.J. Aortic Pulse Pressure Does Not Adequately Index Cardiovascular Risk Factor-Related Changes in Aortic Stiffness and Forward Wave Pressure. Am. J. Hypertens. 2018, 31, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Perry, A.S.; Dooley, E.E.; Master, H.; Spartano, N.L.; Brittain, E.L.; Pettee Gabriel, K. Physical Activity Over the Lifecourse and Cardiovascular Disease. Circ. Res. 2023, 132, 1725–1740. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Ruilope, L.M.; Santos-Lozano, A.; Wilhelm, M.; Krankel, N.; Fiuza-Luces, C.; Lucia, A. Exercise benefits in cardiovascular diseases: From mechanisms to clinical implementation. Eur. Heart J. 2023, 44, 1874–1889. [Google Scholar] [CrossRef]
- Howangyin, K.Y.; Silvestre, J.S. Diabetes mellitus and ischemic diseases: Molecular mechanisms of vascular repair dysfunction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1126–1135. [Google Scholar] [CrossRef]
- Khalafi, M.; Symonds, M.E.; Sakhaei, M.H.; Ghasemi, F. The effects of exercise training on circulating adhesion molecules in adults: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292734. [Google Scholar] [CrossRef]
- Yang, A.L.; Chen, H.I. Chronic exercise reduces adhesion molecules/iNOS expression and partially reverses vascular responsiveness in hypercholesterolemic rabbit aortae. Atherosclerosis 2003, 169, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Linke, A.; Krankel, N.; Erbs, S.; Gielen, S.; Mobius-Winkler, S.; Gummert, J.F.; Mohr, F.W.; Schuler, G.; Hambrecht, R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 2005, 111, 555–562. [Google Scholar] [CrossRef]
- Konigstein, K.; Dipla, K.; Zafeiridis, A. Training the Vessels: Molecular and Clinical Effects of Exercise on Vascular Health-A Narrative Review. Cells 2023, 12, 2544. [Google Scholar] [CrossRef]
- Conraads, V.M.; Beckers, P.; Bosmans, J.; De Clerck, L.S.; Stevens, W.J.; Vrints, C.J.; Brutsaert, D.L. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur. Heart J. 2002, 23, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Munk, P.S.; Breland, U.M.; Aukrust, P.; Ueland, T.; Kvaloy, J.T.; Larsen, A.I. High intensity interval training reduces systemic inflammation in post-PCI patients. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 850–857. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ishii, H.; Kurebayashi, N.; Sato, B.; Hayakawa, S.; Ando, H.; Hayashi, M.; Isobe, S.; Okumura, T.; Hirashiki, A.; et al. Association of cardiorespiratory fitness with characteristics of coronary plaque: Assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int. J. Cardiol. 2013, 162, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Madssen, E.; Moholdt, T.; Videm, V.; Wisloff, U.; Hegbom, K.; Wiseth, R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am. J. Cardiol. 2014, 114, 1504–1511. [Google Scholar] [CrossRef]
- Haskell, W.L.; Sims, C.; Myll, J.; Bortz, W.M.; St Goar, F.G.; Alderman, E.L. Coronary artery size and dilating capacity in ultradistance runners. Circulation 1993, 87, 1076–1082. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Terashima, M.; Fair, J.M.; Varady, A.; Taylor-Piliae, R.E.; Iribarren, C.; Go, A.S.; Haskell, W.L.; Hlatky, M.A.; Fortmann, S.P.; et al. Physical activity in older subjects is associated with increased coronary vasodilation: The ADVANCE study. JACC Cardiovasc. Imaging 2011, 4, 622–629. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Cable, N.T.; Green, D.J. Impact of exercise training on arterial wall thickness in humans. Clin. Sci. (Lond.) 2012, 122, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Constantin-Teodosiu, D.; Cederblad, G.; Bergstrom, M.; Greenhaff, P.L. Maximal-intensity exercise does not fully restore muscle pyruvate dehydrogenase complex activation after 3 days of high-fat dietary intake. Clin. Nutr. 2019, 38, 948–953. [Google Scholar] [CrossRef]
- Terpe, P.; Ruhs, S.; Dubourg, V.; Gekle, M.; Bucher, M. The synergism of cytosolic acidosis and reduced NAD/NADH ratio is responsible for lactic acidosis-induced vascular smooth muscle cell impairment in sepsis. J. Biomed. Sci. 2024, 31, 3. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mathur, J.; Vessieres, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J.; et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173, 762–775.e716. [Google Scholar] [CrossRef]
- Krewson, E.A.; Sanderlin, E.J.; Marie, M.A.; Akhtar, S.N.; Velcicky, J.; Loetscher, P.; Yang, L.V. The Proton-Sensing GPR4 Receptor Regulates Paracellular Gap Formation and Permeability of Vascular Endothelial Cells. iScience 2020, 23, 100848. [Google Scholar] [CrossRef]
- Bolick, D.T.; Skaflen, M.D.; Johnson, L.E.; Kwon, S.C.; Howatt, D.; Daugherty, A.; Ravichandran, K.S.; Hedrick, C.C. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ. Res. 2009, 104, 318–327. [Google Scholar] [CrossRef]
- Chen, L.D.; Zhu, W.T.; Cheng, Y.Y.; Li, Z.H.; Chen, Y.Q.; Yuan, Z.W.; Lin, C.Y.; Jing, D.D.; Liu, Z.Q.; Yan, P.K. T-cell death-associated gene 8 accelerates atherosclerosis by promoting vascular smooth muscle cell proliferation and migration. Atherosclerosis 2020, 297, 64–73. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Gill, P.S.; Ha, T.; Liu, L.; Hall, J.V.; Williams, D.L.; et al. Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis. Sci. Adv. 2022, 8, eabm8965. [Google Scholar] [CrossRef]
- Imenez Silva, P.H.; Wagner, C.A. Physiological relevance of proton-activated GPCRs. Pflugers Arch. 2022, 474, 487–504. [Google Scholar] [CrossRef]
- von Breitenbuch, P.; Kurz, B.; Wallner, S.; Zeman, F.; Brochhausen, C.; Schlitt, H.J.; Schreml, S. Expression of pH-Sensitive GPCRs in Peritoneal Carcinomatosis of Colorectal Cancer-First Results. J. Clin. Med. 2023, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.K.; Hoppe, N.; Huang, X.P.; Macdonald, C.B.; Mehrota, E.; Grimes, P.R.; Zahm, A.; Trinidad, D.D.; English, J.; Coyote-Maestas, W.; et al. Molecular basis of proton-sensing by G protein-coupled receptors. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef]
- Mohanty, I.; Parija, S.C.; Suklabaidya, S.; Rattan, S. Acidosis potentiates endothelium-dependent vasorelaxation and gap junction communication in the superior mesenteric artery. Eur. J. Pharmacol. 2018, 827, 22–31. [Google Scholar] [CrossRef]
- Grogan, A.; Lucero, E.Y.; Jiang, H.; Rockman, H.A. Pathophysiology and pharmacology of G protein-coupled receptors in the heart. Cardiovasc. Res. 2023, 119, 1117–1129. [Google Scholar] [CrossRef]
- Casimir, G.J.; Lefevre, N.; Corazza, F.; Duchateau, J.; Chamekh, M. The Acid-Base Balance and Gender in Inflammation: A Mini-Review. Front. Immunol. 2018, 9, 475. [Google Scholar] [CrossRef]
- de Valliere, C.; Wang, Y.; Eloranta, J.J.; Vidal, S.; Clay, I.; Spalinger, M.R.; Tcymbarevich, I.; Terhalle, A.; Ludwig, M.G.; Suply, T.; et al. G Protein-coupled pH-sensing Receptor OGR1 Is a Regulator of Intestinal Inflammation. Inflamm. Bowel Dis. 2015, 21, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Nassios, A.; Wallner, S.; Haferkamp, S.; Klingelhoffer, C.; Brochhausen, C.; Schreml, S. Expression of proton-sensing G-protein-coupled receptors in selected skin tumors. Exp. Dermatol. 2019, 28, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.B.; Kapolka, N.J.; Taghon, G.J.; Morgan, W.M.; Isom, D.G. The evolution and mechanism of GPCR proton sensing. J. Biol. Chem. 2021, 296, 100167. [Google Scholar] [CrossRef]
- Okajima, F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 2013, 25, 2263–2271. [Google Scholar] [CrossRef]
- Sisignano, M.; Fischer, M.J.M.; Geisslinger, G. Proton-Sensing GPCRs in Health and Disease. Cells 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Galtes, D.; Wang, J.; Rockman, H.A. G protein-coupled receptor signaling: Transducers and effectors. Am. J. Physiol. Cell Physiol. 2022, 323, C731–C748. [Google Scholar] [CrossRef] [PubMed]
- Imenez Silva, P.H.; Camara, N.O.; Wagner, C.A. Role of proton-activated G protein-coupled receptors in pathophysiology. Am. J. Physiol. Cell Physiol. 2022, 323, C400–C414. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98. [Google Scholar] [CrossRef]
- Morales Rodriguez, L.M.; Crilly, S.E.; Rowe, J.B.; Isom, D.G.; Puthenveedu, M.A. Location-biased activation of the proton-sensor GPR65 is uncoupled from receptor trafficking. Proc. Natl. Acad. Sci. USA 2023, 120, e2302823120. [Google Scholar] [CrossRef]
- Huang, X.P.; Kenakin, T.P.; Gu, S.; Shoichet, B.K.; Roth, B.L. Differential Roles of Extracellular Histidine Residues of GPR68 for Proton-Sensing and Allosteric Modulation by Divalent Metal Ions. Biochemistry 2020, 59, 3594–3614. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L. Effects of ovarian cancer G protein coupled receptor 1 on the proliferation, migration, and adhesion of human ovarian cancer cells. Chin. Med. J. 2011, 124, 1327–1332. [Google Scholar]
- Tobo, M.; Tomura, H.; Mogi, C.; Wang, J.Q.; Liu, J.P.; Komachi, M.; Damirin, A.; Kimura, T.; Murata, N.; Kurose, H.; et al. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell Signal 2007, 19, 1745–1753. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, J.; Shawn Xu, X.Z. Mechanosensitive GPCRs and ion channels in shear stress sensing. Curr. Opin. Cell Biol. 2023, 84, 102216. [Google Scholar] [CrossRef]
- Weiss, K.T.; Fante, M.; Kohl, G.; Schreml, J.; Haubner, F.; Kreutz, M.; Haverkampf, S.; Berneburg, M.; Schreml, S. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 2017, 26, 127–132. [Google Scholar] [CrossRef]
- Chen, X.; Jaiswal, A.; Costliow, Z.; Herbst, P.; Creasey, E.A.; Oshiro-Rapley, N.; Daly, M.J.; Carey, K.L.; Graham, D.B.; Xavier, R.J. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 2022, 23, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Tcymbarevich, I.V.; Eloranta, J.J.; Rossel, J.B.; Obialo, N.; Spalinger, M.; Cosin-Roger, J.; Lang, S.; Kullak-Ublick, G.A.; Wagner, C.A.; Scharl, M.; et al. The impact of the rs8005161 polymorphism on G protein-coupled receptor GPR65 (TDAG8) pH-associated activation in intestinal inflammation. BMC Gastroenterol. 2019, 19, 2. [Google Scholar] [CrossRef]
- Liu, J.P.; Nakakura, T.; Tomura, H.; Tobo, M.; Mogi, C.; Wang, J.Q.; He, X.D.; Takano, M.; Damirin, A.; Komachi, M.; et al. Each one of certain histidine residues in G-protein-coupled receptor GPR4 is critical for extracellular proton-induced stimulation of multiple G-protein-signaling pathways. Pharmacol. Res. 2010, 61, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, C.; Li, L.; Xu, G.; Gu, H.; Li, S.; Li, D.; Liu, M.; Han, S.; Zheng, B. Lipid Receptor G2A-Mediated Signal Pathway Plays a Critical Role in Inflammatory Response by Promoting Classical Macrophage Activation. J. Immunol. 2021, 206, 2338–2352. [Google Scholar] [CrossRef]
- de Valliere, C.; Cosin-Roger, J.; Simmen, S.; Atrott, K.; Melhem, H.; Zeitz, J.; Madanchi, M.; Tcymbarevich, I.; Fried, M.; Kullak-Ublick, G.A.; et al. Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68). Cell Mol. Gastroenterol. Hepatol. 2016, 2, 796–810. [Google Scholar] [CrossRef]
- Assimos, D.G. Re: The Proton-Activated Ovarian Cancer G Protein-Coupled Receptor 1 (OGR1) is Responsible for Renal Calcium Loss during Acidosis. J. Urology 2020, 204, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Chen, L.; Becker, J.; Chan, M.; Bushinsky, D.A. Effect of Osteoblast-Specific Deletion of the Proton Receptor OGR1. JBMR Plus 2022, 6, e10691. [Google Scholar] [CrossRef]
- de Valliere, C.; Cosin-Roger, J.; Baebler, K.; Schoepflin, A.; Mamie, C.; Mollet, M.; Schuler, C.; Bengs, S.; Lang, S.; Scharl, M.; et al. pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis. Int. J. Mol. Sci. 2022, 23, 1419. [Google Scholar] [CrossRef]
- Kadowaki, M.; Yamada, H.; Sato, K.; Shigemi, H.; Umeda, Y.; Morikawa, M.; Waseda, Y.; Anzai, M.; Kamide, Y.; Aoki-Saito, H.; et al. Extracellular acidification-induced CXCL8 production through a proton-sensing receptor OGR1 in human airway smooth muscle cells: A response inhibited by dexamethasone. J. Inflamm. 2019, 16, 4. [Google Scholar] [CrossRef]
- de Valliere, C.; Vidal, S.; Clay, I.; Jurisic, G.; Tcymbarevich, I.; Lang, S.; Ludwig, M.G.; Okoniewski, M.; Eloranta, J.J.; Kullak-Ublick, G.A.; et al. The pH-sensing receptor OGR1 improves barrier function of epithelial cells and inhibits migration in an acidic environment. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G475–G490. [Google Scholar] [CrossRef] [PubMed]
- Tomura, H.; Wang, J.Q.; Komachi, M.; Damirin, A.; Mogi, C.; Tobo, M.; Kon, J.; Misawa, N.; Sato, K.; Okajima, F. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J. Biol. Chem. 2005, 280, 34458–34464. [Google Scholar] [CrossRef]
- Liu, J.P.; Komachi, M.; Tomura, H.; Mogi, C.; Damirin, A.; Tobo, M.; Takano, M.; Nochi, H.; Tamoto, K.; Sato, K.; et al. Ovarian cancer G protein-coupled receptor 1-dependent and -independent vascular actions to acidic pH in human aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H731–H742. [Google Scholar] [CrossRef]
- Ding, S.; Xu, J.; Zhang, Q.; Chen, F.; Zhang, J.; Gui, K.; Xiong, M.; Li, B.; Ruan, Z.; Zhao, M. OGR1 mediates the inhibitory effects of acidic environment on proliferation and angiogenesis of endothelial progenitor cells. Cell Biol. Int. 2019, 43, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Othman, F.; Crooks, C.J.; Card, T.R. The risk of Clostridium difficile infection in patients with pernicious anaemia: A retrospective cohort study using primary care database. United European Gastroenterol. J. 2017, 5, 959–966. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, Y.; Wu, X.; Wang, Y.; Liu, F.; Zhang, J.; Qiu, Y.; Zhou, Z.; Wang, Z.; Xia, W.; et al. GPR4 signaling is essential for the promotion of acid-mediated angiogenic capacity of endothelial progenitor cells by activating STAT3/VEGFA pathway in patients with coronary artery disease. Stem Cell Res. Ther. 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Y.; Cai, H.; Ma, H.; Zhao, D.; Zhang, X.; Li, Z.; Wang, S.; Wang, J.; Liu, R.; et al. Human GPR4 and the Notch signaling pathway in endothelial cell tube formation. Mol. Med. Rep. 2016, 14, 1235–1240. [Google Scholar] [CrossRef]
- Wyder, L.; Suply, T.; Ricoux, B.; Billy, E.; Schnell, C.; Baumgarten, B.U.; Maira, S.M.; Koelbing, C.; Ferretti, M.; Kinzel, B.; et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis 2011, 14, 533–544. [Google Scholar] [CrossRef]
- Xue, C.; Shao, S.; Yan, Y.; Yang, S.; Bai, S.; Wu, Y.; Zhang, J.; Liu, R.; Ma, H.; Chai, L.; et al. Association between G-protein coupled receptor 4 expression and microvessel density, clinicopathological characteristics and survival in hepatocellular carcinoma. Oncol. Lett. 2020, 19, 2609–2620. [Google Scholar] [CrossRef]
- Wenzel, J.; Hansen, C.E.; Bettoni, C.; Vogt, M.A.; Lembrich, B.; Natsagdorj, R.; Huber, G.; Brands, J.; Schmidt, K.; Assmann, J.C.; et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 1753–1761. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Fan, Y.; Cao, S.; Zhang, X. Acidosis promotes cell apoptosis through the G protein-coupled receptor 4/CCAAT/enhancer-binding protein homologous protein pathway. Oncol. Lett. 2018, 16, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Krewson, E.A.; Yang, L.V. Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Dong, L.; Leffler, N.R.; Asch, A.S.; Witte, O.N.; Yang, L.V. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS ONE 2011, 6, e27586. [Google Scholar] [CrossRef]
- Weng, Z.; Fluckiger, A.C.; Nisitani, S.; Wahl, M.I.; Le, L.Q.; Hunter, C.A.; Fernal, A.A.; Le Beau, M.M.; Witte, O.N. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 1998, 95, 12334–12339. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, J.P.; Xu, X.Y.; Guo, Z.M.; Bu, B.Y. [Expressions of G2A and OGR1 in peripheral blood cells of patients with hypoxia induced pulmonary hypertension]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 33, 85–88. [Google Scholar] [CrossRef][Green Version]
- Hattori, T.; Obinata, H.; Ogawa, A.; Kishi, M.; Tatei, K.; Ishikawa, O.; Izumi, T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J. Investig. Dermatol. 2008, 128, 1123–1133. [Google Scholar] [CrossRef]
- Kern, K.; Schafer, S.M.G.; Cohnen, J.; Pierre, S.; Osthues, T.; Tarighi, N.; Hohmann, S.; Ferreiros, N.; Brune, B.; Weigert, A.; et al. The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue. Front. Immunol. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef]
- Parks, B.W.; Lusis, A.J.; Kabarowski, J.H. Loss of the lysophosphatidylcholine effector, G2A, ameliorates aortic atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2703–2709. [Google Scholar] [CrossRef]
- Johnson, L.E.; Elias, M.S.; Bolick, D.T.; Skaflen, M.D.; Green, R.M.; Hedrick, C.C. The G protein-coupled receptor G2A: Involvement in hepatic lipid metabolism and gallstone formation in mice. Hepatology 2008, 48, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Kihara, Y.; Shimizu, T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 9083–9087. [Google Scholar] [CrossRef] [PubMed]
- Tomura, H.; Mogi, C.; Sato, K.; Okajima, F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: A novel type of multi-functional receptors. Cell Signal 2005, 17, 1466–1476. [Google Scholar] [CrossRef]
- Kyaw, H.; Zeng, Z.; Su, K.; Fan, P.; Shell, B.K.; Carter, K.C.; Li, Y. Cloning, characterization, and mapping of human homolog of mouse T-cell death-associated gene. DNA Cell Biol. 1998, 17, 493–500. [Google Scholar] [CrossRef]
- Wang, J.Q.; Kon, J.; Mogi, C.; Tobo, M.; Damirin, A.; Sato, K.; Komachi, M.; Malchinkhuu, E.; Murata, N.; Kimura, T.; et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004, 279, 45626–45633. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, A.; Mogi, C.; Ono, H.; Nishi, T.; Horii, Y.; Ohba, Y.; Sato, K.; Nakaya, M.; Okajima, F.; Kurose, H. The proton-sensing G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) shows cardioprotective effects against myocardial infarction. Sci. Rep. 2017, 7, 7812. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Dawson, E.A.; Cable, N.T.; Green, D.J. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010, 55, 312–318. [Google Scholar] [CrossRef]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef]
- Gallardo-Vara, E.; Ntokou, A.; Dave, J.M.; Jovin, D.G.; Saddouk, F.Z.; Greif, D.M. Vascular pathobiology of pulmonary hypertension. J. Heart Lung Transplant. 2023, 42, 544–552. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Lou, J.; Wu, J.; Feng, M.; Dang, X.; Wu, G.; Yang, H.; Wang, Y.; Li, J.; Zhao, Y.; Shi, C.; et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. J. Sport. Health Sci. 2022, 11, 495–508. [Google Scholar] [CrossRef] [PubMed]
- De Ciuceis, C.; Rizzoni, D.; Palatini, P. Microcirculation and Physical Exercise In Hypertension. Hypertension 2023, 80, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; LaRocca, T.J.; Seals, D.R. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv. Physiol. Educ. 2014, 38, 296–307. [Google Scholar] [CrossRef]
- McDermott, M.M.; Spring, B.; Tian, L.; Treat-Jacobson, D.; Ferrucci, L.; Lloyd-Jones, D.; Zhao, L.; Polonsky, T.; Kibbe, M.R.; Bazzano, L.; et al. Effect of Low-Intensity vs High-Intensity Home-Based Walking Exercise on Walk Distance in Patients With Peripheral Artery Disease: The LITE Randomized Clinical Trial. JAMA 2021, 325, 1266–1276. [Google Scholar] [CrossRef]
- Li, Y.; Hanssen, H.; Cordes, M.; Rossmeissl, A.; Endes, S.; Schmidt-Trucksass, A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: A review. Eur. J. Sport. Sci. 2015, 15, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J. Effect of exercise on arterial compliance. Circulation 2000, 102, 1214–1215. [Google Scholar] [CrossRef]
- Okamoto, T.; Min, S.; Sakamaki-Sunaga, M. Arterial compliance and stiffness following low-intensity resistance exercise. Eur. J. Appl. Physiol. 2014, 114, 235–241. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhu, W.; Wu, H.; Yan, S. Acute effects of continuous and interval low-intensity exercise on arterial stiffness in healthy young men. Eur. J. Appl. Physiol. 2014, 114, 1385–1392. [Google Scholar] [CrossRef]
- Siasos, G.; Athanasiou, D.; Terzis, G.; Stasinaki, A.; Oikonomou, E.; Tsitkanou, S.; Kolokytha, T.; Spengos, K.; Papavassiliou, A.G.; Tousoulis, D. Acute effects of different types of aerobic exercise on endothelial function and arterial stiffness. Eur. J. Prev. Cardiol. 2016, 23, 1565–1572. [Google Scholar] [CrossRef]
- Kresnajati, S.; Lin, Y.Y.; Mundel, T.; Bernard, J.R.; Lin, H.F.; Liao, Y.H. Changes in Arterial Stiffness in Response to Various Types of Exercise Modalities: A Narrative Review on Physiological and Endothelial Senescence Perspectives. Cells 2022, 11, 3544. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Mayo, X.; Tai, Y.L.; Fennell, C. Arterial Stiffness and Autonomic Modulation After Free-Weight Resistance Exercises in Resistance Trained Individuals. J. Strength. Cond. Res. 2016, 30, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.D.; Yan, H.; Ranadive, S.M.; Kappus, R.M.; Sun, P.; Cook, M.D.; Harvey, I.; Woods, J.; Wilund, K.; Fernhall, B. Sex differences in ventricular-vascular coupling following endurance training. Eur. J. Appl. Physiol. 2014, 114, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jung, W.S.; Kim, S.W.; Lim, K. Effects of Interval Training Under Hypoxia on the Autonomic Nervous System and Arterial and Hemorheological Function in Healthy Women. Int. J. Womens Health 2022, 14, 79–90. [Google Scholar] [CrossRef]
- Hong, J.; Park, E.; Lee, J.; Lee, Y.; Rooney, B.V.; Park, Y. Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci. Rep. 2021, 11, 15449. [Google Scholar] [CrossRef]
- Zaman, S.; Raj, I.S.; Yang, A.W.H.; Lindner, R.; Denham, J. Exercise training reduces arterial stiffness in women with high blood pressure: A systematic review and meta-analysis. J. Hypertens. 2024, 42, 197–204. [Google Scholar] [CrossRef]
- Tomoto, T.; Liu, J.; Tseng, B.Y.; Pasha, E.P.; Cardim, D.; Tarumi, T.; Hynan, L.S.; Munro Cullum, C.; Zhang, R. One-Year Aerobic Exercise Reduced Carotid Arterial Stiffness and Increased Cerebral Blood Flow in Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 80, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Liu, C.K.; Miao, S.; Fielding, R.; Katzel, L.I.; Giffuni, J.; Well, A.; Seliger, S.L. Effect of Long-term Exercise Training on Physical Performance and Cardiorespiratory Function in Adults With CKD: A Randomized Controlled Trial. Am. J. Kidney Dis. 2023, 81, 59–66. [Google Scholar] [CrossRef]
- DeVallance, E.; Fournier, S.; Lemaster, K.; Moore, C.; Asano, S.; Bonner, D.; Donley, D.; Olfert, I.M.; Chantler, P.D. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur. J. Appl. Physiol. 2016, 116, 899–910. [Google Scholar] [CrossRef]
- Kondo, A.; Yamamoto, S.; Nakaki, R.; Shimamura, T.; Hamakubo, T.; Sakai, J.; Kodama, T.; Yoshida, T.; Aburatani, H.; Osawa, T. Extracellular Acidic pH Activates the Sterol Regulatory Element-Binding Protein 2 to Promote Tumor Progression. Cell Rep. 2017, 18, 2228–2242. [Google Scholar] [CrossRef]
- Soto, E.; Ortega-Ramirez, A.; Vega, R. Protons as Messengers of Intercellular Communication in the Nervous System. Front. Cell Neurosci. 2018, 12, 342. [Google Scholar] [CrossRef]
- Wasserman, K.; Cox, T.A.; Sietsema, K.E. Ventilatory regulation of arterial H(+) (pH) during exercise. Respir. Physiol. Neurobiol. 2014, 190, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Tsukanaka, A.; Harada, T.; Kosaka, M.; Matsui, N. Effect of hypercapnia on changes in blood pH, plasma lactate and ammonia due to exercise. Eur. J. Appl. Physiol. 2005, 95, 400–408. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Kampouras, A.; Hatziagorou, E.; Avramidou, V.; Georgopoulou, V.; Kirvassilis, F.; Hebestreit, H.; Tsanakas, J. Ventilation efficiency to exercise in patients with cystic fibrosis. Pediatr. Pulmonol. 2019, 54, 1584–1590. [Google Scholar] [CrossRef]
- Agostoni, P.; Emdin, M.; De Martino, F.; Apostolo, A.; Mase, M.; Contini, M.; Carriere, C.; Vignati, C.; Sinagra, G. Roles of periodic breathing and isocapnic buffering period during exercise in heart failure. Eur. J. Prev. Cardiol. 2020, 27, 19–26. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Waller, A.P. Physicochemical Analysis of Mixed Venous and Arterial Blood Acid-Base State in Horses at Core Temperature during and after Moderate-Intensity Exercise. Animals 2022, 12, 1875. [Google Scholar] [CrossRef]
- Rossiter, H.B. Exercise: Kinetic Considerations for Gas Exchange. Compr. Physiol. 2011, 1, 203–244. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Weber, R.E. Antagonistic interaction between oxygenation-linked lactate and CO2 binding to human hemoglobin. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 429–434. [Google Scholar] [CrossRef]
- Boning, D.; Klarholz, C.; Himmelsbach, B.; Hutler, M.; Maassen, N. Extracellular bicarbonate and non-bicarbonate buffering against lactic acid during and after exercise. Eur. J. Appl. Physiol. 2007, 100, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Peronnet, F.; Aguilaniu, B. [Pulmonary and alveolar ventilation, gas exchanges and arterial blood gases during ramp exercise]. Rev. Mal. Respir. 2012, 29, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Curl, C.C.; Duong, J.J.; Horning, M.A.; Moreno Santillan, D.D.; Leija, R.G. Lactate as a major myokine and exerkine. Nat. Rev. Endocrinol. 2022, 18, 712. [Google Scholar] [CrossRef]
- Hall, M.M.; Rajasekaran, S.; Thomsen, T.W.; Peterson, A.R. Lactate: Friend or Foe. PM R. 2016, 8, S8–S15. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Luszczyk, M.; Flis, D.J.; Szadejko, I.; Laskowski, R.; Ziolkowski, W. Excess postexercise oxygen consumption and fat oxidation in recreationally trained men following exercise of equal energy expenditure: Comparisons of spinning and constant endurance exercise. J. Sports Med. Phys. Fitness 2018, 58, 1781–1789. [Google Scholar] [CrossRef]
- Keytsman, C.; Hansen, D.; Wens, I.; Eijnde, B.O. Exercise-induced lactate responses in Multiple Sclerosis: A retrospective analysis. NeuroRehabilitation 2019, 45, 99–106. [Google Scholar] [CrossRef]
- Lee, S.; Hong, G.; Park, W.; Lee, J.; Kim, N.; Park, H.; Park, J. The effect of short-term creatine intake on blood lactic acid and muscle fatigue measured by accelerometer-based tremor response to acute resistance exercise. Phys. Act. Nutr. 2020, 24, 29–36. [Google Scholar] [CrossRef]
- Hebisz, P.; Hebisz, R.; Borkowski, J.; Zaton, M. Time of VO(2)max plateau and post-exercise oxygen consumption during incremental exercise testing in young mountain bike and road cyclists. Physiol. Res. 2018, 67, 711–719. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Martin-Rivera, F.; Bonilla, D.A.; Petro, J.L.; Carbone, L.; Romance, R.; deDiego, M.; Schoenfeld, B.J.; Benitez-Porres, J. Comparison of blood lactate and perceived exertion responses in two matched time-under-tension protocols. PLoS ONE 2020, 15, e0227640. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.; So, I.; Sharma, B.; Marzolini, S.; Tartaglia, M.C.; Oh, P.; Green, R. Effects of High-Intensity Interval Training Protocols on Blood Lactate Levels and Cognition in Healthy Adults: Systematic Review and Meta-Regression. Sports Med. 2023, 53, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Lancha Junior, A.H.; Painelli Vde, S.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45 (Suppl. 1), S71–S81. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; Heigenhauser, G.J. Effects of gas exchange on acid-base balance. Compr. Physiol. 2012, 2, 2203–2254. [Google Scholar] [CrossRef]
- Korzeniewski, B. Contribution of proton leak to oxygen consumption in skeletal muscle during intense exercise is very low despite large contribution at rest. PLoS ONE 2017, 12, e0185991. [Google Scholar] [CrossRef]
- Gough, L.A.; Rimmer, S.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F. Post-exercise Supplementation of Sodium Bicarbonate Improves Acid Base Balance Recovery and Subsequent High-Intensity Boxing Specific Performance. Front. Nutr. 2019, 6, 155. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Miller, R.G.; Boska, M.D.; Moussavi, R.S.; Carson, P.J.; Weiner, M.W. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J. Clin. Investig. 1988, 81, 1190–1196. [Google Scholar] [CrossRef]
- Huo, R.X.; Li, W.H.; Wu, H.; He, K.X.; Wang, H.; Zhang, S.; Jiang, S.H.; Li, R.K.; Xue, J.L. Transcription factor ONECUT3 regulates HDAC6/HIF-1α activity to promote the Warburg effect and tumor growth in colorectal cancer. Cell Death Dis. 2025, 16, 149. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017, 36, 252–259. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Deng, T.J.; Ma, L.; Sun, L.; Hao, Y.C.; Yu, H.X.; Yuan, F.; Tian, Y.M.; Wang, S. Acid-sensing ion channel 1 in nucleus tractus solitarii neurons contributes to the enhanced CO2-stimulated cardiorespiratory effect in spontaneously hypertensive rats. Life Sci. 2024, 351, 122853. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Lv, M.Z.; Guo, J.Y.; Mei, A.H.; Qian, H.; Yang, H.D.; Wu, W.W.; Liu, Z.X.; Zhong, J.X.; Wei, Y.; et al. The clinical significance of T-cell regulation in hypertension treatment. Front. Immunol. 2025, 16, 1550206. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Boedtkjer, E.; Pedersen, S.F. How protons pave the way to aggressive cancers. Nat. Rev. Cancer 2023, 23, 825–841. [Google Scholar] [CrossRef]
- Wasserman, K.; Beaver, W.L.; Sun, X.G.; Stringer, W.W. Arterial H+ regulation during exercise in humans. Respir. Physiol. Neurobiol. 2011, 178, 191–195. [Google Scholar] [CrossRef]
- Sietsema, K.E.; Cooper, D.M.; Perloff, J.K.; Child, J.S.; Rosove, M.H.; Wasserman, K.; Whipp, B.J. Control of ventilation during exercise in patients with central venous-to-systemic arterial shunts. J. Appl. Physiol. 1988, 64, 234–242. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Respiratory Acidosis and Respiratory Alkalosis: Core Curriculum 2023. Am. J. Kidney Di.s 2023, 82, 347–359. [Google Scholar] [CrossRef]
- Flacke, J.P.; Kumar, S.; Kostin, S.; Reusch, H.P.; Ladilov, Y. Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression. Apoptosis 2009, 14, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.T.; Yang, L.V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef]
- Namkoong, S.; Kim, C.K.; Cho, Y.L.; Kim, J.H.; Lee, H.; Ha, K.S.; Choe, J.; Kim, P.H.; Won, M.H.; Kwon, Y.G.; et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal 2009, 21, 906–915. [Google Scholar] [CrossRef]
- Gaynullina, D.K.; Tarasova, O.S.; Shvetsova, A.A.; Borzykh, A.A.; Schubert, R. The Effects of Acidosis on eNOS in the Systemic Vasculature: A Focus on Early Postnatal Ontogenesis. Int. J. Mol. Sci. 2022, 23, 5987. [Google Scholar] [CrossRef]
- Foster, S.R.; Hauser, A.S.; Vedel, L.; Strachan, R.T.; Huang, X.P.; Gavin, A.C.; Shah, S.D.; Nayak, A.P.; Haugaard-Kedstrom, L.M.; Penn, R.B.; et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 2019, 179, 895–908 e821. [Google Scholar] [CrossRef] [PubMed]
- Maeyashiki, C.; Melhem, H.; Hering, L.; Baebler, K.; Cosin-Roger, J.; Schefer, F.; Weder, B.; Hausmann, M.; Scharl, M.; Rogler, G.; et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1alpha/JNK Pathway in an Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Xu, H.; Chen, X.; Zhong, Q.; Huang, J.; Zhang, Y.; Guo, W.; Yang, Z.; Ding, S.; Chen, P.; et al. The Proton-Sensing G-Protein Coupled Receptor GPR4 Promotes Angiogenesis in Head and Neck Cancer. PLoS ONE 2016, 11, e0152789. [Google Scholar] [CrossRef]
- Yang, L.V.; Radu, C.G.; Roy, M.; Lee, S.; McLaughlin, J.; Teitell, M.A.; Iruela-Arispe, M.L.; Witte, O.N. Vascular abnormalities in mice deficient for the G protein-coupled receptor GPR4 that functions as a pH sensor. Mol. Cell Biol. 2007, 27, 1334–1347. [Google Scholar] [CrossRef]
- Fukuda, H.; Ito, S.; Watari, K.; Mogi, C.; Arisawa, M.; Okajima, F.; Kurose, H.; Shuto, S. Identification of a Potent and Selective GPR4 Antagonist as a Drug Lead for the Treatment of Myocardial Infarction. ACS Med. Chem. Lett. 2016, 7, 493–497. [Google Scholar] [CrossRef]
- Velcicky, J.; Miltz, W.; Oberhauser, B.; Orain, D.; Vaupel, A.; Weigand, K.; Dawson King, J.; Littlewood-Evans, A.; Nash, M.; Feifel, R.; et al. Development of Selective, Orally Active GPR4 Antagonists with Modulatory Effects on Nociception, Inflammation, and Angiogenesis. J. Med. Chem. 2017, 60, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Ueno, S.; Chen, M.X.; Harvey, J.; Dowell, S.J.; Irving, A.J.; Brown, A.J. N-Palmitoylglycine and other N-acylamides activate the lipid receptor G2A/GPR132. Pharmacol. Res. Perspect. 2019, 7, e00542. [Google Scholar] [CrossRef]
- Nii, T.; Prabhu, V.V.; Ruvolo, V.; Madhukar, N.; Zhao, R.; Mu, H.; Heese, L.; Nishida, Y.; Kojima, K.; Garnett, M.J.; et al. Imipridone ONC212 activates orphan G protein-coupled receptor GPR132 and integrated stress response in acute myeloid leukemia. Leukemia 2019, 33, 2805–2816. [Google Scholar] [CrossRef]
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 42484–42491. [Google Scholar] [CrossRef]
- Lahvic, J.L.; Ammerman, M.; Li, P.; Blair, M.C.; Stillman, E.R.; Fast, E.M.; Robertson, A.L.; Christodoulou, C.; Perlin, J.R.; Yang, S.; et al. Specific oxylipins enhance vertebrate hematopoiesis via the receptor GPR132. Proc. Natl. Acad. Sci. USA 2018, 115, 9252–9257. [Google Scholar] [CrossRef]
- Yin, H.; Chu, A.; Li, W.; Wang, B.; Shelton, F.; Otero, F.; Nguyen, D.G.; Caldwell, J.S.; Chen, Y.A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. 2009, 284, 12328–12338. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S.; Heise, C.E.; Nguyen, T.; O’Dowd, B.F.; Lynch, K.R. Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 2001, 153, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Mogi, C.; Tobo, M.; Tomura, H.; Murata, N.; He, X.D.; Sato, K.; Kimura, T.; Ishizuka, T.; Sasaki, T.; Sato, T.; et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009, 182, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Karpiak, J.; Kroeze, W.K.; Zhu, H.; Chen, X.; Moy, S.S.; Saddoris, K.A.; Nikolova, V.D.; Farrell, M.S.; Wang, S.; et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 2015, 527, 477–483. [Google Scholar] [CrossRef]
- Kleinloog, J.P.D.; Mensink, R.P.; Roodt, J.O.; Thijssen, D.H.J.; Hesselink, M.K.C.; Joris, P.J. Aerobic exercise training improves not only brachial artery flow-mediated vasodilatation but also carotid artery reactivity: A randomized controlled, cross-over trial in older men. Physiol. Rep. 2022, 10, e15395. [Google Scholar] [CrossRef]
- Kleiven, O.; Bjorkavoll-Bergseth, M.F.; Omland, T.; Aakre, K.M.; Froysa, V.; Erevik, C.B.; Greve, O.J.; Melberg, T.H.; Auestad, B.; Skadberg, O.; et al. Endurance exercise training volume is not associated with progression of coronary artery calcification. Scand. J. Med. Sci. Sports 2020, 30, 1024–1032. [Google Scholar] [CrossRef]
- Alali, M.H.; Lucas, R.A.I.; Junejo, R.T.; Fisher, J.P. Impact of acute dynamic exercise and arterial shear rate modification on radial artery low-flow mediated constriction in young men. Eur. J. Appl. Physiol. 2022, 122, 1885–1895. [Google Scholar] [CrossRef]
- Yuan, W.X.; Liu, H.B.; Gao, F.S.; Wang, Y.X.; Qin, K.R. Effects of 8-week swimming training on carotid arterial stiffness and hemodynamics in young overweight adults. Biomed. Eng. Online 2016, 15, 151. [Google Scholar] [CrossRef]
- Sakamoto, R.; Katayose, M.; Yamada, Y.; Neki, T.; Kamoda, T.; Tamai, K.; Yamazaki, K.; Iwamoto, E. High-but not moderate-intensity exercise acutely attenuates hypercapnia-induced vasodilation of the internal carotid artery in young men. Eur. J. Appl. Physiol. 2021, 121, 2471–2485. [Google Scholar] [CrossRef]
- Badrov, M.B.; Freeman, S.R.; Zokvic, M.A.; Millar, P.J.; McGowan, C.L. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur. J. Appl. Physiol. 2016, 116, 1289–1296. [Google Scholar] [CrossRef]
- Hasegawa, N.; Fujie, S.; Kurihara, T.; Homma, T.; Sanada, K.; Sato, K.; Hamaoka, T.; Iemitsu, M. Effects of habitual aerobic exercise on the relationship between intramyocellular or extramyocellular lipid content and arterial stiffness. J. Hum. Hypertens. 2016, 30, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Franklin, N.C.; Norkeviciute, E.; Bian, J.T.; Babana, J.C.; Szczurek, M.R.; Phillips, S.A. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J. Hypertens. 2016, 34, 1309–1316. [Google Scholar] [CrossRef]
- Landers-Ramos, R.Q.; Corrigan, K.J.; Guth, L.M.; Altom, C.N.; Spangenburg, E.E.; Prior, S.J.; Hagberg, J.M. Short-term exercise training improves flow-mediated dilation and circulating angiogenic cell number in older sedentary adults. Appl. Physiol. Nutr. Metab. 2016, 41, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, E.; Bock, J.M.; Casey, D.P. High-Intensity Exercise Enhances Conduit Artery Vascular Function in Older Adults. Med. Sci. Sports Exerc. 2018, 50, 124–130. [Google Scholar] [CrossRef]
- Sherman, S.R.; Lefferts, W.K.; Lefferts, E.C.; Grigoriadis, G.; Lima, N.S.; Fernhall, B.; Baynard, T.; Rosenberg, A.J. The effect of aging on carotid artery wall mechanics during maximal resistance exercise. Eur. J. Appl. Physiol. 2022, 122, 2477–2488. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Tai, Y.L.; Mayo, X.; Glasgow, A.; Marshall, E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur. J. Sport. Sci. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Choi, Y.; Akazawa, N.; Zempo-Miyaki, A.; Ra, S.G.; Shiraki, H.; Ajisaka, R.; Maeda, S. Acute Effect of High-Intensity Eccentric Exercise on Vascular Endothelial Function in Young Men. J. Strength. Cond. Res. 2016, 30, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Fujie, S.; Horii, N.; Miyamoto-Mikami, E.; Tsuji, K.; Uchida, M.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Effects of Different Exercise Modes on Arterial Stiffness and Nitric Oxide Synthesis. Med. Sci. Sports Exerc. 2018, 50, 1177–1185. [Google Scholar] [CrossRef]
- Santos, V.; Massuca, L.M.; Angarten, V.; Melo, X.; Pinto, R.; Fernhall, B.; Santa-Clara, H. Arterial Stiffness following Endurance and Resistance Exercise Sessions in Older Patients with Coronary Artery Disease. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef]
- Campbell, A.K.; Beaumont, A.J.; Hayes, L.; Herbert, P.; Gardner, D.; Ritchie, L.; Sculthorpe, N. Habitual exercise influences carotid artery strain and strain rate, but not cognitive function in healthy middle-aged females. Eur. J. Appl. Physiol. 2023, 123, 1051–1066. [Google Scholar] [CrossRef]
- Dias-Santos, E.G.; Farah, B.Q.; Germano-Soares, A.H.; Correia, M.A.; Souza, A.A.; Hora, J.E.J.; Ritti-Dias, R.M.; Andrade-Lima, A. Effects of Exercise Mode on Arterial Stiffness in Symptomatic Peripheral Artery Disease Patients: A Randomized Crossover Clinical Trial. Ann. Vasc. Surg. 2021, 74, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Marsh, C.E.; Thomas, H.J.; Lester, L.; Scurrah, K.J.; Haynes, A.; Naylor, L.H. Exercise and Artery Function in Twins: Sex Differences in a Cross-Over Trial. Hypertension 2023, 80, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Maldonado, A.; Martinez-Forte, S.; Ferrer-Marquez, M.; Martinez-Rosales, E.; Hernandez-Martinez, A.; Carretero-Ruiz, A.; Villa-Gonzalez, E.; Barranco-Ruiz, Y.; Rodriguez-Perez, M.A.; Torrente-Sanchez, M.J.; et al. Physical Exercise following bariatric surgery in women with Morbid obesity: Study protocol clinical trial (SPIRIT compliant). Medicine 2020, 99, e19427. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Hwang, C.L.; Yoo, J.K.; Hwang, M.H.; Handberg, E.M.; Petersen, J.W.; Nichols, W.W.; Sofianos, S.; Christou, D.D. All-Extremity Exercise Training Improves Arterial Stiffness in Older Adults. Med. Sci. Sports Exerc. 2017, 49, 1404–1411. [Google Scholar] [CrossRef]
- Urabe, J.; Ono, K.; Okagawa, J.; Nakayama, Y.; Yamau, R.; Ishikawa, A. Effect of high-intensity interval exercise on renal artery hemodynamics in healthy young adults. J. Sports Med. Phys. Fitness 2023, 63, 129–135. [Google Scholar] [CrossRef]
- Ogoh, S.; Washio, T.; Suzuki, K.; Iemitsu, M.; Hashimoto, T.; Iwamoto, E.; Bailey, D.M. Greater increase in internal carotid artery shear rate during aerobic interval compared to continuous exercise in healthy adult men. Physiol. Rep. 2021, 9, e14705. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Jia, D.; Wang, R. Proton-Sensing G Protein-Coupled Receptors and Their Potential Role in Exercise Regulation of Arterial Function. Biomolecules 2025, 15, 813. https://doi.org/10.3390/biom15060813
Yu F, Jia D, Wang R. Proton-Sensing G Protein-Coupled Receptors and Their Potential Role in Exercise Regulation of Arterial Function. Biomolecules. 2025; 15(6):813. https://doi.org/10.3390/biom15060813
Chicago/Turabian StyleYu, Fengzhi, Dandan Jia, and Ru Wang. 2025. "Proton-Sensing G Protein-Coupled Receptors and Their Potential Role in Exercise Regulation of Arterial Function" Biomolecules 15, no. 6: 813. https://doi.org/10.3390/biom15060813
APA StyleYu, F., Jia, D., & Wang, R. (2025). Proton-Sensing G Protein-Coupled Receptors and Their Potential Role in Exercise Regulation of Arterial Function. Biomolecules, 15(6), 813. https://doi.org/10.3390/biom15060813






