Expression of FGF23 and α-KLOTHO in Normal Human Kidney Development and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)
Abstract
1. Introduction
2. Materials and Methods
2.1. Kidney Tissue Collection and Preparation
2.2. Separation of Samples Based on Developmental Phases
2.3. Immunofluorescence
2.4. Imaging and Quantitative Analysis
2.5. Statistical Analysis
3. Results
3.1. FGF23 Expression
3.2. FGF23 Expression Is Downregulated in HKs and DKs
3.3. α-KLOTHO Expression
3.4. α-KLOTHO Expression Remains Consistent Across All Tested CAKUT Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walawender, L.; Becknell, B.; Matsell, D.G. Congenital Anomalies of the Kidney and Urinary Tract: Defining Risk Factors of Disease Progression and Determinants of Outcomes. Pediatr. Nephrol. 2023, 38, 3963–3973. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.J.; Butler, M.G. Genetics of Anomalies of the Kidney and Urinary Tract with Congenital Heart Disease: A Review. Clin. Genet. 2024, 106, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Sanna-Cherchi, S.; Westland, R.; Ghiggeri, G.M.; Gharavi, A.G. Genetic Basis of Human Congenital Anomalies of the Kidney and Urinary Tract. J. Clin. Investig. 2018, 128, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kelam, N.; Racetin, A.; Polović, M.; Benzon, B.; Ogorevc, M.; Vukojević, K.; Glavina Durdov, M.; Dunatov Huljev, A.; Kuzmić Prusac, I.; Čarić, D.; et al. Aberrations in FGFR1, FGFR2, and RIP5 Expression in Human Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Int. J. Mol. Sci. 2022, 23, 5537. [Google Scholar] [CrossRef]
- Jain, S.; Chen, F. Developmental Pathology of Congenital Kidney and Urinary Tract Anomalies. Clin. Kidney J. 2019, 12, 382–399. [Google Scholar] [CrossRef]
- Chevalier, R.L. CAKUT: A Pediatric and Evolutionary Perspective on the Leading Cause of CKD in Childhood. Pediatr. Rep. 2023, 15, 143–153. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, Q.; Wu, B.; Wang, H.; Dong, X.; Lu, Y.; Cheng, G.; Wang, L.; Lu, W.; Chen, L.; et al. Genetic Spectrum of Congenital Anomalies of the Kidney and Urinary Tract in Chinese Newborn Genome Project. Kidney Int. Rep. 2023, 8, 2376–2384. [Google Scholar] [CrossRef]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef]
- Rodelo-Haad, C.; Santamaria, R.; Muñoz-Castañeda, J.R.; Pendón-Ruiz De Mier, M.V.; Martin-Malo, A.; Rodriguez, M. FGF23, Biomarker or Target? Toxins 2019, 11, 175. [Google Scholar] [CrossRef]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho Signaling Axis in the Kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef]
- Ali, M.F.; Venkatarayappa, S.K.B.; Benny, M.; Rojas, C.; Yousefi, K.; Shehadeh, L.A.; Kulandavelu, S.; Sharma, M.; Da Silva, N.; Freundlich, M.; et al. Effects of Klotho Supplementation on Hyperoxia-Induced Renal Injury in a Rodent Model of Postnatal Nephrogenesis. Pediatr. Res. 2020, 88, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Mutlu, A.; Bakir, C.N.; Peltek, I.B.; Canbaz, A.A.; Díaz Tocados, J.M.; Haarhaus, M. Klotho in Pregnancy and Intrauterine Development—Potential Clinical Implications: A Review from the European Renal Association CKD-MBD Working Group. Nephrol. Dial. Transplant. 2024, 39, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Little, M.H.; Brennan, J.; Georgas, K.; Davies, J.A.; Davidson, D.R.; Baldock, R.A.; Beverdam, A.; Bertram, J.F.; Capel, B.; Chiu, H.S.; et al. A High-Resolution Anatomical Ontology of the Developing Murine Genitourinary Tract. Gene Expr. Patterns 2007, 7, 680–699. [Google Scholar] [CrossRef]
- Ekblom, P. Kidney Physiology and Pathophysiology; Seldin, D.W., Giebisch, G., Eds.; Raven Press: New York, NY, USA, 1992. [Google Scholar]
- Pavlović, N.; Kelam, N.; Racetin, A.; Filipović, N.; Pogorelić, Z.; Prusac, I.K.; Vukojević, K. Expression Profiles of ITGA8 and VANGL2 Are Altered in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Molecules 2024, 29, 3294. [Google Scholar] [CrossRef]
- Kelam, J.; Kelam, N.; Filipović, N.; Komić, L.; Racetin, A.; Komić, D.; Kostić, S.; Kuzmić Prusac, I.; Vukojević, K. Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes 2024, 15, 702. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Ma, Y.; Kirby, B.J.; Fairbridge, N.A.; Karaplis, A.C.; Lanske, B.; Kovacs, C.S. FGF23 Is Not Required to Regulate Fetal Phosphorus Metabolism but Exerts Effects within 12 Hours after Birth. Endocrinology 2017, 158, 252–263. [Google Scholar] [CrossRef]
- Ma, Y.; Samaraweera, M.; Cooke-Hubley, S.; Kirby, B.J.; Karaplis, A.C.; Lanske, B.; Kovacs, C.S. Neither Absence nor Excess of FGF23 Disturbs Murine Fetal-Placental Phosphorus Homeostasis or Prenatal Skeletal Development and Mineralization. Endocrinology 2014, 155, 1596–1605. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, J.; Chen, R.; Zhou, W.; Geng, H.; Huang, Y.; Shi, S.; Yuan, L.; Wang, Z.; Wang, D. Decreased FGF23 Inhibits Placental Angiogenesis via the ERK1/2-EGR-1 Signaling Pathway in Preeclampsia. Cytokine 2024, 176, 156508. [Google Scholar] [CrossRef]
- Kurtzeborn, K.; Kwon, H.N.; Kuure, S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int. J. Mol. Sci. 2019, 20, 1779. [Google Scholar] [CrossRef]
- Arumugam, S.; Subbiah, N.K.; Senthiappan, A.M. Double Ureter: Incidence, Types, and Its Applied Significance—A Cadaveric Study. Cureus 2020, 12, e7760. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.U.; Ojili, V. Multimodality Imaging Spectrum of Complications of Horseshoe Kidney. Indian. J. Radiol. Imaging 2017, 27, 133. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yu, M.; Ju, H.; Xue, S.; Li, Y.; Wu, X.; Xu, H.; Shen, Q. Inhibition of MAPK/ERK Pathway Activation Rescues Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) in Robo2PB/+ Gen1PB/+ Mice. Biochem. Biophys. Res. Commun. 2023, 653, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Yu, M.; Du, X.; Xue, S.; Ye, N.; Sun, L.; Wu, X.; Xu, H.; Shen, Q. Gestational Diabetes Mellitus Induces Congenital Anomalies of the Kidney and Urinary Tract in Mice by Altering RET/MAPK/ERK Pathway. Biochem. Biophys. Res. Commun. 2024, 714, 149959. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Goetz, R.; Nakada, Y.; Hu, M.C.; Kurosu, H.; Wang, L.; Nakatani, T.; Shi, M.; Eliseenkova, A.V.; Razzaque, M.S.; Moe, O.W.; et al. Isolated C-Terminal Tail of FGF23 Alleviates Hypophosphatemia by Inhibiting FGF23-FGFR-Klotho Complex Formation. Proc. Natl. Acad. Sci. USA 2010, 107, 407–412. [Google Scholar] [CrossRef]
- Johnson, K.; Levine, K.; Sergi, J.; Chamoun, J.; Roach, R.; Vekich, J.; Favis, M.; Horn, M.; Cao, X.; Miller, B.; et al. Therapeutic Effects of FGF23 C-Tail Fc in a Murine Preclinical Model of X-Linked Hypophosphatemia Via the Selective Modulation of Phosphate Reabsorption. J. Bone Min. Res. 2017, 32, 2062–2073. [Google Scholar] [CrossRef]
- Mangos, S.; Amaral, A.P.; Faul, C.; Jüppner, H.; Reiser, J.; Wolf, M. Expression of Fgf23 and Aklotho in Developing Embryonic Tissues and Adult Kidney of the Zebrafish, Danio Rerio. Nephrol. Dial. Transplant. 2012, 27, 4314–4322. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, M.Y.; Kim, Y.J.; Park, S.R.; Kim, J.; Ryu, S.Y.; Jung, J.Y. Developmental Immunolocalization of the Klotho Protein in Mouse Kidney Epithelial Cells. Eur. J. Histochem. 2014, 58, 2256. [Google Scholar] [CrossRef]
- Jung, H.J.; Pham, T.D.; Su, X.T.; Grigore, T.V.; Hoenderop, J.G.; Olauson, H.; Wall, S.M.; Ellison, D.H.; Welling, P.A.; Al-Qusairi, L. Klotho Is Highly Expressed in the Chief Sites of Regulated Potassium Secretion, and It Is Stimulated by Potassium Intake. Sci. Rep. 2024, 14, 10740. [Google Scholar] [CrossRef]
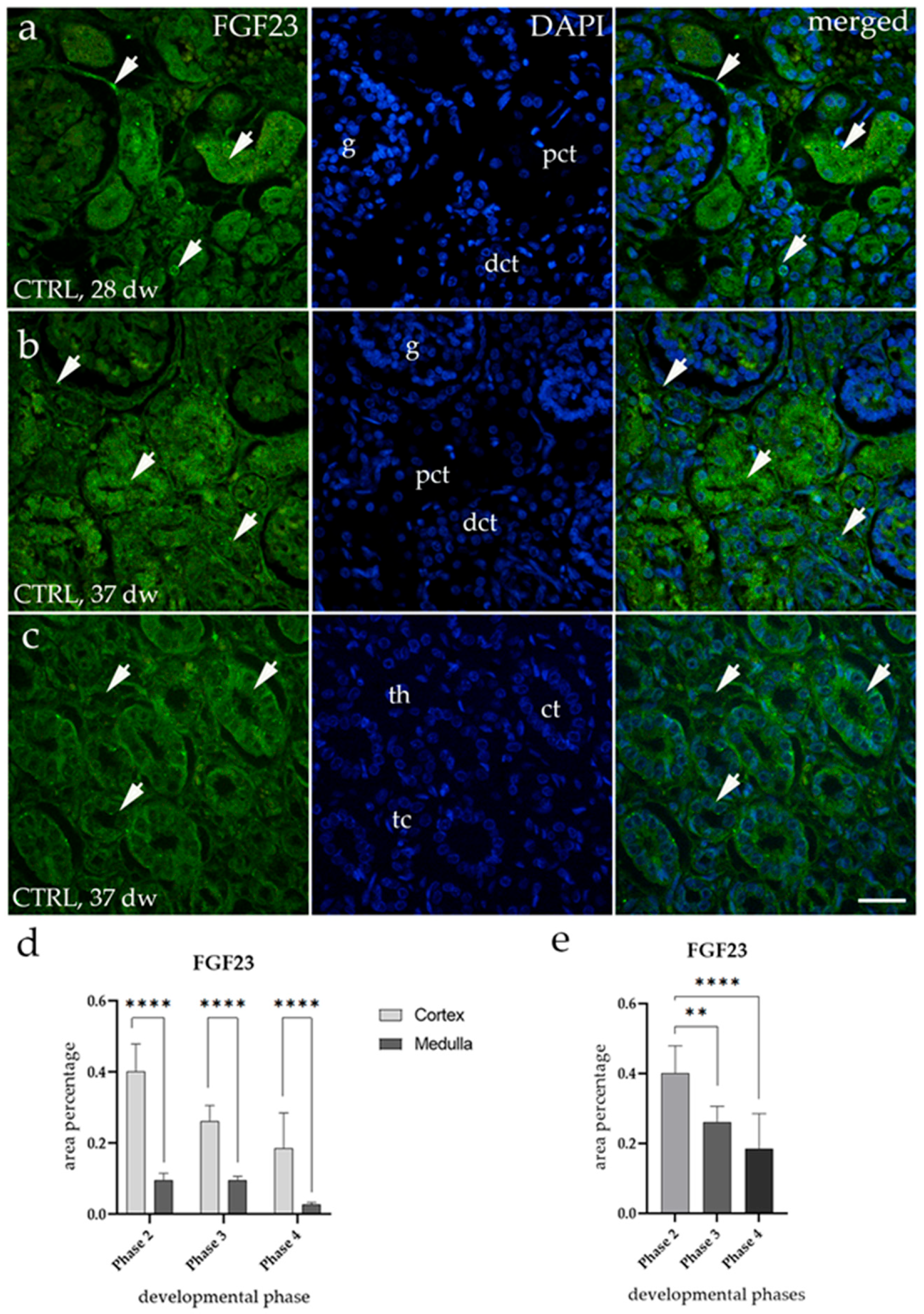
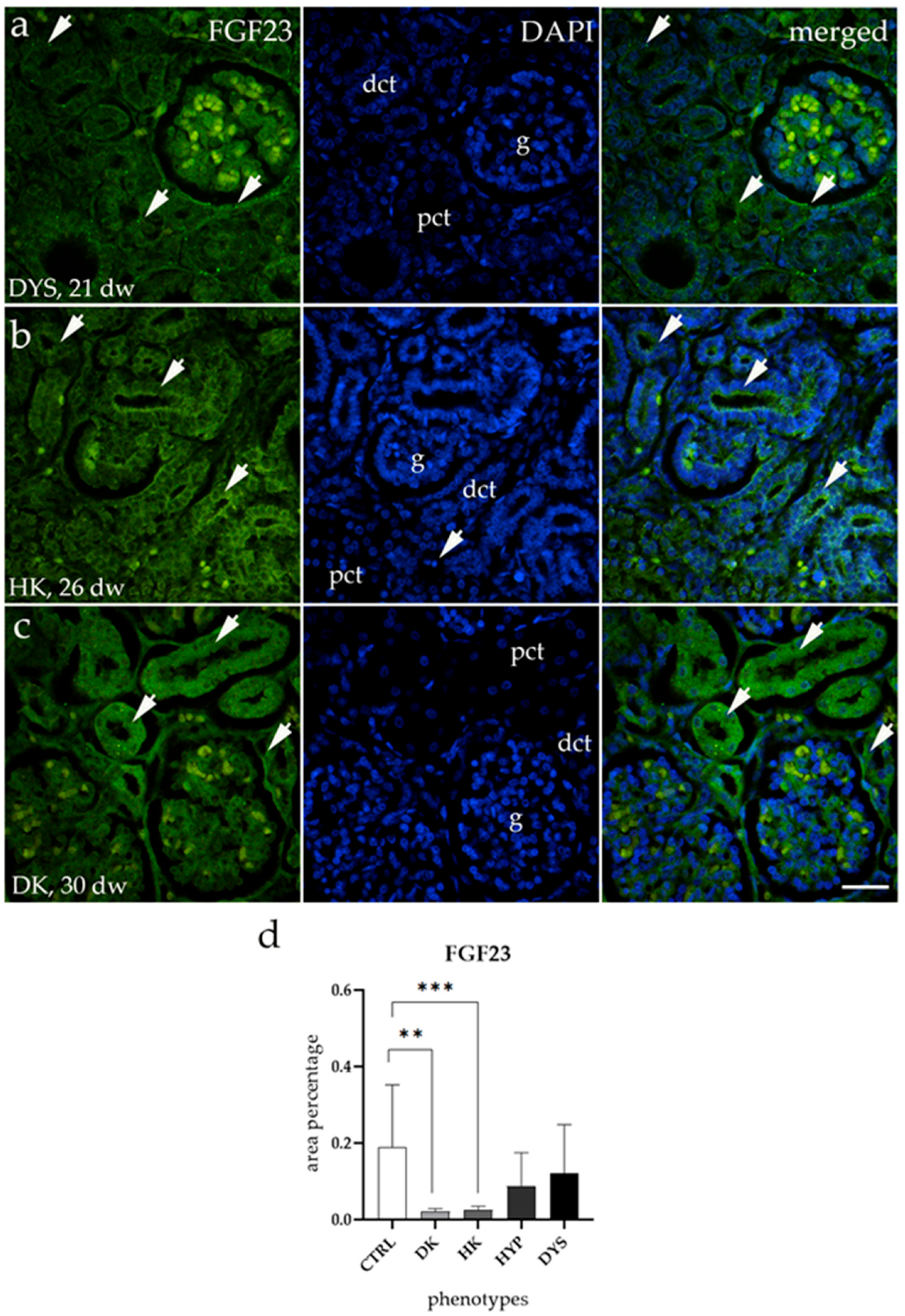
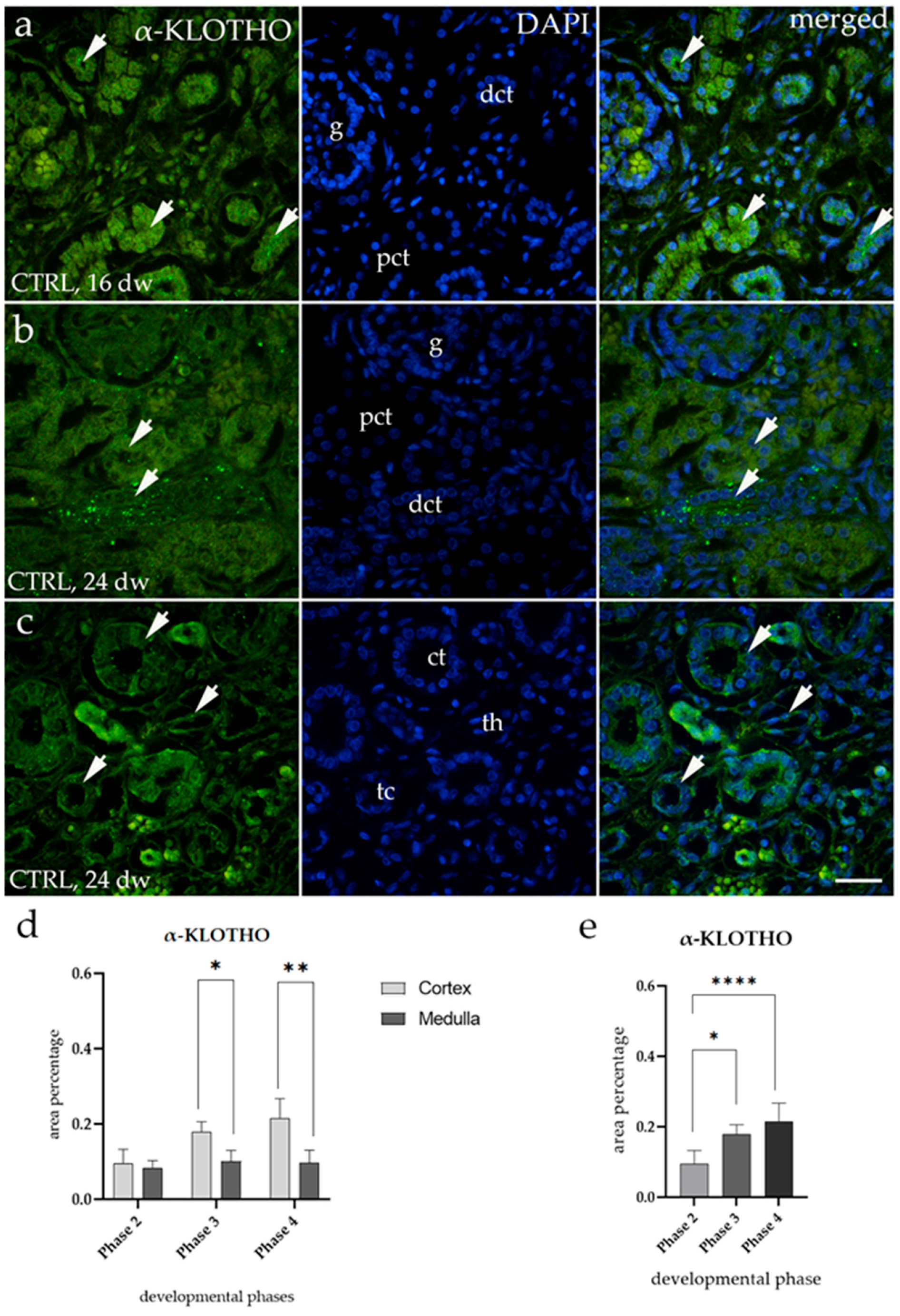
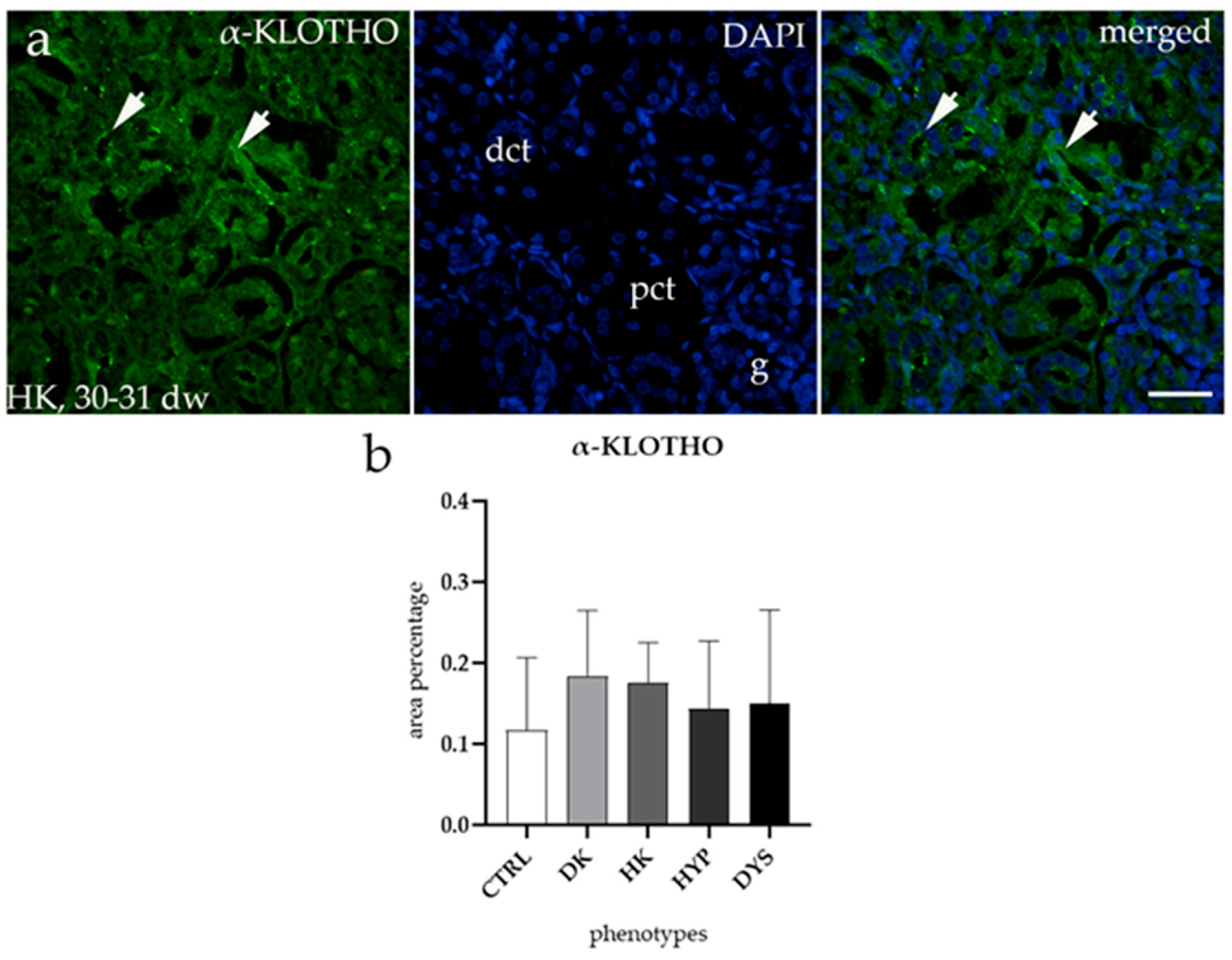
| Groups | Developmental Phase | Renal and Associated Pathology | Gestational Weeks | Number of Kidney Samples |
|---|---|---|---|---|
| Healthy kidneys (CTRLs) | Phase 2 | N/A * | 15 | 1 |
| N/A | 15–16 | 2 | ||
| N/A | 16 | 1 | ||
| N/A | 16–17 | 1 | ||
| N/A | 18 | 1 | ||
| N/A | 18 | 2 | ||
| N/A | 21 | 2 | ||
| Phase 3 | N/A | 24 | 2 | |
| N/A | 28 | 2 | ||
| N/A | 28 | 1 | ||
| N/A | 32 | 2 | ||
| N/A | 35 | 2 | ||
| Phase 4 | N/A | 37 | 2 | |
| N/A | 38 | 1 | ||
| Duplex kidneys (DKs) | Ureter duplex lateris dextri | 24 | 1 | |
| Ureter duplex sinister | 30 | 1 | ||
| Horseshoe kidneys (HKs) | Ren concreatus arcuatus, cystae multiplices corticales | 22 | 1 | |
| Ren concretus arcuatus, tetralogija Fallot | 26 | 1 | ||
| Syndroma Edwards, Ren arcuatus | 30–31 | 1 | ||
| Syndroma Edwards, Ren arcuatus | 34 | 1 | ||
| Hypoplastic kidneys (HYPs) | Hypoplasio renis | 28 | 1 | |
| Hypoplasia renis lateris dextri | 30 | 1 | ||
| Hypoplasio renis sinister | 37 | 1 | ||
| Dysplastic kidneys (DYSs) | Megaureter lateris dextri, dysplasia renalis | 21 | 1 | |
| Agenesia renis dextri, ren unilateralis | 21 | 2 | ||
| Ren sinister cysticus, ren dexter absens | 21–22 | 2 | ||
| Dysplasia mulicystica renis dextri/Cystes parvae focales | 27 | 2 | ||
| Dysplasia Hypoplastica | 33–34 | 1 | ||
| Renes dysplastici cystic, Potter syndroma | 35 | 2 | ||
| Agenesis renis dextri et dysplasia renis sinistri cum ureter duplex, Curranno sindrom | 37 | 1 | ||
| Dysplasia Hypoplastica, renalis bilateralis Down, Potter syndrome | 38 | 1 | ||
| Antibodies | Catalog Number | Host | Source | Dilution | |
|---|---|---|---|---|---|
| Primary | Anti-FGF23 antibody | AF2604 | Goat | R&D Systems (Minneapolis, MN, USA) | 1:200 |
| Anti- α-KLOTHO antibody | 28100-1-AP | Rabbit | Proteintech Group (Planegg-Martinsried, Germany) | 1:200 | |
| Secondary | Anti-Goat IgG (H + L), Alexa Fluor® 488 | 705-545-003 | Donkey | Jackson Immuno Research Laboratories, Inc. (Baltimore, PA, USA) | 1:300 |
| Anti-Rabbit lgG (H + L) Alexa Fluor®488 | 711-545-152 | Donkey | Jackson Immuno Research Laboratories, Inc., (Baltimore, PA, USA) | 1:300 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajt, P.; Racetin, A.; Kelam, N.; Pavlović, N.; Todorović, P.; Korčulanin, M.J.; Filipović, N.; Kuzmić Prusac, I.; Raguž, F.; Vukojević, K. Expression of FGF23 and α-KLOTHO in Normal Human Kidney Development and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Biomolecules 2025, 15, 811. https://doi.org/10.3390/biom15060811
Bajt P, Racetin A, Kelam N, Pavlović N, Todorović P, Korčulanin MJ, Filipović N, Kuzmić Prusac I, Raguž F, Vukojević K. Expression of FGF23 and α-KLOTHO in Normal Human Kidney Development and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Biomolecules. 2025; 15(6):811. https://doi.org/10.3390/biom15060811
Chicago/Turabian StyleBajt, Patricija, Anita Racetin, Nela Kelam, Nikola Pavlović, Petar Todorović, Marinela Jelinčić Korčulanin, Natalija Filipović, Ivana Kuzmić Prusac, Fila Raguž, and Katarina Vukojević. 2025. "Expression of FGF23 and α-KLOTHO in Normal Human Kidney Development and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)" Biomolecules 15, no. 6: 811. https://doi.org/10.3390/biom15060811
APA StyleBajt, P., Racetin, A., Kelam, N., Pavlović, N., Todorović, P., Korčulanin, M. J., Filipović, N., Kuzmić Prusac, I., Raguž, F., & Vukojević, K. (2025). Expression of FGF23 and α-KLOTHO in Normal Human Kidney Development and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Biomolecules, 15(6), 811. https://doi.org/10.3390/biom15060811








